Abstract
Objective
To evaluate incidence of interscalar excursions between round window (RW) and cochleostomy approaches for cochlear implant (CI) insertion.
Methods
This was a retrospective case-comparison. Flat-panel CT (FPCT) scans for 8 CI users with Med-El standard length electrode arrays were collected. Surgical technique was identified by a combination of operative notes and FPCT imaging. Four cochleae underwent round window insertion and 4 cochleae underwent cochleostomy approaches anterior and inferior to the round window.
Results
In our pilot study, cochleostomy approaches were associated with a higher likelihood of interscalar excursion. Within the cochleostomy group, we found 29% of electrode contacts (14 of 48 electrodes) to be outside the scala tympani. On the other hand, 8.5% of the electrode contacts (4 of 47 electrodes) in the round window insertion group were extra-scalar to the scala tympani. These displacements occurred at a mean angle of occurrence of 364° ± 133°, near the apex of the cochlea. Round window electrode displacements tend to localize at angle of occurrences of 400° or greater. Cochleostomy electrodes occurred at an angle of occurrence of 19°–490°.
Conclusions
Currently, the optimal surgical approach for standard CI electrode insertion is highly debated, to a certain extent due to a lack of post-operative assessment of intracochlear electrode contact. Based on our preliminary findings, cochleostomy approach is associated with an increased likelihood of interscalar excursions, and these findings should be further evaluated with future prospective studies.
Keywords: Cochlear implantation, Round window insertion, Cochleostomy, Interscalar excursion, Electrode position, Flat-panel computed tomography, Surgical approach
Introduction
Cochlear implantation is a common surgical procedure used to restore speech perception in adults and children with severe-to-profound hearing loss. The internal component of the surgically implanted device is comprised of a processor and electrode array and works by replacing hair cell function with electrical impulses.
It has been established that one of the main determinants of successful audiological outcome is minimal interscalar excursions.1 In fact, when cochlear implantation was first offered for the hard of hearing, surgical eligibility was limited to individuals with total deafness because this procedure frequently destroyed any remaining residual hearing. Over time, surgical technique has been refined to minimize intracochlear trauma and to optimize placement of electrode contacts within the scala tympani with respect to spiral ganglion neurons. This emphasis on soft surgical technique focuses on preserving residual hearing in the cochlear apex by modifying various components of the operation such as blood and bone dust entry, steroid use, surgical site of insertion, perilymph leakage and suctioning, and depth of insertion.2 Atraumatic surgery has since been of importance to the scientific and medical community as developments in cochlear implant (CI) models (e.g. mid-scala CIs) are increasingly focused on reducing intracochlear trauma.
Round window insertion (RW) and cochleostomy approaches are the two of the most common surgical techniques employed in cochlear implantation (CI). These two approaches are often used interchangeably by otologic surgeons, which may be in part due to a lack of literature comparing these two techniques. It has been previously proposed that cochleostomy approaches may reduce disruption to intracochlear fluid dynamics and the cochlear aqueduct2 and increase electrode contact distance to the osseous spiral lamina and membranes. However, there has been a growing trend towards round window insertion3, 4, 5 in favor over cochleostomy approaches, with reports of less traumatic insertions in cadaveric dissection studies.4, 5
A significant challenge otologic surgeons face at the time of cochlear implantation is the lack of electrode array visualization, which undermines knowledge of final electrode contact position. Standard imaging modalities, such as multislice computed tomography (MSCT), have also been rendered useless in visualizing the electrode array in CI users due to the significant metallic artifact from the electrode contacts. Flat-panel computed tomography (FPCT) is a new imaging technique that provides high resolution images of CI electrode arrays in vivo by overcoming the temporal bone attenuation and metallic noise seen in traditional CT imaging of electrode position.6, 7 The degree of quality is so significant that individual electrode contacts can be delineated, thus permitting assessment of final electrode position.8 In this study, we used high-resolution FPCT imaging to evaluate the impact of cochleostomy approaches and round window insertion on CI electrode interscalar excursion.
Material and methods
Eight subjects underwent previous cochlear implantation with Med-El standard 12-electrode contact arrays (31.5 mm linear insertion length, 2.4 mm between contacts). We evaluated 2 males and 6 females with a mean age of 52 years (range: 21–64 years). One of the research participants was a bilateral CI user. Details regarding the demographics of our research participants can be found in Table 1. A standard posterior tympanotomy approach was used for all cases. The surgical insertion technique was identified using a combination of operative notes and computed tomography visualization in the coronal oblique, sagittal oblique, and axial oblique sections. Implantation approach varied between pure round window (RW) insertions and cochleostomies (COCH) anterior and inferior to the RW. In this study, the term ‘cochleostomy’ refers to a separate opening into the cochlea and not an extension of the round window. The local institutional review board approved the study protocol, and we obtained written informed consent from all participants.
Table 1.
Demographic information for participants with cochlear implants.
| Subject | Sex | Age | Etiology | Implant device | Implant side | RW insertion |
|---|---|---|---|---|---|---|
| COCH.1 | F | 60 | Idiopathic | Med-El Sonata TI100a | Right | COCH |
| COCH.2 | F | 21 | Congenital | Med-El Sonata TI100a | Left | COCH |
| COCH.3 | M | 55 | Anoxic | Med-El Concerto Mi1000a | Right | COCH |
| COCH.4 | F | 54 | Idiopathic | Med-El Concerto Mi1000a | Left | COCH |
| RW.1 | F | 50 | Congenital | Med-El Concerto Mi1000a | Leftb | RW |
| RW.2 | F | 51 | Idiopathic | Med-El Concerto Mi1000a | Right | RW |
| RW.3 | M | 61 | Idiopathic | Med-El Sonata TI100a | Left | RW |
| RW.4 | F | 64 | Antibiotics | Med-El Concerto Mi1000a | Right | RW |
Note: This table summarizes the demographic information for all 8 cochlear implant users with including sex, age, etiology of hearing loss, type of CI, and laterality. Abbreviations: RW, Round window approach; COCH, Cochleostomy approach.
Standard array.
Subject with bilateral CIs.
FPCT datasets were collected between January and August 2013.7 Participants had their FPCT (DynaCT; Siemens, Erlangen, Germany) scans taken on a flat-panel angiography system (Axiom Artis Zee; Siemens) with commercially available software (Syngo DynaCT; Siemens). Collimated 20-s head FPCT scans were taken using the following parameters: 109 kV, small focus, 200° rotation angle, and 0.4° per frame angulation step. A commercially available workstation (Leonardo DynaCT InSpace 3D Software; Siemens) was used for post-processing. High resolution secondary reconstructions were created using the following parameters: manually generated voxels of interest to include only the electrode array; voxel size of 0.07–0.08 mm with the creation of secondary reconstructions, 512 × 512 section matrix; HU kernel types; and normal, auto, and sharp image characteristics. DICOM data processing was performed using open source imaging software for Mac OSX (OsiriX; Pixmeo, Los Angeles, California). We used a curved multi-planar reconstruction (MPR) analysis to visualize and reformat the three dimensional dataset. This platform provided three orthogonal views of the cochlea and the electrode array in the sagittal oblique, coronal oblique, and axial oblique sections.
We conducted all image analyses in a blinded fashion with respect to insertion approaches. For the purposes of this study, we developed standardized steps to evaluate electrode position within the boney labyrinth of the cochlea. First, all three axes were centered on an individual electrode. Second, the three dimensional curved MPR windows were rotated and positioned to provide a coronal oblique, sagittal oblique, and axial oblique cross-sectional view of the cochlea. Next, using the coronal oblique and sagittal oblique windows, we identified the osseous spiral lamina. Within the sagittal oblique cross-sectional window, we noted the bony canal's roof and floor. The sagittal oblique and coronal oblique windows were manipulated to provide visualization of the lateral and medial edges of the bony canal, and the osseous spiral lamina. In the present study, the area above the osseous spiral lamina refers to the extra-scala tympani region (comprised of the scala vestibuli, basilar membrane, and cochlear duct). The area below the osseous spiral lamina was delineated as the scala tympani region. By visualizing of all of these bony landmarks, we were able to establish the scala tympani and extra-scala tympani regions and use them to identify electrode position within the scala (Fig. 1).
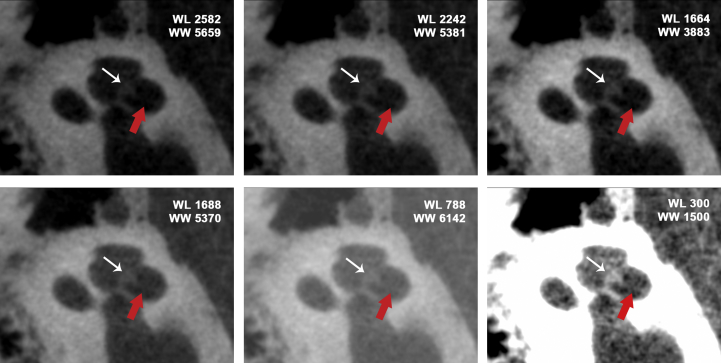
Fig. 1.
Delineation of scala tympani versus scala vestibuli using 3D curved multiplanar reformation analysis and window level/window width settings. The white arrows note the osseous spiral lamina and the red arrows highlight the basilar membrane. A typical WL/WW bone setting on CT is 300/1500. Abbreviations: WW, Window width; W, Window level.
To determine the angle at which interscalar electrode displacement occurred, a line (SCC-V) was drawn from the superior semicircular canal to the middle of the vestibuli. A perpendicular line to the SCC-V line was drawn across the cochlea center core to establish a 0° reference line. The angle of occurrence was determined by the angular position of the individual electrode relative to the 0° reference line. This methodology was adapted from the Xu et al study.9
Results
We evaluated eight FPCT datasets among eight patients; there were four round window insertions and four cochleostomy insertions. The average cochlear duct length for the round window insertion cohort is 35.5 mm (SD 1.43 mm), and the average cochlear duct length for the cochleostomy cohort is 36.7 mm (SD 0.29 mm). For the purposes of this study, we defined ‘interscalar’ as electrode position with respect to the scala vestibuli and the scala tympani and ‘intrascalar’ as position within a given scala. The term ‘interscalar excursion’ was used to describe findings of an electrode array traversing from the scala tympani to the scala vestibuli. In this pilot study, we used a set of secondary reconstructions with high quality resolution (n = 8) to assess interscalar excursion by visualization of the bony cleft surrounding the spiral limbus (Fig. 2). We assessed a total of 96 electrodes (12 electrodes per CI) in this study; one electrode within the RW group was excluded from analysis because it was extracochlear on FPCT imaging.
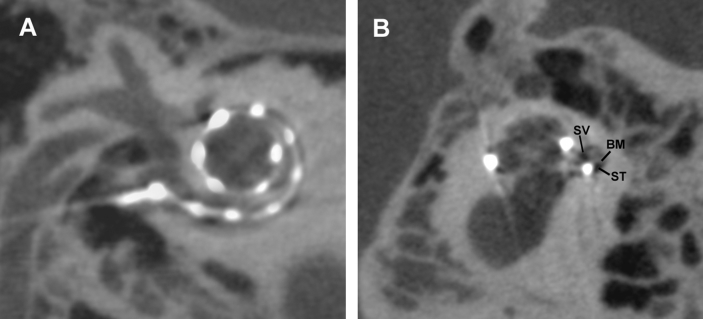
Fig. 2.
Electrode intracochlear position on secondary flat-panel CT reconstructions. (A), Sagittal oblique section of the cochlea (B), and cross section of cochlea at the center of the modiolus. Abbreviations: ST, Scala tympani; SV, Scala vestibuli; BM, Basilar membrane.
Overall, cochleostomy approaches were associated with increased electrode contact placement outside of the scala tympani (Table 2). Within the COCH group, 14 of the 48 electrode contacts (29%) were located outside of the scala tympani. In contrast, 4 of the 47 electrode contacts (8.5%) in the RW group were located outside of the scala tympani. The greatest number of displacements occurred in the latter third of the electrode array with a mean angle of occurrence of 364° ± 133°. Round window electrode displacement occurred at an angle of occurrence of 400° or greater (Table 3), whereas cochleostomy electrodes occurred at a full range of angles of occurrence (range of 19°–490°).
Table 2.
Interscalar excursions between round window and cochleostomy approach.
| Subject | Electrode (1 = Most apical; 12 = Most basal) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDL | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| COCH Group | COCH.1 | 36.3 | ST | NO | NO | NO | ST | NO | ST | ST | ST | ST | ST | ST |
| COCH.2 | 36.8 | ST | NO | NO | NO | ST | ST | ST | ST | ST | NO | ST | ST | |
| COCH.3 | 37.0 | ST | NO | NO | ST | NO | ST | ST | ST | ST | ST | ST | ST | |
| COCH.4 | 36.7 | ST | NO | NO | ST | ST | ST | ST | ST | ST | ST | ST | NO | |
| COCH Group | RW.1 | 36.4 | ST | NO | NO | ST | ST | ST | ST | ST | ST | ST | ST | ST |
| RW.2 | 35.8 | – | ST | NO | ST | ST | ST | ST | ST | ST | ST | ST | ST | |
| RW.3 | 36.4 | ST | NO | ST | ST | ST | ST | ST | ST | ST | ST | ST | ST | |
| RW.4 | 33.4 | ST | ST | ST | ST | NO | ST | ST | ST | ST | ST | ST | ST | |
Note: Electrode positioning outside the scala tympani is more common among the COCH group and predominantly in the most apical third of the electrode array. Abbreviations: RW, Round window approach; COCH, Cochleostomy approach; CDL, Cochlear duct length (in millimeters); ST, Electrode is located within the scala tympani; NO, Electrode is not located within the scala tympani.
Table 3.
Cases of dislocation and the angles at their occurrence.
| Dislocation | Surgical approach | Patient subject | Angle at interscalar excursion (°) |
|---|---|---|---|
| 1 | Cochleostomy | COCHL.4 | 19 |
| 2 | Cochleostomy | COCH.2 | 83 |
| 3 | Cochleostomy | COCH.1 | 227 |
| 4 | Cochleostomy | COCH.3 | 259 |
| 5 | Cochleostomy | COCH.2 | 336 |
| 6 | Cochleostomy | COCH.1 | 342 |
| 7 | Cochleostomy | COCH.4 | 370 |
| 8 | Cochleostomy | COCH.3 | 372 |
| 9 | Round window | RW.1 | 398 |
| 10 | Cochleostomy | COCH.2 | 403 |
| 11 | Cochleostomy | COCH.1 | 407 |
| 12 | Round window | RW.4 | 411 |
| 13 | Cochleostomy | COCHL.4 | 432 |
| 14 | Cochleostomy | COCH.3 | 434 |
| 15 | Round window | RW.1 | 457 |
| 16 | Round window | RW.2 | 458 |
| 17 | Cochleostomy | COCH.1 | 476 |
| 18 | Cochleostomy | COCH.2 | 490 |
| 19 | Round window | RW.2 | 522 |
Abbreviations: 1–17, Numeric identifiers are assigned to each patient; L, Left-sided cochlear implant; R, Right-sided cochlear implant; RW, Round window approach; COCH, Cochleostomy approach.
Discussion
It is well established that minimizing intracochlear trauma during CI electrode array insertion is one of the most efficacious methods in ensuring maximal hearing preservation.5, 10, 11 In fact, there is evidence that suggests a strong association between interscalar excursion and loss of residual hearing following cochlear implantation.12 Consequentially, the interest and popularity in refining surgical approaches to reduce surgically-induced trauma has been growing exponentially each year: recent studies are finding roles for image-guided CI insertions,13 biocompatible polymer-coated electrode arrays,14 and new atraumatic electrode designs. While these new surgical tools and options in device design are relatively elective to the operation, insertion of the electrode array is arguably the most important step in this surgical operation.
Surprisingly, there is no clear consensus on optimal insertion approach in reducing cochlear implantation interscalar to date, despite emerging evidence of differences between round window insertions and cochleostomy approaches. In this case-comparison pilot study, we found preliminary data that suggests round window insertions are associated with fewer incidences of interscalar excursions when compared to cochleostomy approaches. The difference between the two approaches was significant; we found electrode dislocation incidence of 8.5% in RW group versus 29% within the COCH group. These study findings are consistent with the current literature suggesting favorable outcomes with a round window insertion approach.3, 4, 5
Although the sample size is small (n = 8), round window insertions may potentially reduce the likelihood of intracochlear trauma found in cochleostomy insertions. Many operating rooms are now equipped with FPCT imaging (i.e. Siemens Orbic 3D C-arms, Medtronic O-arms), offering the opportunity for real-time imaging during electrode array insertion. As such, FPCT units may be able to prevent episodes of scalar digression by predicting trajectory of the electrode array based on its present curvature. This goal may be particularly important for CI patients with significant residual hearing.
We would like to acknowledge the limitation of this study. Although all cochleostomies were placed anterior and inferior to the round window, the study results do not account for inter-individual differences in the placement of the cochleostomy. We acknowledge that any variability in positioning relative to the round window could influence final intracochlear electrode position and alter the incidence of interscalar excursions reported in this study. Furthermore, insertion velocity was not obtained at the time of the cochlear implantation, and this surgical factor could influence the incidence of interscalar excursions. We also did not confirm interscalar excursions with histology. Previous studies directly evaluated the results of radiographic imaging (multislice CT and FPCT) in CI users with temporal bone gross histology15 and histological micro grinding imaging.16 Moreover, studies have also used FPCT to image the CI in vivo7, 17, 18 with no histology confirmation. Nonetheless, the lack of histological confirmation in our study is an important limitation of this study method and should be taken into consideration when interpreting the study findings.
Despite the limitations described above, this study represents the first effort to use FPCT imaging to perform a quantitative comparison of electrode interscalar excursions between round window and cochleostomy approaches. This study provides new evidence that round window insertions may reduce intracochlear trauma. Further studies are necessary to examine the relationship between surgical approach and electrode positioning within the scala tympani, with respect to optimal CI insertion outcomes.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Shepherd R.K., Hatsushika S., Clark G.M. Electrical stimulation of the auditory nerve: the effect of electrode position on neural excitation. Hear Res. 1993;66:108–120. doi: 10.1016/0378-5955(93)90265-3. [DOI] [PubMed] [Google Scholar]
- 2.Lehnhardt E. Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO. 1993;41:356–359. [PubMed] [Google Scholar]
- 3.Richard C., Fayad J.N., Doherty J., Linthicum F.H. Round window versus cochleostomy techniques in cochlear implantation: histological findings. Otol Neurotol. 2012;33:1181–1187. doi: 10.1097/MAO.0b013e318263d56d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adunka O., Unkelbach M.H., Mack M., Hambek M., Gstoettner W., Kiefer J. Cochlear implantation via the round window membrane minimizes trauma to cochlear structures: a histologically controlled insertion study. Acta Otolaryngol. 2004;124:807–812. doi: 10.1080/00016480410018179. [DOI] [PubMed] [Google Scholar]
- 5.Wanna G.B., Noble J.H., Carlson M.L. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope. 2014;124(suppl 6):S1–S7. doi: 10.1002/lary.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassepass F., Aschendorff A., Bulla S. Radiologic results and hearing preservation with a straight narrow electrode via round window versus cochleostomy approach at initial activation. Otol Neurotol. 2015;36:993–1000. doi: 10.1097/MAO.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 7.Pearl M.S., Roy A., Limb C.J. High-resolution secondary with the use of flat panel CT in the clinical assessment of patients with cochlear implants. AJNR Am J Neuroradiol. 2014;35:1202–1208. doi: 10.3174/ajnr.A3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiam N.T., Jiradejvong P., Pearl M.S., Limb C.J. The effect of round window vs cochleostomy surgical approaches on cochlear implant electrode position: a flat-panel computed tomography study. JAMA Otolaryngol Head Neck Surg. 2016;142:873–880. doi: 10.1001/jamaoto.2016.1512. [DOI] [PubMed] [Google Scholar]
- 9.Xu J., Xu S.A., Cohen L.T., Clark G.M. Cochlear view: postoperative radiography for cochlear implantation. Am J Otol. 2000;21:49–56. [PubMed] [Google Scholar]
- 10.Zhou L., Friedmann D.R., Treaba C., Peng R., Roland J.T., Jr. Does cochleostomy location influence electrode trajectory and intracochlear trauma. Laryngoscope. 2015;125:966–971. doi: 10.1002/lary.24986. [DOI] [PubMed] [Google Scholar]
- 11.Carlson M.L., Driscoll C.L., Gifford R.H. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol. 2011;32:962–968. doi: 10.1097/MAO.0b013e3182204526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wanna G.B., Noble J.H., Gifford R.H. Impact of intrascalar electrode location, electrode type, and angular insertion depth on residual hearing in cochlear implant patients: preliminary results. Otol Neurotol. 2015;36:1343–1348. doi: 10.1097/MAO.0000000000000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohani P., Pile J., Kahrs L.A. Forces and trauma associated with minimally invasive image-guided cochlear implantation. Otolaryngol Head Neck Surg. 2014;150:638–645. doi: 10.1177/0194599813519747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinoshita M., Kikkawa Y.S., Sakamoto T. Safety, reliability, and operability of cochlear implant electrode arrays coated with biocompatible polymer. Acta Otolaryngol. 2015;135:320–327. doi: 10.3109/00016489.2014.990580. [DOI] [PubMed] [Google Scholar]
- 15.Aschendorff A., Klenzner T., Richter B., Kubalek R., Nagursky H., Laszig R. Evaluation of the hifocus electrode array with positioner in human temporal bones. J Laryngol Otol. 2003;117:527–531. doi: 10.1258/002221503322112932. [DOI] [PubMed] [Google Scholar]
- 16.Zeitler D.M., Wang K.H., Prasad R.S., Wang E.Y., Roland J.T. Flat-panel computed tomography versus multislice computed tomography to evaluate cochlear implant positioning. Cochlear Implants Int. 2011;12:216–222. doi: 10.1179/146701011X12962268235742. [DOI] [PubMed] [Google Scholar]
- 17.Bartling S.H., Gupta R., Torkos A. Flat-panel volume computed tomography for cochlear implant electrode array examination in isolated temporal bone specimens. Otol Neurotol. 2006;27:491–498. doi: 10.1097/01.mao.0000194816.15298.50. [DOI] [PubMed] [Google Scholar]
- 18.Trieger A., Schulze A., Schneider M., Zahnert T., Mürbe D. In vivo measurements of the insertion depth of cochlear implant arrays using flat-panel volume computed tomography. Otol Neurotol. 2011;32:152–157. doi: 10.1097/MAO.0b013e3181fcf04d. [DOI] [PubMed] [Google Scholar]