ABSTRACT
Cryptococcus neoformans is a major fungal pathogen that disseminates to the central nervous system (CNS) to cause fatal meningoencephalitis, but little is known about immune responses within this immune-privileged site. CD4+ T cells have demonstrated roles in anticryptococcal defenses, but increasing evidence suggests that they may contribute to clinical deterioration and pathology in both HIV-positive (HIV+) and non-HIV patients who develop immune reconstitution inflammatory syndrome (IRIS) and post-infectious inflammatory response syndrome (PIIRS), respectively. Here we report a novel murine model of cryptococcal meningoencephalitis and a potential damaging role of T cells in disseminated cryptococcal CNS infection. In this model, fungal burdens plateaued in the infected brain by day 7 postinfection, but activation of microglia and accumulation of CD45hi leukocytes was significantly delayed relative to fungal growth and did not peak until day 21. The inflammatory leukocyte infiltrate consisted predominantly of gamma interferon (IFN-γ)-producing CD4+ T cells, conventionally believed to promote fungal clearance and recovery. However, more than 50% of mice succumbed to infection and neurological dysfunction between days 21 and 35 despite a 100-fold reduction in fungal burdens. Depletion of CD4+ cells significantly impaired IFN-γ production, CD8+ T cell and myeloid cell accumulation, and fungal clearance from the CNS but prevented the development of clinical symptoms and mortality. These findings conclusively demonstrate that although CD4+ T cells are necessary to control fungal growth, they can also promote significant immunopathology and mortality during CNS infection. The results from this model may provide important guidance for development and use of anti-inflammatory therapies to minimize CNS injury in patients with severe cryptococcal infections.
KEYWORDS: Cryptococcus, Cryptococcus neoformans, IRIS, PIIRS, T cells, central nervous system infections, encephalitis, fungi, immunopathology, meningitis, opportunistic infections
IMPORTANCE
CNS infection with the fungal pathogen Cryptococcus neoformans often results in debilitating brain injury and has a high mortality rate despite antifungal treatment. Treatment is complicated by the fact that immune responses needed to eliminate infection are also thought to drive CNS damage in a subset of both HIV+ and non-HIV patients. Thus, physicians need to balance efforts to enhance patients’ immune responses and promote microbiological control with anti-inflammatory therapy to protect the CNS. Here we report a novel model of cryptococcal meningoencephalitis demonstrating that fungal growth within the CNS does not immediately cause symptomatic disease. Rather, accumulation of antifungal immune cells critically mediates CNS injury and mortality. This model demonstrates that antifungal immune responses in the CNS can cause detrimental pathology and addresses the urgent need for animal models to investigate the specific cellular and molecular mechanisms underlying cryptococcal disease in order to better treat patients with CNS infections.
INTRODUCTION
Cryptococcus neoformans is an environmental fungal pathogen that causes substantial morbidity and mortality worldwide, accounting for an estimated 1 million infections and 200,000 to 600,000 deaths per year (1–3). Although inhalation is considered to be a primary route of infection, the majority of symptomatic disease and deaths are due to fungal dissemination to the central nervous system (CNS) and subsequent meningoencephalitis. Accordingly, the majority of patients do not display overt pulmonary symptoms but present with neurological sequelae. Treating cryptococcal CNS infections is challenging due to the high toxicity and poor blood-brain barrier (BBB) penetration of antifungal drugs in addition to increased development of drug resistance (4–6). Hence, therapeutic failures are common. Acute mortality for CNS infections is up to 30 to 50% despite treatment in both HIV-positive (HIV+) and -negative patients, and relapse is common (6–11). Many surviving patients require life-long therapy and may be left with lasting disabilities, including memory loss, vision deficiencies, hearing and speech impairments, and motor deficits. Thus, there is an urgent need for studies to elucidate the cellular and molecular processes underlying the pathogenesis of cryptococcal CNS disease to develop more effective therapeutic strategies.
Prior studies have established a central role for CD4+ and CD8+ T cells in fungal clearance from the lungs in mice (12–19) and suggest that similar Th1-biased cellular immunity is beneficial for fungal clearance in the CNS (20–24). Similarly, the susceptibility to cryptococcal disease in T cell-deficient human patients, such as due to HIV/AIDS or T cell depletion therapies, including transplant conditioning, supports a paradigm that clinical failures are predominantly due to a deficiency in microbiological control. This paradigm has led to recommendations for T cell adjunctive therapies, such as recombinant gamma interferon (IFN-γ), to treat clinical failures of the disease in both HIV-positive and HIV-negative individuals (6, 25). However, accumulating evidence supports that control of fungal growth is only one aspect of disease, while immune inflammatory factors can significantly contribute to CNS pathology in certain subsets of patients with complicated CNS disease (26–28). Up to one-third of cryptococcus-infected HIV+ patients initiating antiretroviral therapy develop immune reconstitution inflammatory syndrome (IRIS) upon restoration of T cell counts (29). These patients develop severe neurological sequela and morbidity, associated with high levels of proinflammatory markers, often despite fungal eradication (30). These findings were reproduced in a C. neoformans-related IRIS model, in which transfer of CD4+ T cells into the infected Rag-1−/− mice produced inflammatory reactions, including systemic increases in IFN-γ and inflammatory lesions in the lungs, liver, and brain (31).
A similar phenomenon occurs among non-HIV patients with severe cryptococcal CNS disease in the setting of microbiological control, termed post-infectious immune response syndrome (PIIRS) (32–35). Studies confirm that cryptococcal PIIRS and IRIS share many characteristic immunological features, including elevated CD4+ and CD8+ T cell counts in cerebrospinal fluid (CSF) and elevated IFN-γ (32, 33, 36–40), suggesting that the Th1 immune responses necessary for fungal clearance simultaneously contribute to clinical disease. A potential detrimental role for host inflammation in these patients is further supported by the benefits of selective and judicious use of corticosteroid therapy in ameliorating IRIS and PIIRS symptoms and by observations that premature or abrupt steroid weaning potentiates relapse in CNS lesion formation and redeterioration in clinical symptoms (6, 11, 27, 32–34, 41, 42). Thus, traditionally recognized hallmarks of the protective immune response can be important contributors to pathogenesis of CNS injury and its lasting consequences during CNS cryptococcosis.
While several models of cryptococcal CNS infection have been described, these studies have not focused on the role of CNS inflammation in pathogenesis of cryptococcal CNS disease, such as the cryptococcal CNS disease that occurs in IRIS and PIIRS patients (20–24, 28, 43–47), or report on only a few selected inflammatory parameters for the CNS (31). The goal of the present study was to develop a robust, physiologically relevant murine model of C. neoformans CNS infection that reproduces aspects of human cryptococcal disease pathology and that is suitable for detailed immunological analysis. Here, using this newly established model of disseminated CNS infection in C57BL/6 mice, we demonstrate that Th1-biased CD4+ T cell-driven inflammation in the CNS can be the primary cause of detrimental pathology at least in a subset of patients with severe or refractory cryptococcal meningoencephalitis. These studies provide new insights regarding clinical outcomes observed in IRIS and PIIRS patients and support the development of therapeutic strategies that limit aspects of inflammation to prevent CNS damage in suitably selected patients with CNS cryptococcosis.
RESULTS
Intravenous infection with C. neoformans 52D causes fatal meningoencephalitis, but symptomatic disease is independent of peak fungal burden.
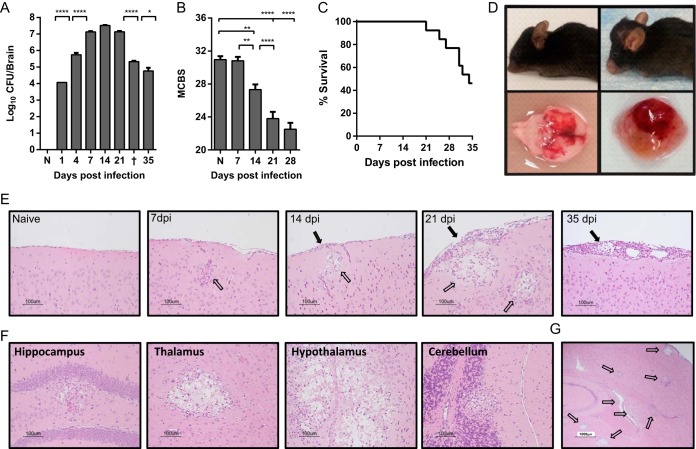
In order to establish a reproducible, controlled-onset mouse model of cryptococcal meningoencephalitis, we infected C57BL/6 mice with 106 CFU of C. neoformans strain 52D via intravenous (i.v.) injection. This strain was chosen for these studies because other highly virulent strains (such as H99) induce rapid 100% mortality by 4 to 14 days postinfection before a measurable adaptive immunological response can develop (21, 23, 24, 43, 45, 48) and thus are less suited to study adaptive responses within the CNS. At selected time points, C. neoformans 52D-infected mice were euthanized, and fungal growth was assessed in perfused, homogenized brains. In separate experiments, animals were monitored and evaluated for symptoms of neurological dysfunction and then euthanized when endpoint symptoms of disease were present. Using this model, we found that abundant fungal organisms were present in the brains by 24 h postinfection, indicating that C. neoformans rapidly crossed from circulation into the CNS (Fig. 1A). Fungal burdens continued to rise sharply through day 7, after which they remained relatively stable through day 21 postinfection. From days 21 to 35 postinfection, fungal burdens progressively declined, decreasing by an average of 2 orders of magnitude on day 35 compared to the earlier peak. Throughout this time, mice were systematically examined for symptoms of disease and multiple measurements of body condition, behavior, and neurological status were quantified using a murine coma and behavior score (MCBS) (see Table S1 in the supplemental material) modified from the MCBS of Carroll et al. (49). Despite extremely high CNS fungal burdens, which peaked at 7 to 14 days postinfection, mice remained relatively asymptomatic as assessed by MCBS at these time points (Fig. 1B). In fact, MCBS scores of infected mice did not reach a nadir indicative of highly symptomatic disease until day 21 to 28 postinfection, which corresponded with the onset of fungal clearance and mortality. During the same period, mice began displaying overt and severe clinical symptoms of disease, including significant weight loss despite dietary supplementation and/or neurological sequelae, such as cranial swelling, seizures, ataxia, and limb paralysis. Overall, 50% animals succumbed to infection and required euthanasia between days 21 and 35 postinfection (Fig. 1C). Cranial swelling and tissue edema suggestive of raised intracranial pressure (11) and gross injury were frequently evident (Fig. 1D). However, even animals that succumbed to infection had substantially decreased fungal burdens (Fig. 1A), suggesting that clinical deterioration occurred despite the onset of fungal clearance. Thus, these data demonstrate that the magnitude of CNS fungal burden does not directly correlate with the intensity of clinical symptoms or mortality during cryptococcal meningoencephalitis.
FIG 1 .
Infection with Cryptococcus neoformans 52D causes a highly lethal meningoencephalitis in C57BL/6 mice despite fungal clearance. C57BL/6 mice were infected with 106 CFU of C. neoformans 52D via retro-orbital intravenous inoculation. (A) Fungal burdens were measured in whole-brain homogenates at the indicated time points postinfection. Naive mice (N) and animals that succumbed to infection and were euthanized between days 31 and 33 postinfection (†) are indicated. (B) Overall health and neurological status was assessed using a modified murine coma and behavioral scale (MCBS), out of a maximum score of 36 points. Data shown in panels A and B are the means plus standard errors of the means (SEM) (error bars) from a representative experiment of 2 to 5 independent experiments with 3 to 8 mice per time point. Values that are statistically significantly different are indicated by brackets and asterisks as follows: *, P < 0.05; **, P < 0.01; ****, P < 0.0001. (C) In separate experiments, survival was monitored through day 35 postinfection. Infection was considered fatal, and animals were euthanized when they lost 20% body weight, had persistent cranial swelling, and/or developed neurological sequelae such as seizures, tremors, limb weakness or paralysis. (D) Representative images of severe cranial swelling and CNS tissue injury in infected mice. (E to G) Brains from perfused mice were paraffin embedded, coronally sectioned, and stained with hematoxylin and eosin (H&E). Note that stained C. neoformans organisms occupy a relatively small area compared to areas of displaced cerebral tissue by accumulating polysaccharide capsule material, leaving behind a characteristic “Swiss cheese” pattern. Solid black arrows indicate inflammation and cryptococcal organisms in the meninges; clear arrows indicate inflammation and cryptococcal organisms in the parenchyma. (E to G) Representative images shown are from the cortex at various time points postinfection (in days postinfection [dpi]) in panel E (20×), various anatomical structures at 21 days postinfection (20×) (F), and whole brain at 21 days postinfection (4×) (G).
Modified murine coma and behavioral scale (MCBS) score chart. A modified MCBS scoring system was adapted and expanded from the MCBS scoring system of Carroll et al. (R. W. Carroll et al, PLoS One 5:e13124, 2010, https://doi.org/10.1371/journal.pone.0013124). Individual mice were placed into the corner of a clean, overturned microisolator cage lid. Each mouse was allowed to freely explore the lid while the above observations on gait, balance, exploration, body position, and coat condition were scored over the course of 90 s. For neck, body, and pinna touch scoring, the appropriate body area was touched with a pen or open forceps while the animal was free in the cage lid. Next, each mouse was placed atop wire cage hoppers, and each hind limb footpad was gently grasped with forceps to score toe touch reflexes. blink reflexes in response to an approaching pen or forceps. Grasp strength and sense of smell were measured by investigation with a coffee-soaked cotton swab were also assessed while the mouse was on the wire hopper. We note that the phenotype of most parameters we observed in mice with C. neoformans meningoencephalitis were more subtle than observed in Carroll et al., so our scoring criteria were accordingly adjusted as described in Materials and Methods. Download TABLE S1, DOCX file, 0.02 MB (16.9KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Fungal growth and inflammation are found throughout the brains of mice with cryptococcal meningoencephalitis.
Clinical evidence increasingly suggests that the host inflammatory response, rather than pathogen burden, may lead to the development of pathology, neuronal injury, and deteriorating status, especially in IRIS and PIIRS patients (28, 30, 32, 33, 36–40). Thus, our next goal was to evaluate CNS pathology during the progression of C. neoformans infection and to determine a possible link to the accumulation of a cellular inflammatory response within the CNS. Histological analysis of brains from infected mice on day 7 postinfection showed that cryptococci were found in meninges, perivascular spaces, and brain parenchyma proper, confirming that C. neoformans crossed the blood-brain barrier to colonize the CNS relatively early and in multiple locations (Fig. 1E and data not shown). However, an inflammatory response around areas of cryptococcal growth did not develop immediately. Chronological analysis of infected brains (Fig. 1E) showed minimal inflammation through day 14 postinfection despite the presence of numerous microfocal areas of cryptococcal growth and overall high fungal burdens within the CNS (Fig. 1A). However, on day 21 postinfection, there were marked increases in cellular inflammation surrounding areas of cryptococci in the parenchyma and in the meninges, signaling the development of fulminant meningoencephalitis. At this time and thereafter, cryptococcal organisms continued to be identified in foci throughout all regions of the brains (Fig. 1F and G), including the cortex, hippocampus, hypothalamus, pons, and cerebellum. Fungal growth was evident throughout the brains of all infected mice with no discernible predilection for specific areas and appeared as larger areas with centrally abundant fungi, resembling characteristic mucoid pseudocysts (32, 43). Notably, the timing of cellular inflammation was synchronized with the onset of mortality and neurological deficits we measured in animals between days 21 and 35 postinfection (Fig. 1B to D). Together, these results suggest that fungal invasion and growth in the CNS alone were not significant factors mediating severe neuronal injury. Rather, progressive cellular inflammation, responsible for driving fungal clearance, correlated with the onset of clinical symptoms and mortality.
Brain-infiltrating leukocyte accumulation is significantly delayed relative to fungal burden but is synchronized with mortality in mice with cryptococcal meningoencephalitis.
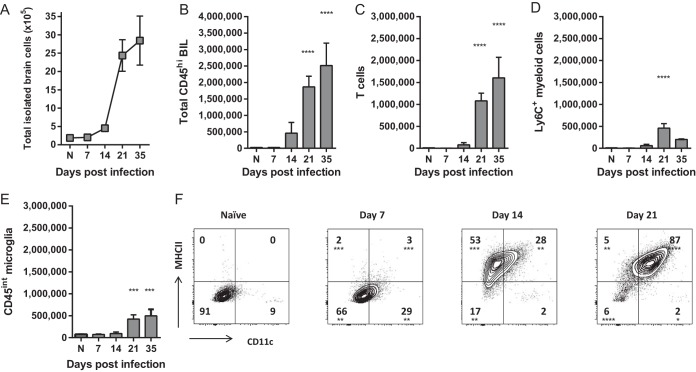
To quantify and determine the phenotypes of the immune cells in the brains of C. neoformans-infected mice, we isolated the leukocyte-enriched brain cell fraction, consisting predominantly of brain-infiltrating leukocytes (BIL) and microglia, containing few structural cells, from brains that had been generously perfused and processed as described in Materials and Methods. These total leukocyte-enriched brain cells were then enumerated and their phenotype was determined using flow cytometry. To correlate the dynamics of resident versus recruited immune cell subsets within the infected CNS and their roles in fungal clearance versus pathology, we extensively analyzed these cells by flow cytometry using the expression level of CD45 to differentiate between microglia (CD45lo) and BIL (CD45hi) at various times throughout the course of infection. Approximately 1 × 105 cells were isolated from the brains of naive mice, and consistent with the appearance of histological sections, the total number of cells remained stable in the brains of C. neoformans-infected mice through day 14 postinfection (Fig. 2A). On day 21, however, the total number of leukocyte-enriched cells recovered from the CNS rose sharply, with a 25-fold increase in cell numbers. The majority of this increase was attributable to an influx of CD45hi recruited BIL (Fig. 2B) consisting primarily of T cells (Fig. 2C) and CD11b+ Ly6C+ myeloid cells (Fig. 2D), which continued to accumulate through day 35 postinfection. Consistent with histological appearance, these data demonstrate that the influx of the cellular inflammatory response was delayed by approximately 2 to 3 weeks relative to the onset of infection and peak fungal burden. Furthermore, the influx of BIL on day 21 postinfection was synchronized with the onset of fungal clearance and mortality (Fig. 1A and D).
FIG 2 .
Recruitment of brain-infiltrating leukocytes and activation of microglia is delayed relative to early fungal growth in the CNS. (A) The total number of cells isolated from the leukocyte-enriched fraction of brains, containing predominantly leukocytes and microglia, was quantified at various time points postinfection. (B to D) The number of CD45hi brain-infiltrating leukocytes (BIL) (B), including T cells (C) and CD11b+ LyC+ myeloid cells (D) was determined by flow cytometry. (E and F) Numbers of CD45int microglia (E) and expression of surface activation markers CD11c and MHCII (F) were also quantified. Note that there was not significant accumulation of BIL or complete activation of microglia until late on day 21 postinfection, delayed relative to peak fungal growth but coinciding with the onset of mortality (Fig. 1). Data shown are the mean ± SEM from a representative experiment of two to four independent experiments with three to eight animals per time point. Values that are statistically significantly different from the means for naive (N) animals are indicated by asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
We also quantified and determined the phenotypes of CD45int microglia throughout infection. As brain resident macrophages, microglia are among the first cells to encounter C. neoformans during CNS infection and are capable of antimicrobial effector responses (22, 45, 50–53). Thus, we initially anticipated that these cells would become activated in response to C. neoformans within the infected CNS early during infection prior to the accumulation of BIL. The number of CD45int microglia remained relatively stable throughout the course of infection (Fig. 2E). However, these cells were not immediately activated by rapid expansion of C. neoformans in the infected brains. Resting microglia from naive animals expressed low levels of CD11c and major histocompatibility complex class II (MHCII) markers of activation, and expression of these activation markers in infected animals occurred gradually, continuing through day 21 postinfection, at which point nearly all microglia had acquired a CD11chi MHCIIhi phenotype (Fig. 2F). Together, these data support the idea that the CNS inflammatory response involving both resident and infiltrating cells does not develop immediately during cryptococcal infection but is delayed by approximately 3 weeks and aligns with the onset of neurological injury and mortality.
Production of inflammatory cytokines in the C. neoformans-infected brain is significantly delayed.
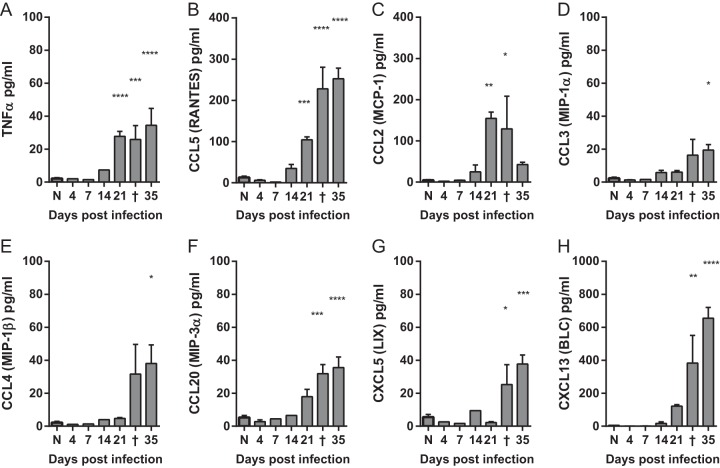
We also asked whether inflammatory cytokines were produced by early resident cells in response to CNS infection and found that the early response to C. neoformans infection in the CNS was relatively quiescent. The levels of tumor necrosis factor alpha (TNF-α) remained at basal levels through day 14 and only showed statistically significant increases on day 21 postinfection and beyond (Fig. 3A). Chemokines CCL5 (CC chemokine ligand 5) (RANTES [regulated on activation, normal T cell expressed and secreted]) and CCL2 (macrophage chemoattractant 1 [MCP-1]) also did not increase until day 21 (Fig. 3B and C), whereas CCL3 (macrophage inflammatory protein 1α [MIP-1α]), CCL4 (MIP-1β), CCL20 (MIP-3α), CXCL5 (CXC chemokine ligand 5) (lipopolysaccharide-induced CSC chemokine [LIX]), and CXCL13 (B lymphocyte chemoattractant [BLC]) were even further delayed (Fig. 3D to H). Interestingly, we did not find significantly elevated levels of interleukin 12p70 (IL-12p70), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), or CCL17 (thymus and activation-regulated chemokine [TARC]) at any time point throughout the course of infection (data not shown). These data further support that molecular signals consistent with antigen recognition are not immediately correlated with increasing fungal burdens early during infection but are delayed and synchronized with the leukocyte recruitment (Fig. 2A to D) beginning on day 21 postinfection.
FIG 3 .
Production of inflammatory cytokines in the brains of mice with cryptococcal meningoencephalitis is delayed but coincides with leukocyte recruitment. (A to H) Levels of inflammatory cytokines were measured in the supernatants of whole-brain homogenates. Each brain was homogenized in 5 ml of medium. Naive mice (N) and animals that succumbed to infection and were euthanized between days 31 to 33 postinfection (†) are indicated. Note that there was not significant induction of any cytokines measured until late on day 21 postinfection. Data shown are the means ± SEM from a representative of two to four independent experiments with three to eight mice per time point. Values that are statistically significantly different from the means for naive animals are indicated by asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Brain-infiltrating leukocytes consist primarily of antigen-experienced CD4+ and CD8+ T cells in mice with cryptococcal meningoencephalitis.
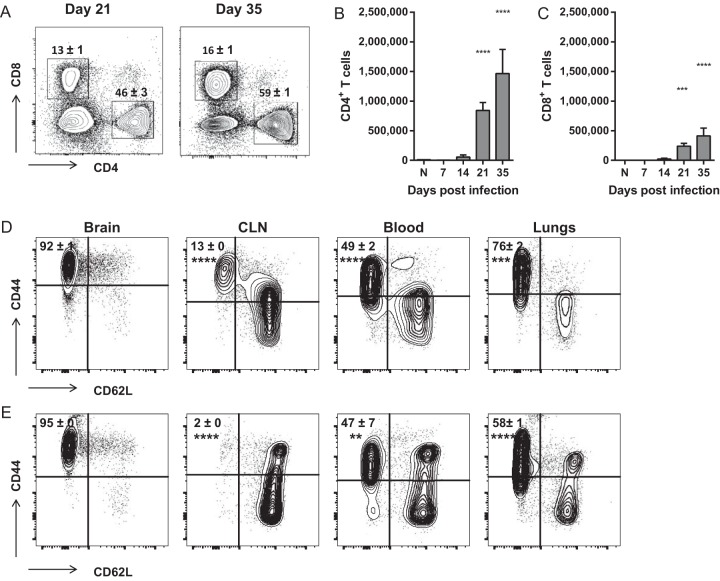
To determine the dynamics and immune phenotypes of BIL entering the CNS on day 21 and beyond, we extensively analyzed the CD45hi recruited population by flow cytometry. CD4+ T cells composed the majority of the CD45hi cellular infiltrate, overall accounting for 40 to 50% of the total recruited cells from days 21 and 35 postinfection (Fig. 4A and B). CD8+ T cells composed a smaller fraction of approximately 15% of recruited cells (Fig. 4C). Nearly all (>90%) of both CD4+ and CD8+ T cells isolated from the C. neoformans-infected CNS were antigen-experienced (CD44hi CD62Llo [CD62L stands for CD62 ligand]) (Fig. 4D and E). These frequencies were significantly greater than those found in the peripheral tissues, including the cervical lymph nodes, blood, and lungs for both CD4+ and CD8+ T cells, suggesting that these antigen-experienced cells have preferential access to the C. neoformans-infected CNS.
FIG 4 .
CD4+ and CD8+ T cells that accumulate in the brains of mice with cryptococcal meningoencephalitis are antigen-experienced cells. (A to C) The percentages (of total CD45hi BIL) (A) and total numbers of CD4+ and CD8+ T cells (B and C) that accumulate in the brains of C. neoformans-infected mice. (D and E) The frequencies of antigen-experienced (CD44hi CD62Llo) CD4+ (D) and CD8+ (E) T cells isolated from the brains, cervical lymph nodes (CLN), blood, and lungs of infected mice on day 35 postinfection. Similar frequencies were observed on day 21. Data shown are the mean ± SEM from a representative experiment of two to four independent experiments with three to eight mice per time point. Representative flow cytometry plots are shown. Values that are statistically significantly different from the means for naive (N) animals (B and C) or brain-infiltrating cells (D and E) are indicated by asterisks as follows: **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
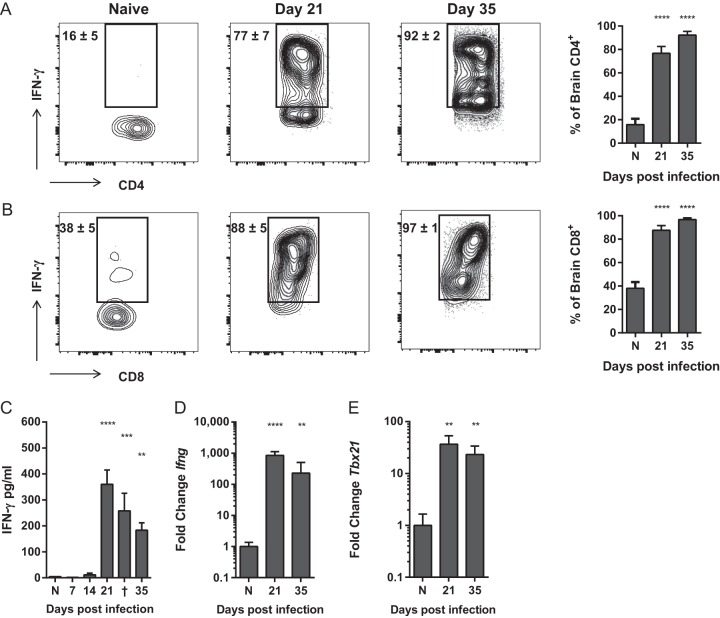
T cell inflammatory response in the CNS during cryptococcal meningoencephalitis is highly Th1 polarized, and nearly all CD4+ and CD8+ T cells produce IFN-γ.
The subsets of patients with paradoxical inflammatory responses to cryptococcal infection (IRIS and PIIRS) display dominant Th1 T cell polarization with elevated levels of IFN-γ in the serum and CSF (32, 33, 36–40). Thus, we sought to determine whether murine brain-infiltrating T cells would mimic this phenotype by analyzing T cell cytokine production and expression of polarizing Th-associated transcription factors in the C. neoformans-infected CNS. Brain-infiltrating T cells demonstrated an overwhelming Th1 bias, as nearly all (>90%) CD4+ and CD8+ cells isolated from the brains of C. neoformans-infected mice on days 21 to 35 postinfection produced IFN-γ (Fig. 5A). This influx of IFN-γ-producing T cells correlated with significant elevation of secreted IFN-γ in whole-brain tissue samples (Fig. 5B), robust increases in IFN-γ transcript and induction of the Th1-associated transcription factor Tbet (Fig. 5D and E). Remarkably, there was no evidence of either a Th17 or Th2 response in the infected CNS, either at the level of protein or transcript (Fig. S2), indicating that virtually all of the immune response was Th1 driven. Cumulatively, these data corroborate the increased T cell responses and IFN-γ levels found in patients with IRIS and PIIRS and suggest that overexuberant Th1-biased CD4+ T cell responses may contribute to detrimental clinical outcomes during cryptococcal CNS infections.
FIG 5 .
CD4+ and CD8+ T cells that accumulate in the brains of mice with cryptococcal meningoencephalitis are uniformly Th1 polarized and produce IFN-γ. (A and B) Frequency of IFN-γ-producing CD4+ (A) and CD8+ (B) T cells isolated from the brains of naive (N) and infected mice on day 21 and 35 postinfection. (C to E) Levels of IFN-γ protein and expression of IFN-γ (Ifng) (D) and Tbet (Tbx21) transcript (E) were measured in whole-brain homogenates. Note that fold expression data are on a log scale. Data shown are the means ± SEM from a representative of two to four independent experiments with three to eight mice per time point. Representative flow cytometry plots are shown. Values that are statistically significantly different from the means for naive (N) animals are indicated by asterisk as follows: **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Depletion of CD4+ T cells prevents mortality of mice with cryptococcal meningoencephalitis despite impaired fungal clearance.
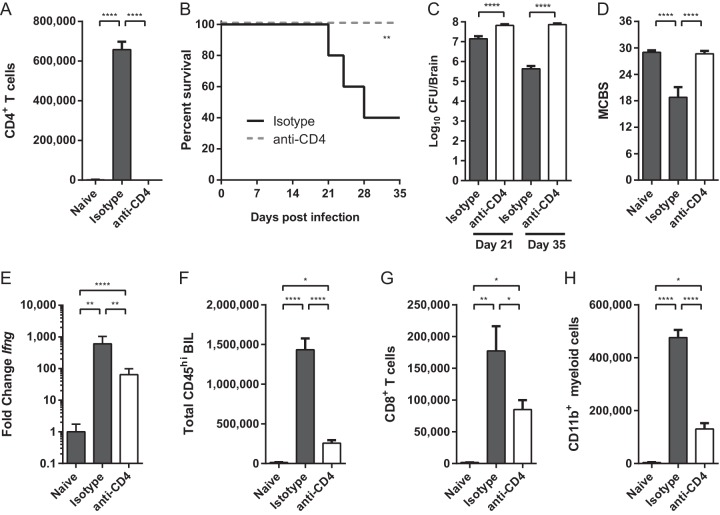
To conclusively determine whether CD4+ T cells contribute to CNS damage and mortality in our murine model of cryptococcal meningoencephalitis, we depleted CD4+ T cells from mice beginning 2 days prior and throughout the course of infection. This depletion regimen successfully eliminated >99% of CD4+ T cells from the brains of C. neoformans-infected mice (Fig. 6A) and in the periphery (data not shown). Unlike their isotype-treated counterparts, nearly 100% of CD4-depleted mice survived to day 35 post C. neoformans infection and did not succumb to immune pathology (Fig. 6B). Notably, the survival of CD4-depleted mice occurred despite their failure to clear fungal organisms. The brain fungal burdens of CD4-depleted mice peaked at levels 10-fold higher than isotype-treated animals (Fig. 6C) and remained elevated out to day 35 without apparent symptoms of CNS disease. CD4-depleted infected mice also demonstrated behavioral responses indistinguishable from naive animals (Fig. 6D), whereas isotype-treated animals had worse MCBS scores and succumbed to disease despite fungal clearance (Fig. 6C and D). These data demonstrate that while CD4+ T cells are necessary to control fungal burden, they ultimately contribute to disease pathology and clinical deterioration. Further supporting this conclusion, animals treated with a partial-depletion regimen consisting of only two anti-CD4 treatments beginning the day of infection, which reduced CNS CD4+ T cells by only approximately 30%, still exhibited delayed mortality and a trend toward increased survival (Fig. S3).
FIG 6 .
Depletion of CD4+ T cells rescues survival despite failure to clear infection during cryptococcal meningoencephalitis. (A) Treatment with anti-CD4 depleting antibody (white bar) reduces the total number of brain-infiltrating CD4+ T cells by >99% compared to infected isotype-treated controls (dark gray bar). (B) Survival of infected CD4-depleted (broken line) and isotype-treated mice through day 35 postinfection. (C and D) Brain fungal burdens were calculated on day 21 and 35 postinfection (C), and MCBS scores were calculated on day 21 (D). (E) Expression of IFN-γ transcript was quantified in whole-brain homogenates on day 35. Note that fold expression data are on a log scale. (F to H) The total numbers of CD45hi BIL (F), CD8+ T cells (G), and CD11b+ Ly6C+ myeloid cells (H) were quantified by flow cytometry on day 35 postinfection. Similar reductions were also observed on day 21. Data shown are the means ± SEM from a representative experiment of three independent experiments with 5 to 10 mice per group. Values that are statistically significantly different are indicated by brackets and asterisks as follows: *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
Finally, depletion of CD4+ T cells during C. neoformans meningoencephalitis also broadly inhibited all other aspects of the CNS inflammatory response. Expression of IFN-γ was dramatically reduced in CD4-depleted mice (Fig. 6E) consistent with CD4+ T cells being an abundant source of this cytokine in the CNS during infection. Depletion of CD4+ T cells also led to a striking reduction in the accumulation of total CD45hi BIL (Fig. 6F), including CD8+ T cells (Fig. 6G) and CD11b+ Ly6C+ myeloid effector cells (Fig. 6H). Taken together, these data strongly support the idea that CD4+ T cells exert dual but opposing roles: promoting the accumulation of immune cells and driving fungal clearance but simultaneously orchestrating lethal pathology during cryptococcal meningoencephalitis.
DISCUSSION
In this study, we established an intravenous infection model using C. neoformans strain 52D in C57BL/6 mice that reproduces features of highly lethal cryptococcal meningoencephalitis occurring in human patients with severe CNS manifestations in the setting of microbiological control, including immune reconstitution inflammatory syndrome (IRIS) and post-infectious inflammatory response syndrome (PIIRS). Using this model, we showed that although C. neoformans rapidly enters and expands within the murine CNS within a few days postinfection, it does not induce an immediate inflammatory response at this site. Rather, the cellular inflammatory responses did not become pronounced until day 21 postinfection, which coincided with the onset of fungal clearance, development of neurological symptoms, and progressive mortality. The major immune cell subset that accumulated within the infected CNS were Th1-polarized IFN-γ-producing CD4+ T cells. Importantly, the development of lethal CNS symptoms could be prevented by depletion of CD4+ T cells, despite the persistent and increased growth of fungus within the CNS. Thus, despite being required for fungal clearance, Th1 cells are a central mediator of detrimental CNS pathology. These results support the paradigm that regulation of T cell responses within the CNS needs to be considered part of the therapeutic strategy to prevent CNS damage in certain patients with cryptococcal CNS infection.
The treatment of cryptococcal CNS infections presents many challenges due to the need to balance microbial control with a patient’s own potentially paradoxical responses during therapy (26, 27, 29, 30, 32). In these patients, treatment strategies must strike a balance between activation of antifungal T cell responses and limiting CNS damage due to inflammation. Although indiscriminate use of steroids is not universally justified for all cases of cryptococcal meningitis (54), including initial treatment of most HIV-infected patients where the immune response is generally poor and microbiological control has not been achieved (55), steroid therapy has been used with some success in refractory cases where elevated levels of IFN-γ, CNS T cell reactivity, and neuronal injury are predominate features of disease, such as in IRIS and PIIRS (6, 11, 27, 28, 32–34, 41, 42). However, a more complete understanding of immune pathogenesis is desperately needed to develop more precise and effective therapies. Much of the current understanding regarding immune defense against cryptococcal infection results from studies using models of pulmonary infection aimed at deciphering mechanisms preventing fungal escape from the lungs (12–18), which were presumed to be beneficial in the CNS (20–24). However, to date, little has been shown about either the pathogenesis or the cellular and molecular mechanisms mediating anticryptococcal defenses within the CNS itself. The data we present here using our CNS infection model demonstrates dramatic and significant distinctions between immune responses within the CNS compared to the lungs, which has important implications for clinical treatment of CNS infections.
First, we observed that there is a surprisingly long quiescent period between entry of C. neoformans into the CNS before evidence of cryptococcal antigen recognition and subsequent development of the immune response. We did not measure significant chemokine production or inflammatory cell recruitment to the infected CNS until 21 days postinfection with high inoculum (106 CFU i.v. seeding 104 CFU in the CNS within 24 h) (Fig. 2 and 3), unlike in the lungs where the robust cytokine responses and leukocyte accumulation occur by 7 days postinfection, even after a relatively small dose (104 CFU) of C. neoformans is instilled (13, 56, 57). This delay occurred despite the fact that the fungus rapidly expanded within CNS, at rates comparable to that reported in the lungs; expansion of 104 organisms at 24 h to 107 organisms within 7 days. This is likely due to the poor response of CNS resident cells in recognizing and responding to C. neoformans. The fact that we did not observe full activation of microglia until 21 days postinfection is supported by previous studies showing that C. neoformans can be phagocytosed and induce cytokine and chemokine production by microglia, including CCL3, CCL4, and CCL5, but these effects are severely hindered in the absence of opsonizing antibody or additional stimulatory factors (45, 47, 50, 51, 53). Thus, it appears that substantial time is required to overcome the poor activation and impaired phagocytosis of C. neoformans by microglia and to provide significant antigenic stimulation to prompt local reactivation and proliferation of peripherally licensed T cells surveilling the CNS. Likewise, we did not measure any significant breakdown in blood-brain barrier function through at least day 21 postinfection (by Evans blue dye exclusion [data not shown]) that would have permitted unrestricted immune cell entry. Furthermore, even at its peak, the relative abundance of accumulated leukocytes was at least 1 order of magnitude lower in the CNS than in the lungs despite relatively similar fungal loads achieved in both tissues (13, 56, 57).
The second, but perhaps most important, distinction was the unique compartmentalization of polarized T cell responses in the CNS compared to the lungs. Notably, we observed a total dominance of Th1 polarization in the CNSs of C. neoformans 52D-infected mice; nearly all CD4+ and CD8+ T cells were found to be antigen experienced, in contrast to the periphery, and virtually all produced IFN-γ. This is in stark contrast to years of data gained from studies of pulmonary and systemic responses where the balance of Th1/Th2/Th17/Treg (regulatory T cell) responses ultimately shape disease outcome. Data generated by multiple labs, using multiple strains of mice and of C. neoformans, uniformly support the idea that Th1 responses are protective, promoting fungal clearance and recovery, while Th2 responses allow for fungal persistence and drive immune pathology in the infected lungs. In particular, C57BL/6 mice develop a mixed Th2/Th1/Th17 response to pulmonary infection with C. neoformans 52D, resulting in immunological stalemate and persistent infection, but outcomes can be improved by provoking a greater Th1 bias (12, 58, 59) or worsened when stronger Th2 bias is induced (44, 60, 61). Our data significantly differ from this balanced Th polarization paradigm, in that we did not observe any hallmarks of Th2 or Th17 response within the infected CNS of C57BL/6 mice at any time point.
Rather, the seminal finding of our study is that antifungal Th1-polarized IFN-γ-producing CD4+ T cells have immense pathogenic potential within the CNS. The timing of IFN-γ-producing T cell accumulation in C. neoformans-infected CNS just prior to the onset of fatalities and declining neurological function, despite fungal clearance, strongly suggests that these cells contribute to mortality in certain patients with severe or refractory CNS disease despite successful microbiological control. This notion is further supported by our results using CD4+ T cell depletion in C. neoformans-infected mice. CD4-depleted mice uniformly survived to day 35 after CNS infection despite fungal burdens maintained for weeks at levels 10- to 1,000-fold higher than controls, which develop neurological sequelae and succumb to infection despite fungal clearance. These data conclusively show that Th1-polarized CD4+ T cells are required to directly or indirectly orchestrate a catastrophic inflammatory cascade that can lead to the CNS damage independent of fungal burden during cryptococcal CNS infection. On the other hand, the high susceptibility and mortality of HIV-associated cryptococcal meningitis, along with increased fungal burdens in CD4-depleted mice, support the idea that restoration of CD4+ T cells and treatment with antifungal drugs are still required for pathogen clearance and long-term patient survival. Thus, the goal of the present study is to begin to dissect microbiologically from immunologically induced pathological effects in order to minimize overall CNS damage (62). While our data emphasize that inhibition of T cell responses in the CNS may be protective against severe inflammatory damage in select cases of CNS cryptococcosis, such as IRIS and PIIRS, long-term inhibition may pose other risks due to microbiological factors (i.e., unrestricted growth of the fungus). Thus, future T cell-targeted therapies for patients with severe cryptococcal CNS disease will need to identify critical windows of T cell functions and carefully weigh their pathological versus beneficial effects.
These data showing the pathological potential of IFN-γ-producing T cells in the CNS during C. neoformans meningoencephalitis are supported by notable damaging roles of CD4+ and CD8+ T cells in the CNS during infections, including cerebral malaria (63–65), viral encephalitis (66), toxoplasmosis (67–69), tuberculosis meningitis (27), and immunological disorders such as multiple sclerosis (70). However, a similar paradigm exalting paradoxical roles for T cells has only recently begun to emerge in the study of cryptococcal infections. Clinical review of complex and refractory human infections increasingly implicate T cells in immune-driven CNS pathology in both HIV+ and non-HIV patients. In HIV+ patients, IRIS frequently arises following restoration of T cell-mediated immunity, and in non-HIV individuals, PIIRS is correlated with elevated CSF CD4+ T cell counts. Both are associated with elevated IFN-γ and often occur despite successful antifungal therapy and clearance supporting the idea that, as in our murine model, immune pathology is Th1 cell driven and is not solely determined by fungal burden (32, 33, 36–40). How the overexuberant Th1 responses develop in these patients is unknown, but they are possibly linked to myeloid cell dysfunction leading to a persistent overly trigger-sensitive (low-threshold) and hyperactivated state (35, 39, 71).
Finally, our findings that CD4 depletion conferred a survival benefit during severe CNS cryptococcosis in mice correlate with cases of severe paradoxical or refractory cryptococcal CNS disease in humans, where the use of steroids has been shown to aid or even be essential for the resolution of clinical symptoms (6, 11, 27, 32–34, 41). Together, these studies clearly demonstrate that therapies to control or fine-tune the antifungal immune response within CNS can be invaluable to preventing detrimental brain injury. However, a recent report found that the use of steroids, which have broad immunosuppressive properties but many detrimental side effects, is not universally justified in broadly treating HIV+ patients with cryptococcal meningitis (54). Even in subsets of patients initially benefitting from steroid therapy, the risks associated with increased cumulative dosing limit their long-term use. Next-generation therapies must overcome these limitations in order to develop more precisely targeted and safe treatments for this devastating CNS infection. However, our findings crucially reenforce that therapies targeting the inflammatory processes have definitive use in cases such as IRIS and PIIRS and suggest that approaches targeting more-specific immune pathways can be refined in order to tailor the most appropriate therapies for treating CNS injury in these patients. While optimal treatment strategies for CNS cryptococcosis still need to be elaborated, our study provides a robust tool to systematically investigate these processes and shows that the ultimate goal of these approaches will be to balance fungal control with immunomodulation to minimize inflammatory damage within the CNS.
MATERIALS AND METHODS
Mice.
C57BL/6 mice (Jackson Laboratory) were housed under specific-pathogen-free (SPF) conditions in microisolator cages at the Ann Arbor Veterans Affairs Medical Center and were provided with food and water ad libitum. Animals were 8 to 12 weeks old at the time of infection. Mice were euthanized using CO2 inhalation followed by exsanguination. All experiments were approved by the Veterans Affairs Institutional Animal Care and Use Committee under protocol 1408-004 and were performed in accordance with NIH guidelines and the Guide for the Care and Use of Laboratory Animals. In mortality studies, animals were euthanized when they lost 20% body weight, had persistent cranial swelling, and/or developed neurological symptoms.
C. neoformans infection.
Cryptococcus neoformans strain 52D was grown for 4 days in Sabouraud dextrose broth (Difco). Fungal cells were washed in phosphate-buffered saline (PBS), counted on a hemocytometer with trypan blue, and adjusted to a concentration of 5 × 106/ml just prior to infection. Mice were infected with 106 organisms (in 200 μl PBS) via retro-orbital intravenous injection under inhaled isoflurane anesthesia. Serial dilutions of the C. neoformans suspension were plated on Sabouraud dextrose agar to confirm the number of viable fungi in the inoculum.
Inoculation with a substantially reduced infectious dose of C. neoformans 52D, such as the 104 CFU typically used to establish pulmonary infection of the lungs (14, 72), failed to induce substantial fungal growth and immune cell accumulation within the CNS on day 21 postinfection (see Fig. S1 in the supplemental material) and therefore was not suitable as a model of meningoencephalitis. Thus, the inoculum dose of 106 CFU was used for all experiments.
Effect of inoculum size on establishment and cellular inflammation during C. neoformans meningoencephalitis. (A and B) C57BL/6 mice were infected with 104 or 106 CFU of C. neoformans 52D, and then fungal burdens (A) and the total numbers of leukocyte-enriched brain cells (B) were determined at 21 days postinfection. Naive animals (N) are shown as controls. Data shown are means ± SEM from three to six mice per group. Values that are statistically significantly different are indicated by asterisks as follows: ***, P < 0.001; **** P < 0.0001. Download FIG S1, EPS file, 0.5 MB (576.8KB, eps) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Absence of Th17 and Th2 polarization in the CNSs of mice with cryptococcal meningoencephalitis. (A and B) C57BL/6 mice were infected with C. neoformans 52D, and the levels of Th17-associated IL-17A in the supernatant (A) and expression of RORγt (Rorc) (B) was measured in whole-brain homogenates. (C to G) Levels of Th2-associated cytokines IL-4, IL-5, IL-10, and IL-13 (C to F) and expression of Gata3 (G) were measured at the same time points. Each brain was homogenized in 5 ml of medium. Naive mice (N) and animals that succumbed to infection and were euthanized between days 31 to 33 postinfection (†) are indicated. There was not significant induction of any cytokines measured. Data shown are the means ± SEM from a representative experiment of two to four independent experiments with three to eight mice per time point. Download FIG S2, EPS file, 1.1 MB (1.1MB, eps) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Partial depletion of CD4+ T cells in mice with cryptococcal meningoencephalitis. C57BL/6 mice received two doses of anti-CD4+ depleting antibody (300 μg; GK1.5; BioXCell) on day 0 and day 10 postinfection with Cryptococcus neoformans 52D which resulted in only partial (~30%) depletion of CD4+ T cells through day 35 postinfection. (A and B) The total number of CD4+ T cells (A) was calculated from surviving CD4-depleted (white) animals compared to control (dark) animals on day 35 postinfection (B). (C and D) Brain fungal burdens (C) and MCBS scores (D) were also determined on day 35 postinfection. Data shown are the means ± SEM from one experiment with four to six mice per group. Download FIG S3, EPS file, 0.8 MB (841.5KB, eps) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Murine coma and behavioral scale.
A modified murine coma and behavioral scale (MCBS) to assess the overall physical and neurological condition of infected mice was adapted from the MCBS of Carroll et al. (49) using expanded scoring criteria to reflect the more subtle degree of symptoms observed in mice with cryptococcal infection. Additional smell, vision, and touch reflexes were also incorporated. Briefly, mice were scored on a scale of 0 to 3 for exploration, balance, gait, body posture, coat condition, grip strength, reflexes (body, neck, pinna and footpad reflexes), and response to visual and olfactory stimuli. Scores are reported with a maximum of 36 possible points, with lower scores reflecting more-pronounced symptoms. Detailed scoring criteria are in Table S1.
Cell isolation.
Mice were euthanized with CO2 and then perfused with PBS. The brains were aseptically removed, transferred to GentleMACs C tubes containing 5 ml of sterile complete RPMI 1640 (with 5% fetal bovine serum [FBS], 25 mM HEPES, GlutaMAX, penicillin-streptomycin, nonessential amino acids, sodium pyruvate, and beta-mercaptoethanol) supplemented with collagenase (50 μg/ml; Roche) and DNase (100 U/ml; Worthington). The tissue was minced and then processed on a GentleMACs homogenizer (Miltenyi). Small samples of the homogenate were collected immediately after processing for whole-brain fungal burden and RNA and cytokine measurements. The remaining homogenate was washed with RPMI 1640 and filtered through a 70-μm cell strainer. A discontinuous 30%/70% Percoll (GE Healthcare) gradient was used to remove cell debris, myelin, and neurons, and then microglia and brain-infiltrating leukocytes (BIL) were recovered from the interface. Isolated cells were washed twice to remove residual Percoll before use in assays. Total cell numbers were determined by counting live cells on a hemocytometer with trypan blue.
Flow cytometry.
Isolated cells were stained with fixable live/dead dye (Life Technologies), blocked with anti-CD16/32, and stained with antibodies for CD45, CD3, CD4, CD8, CD11b, CD11c, Ly6C, Ly6G, major histocompatibility complex class II (MHCII), and gamma interferon (IFN-γ). All antibodies were purchased from BioLegend. For IFN-γ production, the cells were stimulated for 6 h with phorbol myristate acetate (PMA) and ionomycin in the presence of brefeldin A and monensin for the final 4 h. The cells were stained for extracellular markers and then fixed with fixation/permeabilization buffer (eBioscience), and intracellular staining was performed in permeabilization buffer. Fluorescence minus one (FMO) controls were used for all experiments. Data were collected on an LSRII cytometer (BD) and analyzed using FlowJo (Treestar).
Fungal burdens.
To enumerate fungal burdens, samples of whole-brain homogenate were serially diluted in distilled water, and 10-μl aliquots were deposited on Sabouraud dextrose agar. The number of CFU per whole brain was calculated from the average of replicate counts.
CD4+ T cell depletion.
Mice were depleted of CD4+ T cells via intraperitoneal injection of monoclonal anti-CD4 antibody (GK1.5; 300 μg) on days −2, 0, and 5 postinfection and then weekly thereafter. Control animals received corresponding anti-keyhole limpet hemocyanin (anti-KLH) isotype antibody (LTF-2; 300 μg). All depleting antibodies were purchased in in vivo grade purity from BioXCell, West Lebanon, NH. This regimen resulted in >99% depletion of CD4+ T cells in the brains, blood, and spleens of infected mice.
Cytokine and gene expression.
Cytokine protein levels were measured from the supernatants of whole-brain homogenate after centrifugation (600 × g, 5 min) using LegendPlex cytometric bead assays (BioLegend) according to the manufacturer’s instructions. For gene expression, RNA was isolated from the whole-brain cell pellets using TRIzol (Life Technologies) and converted to cDNA using Quantitech reverse-transcription kit (Qiagen). Gene expression was calculated relative to Gapdh using SYBR-based amplification (Radiant Green master mix; Alkali Science) on a LightCycler 96 thermocycler (Roche) and the 2−ΔΔCt method.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism v6 software with Student’s t test or analysis of variance (ANOVA) plus Tukey’s posthoc test for multiple comparisons. Asterisks on figures (in graphs or in the corners of flow cytometry plots) indicate statistical significance as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
ACKNOWLEDGMENTS
We thank Alison J. Eastman and Benjamin H. Singer for thoughtful advice and discussions.
This work was supported by Veterans Administration Merit Review Awards to M.A.O. (1I01BX000656), B.M.S. (1I01RX000416 and 1I01BX001387), and J.J.O. (BX002120-01), VA RCS Award M.A.O. (1IK6BX003615), as well as funding from NIH to B.M.S. (NINDS, R01 NS057670) and L.M.N. (NHLBI, T32 HL007749). P.R.W. was supported by the Intramural Research Program of the NIAID (AI001123 and AI001124) and extramural awards (U01 AI109657). We also thank the University of Michigan UROP for supporting E.X. and J.L.K.
The funding agencies had no role in study design, data collection or interpretation, or decision to publish the manuscript.
Footnotes
Citation Neal LM, Xing E, Xu J, Kolbe JL, Osterholzer JJ, Segal BM, Williamson PR, Olszewski MA. 2017. CD4+ T cells orchestrate lethal immune pathology despite fungal clearance during Cryptococcus neoformans meningoencephalitis. mBio 8:e01415-17. https://doi.org/10.1128/mBio.01415-17.
REFERENCES
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Pappas PG. 2013. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc 124:61–79. [PMC free article] [PubMed] [Google Scholar]
- 3.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. 2017. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. 2006. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis 43:1069–1073. doi: 10.1086/507895. [DOI] [PubMed] [Google Scholar]
- 5.Saag MS, Graybill RJ, Larsen RA, Pappas PG, Perfect JR, Powderly WG, Sobel JD, Dismukes WE. 2000. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis 30:710–718. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 6.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saag MS, Powderly WG, Cloud GA, Robinson P, Grieco MH, Sharkey PK, Thompson SE, Sugar AM, Tuazon CU, Fisher JF, Hyslop N, Jacobson JM, Hafner R, Dismukes WE. 1992. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med 326:83–89. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- 8.Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, Lancaster DJ, Henderson H, Kauffman CA, Haas DW, Saccente M, Hamill RJ, Holloway MS, Warren RM, Dismukes WE. 2001. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis 33:690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 9.Lee YC, Wang JT, Sun HY, Chen YC. 2011. Comparisons of clinical features and mortality of cryptococcal meningitis between patients with and without human immunodeficiency virus infection. J Microbiol Immunol Infect 44:338–345. doi: 10.1016/j.jmii.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. 2013. Epidemiology of cryptococcal meningitis in the US: 1997-2009. PLoS One 8:e56269. doi: 10.1371/journal.pone.0056269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musubire AK, Boulware DR, Meya DB, Rhein J. 2013. Diagnosis and management of cryptococcal relapse. J AIDS Clin Res Suppl 3:S3-003. doi: 10.4172/2155-6113.S3-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arora S, Olszewski MA, Tsang TM, McDonald RA, Toews GB, Huffnagle GB. 2011. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infect Immun 79:1915–1926. doi: 10.1128/IAI.01270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen GH, McNamara DA, Hernandez Y, Huffnagle GB, Toews GB, Olszewski MA. 2008. Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model. Infect Immun 76:2379–2391. doi: 10.1128/IAI.01143-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain AV, Zhang Y, Fields WB, McNamara DA, Choe MY, Chen GH, Erb-Downward J, Osterholzer JJ, Toews GB, Huffnagle GB, Olszewski MA. 2009. Th2 but not Th1 immune bias results in altered lung functions in a murine model of pulmonary Cryptococcus neoformans infection. Infect Immun 77:5389–5399. doi: 10.1128/IAI.00809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murdock BJ, Huffnagle GB, Olszewski MA, Osterholzer JJ. 2014. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and gamma interferon production. Infect Immun 82:937–948. doi: 10.1128/IAI.01477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Wang F, Tompkins KC, McNamara A, Jain AV, Moore BB, Toews GB, Huffnagle GB, Olszewski MA. 2009. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol 175:2489–2500. doi: 10.2353/ajpath.2009.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardison SE, Ravi S, Wozniak KL, Young ML, Olszewski MA, Wormley FL Jr. 2010. Pulmonary infection with an interferon-gamma-producing Cryptococcus neoformans strain results in classical macrophage activation and protection. Am J Pathol 176:774–785. doi: 10.2353/ajpath.2010.090634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wozniak KL, Ravi S, Macias S, Young ML, Olszewski MA, Steele C, Wormley FL. 2009. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PLoS One 4:e6854. doi: 10.1371/journal.pone.0006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wozniak KL, Young ML, Wormley FL Jr. 2011. Protective immunity against experimental pulmonary cryptococcosis in T cell-depleted mice. Clin Vaccine Immunol 18:717–723. doi: 10.1128/CVI.00036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huffnagle GB, McNeil LK. 1999. Dissemination of C. neoformans to the central nervous system: role of chemokines, Th1 immunity and leukocyte recruitment. J Neurovirol 5:76–81. doi: 10.3109/13550289909029748. [DOI] [PubMed] [Google Scholar]
- 21.Uicker WC, McCracken JP, Buchanan KL. 2006. Role of CD4+ T cells in a protective immune response against Cryptococcus neoformans in the central nervous system. Med Mycol 44:1–11. doi: 10.1080/13693780500088424. [DOI] [PubMed] [Google Scholar]
- 22.Aguirre K, Crowe J, Haas A, Smith J. 2004. Resistance to Cryptococcus neoformans infection in the absence of CD4+ T cells. Med Mycol 42:15–25. doi: 10.1080/378032000141732-1. [DOI] [PubMed] [Google Scholar]
- 23.Uicker WC, Doyle HA, McCracken JP, Langlois M, Buchanan KL. 2005. Cytokine and chemokine expression in the central nervous system associated with protective cell-mediated immunity against Cryptococcus neoformans. Med Mycol 43:27–38. doi: 10.1080/13693780410001731510. [DOI] [PubMed] [Google Scholar]
- 24.Buchanan KL, Doyle HA. 2000. Requirement for CD4+ T lymphocytes in host resistance against Cryptococcus neoformans in the central nervous system of immunized mice. Infect Immun 68:456–462. doi: 10.1128/IAI.68.2.456-462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvis JN, Meintjes G, Rebe K, Williams GN, Bicanic T, Williams A, Schutz C, Bekker LG, Wood R, Harrison TS. 2012. Adjunctive interferon-gamma immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS 26:1105–1113. doi: 10.1097/QAD.0b013e3283536a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, Harrison TS. 2017. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol 13:13–24. doi: 10.1038/nrneurol.2016.167. [DOI] [PubMed] [Google Scholar]
- 27.Panackal AA, Williamson KC, van de Beek D, Boulware DR, Williamson PR. 2016. Fighting the monster: applying the host damage framework to human central nervous system infections. mBio 7:e01906-15. doi: 10.1128/mBio.01906-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammoud DA, Mahdi E, Panackal AA, Wakim P, Sheikh V, Sereti I, Bielakova B, Bennett JE, Williamson PR. 2017. Choroid plexitis and ependymitis by magnetic resonance imaging are biomarkers of neuronal damage and inflammation in HIV-negative cryptococcal meningoencephalitis. Sci Rep 7:9184. doi: 10.1038/s41598-017-09694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shelburne SA III, Darcourt J, White AC Jr, Greenberg SB, Hamill RJ, Atmar RL, Visnegarwala F. 2005. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis 40:1049–1052. doi: 10.1086/428618. [DOI] [PubMed] [Google Scholar]
- 30.Wiesner DL, Boulware DR. 2011. Cryptococcus-related immune reconstitution inflammatory syndrome (IRIS): pathogenesis and its clinical implications. Curr Fungal Infect Rep 5:252–261. doi: 10.1007/s12281-011-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eschke M, Piehler D, Schulze B, Richter T, Grahnert A, Protschka M, Müller U, Köhler G, Höfling C, Rossner S, Alber G. 2015. A novel experimental model of Cryptococcus neoformans-related immune reconstitution inflammatory syndrome (IRIS) provides insights into pathogenesis. Eur J Immunol 45:3339–3350. doi: 10.1002/eji.201545689. [DOI] [PubMed] [Google Scholar]
- 32.Panackal AA, Wuest SC, Lin YC, Wu T, Zhang N, Kosa P, Komori M, Blake A, Browne SK, Rosen LB, Hagen F, Meis J, Levitz SM, Quezado M, Hammoud D, Bennett JE, Bielekova B, Williamson PR. 2015. Paradoxical immune responses in non-HIV cryptococcal meningitis. PLoS Pathog 11:e1004884. doi: 10.1371/journal.ppat.1004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panackal AA, Komori M, Kosa P, Khan O, Hammoud DA, Rosen LB, Browne SK, Lin YC, Romm E, Ramaprasad C, Fries BC, Bennett JE, Bielekova B, Williamson PR. 2017. Spinal arachnoiditis as a complication of cryptococcal meningoencephalitis in non-HIV previously healthy adults. Clin Infect Dis 64:275–283. doi: 10.1093/cid/ciw739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima H, Takayama A, Fujiki Y, Ito T, Kitaoka H. 2015. Refractory Cryptococcus neoformans meningoencephalitis in an immunocompetent patient: paradoxical antifungal therapy-induced clinical deterioration related to an immune response to cryptococcal organisms. Case Rep Neurol 7:204–208. doi: 10.1159/000440948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williamson PR. 2015. Post-infectious inflammatory response syndrome (PIIRS): dissociation of T-cell-macrophage signaling in previously healthy individuals with cryptococcal fungal meningoencephalitis. Macrophage 2:e1078. doi: 10.14800/Macrophage.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boulware DR, Bonham SC, Meya DB, Wiesner DL, Park GS, Kambugu A, Janoff EN, Bohjanen PR. 2010. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis 202:962–970. doi: 10.1086/655785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulware DR, Meya DB, Bergemann TL, Wiesner DL, Rhein J, Musubire A, Lee SJ, Kambugu A, Janoff EN, Bohjanen PR. 2010. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med 7:e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang CC, Omarjee S, Lim A, Spelman T, Gosnell BI, Carr WH, Elliott JH, Moosa MY, Ndung'u T, French MA, Lewin SR. 2013. Chemokine levels and chemokine receptor expression in the blood and the cerebrospinal fluid of HIV-infected patients with cryptococcal meningitis and cryptococcosis-associated immune reconstitution inflammatory syndrome. J Infect Dis 208:1604–1612. doi: 10.1093/infdis/jit388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meya DB, Manabe YC, Boulware DR, Janoff EN. 2016. The immunopathogenesis of cryptococcal immune reconstitution inflammatory syndrome: understanding a conundrum. Curr Opin Infect Dis 29:10–22. doi: 10.1097/QCO.0000000000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meya DB, Okurut S, Zziwa G, Rolfes MA, Kelsey M, Cose S, Joloba M, Naluyima P, Palmer BE, Kambugu A, Mayanja-Kizza H, Bohjanen PR, Eller MA, Wahl SM, Boulware DR, Manabe YC, Janoff EN. 2015. Cellular immune activation in cerebrospinal fluid from Ugandans with cryptococcal meningitis and immune reconstitution inflammatory syndrome. J Infect Dis 211:1597–1606. doi: 10.1093/infdis/jiu664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips P, Chapman K, Sharp M, Harrison P, Vortel J, Steiner T, Bowie W. 2009. Dexamethasone in Cryptococcus gattii central nervous system infection. Clin Infect Dis 49:591–595. doi: 10.1086/603554. [DOI] [PubMed] [Google Scholar]
- 42.Mehta GU, Panackal AA, Murayi R, Bennett JE, Williamson PR, Chittiboina P 26 May 2017. Corticosteroids for shunted previously healthy patients with non-HIV cryptococcal meningoencephalitis. J Neurol Neurosurg Psychiatry doi: 10.1136/jnnp-2017-315830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chrétien F, Lortholary O, Kansau I, Neuville S, Gray F, Dromer F. 2002. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J Infect Dis 186:522–530. doi: 10.1086/341564. [DOI] [PubMed] [Google Scholar]
- 44.Olszewski MA, Noverr MC, Chen GH, Toews GB, Cox GM, Perfect JR, Huffnagle GB. 2004. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am J Pathol 164:1761–1771. doi: 10.1016/S0002-9440(10)63734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Q, Gault RA, Kozel TR, Murphy WJ. 2007. Protection from direct cerebral Cryptococcus infection by interferon-gamma-dependent activation of microglial cells. J Immunol 178:5753–5761. doi: 10.4049/jimmunol.178.9.5753. [DOI] [PubMed] [Google Scholar]
- 46.Stenzel W, Müller U, Köhler G, Heppner FL, Blessing M, McKenzie AN, Brombacher F, Alber G. 2009. IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. Am J Pathol 174:486–496. doi: 10.2353/ajpath.2009.080598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aguirre K, Miller S. 2002. MHC class II-positive perivascular microglial cells mediate resistance to Cryptococcus neoformans brain infection. Glia 39:184–188. doi: 10.1002/glia.10093. [DOI] [PubMed] [Google Scholar]
- 48.Pool A, Lowder L, Wu Y, Forrester K, Rumbaugh J. 2013. Neurovirulence of Cryptococcus neoformans determined by time course of capsule accumulation and total volume of capsule in the brain. J Neurovirol 19:228–238. doi: 10.1007/s13365-013-0169-7. [DOI] [PubMed] [Google Scholar]
- 49.Carroll RW, Wainwright MS, Kim KY, Kidambi T, Gómez ND, Taylor T, Haldar K. 2010. A rapid murine coma and behavior scale for quantitative assessment of murine cerebral malaria. PLoS One 5:e13124. doi: 10.1371/journal.pone.0013124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldman D, Song X, Kitai R, Casadevall A, Zhao ML, Lee SC. 2001. Cryptococcus neoformans induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta in human microglia: role of specific antibody and soluble capsular polysaccharide. Infect Immun 69:1808–1815. doi: 10.1128/IAI.69.3.1808-1815.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee SC, Kress Y, Zhao ML, Dickson DW, Casadevall A. 1995. Cryptococcus neoformans survive and replicate in human microglia. Lab Invest 73:871–879. [PubMed] [Google Scholar]
- 52.Lipovsky MM, Juliana AE, Gekker G, Hu S, Hoepelman AI, Peterson PK. 1998. Effect of cytokines on anticryptococcal activity of human microglial cells. Clin Diagn Lab Immunol 5:410–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Redlich S, Ribes S, Schütze S, Eiffert H, Nau R. 2013. Toll-like receptor stimulation increases phagocytosis of Cryptococcus neoformans by microglial cells. J Neuroinflamm 10:71. doi: 10.1186/1742-2094-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beardsley J, Wolbers M, Kibengo FM, Ggayi AB, Kamali A, Cuc NT, Binh TQ, Chau NV, Farrar J, Merson L, Phuong L, Thwaites G, Van Kinh N, Thuy PT, Chierakul W, Siriboon S, Thiansukhon E, Onsanit S, Supphamongkholchaikul W, Chan AK, Heyderman R, Mwinjiwa E, van Oosterhout JJ, Imran D, Basri H, Mayxay M, Dance D, Phimmasone P, Rattanavong S, Lalloo DG, Day JN, CryptoDex Investigators . 2016. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med 374:542–554. doi: 10.1056/NEJMoa1509024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siddiqui AA, Brouwer AE, Wuthiekanun V, Jaffar S, Shattock R, Irving D, Sheldon J, Chierakul W, Peacock S, Day N, White NJ, Harrison TS. 2005. IFN-gamma at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J Immunol 174:1746–1750. doi: 10.4049/jimmunol.174.3.1746. [DOI] [PubMed] [Google Scholar]
- 56.Olszewski MA, Huffnagle GB, Traynor TR, McDonald RA, Cook DN, Toews GB. 2001. Regulatory effects of macrophage inflammatory protein 1alpha/CCL3 on the development of immunity to Cryptococcus neoformans depend on expression of early inflammatory cytokines. Infect Immun 69:6256–6263. doi: 10.1128/IAI.69.10.6256-6263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guillot L, Carroll SF, Homer R, Qureshi ST. 2008. Enhanced innate immune responsiveness to pulmonary Cryptococcus neoformans infection is associated with resistance to progressive infection. Infect Immun 76:4745–4756. doi: 10.1128/IAI.00341-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hernandez Y, Arora S, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. 2005. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J Immunol 174:1027–1036. doi: 10.4049/jimmunol.174.2.1027. [DOI] [PubMed] [Google Scholar]
- 59.Murdock BJ, Teitz-Tennenbaum S, Chen GH, Dils AJ, Malachowski AN, Curtis JL, Olszewski MA, Osterholzer JJ. 2014. Early or late IL-10 blockade enhances Th1 and Th17 effector responses and promotes fungal clearance in mice with cryptococcal lung infection. J Immunol 193:4107–4116. doi: 10.4049/jimmunol.1400650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arora S, Hernandez Y, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J Immunol 174:6346–6356. doi: 10.4049/jimmunol.174.10.6346. [DOI] [PubMed] [Google Scholar]
- 61.Wiesner DL, Smith KD, Kotov DI, Nielsen JN, Bohjanen PR, Nielsen K. 2016. Regulatory T cell induction and retention in the lungs drives suppression of detrimental type 2 Th cells during pulmonary cryptococcal infection. J Immunol 196:365–374. doi: 10.4049/jimmunol.1501871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casadevall A, Pirofski LA. 2003. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol 1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hermsen C, van de Wiel T, Mommers E, Sauerwein R, Eling W. 1997. Depletion of CD4+ or CD8+ T-cells prevents Plasmodium berghei induced cerebral malaria in end-stage disease. Parasitology 114:7–12. doi: 10.1017/S0031182096008293. [DOI] [PubMed] [Google Scholar]
- 64.Campanella GS, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA, Colvin RA, Luster AD. 2008. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci U S A 105:4814–4819. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miu J, Mitchell AJ, Müller M, Carter SL, Manders PM, McQuillan JA, Saunders BM, Ball HJ, Lu B, Campbell IL, Hunt NH. 2008. Chemokine gene expression during fatal murine cerebral malaria and protection due to CXCR3 deficiency. J Immunol 180:1217–1230. doi: 10.4049/jimmunol.180.2.1217. [DOI] [PubMed] [Google Scholar]
- 66.Kim JV, Kang SS, Dustin ML, McGavern DB. 2009. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaddi PJ, Yap GS. 2007. Cytokine regulation of immunopathology in toxoplasmosis. Immunol Cell Biol 85:155–159. doi: 10.1038/sj.icb.7100038. [DOI] [PubMed] [Google Scholar]
- 68.Lu F, Huang S, Kasper LH. 2004. CD4+ T cells in the pathogenesis of murine ocular toxoplasmosis. Infect Immun 72:4966–4972. doi: 10.1128/IAI.72.9.4966-4972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rb-Silva R, Nobrega C, Reiriz E, Almeida S, Sarmento-Castro R, Correia-Neves M, Horta A. 2017. Toxoplasmosis-associated IRIS involving the CNS: a case report with longitudinal analysis of T cell subsets. BMC Infect Dis 17:66. doi: 10.1186/s12879-016-2159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carbajal KS, Mironova Y, Ulrich-Lewis JT, Kulkarni D, Grifka-Walk HM, Huber AK, Shrager P, Giger RJ, Segal BM. 2015. Th cell diversity in experimental autoimmune encephalomyelitis and multiple sclerosis. J Immunol 195:2552–2559. doi: 10.4049/jimmunol.1501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scriven JE, Rhein J, Hullsiek KH, von Hohenberg M, Linder G, Rolfes MA, Williams DA, Taseera K, Meya DB, Meintjes G, Boulware DR, COAT Team . 2015. Early ART after cryptococcal meningitis is associated with cerebrospinal fluid pleocytosis and macrophage activation in a multisite randomized trial. J Infect Dis 212:769–778. doi: 10.1093/infdis/jiv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neal LM, Qiu Y, Chung J, Xing E, Cho W, Malachowski AN, Sandy-Sloat AR, Osterholzer JJ, Maillard I, Olszewski MA. 2017. T cell-restricted notch signaling contributes to pulmonary Th1 and Th2 immunity during Cryptococcus neoformans infection. J Immunol 199:643–655. doi: 10.4049/jimmunol.1601715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Modified murine coma and behavioral scale (MCBS) score chart. A modified MCBS scoring system was adapted and expanded from the MCBS scoring system of Carroll et al. (R. W. Carroll et al, PLoS One 5:e13124, 2010, https://doi.org/10.1371/journal.pone.0013124). Individual mice were placed into the corner of a clean, overturned microisolator cage lid. Each mouse was allowed to freely explore the lid while the above observations on gait, balance, exploration, body position, and coat condition were scored over the course of 90 s. For neck, body, and pinna touch scoring, the appropriate body area was touched with a pen or open forceps while the animal was free in the cage lid. Next, each mouse was placed atop wire cage hoppers, and each hind limb footpad was gently grasped with forceps to score toe touch reflexes. blink reflexes in response to an approaching pen or forceps. Grasp strength and sense of smell were measured by investigation with a coffee-soaked cotton swab were also assessed while the mouse was on the wire hopper. We note that the phenotype of most parameters we observed in mice with C. neoformans meningoencephalitis were more subtle than observed in Carroll et al., so our scoring criteria were accordingly adjusted as described in Materials and Methods. Download TABLE S1, DOCX file, 0.02 MB (16.9KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Effect of inoculum size on establishment and cellular inflammation during C. neoformans meningoencephalitis. (A and B) C57BL/6 mice were infected with 104 or 106 CFU of C. neoformans 52D, and then fungal burdens (A) and the total numbers of leukocyte-enriched brain cells (B) were determined at 21 days postinfection. Naive animals (N) are shown as controls. Data shown are means ± SEM from three to six mice per group. Values that are statistically significantly different are indicated by asterisks as follows: ***, P < 0.001; **** P < 0.0001. Download FIG S1, EPS file, 0.5 MB (576.8KB, eps) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Absence of Th17 and Th2 polarization in the CNSs of mice with cryptococcal meningoencephalitis. (A and B) C57BL/6 mice were infected with C. neoformans 52D, and the levels of Th17-associated IL-17A in the supernatant (A) and expression of RORγt (Rorc) (B) was measured in whole-brain homogenates. (C to G) Levels of Th2-associated cytokines IL-4, IL-5, IL-10, and IL-13 (C to F) and expression of Gata3 (G) were measured at the same time points. Each brain was homogenized in 5 ml of medium. Naive mice (N) and animals that succumbed to infection and were euthanized between days 31 to 33 postinfection (†) are indicated. There was not significant induction of any cytokines measured. Data shown are the means ± SEM from a representative experiment of two to four independent experiments with three to eight mice per time point. Download FIG S2, EPS file, 1.1 MB (1.1MB, eps) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Partial depletion of CD4+ T cells in mice with cryptococcal meningoencephalitis. C57BL/6 mice received two doses of anti-CD4+ depleting antibody (300 μg; GK1.5; BioXCell) on day 0 and day 10 postinfection with Cryptococcus neoformans 52D which resulted in only partial (~30%) depletion of CD4+ T cells through day 35 postinfection. (A and B) The total number of CD4+ T cells (A) was calculated from surviving CD4-depleted (white) animals compared to control (dark) animals on day 35 postinfection (B). (C and D) Brain fungal burdens (C) and MCBS scores (D) were also determined on day 35 postinfection. Data shown are the means ± SEM from one experiment with four to six mice per group. Download FIG S3, EPS file, 0.8 MB (841.5KB, eps) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.