Abstract
Objective
To explore the feasibility of the submental island flap in the repair of hypopharyngeal defects.
Methods
We collected wet specimens of fresh cadaveric heads from the Han Chinese adult population for applied anatomy of the submental island flap, and followed five patients with pyriform sinus carcinoma after reconstruction surgery using submental island flaps.
Results
We found that the average length and width of the submental island flaps were (65.20 ± 11.69) mm and (46.70 ± 6.59) mm, respectively. The skin flap in all five patients survived after surgery, and tracheal tubes and gastric tubes were removed 7–36 days after surgery. Patients were followed up for 24–42 months, pharyngeal flaps grew well, and speech and swallowing functions were satisfactory.
Conclusion
The submental island flap is a preferred material for the repair of hypopharyngeal defects after hypopharyngeal carcinoma resection, because of good blood supply, easy harvesting, and high survival rate.
Keywords: Submental island flap, Submental artery, Submental vein, Hypopharyngeal neoplasms, Reconstructive surgical procedures
Introduction
Maintenance and reconstruction of function are hot topics in head and neck surgery, especially functional reconstruction following the resection of head and neck malignant tumors, such as laryngeal carcinoma and hypopharyngeal carcinoma. Martin et al.1 first reported the advantages of the submental island flap applied for postoperative repair of orofacial defects, with respect to highly consistent color and flexibility compared with the head and neck skin, simple harvesting, high survival rate, easy suturing at the donor site, and small scars. With advances in anatomy and surgical techniques, submental island flaps have been increasingly used to repair various types of head and neck defects. However, current clinical research focuses on the use of flaps in maxillofacial defects, and to our knowledge, there has been no systematic study addressing repair and reconstruction in laryngeal and hypopharyngeal defects. We studied the local anatomy of the submental artery and accompanying veins, observed vascular paths and possible variations, as well as links with adjacent organs, to explore the dominant region supplied by the submental artery and investigate the feasibility of the submental island flap in the repair of hypopharyngeal defects after radical resection of hypopharyngeal carcinoma under the premise of maintaining laryngeal function.
Materials and methods
Applied anatomy of the submental island flap
Path of the submental artery and accompanying veins
Ten head specimens (20 sides) were collected from Han adult cadavers. The arterial system was perfused with red dye emulsion (Fig. 1), while the venous system was infused with blue dye emulsion (Fig. 2). The specimens were then subjected to gross observation and microscopic anatomy. Measurement parameters included the initial diameter of the submental artery and accompanying veins, the length of the submental artery (Fig. 3), and the correlation with the primary body surface landmarks.
Fig. 1.

Submental artery (red) after perfusion.
Fig. 2.

Submental vein (blue) accompanying the submental artery.
Fig. 3.

Submental artery branches.
Dominant perfusion area of the submental artery
Five head specimens (10 sides) were collected from Han adult cadavers. The submental artery was dissected and perfused with black ink via the artery to observe the staining scope of the submental skin. The maximal length and width of the submental island flap were measured. Surgical procedures were simulated, as follows, briefly: the specimens were cut along the stained edges, and separated from the beginning of the submental artery to the external carotid artery, then the maximal length of the vascular pedicle was measured, and the anatomic range of the flap covered was assessed.
Clinical application of the submental island flap in the repair of hypopharyngeal defects
Patient demographics and clinical information
Five patients (Table 1) were males aged 49–70 years, with a mean of 61.2 years. Three cases were over 60 years of age, and the tumor originated in the lateral wall of the unilateral pyriform fossa, involving the ipsilateral half of the larynx. The vocal cords were fixed and the ipsilateral pyriform tip was uninvolved; no abnormalities were found by esophageal lipiodol radiography. The preoperative pathology report suggested T3N1M0 stage squamous cell carcinoma. One 53-year-old patient was diagnosed as having T4N1M0 stage squamous cell carcinoma of the lateral wall of the pyriform fossa unilaterally, affecting the cervical esophagus. In these four patients, lymph nodes (diameter < 3 cm) were palpable in the ipsilateral neck and none received other treatment before surgery. Another patient (aged 49 years) underwent unilateral pyriform sinus carcinoma resection and local mucosal repair for anastomotic stenosis. All of the involved patients received chest X-ray and abdominal ultrasound, and the findings revealed no metastases. Surgery was performed between February 2011 and August 2012, with written informed consent obtained from all patients. The research protocol was approved by our institutional review board.
Table 1.
Clinical information of cases undergoing hypopharyngeal defect repair using a submental island flap.
| No. Gender | Age (years) | TNM stage | Flap size (cm × cm) | Flap survival | Recovery time of deglutition (days) | Operative complications | Follow-up time (months) |
|---|---|---|---|---|---|---|---|
| 1 male | 49 | – | 7 × 3 | Survived | 7 | None | 42 |
| 2 male | 70 | T3N1M0 | 7 × 4 | Survived | 10 | None | 39 |
| 3 male | 70 | T3N1M0 | 7 × 5 | Survived | 36 | None | 36 |
| 4 male | 53 | T4N1M0 | 8 × 5 | Survived | 15 | None | 32 |
| 5 male | 61 | T3N1M0 | 7 × 5 | Survived | 14 | None | 24 |
Surgical procedure
All patients underwent unilateral or bilateral selective neck dissection (regions II, III, IV, V) under general anesthesia following prophylactic tracheotomy. Three cases with a fixed ipsilateral vocal cord underwent laryngofission, with vertical hemilaryngectomy and complete ipsilateral pyriform fossa resection 2 cm away from the carcinoma. One patient with cervical esophageal invasion underwent the removal of both the pyriform sinus unilaterally, and the cervical esophageal lateral wall (approximately 2 cm), leaving the larynx intact. Intraoperative pathology consultation revealed negative margins.
Based on the result of pinch test and the lesion of the primary tumor, the incisions of the submental island flap were designed and marked (Fig. 4). An appropriately-sized submental island flap was selected for the repair, which was determined by the scope of the tissue defects (Fig. 5). The flap size ranged from 7 cm × 4 cm–8 cm × 5 cm. In another case, anastomotic stenosis was observed following pyriform sinus carcinoma resection, which was repaired using a submental island flap (7 cm × 3 cm) trimmed with a lingual contour.
Fig. 4.
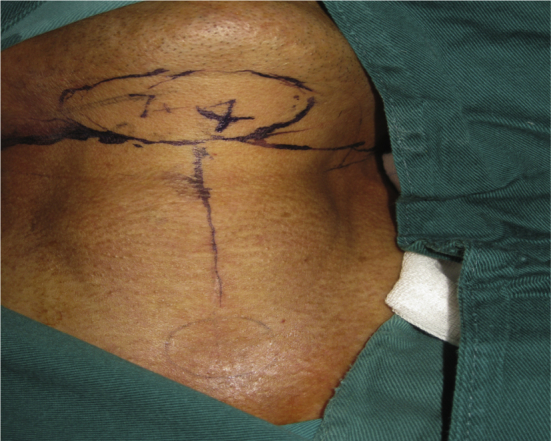
Design of the submental island flap.
Fig. 5.

Tissue defects (blue arrow) after resection of the hypopharyngeal lesion.
When the flap was produced, the facial artery on the affected side was dissected free to the submental artery bifurcation, then the distal end of the facial artery was ligated. The flap was then revised accordingly, parallel with the inferior border of the mandible and the free edge of the skin flap. The skin, subcutaneous tissue, and platysma were then dissected, and the skin and platysma were sutured in an interrupted pattern to prevent skin flap slippage. The flap below the platysma was left free to protect the marginal mandibular branch of the facial nerve traveling toward the pedicle with the anterior belly of the digastric muscle. The mylohyoid muscle branch, mandibular periosteal branch, and submandibular gland branch of the submental artery were ligated. The submandibular gland was dissected and left free. The vascular pedicle (Fig. 6) could then be separated to the origin of the facial artery, if necessary. The skin flap (Fig. 7) was extended downward to the hypopharyngeal defect and sutured in an interrupted pattern. Finally, the pharyngeal cavity was closed after placing an indwelling stomach tube. The skin at the donor site was sutured directly. Adjuvant radiotherapy at a dose of 65 Gy was begun 6 weeks after surgery.
Fig. 6.

The pedicle (blue arrow) of the submental flap.
Fig. 7.

Skin flap (blue arrow) for the repair of tissue defects.
Results
Applied anatomy
The submental artery originated from the facial artery, with a length ranging from 58.0 to 72.2 mm [mean, (69.10 ± 7.47) mm]. The diameter of the artery where it originated from the facial artery ranged from 1.0 to 2.2 mm [mean, (1.60 ± 0.47) mm] and there were 5–8 submental artery branches (Fig. 3), with a mean of (6.50 ± 1.09) mm branches. The majority of the branches were thick and responsible for supplying the anterior belly of the digastric muscle, and had a diameter of 0.8–1.6 mm [mean, (1.10 ± 0.25) mm]. The distance from the inferior border of the mandible to the submental artery was 2.2–10.7 mm (mean, 5.4 ± 1.63 mm) and among the specimens, only one submental vein was accompanied by a submental artery, which was returned to the facial veins. The initial diameter of the vein ranged from 1.6 to 2.9 mm [mean, (2.30 ± 0.58) mm], and the distance from the inferior border of the mandible to the submental vein was 5.0–22.1 mm [mean, (15.40 ± 3.29) mm].
After fresh cadaver specimens were stained with black dye via the submental artery, the skin staining showed significant differences. The staining length of the submental island flap ranged 52.5–84.6 mm [mean, (65.20 ± 11.69) mm], and the staining width ranged from 35.9 to 53.5 mm [mean, (46.70 ± 6.59) mm].
Clinical application
Five patients achieved successful surgical results: the submental island flap survived with no local necrosis or infection, and the incision at the donor site healed without complications. Patients restarted oral feeding 7 days after surgery, but with varying degrees of aspiration. Swallowing and vocal functions were restored at 7–36 days, respectively, at which time the gastric tube and tracheal cannula were disconnected. Patients were rechecked by laryngoscope 1 month postoperatively, and the findings revealed that the flaps were pale and the flap in one patient had a small amount of hair (Fig. 8). Laryngoscopy at 3 months showed that the flap color was similar to the mucous membranes and that there was basically no hair. Patients were followed up for 24–42 months (Table 1), with no local recurrences or complications. The pharyngeal flaps survived and grew well, speech and swallowing functions were satisfactory, but patients' voices were hoarse. There was no visible neck scar.
Fig. 8.

Laryngoscopic view of the flap (blue arrow) one month after surgery.
Discussion
Because of the hidden location of the hypopharynx, early hypopharyngeal carcinoma has no characteristic symptoms and is difficult to diagnose. The carcinoma may have already affected the cervical esophagus when symptoms are noted, and the 5-year survival rate is only 15%–47%.2 It is reported that wide resection of the hypopharynx and cervical esophagus, together with postoperative chemoradiation, could prolong 5-year survival rates. However, resection also produces speech and swallowing dysfunction, seriously affecting patients' quality of life. Therefore, adequate repair of tissue defects after hypopharyngeal resection is crucial, because it requires both morphological restoration and functional reconstruction. The challenges facing head and neck surgeons treating these patients are repairing the laryngeal and hypopharyngeal defects after surgery, and restoring swallowing and speech function.
Martin et al.1 first proposed the use of a submental island flap in the reconstruction of orofacial defects in 1993. Submental island flap is an axial skin flap pedicled on the submental artery and vein. The submental artery is a constant branch of the facial artery3 with a length of approximately 6–7 cm and evidence4, 5 has shown that, from its origin in the facial artery, the submental artery travels forward between the two lobes of the submaxillary gland or between the submandibular gland and the mandible, entering the deep (70%) or superficial layer (30%) of the anterior belly of the digastric muscle, then piercing the deep fascia and entering the skin. The initial diameter of the submental artery is 1.0–2.0 mm (mean, 1.7 mm), and it elicits several branches to supply the lower lip, submandibular gland, platysma, the anterior belly of the digastric muscle, as well as 1–4 perforating branches. The terminal branches coincide with the contralateral branches, and supply the upper neck skin. The venous diameter of the submental island flap vein ranges from 1.0 to 2.9 mm (mean, 2.2 mm). The submental venous system is often classified into two groups: 1) tight accompanying: the veins accompany the submental artery with reflux into the facial vein; 2) non-tight accompanying: the veins are located superficially, and share 2–3 communicating branches with tight accompanying veins, then flow into the anterior jugular vein. Our anatomical findings showed that the submental artery provided the blood supply to the submental island flap, and it was located in a constant position. The average length was 69.1 mm; and the average diameter of the arteries originating from the facial artery was 1.6 mm, with 6.5 branches on average, and 5.4 mm away from the inferior border of the mandible. When a submental vein accompanied the submental artery, it returned to the facial veins, and had an average diameter of 2.3 mm, and an average distance from the inferior border of the mandible of 15.4 mm. Our findings were consistent with previous studies.
The size of the submental island flap varies greatly and is maximized at 18 cm × 7 cm, with a vascular pedicle length of up to 8 cm.6 If dissected to its origin in the facial artery, the pedicle can be extended 1–2 cm, reaching the canthus and the zygomatic arch, which provides the possibility of repairing defects in the upper face and the lower neck.7 The present study showed that the length and width of the stained submental island flap were 65.2 mm and 46.7 mm, respectively, and the maximum size was 84.6 mm × 53.5 mm, which differed from previously reported flap sizes.8 The difference largely depends on the population race and the embolism of terminal branches of the submental artery and vein, which may affect the traveling of dyes. A larger number of cadaver specimens is needed for further observation and discussion.
Sebastian et al.9 described a method of harvesting submental island flaps by isolating from the free edge of the flap toward the pedicle. We modified this method by first dissecting the facial artery and vein to the submental artery and vein, and cutting the skin flap after ensuring arterial supply and venous return. This modification can ensure the survival of the flap. Our anatomical results showed that there were many thick submental artery branches involving the anterior belly of the digastric muscle, with an average diameter of 1.1 mm; therefore, we recommend removing the anterior belly of the digastric muscle when cutting the flaps to effectively guarantee flap blood supply.
Restoration of speech and swallowing after hypopharyngeal surgery is currently a crucial challenge for head and neck surgeons. Lee et al.10 achieved success repairing hypopharyngeal defects using a submental island flap in six cases, but all patients underwent total laryngectomy, which seriously affected the patients' quality of life. In our study, the size of the skin flap was adjusted according to intraoperative observation, ranging from 7 cm × 3 cm–8 cm × 5 cm, the skin incision at the donor site was successfully sutured, and the scars were concealed. We speculate that laryngeal function can be preserved in the majority of T3, and even in T4 pyriform sinus carcinomas. The patients in our study completely or partially retained their laryngeal function, which significantly improved their quality of life after surgery. Although there is a risk of aspiration, tracheal tubes and nasogastric feeding tubes can be successfully removed as patients gradually recover swallowing function.
Previous reports11, 12, 13 summarized the criteria for repairing hypopharyngeal and cervical esophageal defects: 1) ensure adequate safe margins; 2) achieve first-stage reconstruction; 3) small scar at donor site; 4) high success rate; 5) short operation time; 6) low incidence of stenosis and pharyngeal fistula; and 7) physicians experienced in treating complications. Free flaps have been considered the preferred method for repairing tissue defects after resection of hypopharyngeal tumors. With advances in microvascular surgical techniques, this flap has become widely used. Recently, Paydarfar et al.14 compared submental island pedicled flaps and radial forearm free flaps and found that there was no significant difference between the two flaps in the restoration of swallowing and speech functions as well as in local recurrence rate after the surgical repair of oral defects, but that the submental island flap effectively shortened operation time and postoperative hospital stay. The submental island flap has a rich blood supply, survives well, and produces an invisible scar; while a forearm free flap produces visible scars at the donor site, which then require free skin grafts. Another consideration is that the metastasis rate of submental and submandibular lymph nodes is very low in patients with hypopharyngeal carcinoma, and the donor site of the submental island flap is in the non-lymphatic drainage area; therefore, the donor site can be simultaneously removed with cervical gland dissection, if necessary. As a disadvantage, submental island flaps have a smaller dominant area (36 cm2) than forearm free flaps (50 cm2), so they cannot be used to treat circumferential or near-circumferential defects in the hypopharynx. Similarly, submental island flaps are not ideal for patients who undergo local radiation therapy before surgery.
In summary, the submental island flap is an optimal choice for hypopharyngeal defects following tumor resection, because of the abundant blood supply, ease of harvest, high survival rate, unique flexibility, and advantage in retaining laryngeal function. The limitations of our study are the small sample size and short-term follow-up visits, and the lack of clinical observation for long-term effects.
Conflicts of interest
All authors have none to declare.
Acknowledgments
This work was supported by grants from the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231801), and the Jiangsu Provincial Department of Health (XK200719, H201003 and RC2011071), the People's Republic of China.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Martin D., Pascal J., Baudet J. The submental island flap: a new donor site. Anatomy and clinical applications as a free or pedicled flap. Plast Reconstr Surg. 1993;92:867–873. [PubMed] [Google Scholar]
- 2.Keereweer S., de Wilt J.H., Sewnaik A. Early and long-term morbidity after total laryngopharyngectomy. Head Neck. 2010;267:1437–1444. doi: 10.1007/s00405-010-1244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magden O., Edizer M., Tayfur V. Anatomic study of the vasculature of the submental artery flap. Plast Reconstr Surg. 2004;114:1719–1723. doi: 10.1097/01.prs.0000142479.52061.7d. [DOI] [PubMed] [Google Scholar]
- 4.Multinu A., Ferrari S., Bianchi B. The submental island flap in head and neck reconstruction. Int J Oral Maxillofac Surg. 2007;36:716–720. doi: 10.1016/j.ijom.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Tan O., Atik B., Parmaksizoglu D. Soft-tissue augmentation of the middle and lower face using the deepithelialized submental flap. Plast Reconstr Surg. 2007;119:873–879. doi: 10.1097/01.prs.0000252002.76466.cf. [DOI] [PubMed] [Google Scholar]
- 6.Genden E.M., Buchbinder D., Urken M.L. The submental island flap for palatal reconstruction: a novel technique. J Oral Maxillofac Surg. 2004;62:387–390. doi: 10.1016/j.joms.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Akan I., Ozdemir R., Uysal A. The submental artery flap. Eur J Plast Surg. 2001;24:134–139. [Google Scholar]
- 8.Li G.P., Li X.J., Sui J. Applied anatomy study on the reversed submental island flap. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;42:268–272. [PubMed] [Google Scholar]
- 9.Sebastian P., Thomas S., Varghese B.T. The submental island flap for reconstruction of intraoral defects in oral cancer patients. Oral Oncol. 2008;44:1014–1018. doi: 10.1016/j.oraloncology.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.C., Chu Y.H., Lin Y.S. Reconstruction of hypopharyngeal defects with submental flap after laryngopharyngectomy. Eur Arch Otorhinolaryngol. 2013;270:319–323. doi: 10.1007/s00405-012-2033-4. [DOI] [PubMed] [Google Scholar]
- 11.Couch M.E. Laryngopharyngectomy with reconstruction. Otolaryngol Clin North Am. 2002;35:1097–1114. doi: 10.1016/s0030-6665(02)00034-8. [DOI] [PubMed] [Google Scholar]
- 12.Murray D.J., Gilbert R.W., Vesely M.J. Functional outcomes and donor site morbidity following circumferential pharyngoesophageal reconstruction using an anterolateral thigh flap and salivary bypass tube. Head Neck. 2007;29:147–154. doi: 10.1002/hed.20489. [DOI] [PubMed] [Google Scholar]
- 13.Xuwei D., Jian X., Xueqin L. The reconstruction of head and neck defects with the submental island flap. Head Neck Oncol. 2013;5:19. [Google Scholar]
- 14.Paydarfar J.A., Patel U.A. Submental island pedicled flap vs radial forearm free flap for oral reconstruction: comparison of outcomes. Arch Otolaryngol Head Neck Surg. 2011;137:82–87. doi: 10.1001/archoto.2010.204. [DOI] [PubMed] [Google Scholar]


