Abstract
Objective
To report T1-2N0 tongue cancer recurrences initially treated with surgery alone.
Methods
Between 1990 and 2010, 27 patients at tertiary hospital referral center institution were treated with curative intent for locoregional recurrence after initial glossectomy with or without neck dissection for T1-2N0 tongue cancer. None had received adjuvant postoperative radiation as a component of the original treatment.
Results
Median time to locoregional recurrence was 12 months (range 5–39 months) and 78% of failures occurred in the first 2 years. Most treatment failures were local (63%). Salvage strategy was risk-adapted by individual patient. The 5-year disease specific survival (DSS) was 61%. Patients with local recurrences alone fared significantly better than those with regional recurrences (5-yr DSS: 86% vs. 22%, P = 0.0018). Local recurrences were usually treated by surgery alone, while regional recurrences were more commonly treated with combined modality treatment (P = 0.005).
Conclusions
Recurrence of early stage oral tongue cancer can be successfully salvaged in a majority of cases. Patients developing regional recurrence have significantly worse prognosis than those with local failures.
Keywords: Oral cancer, Head and neck cancer, Tongue neoplasms, Salvage therapy
Introduction
Primary surgery is the standard of care for early stage (T1-2N0-1) squamous cell carcinoma of the oral tongue.1, 2 The clinically and/or radiographically N0 neck is managed according to tumor thickness: patients with thin tumors are observed, while elective neck dissection is performed for thick tumors.3, 4, 5 Post-operatively, patients with risk factors for local-regional recurrence are recommended adjuvant radiation while those without are observed.6 The results of this management technique are effective for both Stage I/II oral tongue tumors managed either with surgery alone7 or combined modality therapy.8
Since local and regional recurrences of early oral tongue cancers are uncommon and the local recurrences are unique, management of recurrent disease is individualized. The treatment of a tongue cancer relapse is influenced by management of the original tumor. Whether the neck was dissected or observed, the administration of adjuvant radiation, the local extent of the local or regional disease, and the duration between initial treatment and diagnosis of recurrence may all affect management decisions. Thus, a detailed analysis of the salvage early oral tongue cancer failures needs a detailed analysis of the prior oral tongue cancer management.
This series examines a single institution experience with attempted salvage for isolated local-regional oral tongue cancer recurrences after initial management with surgery alone.
Materials and methods
We retrospectively identified all patients with early stage squamous cell carcinoma of the oral tongue who were treated with primary surgical resection alone from 1990 to 2010 at Fox Chase Cancer Center. Inclusion criteria were American Joint Committee and Cancer (AJCC) Stage I–II T1-2N0 squamous cell carcinoma of the oral tongue with definitive surgical resection performed at a single institution. The decision to perform glossectomy versus glossectomy/neck dissection in initial management was determined by the treating surgeon.
Patients who received post-operative radiation, had involved neck lymph nodes on pathology that were not clinically evident, and patients with involved surgical margins were considered representative of a higher risk population and were excluded from this analysis. Patient demographics, tumor characteristics, and treatment related outcomes were abstracted from the relevant medical records in accordance with a Fox Chase Cancer Center Institutional Review Board approved protocol and the Health Insurance Portability and Accountability Act.
All patients underwent glossectomy for definitive management of their presenting tongue cancer diagnosis. Patients presenting from an outside institution after a diagnostic excisional biopsy were submitted to additional wide local resection/glossectomy to confirm adequacy of resection of the lesion.
After the initial operation, patients were followed in clinic according to standard practice.9 Recurrences of squamous cancer in the tongue/floor of the mouth or neck within 5 years of the initial operation were considered recurrences. Local recurrence was defined as an oral cavity recurrence at the primary oral cavity site; regional recurrence was defined as a recurrence within the neck. Patients diagnosed with a second primary cancer of the upper aerodigestive tract during followup were excluded. Upon appreciation of local-regional recurrence, biopsy and staging studies were pursued. Patients with synchronous distant metastasis were excluded. Treatment of the recurrence was recommended following discussion at a multidisciplinary tumor board; in general salvage resection followed by risk adapted adjuvant radiation was favored. In the salvage operations for isolated local recurrence, ipsilateral neck node basins that had been managed expectantly at the initial presentation were electively dissected while those that were electively dissected at the primary presentation were observed. Isolated regional recurrence was treated with therapeutic node dissection followed by risk adapted adjuvant radiation.
Stata (College Station, TX) was used to perform statistical analysis. Univariate analysis was performed by logistic regression, and multivariate analysis was performed using a Cox proportional hazards model. Fisher's exact test was applied for 2 × 2 comparisons. Kaplan Meier curves and tables were also constructed to analyze outcomes.
Results
A total of 27 patients developed isolated locoregional recurrence. Most of the recurrences were from tumors that were originally T1N0 (81%) and 59% of the overall cohort was initially managed with glossectomy alone. The median interval to locoregional relapse was 12.4 months (range 5–39 months) and almost 78% of recurrences occurred in the first 2 years, with 44% recurring within the first year. There were no synchronous recurrences in both the tongue and neck (Table 1). Two patients had perineural invasion on final pathology but declined radiation therapy.
Table 1.
Patient demographics and treatment.
| Local recurrence (n = 17%) | Regional recurrence (n = 10%) | All patients (n = 27%) | P value | |
|---|---|---|---|---|
| Age at diagnosis (yr) | ||||
| <50 | 6 (35) | 2 (20) | 8 (30) | P = 0.67 |
| >50 | 11 (65) | 8 (80) | 19 (70) | |
| Average (yr) | 56.3 | 65.9 | 60.0 | |
| Median (yr) | 55.1 | 72.2 | 61.0 | |
| Sex | ||||
| Male | 9 (53) | 7 (70) | 16 (59) | P = 0.45 |
| Female | 8 (47) | 3 (30) | 11 (41) | |
| Smoking status | ||||
| Yes | 11 (65) | 6 (60) | 17 (63) | P = 0.69 |
| No | 5 (29) | 4 (40) | 9 (33) | |
| Unknown | 1 (6) | – | 1 (4) | |
| Initial tumor T stage | ||||
| T1 | 14 (82) | 8 (80) | 22 (81) | P = 1.00 |
| T2 | 3 (18) | 2 (20) | 5 (19) | |
| Initial treatment | ||||
| Glossectomy alone | 8 (47) | 8 (80) | 16 (59) | P = 0.12 |
| Glossectomy with neck dissection | 9 (53) | 2 (20) | 11 (41) | |
The majority of locoregional recurrences were confined to the tongue (n = 17, 63%) and a majority were managed with surgery (n = 20, 75%) followed by risk adapted therapy. Most node-negative recurrences were rT1-2 (85%) and all of these local recurrences were addressed by primary surgery.
Adjuvant therapy was determined according to accepted practice for the de novo presentation of oral tongue cancer. Tumor recurrence alone was not considered an independent indication for adjuvant radiation. Slightly more than half of the patients were treated with adjuvant therapy – radiation (45%) and chemoradiation (10%). Three patients, all with local failures, refused any therapy and were excluded from the survival analysis (Table 2).
Table 2.
Salvage therapy following local or regional recurrence.
| Surgery alone | Radiation alone | Surgery + Radiation | Chemo-radiation | Surgery + Chemoradiation | No treatment | Total | |
|---|---|---|---|---|---|---|---|
| Local recurrence | 8 | 2 | 3 | 1 | 0 | 3 | 17 |
| Regional recurrence | 1 | 0 | 6 | 1 | 2 | 0 | 10 |
| Total | 9 | 2 | 9 | 2 | 2 | 3 | 27 |
All regional recurrences had high risk features and were recommended combined modality therapy. Most regional recurrences ensued in a neck that was originally managed expectantly (n = 8, 80%); all were ipsilateral. One patient refused adjuvant radiation and was thus managed with neck dissection alone for a regional recurrence. That patient developed disease in the operated neck and succumbed to recurrent cancer.
Four patients declined surgical salvage and were treated with either radiation alone or by chemoradiation; half of these patients achieved disease control at last followup.
The 2-year and 5-year overall survival (OS) for the cohort was 83% and 50%. The 2-year and 5-year disease specific survival (DSS) for the cohort was 87% and 61%. Gender and age were significant (P = 0.05, P = 0.015 respectively) for OS they were not significant on DSS. The median follow-up for patients still alive was 61 months. Median survival after second progression was 18 months (3–39 months).
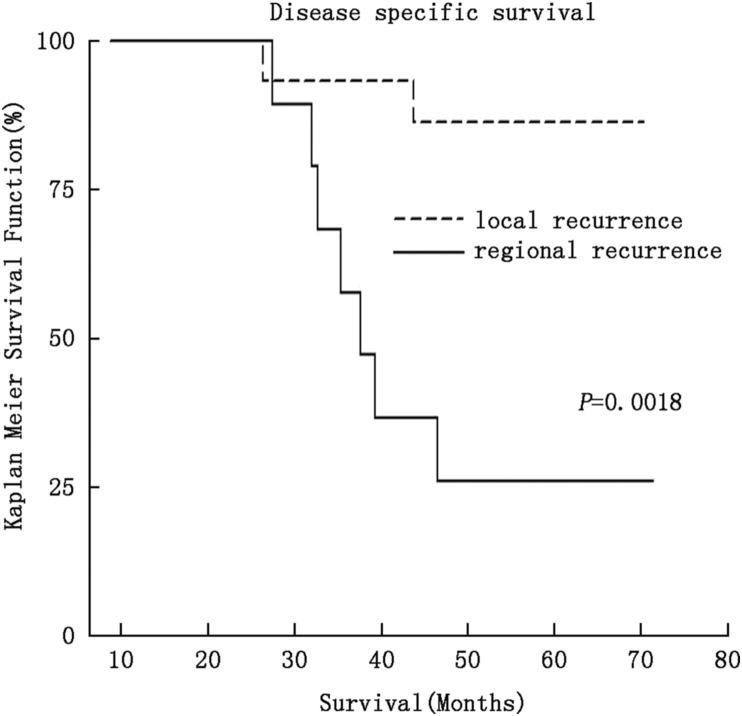
Multiple factors associated with both the original presentation of oral tongue cancer and the recurrence were evaluated for DSS. Tobacco use, initial T-stage, interval between initial cancer treatment and recurrence, and neck dissection as part initial treatment were all not significant for DSS (data not shown). The single significant prognostic factor was the type of recurrence, local vs. regional, in both univariate and multivariate analysis (Table 3). The 5 year DSS stratified by site of recurrence was 86% for local versus 22% regional (P = 0.0018) (Fig. 1). A majority of local recurrences (57%) were managed with surgery alone and a majority of patients with a localized rT1T2N0recurrence (n = 12, 71%) survived their disease. Survival following regional recurrence was poor, regardless of whether the patient was initially treated with surgery or observation of the neck (0% and 38%, respectively).
Table 3.
Univariate and Multivariate analysis for disease specific survival.
| Unadjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | |
|---|---|---|
| Under age 60 | 0.25 (0.05–1.19) | 0.41 (0.08–2.17) |
| Male gender | 0.44 (0.18–2.50) | 0.90 (0.21–3.96) |
| Local vs. regional disease | 8.50 (1.74–42.10) | 6.70 (1.30–34.40) |
The significance values are in bold.
Fig. 1.
Kaplan–Meier survival plot of disease specific survival and recurrence type.
Discussion
Surgery is the recommended treatment strategy for most early stage tongue cancer.9 Although initial treatment with an operationis often successful, isolated locoregional recurrences constitute management problems.10 This analysis reports the results from treatment of 27 patients initially treated with surgery alone at a single tertiary referral center who developed locoregional recurrence. Similar to other analyses, 80% of these recurrences occurred in the first 2 years. The reported overall survival after locoregional recurrence in such patients is approximately 20%–50%.10, 11, 12 Our 60% DSS salvage survival is slightly higher than the reported literature. This is possibly due to the high percentage of rT1T2N0 patients in our series.11
The difference between a local and regional recurrence is substantial. Local recurrences in this series had a 5-year DSS of 86% and the local recurrences who could be managed by surgery alone (57% of all failures) represented a favorable subgroup. By contrast, only 22% of patients with regional metastasis could be salvaged, despite multimodality therapy. Although the historical reports of recurrent oral tongue cancer suggest that DSS is poor,13 isolated T1-2 local recurrence can be managed successfully with single modality therapy. This supports the need for timely surveillance, especially following the first two years after resection. Routine adjuvant radiation in the salvage plan for locoregional recurrence is not supported for local favorable recurrences.
This analysis is not designed to define the benefits of staging neck dissection by comparison to an expectant approach in the initial management early stage oral cancer. The literature has reported on this question.5, 14 In this series, most regional failures developed in a nodal basin that was originally approached expectantly. The recommendation for an elective neck dissection in patients with thin and intermediate thickness lesions is complex. Thicker tongue lesions, especially those measuring more than 4 mm are at higher risk for locoregional metastasis and typically receive elective neck dissection.15 Primary tumor features like perineural invasion16 and high risk pathological islands of tongue invasion17 are more likely to undergo initial neck dissection, although sometimes these factors are not appreciated until final surgical pathology is available. A prospective study to determine whether elective neck radiation would adequately control regional nodal disease closed early due to unacceptable regional progression.18 The best strategy for considering a staging neck dissection vs. Observation for cT1-2N0 tongue cancer remains to be defined. Regardless of whether patients undergo neck dissection in initial management, the type of locoregional recurrence is the primary driver of outcome following locoregional recurrence of T1T2N0 oral cavity cancer.
Conclusion
Locoregional recurrence following treatment of Stage I–II tongue cancer by surgery alone poses a treatment challenge. With appropriate risk-adapted therapy approximately 60% of patients will survive the disease. Local recurrences are significantly easier to salvage than regional recurrences, arguing for the value of careful close surveillance. Regional recurrences have poor outcomes despite multi-modality therapy.
Conflict of interest
No conflict of interest or disclosures for any authors.
Edited by Jing Li
Footnotes
Presented as a poster at the American Radium Society, April 27-May 1, 2014. St Thomas.
Peer review under responsibility of Chinese Medical Association.
References
- 1.Ganly I., Patel S., Shah J. Early stage squamous cell cancer of the oral tongue–clinicopathologic features affecting outcome. Cancer. 2012;118:101–111. doi: 10.1002/cncr.26229. [DOI] [PubMed] [Google Scholar]
- 2.Preis M., Hadar T., Soudry E. Early tongue carcinoma: analysis of failure. Head Neck. 2012;34:418–421. doi: 10.1002/hed.21754. [DOI] [PubMed] [Google Scholar]
- 3.Fukano H., Matsuura H., Hasegawa Y., Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck. 1997;19:205–210. doi: 10.1002/(sici)1097-0347(199705)19:3<205::aid-hed7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Matsuura K., Hirokawa Y., Fujita M., Akagi Y., Ito K. Treatment results of stage I and II oral tongue cancer with interstitial brachytherapy: maximum tumor thickness is prognostic of nodal metastasis. Int J Radiat Oncol Biol Phys. 1998;40:535–539. doi: 10.1016/s0360-3016(97)00811-0. [DOI] [PubMed] [Google Scholar]
- 5.Yuen A.P., Ho C.M., Chow T.L. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck. 2009;31:765–772. doi: 10.1002/hed.21033. [DOI] [PubMed] [Google Scholar]
- 6.Hinerman R.W., Mendenhall W.M., Morris C.G., Amdur R.J., Werning J.W., Villaret D.B. Postoperative irradiation for squamous cell carcinoma of the oral cavity: 35-year experience. Head Neck. 2004;26:984–994. doi: 10.1002/hed.20091. [DOI] [PubMed] [Google Scholar]
- 7.Ganly I., Goldstein D., Carlson D.L. Long-term regional control and survival in patients with “low-risk”, early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: the importance of tumor thickness. Cancer. 2013;119:1168–1176. doi: 10.1002/cncr.27872. [DOI] [PubMed] [Google Scholar]
- 8.Iyer N.G., Kim L., Nixon I.J. Outcome of patients with early T1 and T2 squamous cell carcinoma of the base of tongue managed by conventional surgery with adjuvant postoperative radiation. Head Neck. 2013;35:999–1006. doi: 10.1002/hed.23071. [DOI] [PubMed] [Google Scholar]
- 9.NCCN Guidelines [NCCN Guidelines]. Available at: http://www.nccn.org/. Accessed 01.01.15.
- 10.Kernohan M.D., Clark J.R., Gao K., Ebrahimi A., Milross C.G. Predicting the prognosis of oral squamous cell carcinoma after first recurrence. Arch Otolaryngol Head Neck Surg. 2010;136:1235–1239. doi: 10.1001/archoto.2010.214. [DOI] [PubMed] [Google Scholar]
- 11.Kowalski L.P. Results of salvage treatment of the neck in patients with oral cancer. Arch Otolaryngol Head Neck Surg. 2002;128:58–62. doi: 10.1001/archotol.128.1.58. [DOI] [PubMed] [Google Scholar]
- 12.Ord R.A., Kolokythas A., Reynolds M.A. Surgical salvage for local and regional recurrence in oral cancer. J Oral Maxillofac Surg. 2006;64:1409–1414. doi: 10.1016/j.joms.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin W.J., Jr. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. 2000;110:1–18. doi: 10.1097/00005537-200003001-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kelner N., Vartanian J.G., Pinto C.A., Coutinho-Camillo C.M., Kowalski L.P. Does elective neck dissection in T1/T2 carcinoma of the oral tongue and floor of the mouth influence recurrence and survival rates? Br J Oral Maxillofac Surg. 2014;52:590–597. doi: 10.1016/j.bjoms.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Huang S.H., Hwang D., Lockwood G., Goldstein D.P., O'Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. 2009;115:1489–1497. doi: 10.1002/cncr.24161. [DOI] [PubMed] [Google Scholar]
- 16.Chinn S.B., Spector M.E., Bellile E.L. Impact of perineural invasion in the pathologically N0 neck in oral cavity squamous cell carcinoma. Otolaryngol Head Neck Surg. 2013;149:893–899. doi: 10.1177/0194599813506867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandwein-Gensler M., Teixeira M.S., Lewis C.M. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 18.Brennan S., Corry J., Kleid S. Prospective trial to evaluate staged neck dissection or elective neck radiotherapy in patients with CT-staged T1-2 N0 squamous cell carcinoma of the oral tongue. Head Neck. 2010;32:191–198. doi: 10.1002/hed.21167. [DOI] [PubMed] [Google Scholar]