Abstract
We undertook an exclusive meta‐analysis of cohort studies investigating the possible link between prenatal selective serotonin reuptake inhibitor (SSRI) exposure and autism spectrum disorders (ASD) in children to further investigate our previous suggestion of confounding by indication. The point estimates regarding the following cohorts were extracted and pooled: (1) pregnant women who discontinued SSRI until 3 months before pregnancy; (2) pregnant women who were exposed to SSRI during pregnancy; and (3) pregnant women with maternal psychiatric disorder but no exposure to SSRI during pregnancy. Although the pooled point estimate of the first cohort showed a trend for increase, it did not reach significance. The pooled point estimates of the latter cohorts showed a significant association with ASD which strengthens our previous suggestion of confounding by indication. Future studies should be adequately designed to differentiate whether the previously suggested association is a result of maternal psychiatric disorder or SSRI exposure or both.
Keywords: antidepressants, autism, maternal psychiatric disorder, pregnancy, selective serotonin reuptake inhibitors
What is Already Known about this Subject
The question whether prenatal SSRI exposure is linked to autism spectrum disorders (ASD) in children remains inconclusive.
Most of the case–control studies, and their pooled results in relevant meta‐analyses, suggest an association.
Some cohort studies, however, report negative or inconsistent findings and suggest confounding by indication.
What this Study Adds
Data from cohort studies, exclusively, were pooled.
Maternal psychiatric disorder but no SSRI exposure during pregnancy was also found to be associated with a significantly increased risk of ASD, as well as SSRI exposure.
The similar effect sizes and largely overlapping confidence intervals make the previously suggested associations questionable.
Introduction
The question whether selective serotonin reuptake inhibitor (SSRI) use during pregnancy is associated with the risk of autism spectrum disorders (ASD) in children has been an increasing focus of research in the last decade 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18. The statistically significant association between SSRI use during pregnancy and risk of ASD in children was demonstrated by several studies 1, 4, 10, 12, yet these results were challenged by the negative findings in some others 6, 9, raising the possibility of confounding by indication, which is the term used when the clinical indication (e.g. depression) for choosing a treatment (e.g. SSRI) also affects the measured outcome (e.g. ASD) 19. There is still no conclusive answer even after the publication to‐date of eight case–control studies 1, 2, 3, 4, 5, 6, 7, 8, five cohort studies 9, 10, 11, 12, 13 and five meta‐analyses 14, 15, 16, 17, 18.
In our recent previous meta‐analysis of case–control studies, we demonstrated a significant association between SSRI exposure during the first (OR, 1.90; 95% CI 1.28–2.83), second (OR, 1.73; 95% CI 1.15–2.61) and anytime during pregnancy (OR, 1.66; 95% CI 1.23–2.23) and risk of ASD in children 17. However, we also detected an unanticipated yet significant association with preconception‐only SSRI exposure (use within the 3 months or 90 days prior to the last menstrual period (LMP) or estimated date of conception) with an effect size similar to the gestational exposure 17. In addition, our qualitative review of the four cohort studies 9, 10, 11, 12, 13 yielded some inconsistent/negative findings, which further challenged and weakened the association that we detected in the meta‐analysis of case–control studies. We suggested that a confounding by indication could not be ruled out 17.
The objective of this short report is to explore this suggestion with a meta‐analysis pooling exclusively cohort studies by including the recently published data by Malm et al. 13. This time we would like to test our previous hypothesis of confounding by indication through assessing whether the combined point estimate regarding the risk of ASD in children would suggest an association among the following three separate cohorts of pregnant women enrolled in cohort studies 9, 10, 11, 12, 13: (1) pregnant women who discontinued SSRI until 3 months before pregnancy; (2) pregnant women who were exposed to SSRI during pregnancy; and (3) pregnant women with maternal psychiatric disorder but no exposure to SSRI during pregnancy.
Methods
Search strategy
The search strategy was described previously 17. However, because the previous search was conducted from inception to 26 December 2015, we ran an additional search from 26 December 2015 to 4 February 2017 using the same strategy 17 in PubMed/MedLine, Cochrane Central Register of Controlled Trials and Reprotox®. No language or date restrictions were applied. The flow chart was prepared in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 20 and is presented in Figure 1.
Figure 1.
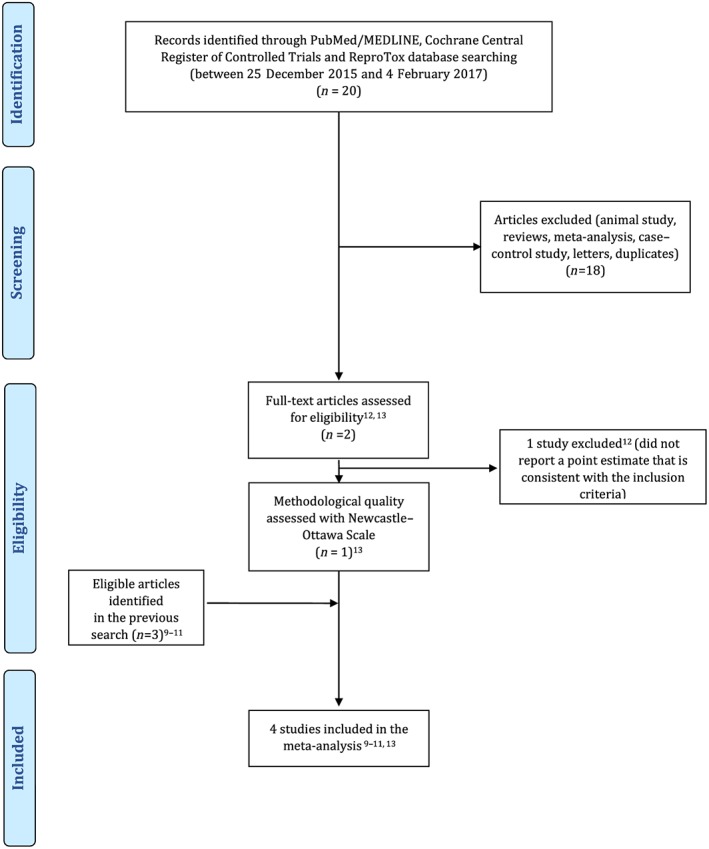
PRISMA flow diagram
Inclusion and exclusion criteria
Because we previously combined the data from the case–control studies 17, this time we only included the cohort studies investigating the link between prenatal exposure to SSRIs and ASD. A study was considered eligible if it met the following criteria: (1) a cohort of pregnant women with exposure to SSRIs any time during pregnancy was included; (2) a cohort of pregnant women who discontinued the SSRIs until 3 months before pregnancy or a cohort of pregnant women with maternal psychiatric disorder but no exposure to SSRIs during pregnancy was included; (3) a control (unexposed) group was included; (4) one of an odds ratio (OR), risk ratio (RR) or hazard ratio (HR) was reported; and (5) the data reported were not overlapping with another study. If an overlap between two studies was detected, the most recent one was included, after ensuring that both had similar methodological qualities. The exclusion criteria were case–control studies, case reports and series, animal studies, editorials and reviews.
Quality assessment
The Newcastle–Ottawa scale 21 was used for quality assessment of the study methodologies.
Outcome measures
The main outcome of interest for this meta‐analysis was ASD.
Data extraction
Two authors (EK‐A and SA) independently undertook the screening and data extraction process. The recently published data 13 were extracted using a standardized data extraction form and combined with our previously extracted data 17 and are presented in Table 1. Any disagreements were resolved by consulting with another author (YCK).
Table 1.
Characteristics of the cohort studies considered for this review
| Hviid et al. 2013 | Sorensen et al. 2013 | El Marroun et al. 2014 | Boukhris et al. 2015 a | Malm et al. 2016 | |
|---|---|---|---|---|---|
| Country | Denmark | Denmark | Netherlands | Canada | Finland |
| Study period | 1996–2005 | 1996–2006 | 2002–2006 | 1998–2009 | 1996–2010 |
| Design/setting | Registry‐based cohort | Registry‐based cohort | Registry‐based cohort | Registry‐based cohort | Registry‐based cohort |
| Data source | Danish National Prescription Registry, Danish Medical Birth Registry, Danish Psychiatric Central Register, Danish Civil Registration System | Danish National Prescription Registry, Danish Medical Birth Registry, Danish Psychiatric Central Register, Danish Civil Registration System, Danish National Hospital Register | Generation R Study Population‐Based Cohort | Québec Pregnancy/Children Cohort. Régiede l'assurance maladie du Québec, Québec centralized hospitalization archives databases, Public Prescription Drug Insurance database of Québec, Québec Statistics database |
National Medical Birth Register, The Drug Reimbursement Register, The Hospital Discharge Register, National Population Register The Special Reimbursement Register |
| Number of participants | 626 875 | 655 615 | 5976 | 145 456 | 845 345 |
| Number of events | |||||
| SSRI‐exposed: | Total: 6068 | Total: 7506 | Total: 69 | Total: 1583 | Total: 15 729 |
| ASD: 52 | ASD: 91 | ASD: Not reported | ASD: 22 | ASD: 88 | |
| Normal: 6016 | Normal: 7415 | Normal: Not reported | Normal: 1561 | Normal: 15 641 | |
| Unexposed: | Total: 620 807 | Total: 646 782 | Total: 5531 | Total: 142 924 | Total: 31 394 |
| ASD: 3752 | ASD: 5333 | ASD: Not reported | ASD: 1023 | ASD: 100 | |
| Normal: 617 055 | Normal: 641 449 | Normal: Not reported | Normal: 141 901 | Normal: 31 294 | |
| Inclusion criteria |
Live births in Denmark between 1 January 1996 and 31 December 2005 Known gestational age Singleton births |
Live births between 1 January 1996 and 31 December 2006 in the Danish Civil Registration System (CRS) |
All pregnant women residing in Rotterdam; delivered between April 2002 and January 2006 Children participated in the pre‐ and postnatal follow‐up and with information regarding behavioural and emotional problems |
Live births between 1 January 1998 and 31 December 2009 All full‐term (≥37 weeks' gestation) singleton infants whose mothers were covered by the Régiede l'assurance maladie du Québec drug plan for at least 12 months before and during pregnancy |
Live births in Finland between 1 January 1996 and 31 December 2010Singleton births For outcome variables (depression, anxiety, autism spectrum disorder, attention‐deficit/hyperactivity disorder) included only ICD codes after the diagnosis established |
| Exclusion criteria | Offspring with any of the following genetic conditions: fragile X syndrome, tuberous sclerosis, Angelman's syndrome, Down's syndrome, DiGeorge's syndrome, neurofibromatosis, and Prader–Willi syndrome) and congenital rubella syndrome |
Children with missing or extreme values of gestational age (<23 weeks and >45 weeks) Missing information about the mothers Adopted children Children who died during the first year of life |
If maternal SSRI use was unavailable If the use of SSRIs were before pregnancy only |
Multiple pregnancies Preterm live births |
Rett syndrome ICD codes which were used in the evaluation process Children with a depression diagnosis only during the first 2 years of life if the diagnosis was not recorded at later stages |
| Exposure | SSRIs; citalopram, fluoxetine, sertraline, paroxetine, escitalopram and fluvoxamine | SSRIs, TCA, SNRIs | SSRIs; paroxetine, fluoxetine, sertraline, fluvoxamine and citalopram | SSRIs, TCA, SNRIs, MAOI, other antidepressants; bupropion, amoxapine, maprotiline, mirtazapine, trazodone, and nefazodone | sertraline, fluvoxamine, escitalopram |
| Exposure time window | SSRIs with the ATC code N06AB that were filled during the period from 2 years before the beginning of the pregnancy until delivery | Women with antidepressant (ATC code N06A) prescriptions from 30 days before conception to the day of birth | Exposure to SSRIs during pregnancy | AD exposure as having at least one prescription filled at any time during pregnancy or a prescription filled before pregnancy that overlapped the first day of gestation or prescription filled 1 year before first day of gestation | At least one purchase of SSRIs during 1 year before pregnancy until 3 months before pregnancy and the period from 30 days before pregnancy until the end of pregnancy. |
| Control | Women with no SSRI prescriptions from 2 years before pregnancy through delivery | Women with no SSRI prescriptions from 30 days before conception to the day of birth |
No exposure to SSRIs and a low score of maternal depressive symptoms by Brief Symptom Inventory |
Infants with no in utero exposure to ADs |
Women with no purchases of antidepressants or antipsychotics, and no depression or related psychiatric disorder at any time before or during pregnancy Two controls matched with one participant exposed to SSRI according to offspring date of birth within 6 months |
| Method of ASD diagnosis | International Classification of Diseases, 10th Revision (ICD‐10) | International Classification of Diseases, 10th Revision (ICD‐10) | Child Behaviour Checklist, Social Responsiveness Scale |
International Classification of Diseases, Ninth Revision (ICD‐9) OR International Classification of Diseases, 10th Revision |
International Classification of Diseases, 10th Revision (ICD‐10) |
| Covariates for adjustment | Age and calendar period, the mother's age at birth, country of origin, place of residence, parity, psychiatric diagnoses before delivery, other drug use during pregnancy, smoking status during pregnancy, employment status, and level of education | Maternal and paternal age at conception, parental psychiatric history (except maternal affective disorder), gestational age, birth weight, sex, and parity | Maternal age at intake, gender of the child, maternal education, ethnicity, maternal smoking habits and gestational age at birth | Use of ADs 1 year before the first day of gestation, use of ADs in the first trimester, infant sex and year of birth, and maternal variables; maternal age at first day of gestation, high school completed [≥12 y], recipient of social assistance, living alone, chronic or gestational hypertension, chronic or gestational diabetes, and other psychiatric disorders | Sex, maternal age, socioeconomic status, maternal history of other psychiatric diagnosis, entitlement to special reimbursement for chronic disease (ever), preterm birth, neonatal care unit |
| Results relevant to this meta‐analysis |
SSRI discontinuation before pregnancy and the risk of autism: Use of SSRI before pregnancy (2 year to 6 months) but not use during pregnancy aRR: 1.46; 95% CI (1.17–1.81) SSRI exposure during pregnancy and the risk of autism: Use of SSRIs during pregnancy but not before pregnancy a RR: 1.40; 95% CI (0.92–2.13) Maternal psychiatric disorder but no SSRI exposure during pregnancy and the risk of autism: N/A |
SSRI discontinuation before pregnancy and the risk of ASD: Use of SSRI 180 days prior to conception a HR: 1.63; 95% CI (1.36–1.95) SSRI exposure during pregnancy and the risk of ASD: aHR: 1.6; 95% CI (1.3–2.0) Maternal psychiatric disorder but no SSRI exposure during pregnancy and the risk of ASD: N/A |
SSRI discontinuation before pregnancy and the risk of PDD: N/A SSRI exposure during pregnancy and the risk of PDD: OR: 2.58; 95% CI (1.46–4.54) Maternal psychiatric disorder but no SSRI exposure during pregnancy and the risk of PDD: OR: 2.02; 95% CI (1.53–2.66) |
SSRI discontinuation before pregnancy and the risk of ASD: N/A SSRI exposure during pregnancy and the risk of ASD: N/A for any time during pregnancy or first trimester, only available for the exposure during second and/or third trimester Maternal psychiatric disorder but no SSRI exposure during pregnancy and the risk of ASD: N/A |
SSRI discontinuation before pregnancy and the risk of ASD: aHR: 1.08; 95%CI (0.74–1.56) |
| SSRI exposure during pregnancy and the risk of ASD: aHR: 1.40; 95%CI (1.02–1.92) | |||||
| Maternal psychiatric disorder but no SSRI exposure during pregnancy and the risk of ASD: aHR:1.59; 95%CI (1.16–2.18) | |||||
|
Quality assessment
(Newcastle– Ottawa scale) |
****/**/*** | ****/**/*_* | ****/**/_** | ****/**/*_* | ****/**/*‐* |
| 9 | 8 | 8 | 8 | 8 | |
Excluded for not including a point estimate consistent with our inclusion criteria.
AD: antidepressant; MAOI: monoamine‐oxidase inhibitors; N/A: not available; PDD: pervasive developmental problems; SNRI: serotonin–norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressants.
Meta‐analytic methods
Point estimates (adjusted when available) were extracted from eligible cohort studies and combined using generic inverse variance method and random‐effects model in RevMan 5.3 (Review Manager 5.3; Cochrane Collaboration, Oxford, UK) 22. Heterogeneity was assessed utilizing the Q and I‐square statistic. An I‐square value between 25% and 50% signified low heterogeneity, between 50% and 75% moderate heterogeneity and >75% signified high heterogeneity 23. Publication bias was not assessed because the number of included studies was less than 10 24.
Results
Our search gleaned one additional cohort study by Malm et al. 13 published since our previous search. Because Boukhris et al. 12 did not report a point estimate consistent with our inclusion criteria, it was excluded. Four cohort studies were identified as eligible 9, 10, 11, 13. The data from Hviid et al. 9 and Sorensen et al. 10 were largely overlapping. We chose to progress with Hviid et al. 9 in our primary analysis as it was methodologically superior and included an SSRI‐discontinued group (use of SSRI starting from 2 years to 6 months before pregnancy) that is consistent with our inclusion criteria. However, we also extracted the point estimate of the SSRI‐exposed group from the published data of Sorensen et al. 10 and retrieved the unpublished point estimate of the SSRI‐discontinued group (use of SSRIs starting from 2 years to 6 months before pregnancy) from Sorensen et al. 10 through personal communication sensitivity analysis. Importantly, because El Marroun et al. did not measure and therefore did not provide an OR regarding ASD but did so regarding pervasive developmental problems (PDD), we extracted the OR regarding PDD (Model I) from this study 11. Because the reported point estimates differed across the studies, the combined point estimates were reported either as HR, RR or OR with regard to the particular analysis.
Meta‐analysis of cohort studies regarding SSRI‐discontinuation until 3 months before pregnancy and risk of ASD in children
Three studies were eligible for this meta‐analysis 9, 10, 13. Hviid et al. 9 reported a cohort of pregnant women who discontinued SSRI until 6 months before pregnancy while Malm et al. 13 included a cohort of pregnant women who discontinued SSRI until 3 months before pregnancy. The discontinuation of SSRIs until 3 months before pregnancy was not significantly associated with an increased risk of ASD in children when adjusted point estimate from each study was pooled (aRR, 1.31; 95% CI 0.98–1.74). No significant heterogeneity was detected among the studies (P = 0.17, I‐square = 46%) (Figure 2A). The point estimate remained non‐significant when Sorensen et al. 10 wsa included instead of Hviid et al. 9 with a non‐significant moderate heterogeneity (aHR, 1.37; 95% CI 0.92–2.04; P = 0.051, I‐square = 74%) (Figure 2B).
Figure 2.
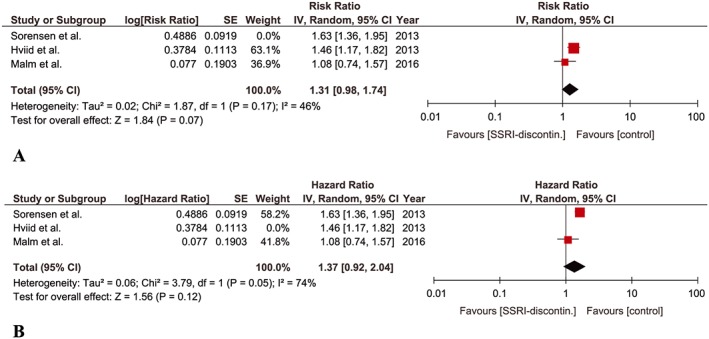
Meta‐analysis of cohort studies regarding SSRI‐discontinuation until 3 months before pregnancy and risk of ASD in children: (A) Forest plot of pooled adjusted risk ratios including Hviid et al. (B) Forest plot of pooled adjusted risk ratios including Sorensen et al.
Meta‐analysis of cohort studies regarding SSRI exposure during pregnancy and risk of ASD in children
Four studies were found eligible for this meta‐analysis 9, 10, 11, 13. The combined point estimate yielded a significant association between SSRI exposure during pregnancy and risk of ASD in children (aHR, 1.61; 95% CI 1.16–2.25). A low yet insignificant heterogeneity was detected among the studies (P = 0.15, I‐square = 46%) (Figure 3A). The point estimate remained significant when Sorensen et al. 10 was included instead of Hviid et al. 9 (aHR, 1.65; 95% CI 1.28–2.11; P = 0.18, I‐square = 41%, insignificant low heterogeneity) (Figure 3B).
Figure 3.
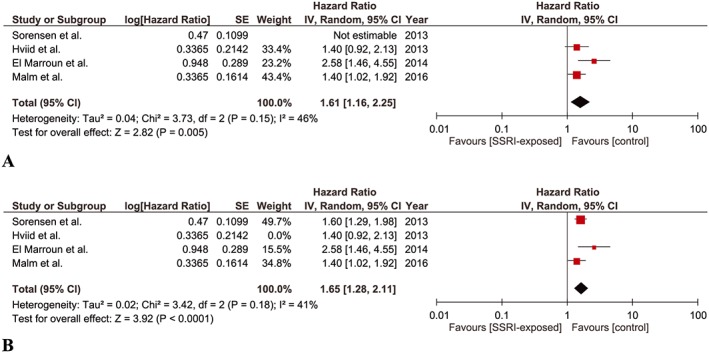
Meta‐analysis of cohort studies regarding SSRI exposure during pregnancy and risk of ASD in children: (A) Forest plot of pooled adjusted hazard ratios including Hviid et al. (B) Forest plot of pooled adjusted hazard ratios including Sorensen et al.
Meta‐analysis of cohort studies regarding maternal psychiatric disorder but no SSRI exposure during pregnancy and risk of ASD in children
Two studies were found eligible for this meta‐analysis 11, 13. We detected a significant association between maternal psychiatric disorder but no SSRI exposure during pregnancy and risk of ASD in children (aOR, 1.81; 95% CI 1.44–2.29). No significant heterogeneity was detected among the studies (P = 0.26, I‐square = 20%) (Figure 4).
Figure 4.

Meta‐analysis of cohort studies regarding maternal psychiatric disorder but no SSRI exposure during pregnancy and risk of ASD in children. Forest plot of pooled adjusted odds ratios
Discussion
In this meta‐analysis, both SSRI exposure any time during pregnancy and maternal psychiatric disorder but no SSRI exposure during pregnancy was demonstrated to be significantly associated with the risk of ASD in children. Although the combined point estimate for SSRI discontinuation until 3 months before pregnancy indicated a small increase in the risk of ASD, the trend was not significant.
The pooled effect size regarding SSRI exposure any time during pregnancy in this meta‐analysis of cohort studies was very similar to the pooled effect size of SSRI exposure any time during pregnancy of the case–control studies we computed in our previous meta‐analysis (aHR, 1.61; 95% CI 1.16–2.25 vs. aOR, 1.66; 95% CI 1.23–2.23) 17. This effect size was also very close to the reported effect size in a recent meta‐analysis investigating in utero SSRI exposure and the risk of ASD in children by Kobayashi et al. (aHR, 1.61; 95% CI 1.16–2.25 vs. aOR, 1.45; 95% CI 1.15–1.82) 16. Nevertheless, Kobayashi et al. reported that this significant association did not persist when the analysis was restricted to the mothers with psychiatric disorders 16, which is also corroborated by the significant association we detected between maternal psychiatric disorder but no SSRI exposure during pregnancy and the risk of ASD in children in our meta‐analysis. Of interest, in our meta‐analysis, the pooled effect size in maternal psychiatric disorder but no SSRI exposure cohort regarding ASD risk in children was slightly bigger than that of the pooled effect size in SSRI exposure during pregnancy cohort (aOR, 1.81 vs. 1.61) and the CI of both effects sizes were largely overlapping (1.44–2.29 vs. 1.16–2.25). Our meta‐analysis provides some advantages over the meta‐analysis by Kobayashi et al. 16 in terms of exclusively pooling cohort studies and comprising a recent cohort study 13 which was not available to them.
A combined summary of the findings of this and our previous meta‐analysis 17 is as follows:
The pooled results of cohort studies in this meta‐analysis suggest a trend for a mild increase in rişk of ASD with SSRI exposure until 3 months before pregnancy; however, it was not significant.
The pooled results of case–control studies suggest a significant association between preconception‐only (within 3 months or 90 days prior to the LMP or estimated date of conception) SSRI exposure and risk of ASD in children 17.
The pooled results of case–control studies suggest a significant association between SSRI exposure during first, second trimester and anytime during pregnancy and risk of ASD in children 17, while pooled results of the cohort studies in this meta‐analysis also suggest a significant moderate increase in risk of ASD with SSRI exposure at any time during pregnancy. The effect sizes were very similar.
The pooled results of the cohort studies in this study suggest a significant moderate increase in the risk of ASD with maternal psychiatric disorder but no exposure to SSRI during pregnancy with a slightly bigger effect size than that of SSRI exposure and with a largely overlapping confidence interval.
The most important limitation of our meta‐analysis is the low number of included studies. Nevertheless, a major strength of our meta‐analysis is the exclusive inclusion of cohort studies, which are less prone to bias in terms of assessing exposures during pregnancy. Our study is also the first meta‐analysis to pool the point estimates of SSRI‐discontinued and maternal psychiatric disorder but no SSRI exposure groups vs. SSRI exposure during pregnancy group.
In conclusion, this meta‐analysis showed that maternal psychiatric disorder but no SSRI exposure is also associated with increased risk of ASD in children. The similar effect size of this group to that of SSRI exposure any time during pregnancy group with a largely overlapping confidence interval strengthens our previous suggestion that the maternal psychiatric disorder may be a strong confounder in studies assessing the connection between maternal SSRI use and ASD risk in children 17. Although sought by an increasing number of studies and considered as biologically plausible 25, 26, 27, 28, 29, 30, the suggested association of SSRI exposure during pregnancy and risk of ASD in children still remains to be adequately substantiated.
Competing Interests
All authors have completed the Unified Competing Interest Form (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
We would like to express our sincere thanks to Dr. Merete Juul Sørensen for providing details of her study and providing unpublished data.
Kaplan, Y. C. , Keskin‐Arslan, E. , Acar, S. , and Sozmen, K. (2017) Maternal SSRI discontinuation, use, psychiatric disorder and the risk of autism in children: a meta‐analysis of cohort studies. Br J Clin Pharmacol, 83: 2798–2806. doi: 10.1111/bcp.13382.
References
- 1. Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68: 1104–1112. [DOI] [PubMed] [Google Scholar]
- 2. Eriksson MA, Westerlund J, Anderlid BM, Gillberg C, Fernell E. First‐degree relatives of young children with autism spectrum disorders: some gender aspects. Res Dev Disabil 2012; 33: 1642–1648. [DOI] [PubMed] [Google Scholar]
- 3. Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case–control study. BMJ 2013; 346: f2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gidaya NB, Lee BK, Burstyn I, Yudell M, Mortensen EL, Newschaffer CJ. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J Autism Dev Disord 2014; 44: 2558–2567. [DOI] [PubMed] [Google Scholar]
- 5. Harrington RA, Lee LC, Crum RM, Zimmerman AW, Hertz‐Picciotto I. Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics 2014; 133: e1241–e1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, et al Prenatal antidepressant exposure is associated with risk for attention‐deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20: 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Man K, Chan E, Coghill D, Ip P, Simonoff E, Lau W, et al Prenatal antidepressant exposure and the risk of autism spectrum disorder and attention‐deficit hyperactivity disorder. Pharmacoepidemiol Drug Saf 2015; 24 (Suppl.1): 234. [Google Scholar]
- 8. Castro VM, Kong SW, Clements CC, Brady R, Kaimal AJ, Doyle AE, et al Absence of evidence for increase in risk for autism or attention‐deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Transl Psychiatry 2016; 6: e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med 2013; 369: 2406–2415. [DOI] [PubMed] [Google Scholar]
- 10. Sørensen MJ, Grønborg TK, Christensen J, Parner ET, Vestergaard M, Schendel D, et al Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol 2013; 5: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Marroun H, White TJ, van der Knaap NJ, Homberg JR, Fernández G, Schoemaker NK, et al Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population‐based study of young children. Br J Psychiatry 2014; 205: 95–102. [DOI] [PubMed] [Google Scholar]
- 12. Boukhris T, Sheehy O, Mottron L, Bérard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr 2016; 170: 117–124. [DOI] [PubMed] [Google Scholar]
- 13. Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka‐Yli‐Salomäki S, McKeague IW, et al Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register‐based study. J Am Acad Child Adolesc Psychiatry 2016; 55: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Man KK, Tong HH, Wong LY, Chan EW, Simonoff E, Wong IC. Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder inchildren: a systematic review and meta‐analysis of observational studies. Neurosci Biobehav Rev 2015; 49: 82–89. [DOI] [PubMed] [Google Scholar]
- 15. Healy D, Le Noury J, Mangin D. Links between serotonin reuptake inhibition during pregnancy and neurodevelopmental delay/spectrum disorders: a systematic review of epidemiological and physiological evidence. Int J Risk Saf Med 2016; 28: 125–141. [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi T, Matsuyama T, Takeuchi M, Ito S. Autism spectrum disorder and prenatal exposure to selective serotonin reuptake inhibitors: a systematic review and meta‐analysis. Reprod Toxicol 2016; 65: 170–178. [DOI] [PubMed] [Google Scholar]
- 17. Kaplan YC, Keskin‐Arslan E, Acar S, Sozmen K. Prenatal selective serotonin reuptake inhibitor use and the risk of autism spectrum disorder in children: a systematic review and meta‐analysis. Reprod Toxicol 2016; 66: 31–43. [DOI] [PubMed] [Google Scholar]
- 18. Brown HK, Hussain‐Shamsy N, Lunsky Y, Dennis CE, Vigod SN. The association between antenatal exposure to selective serotonin reuptake inhibitors and autism: a systematic review and meta‐analysis. J Clin Psychiatry 2017; 78: e48–e58. [DOI] [PubMed] [Google Scholar]
- 19. Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA 2016; 316: 1818–1819. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 11 August 2017).
- 22. The Cochrane Collaboration . Cochrane handbook for systematic reviews of interventions, version 5.1.0. Available from http://handbook.cochrane.org/chapter_9/9_4_3_2_the_generic_inverse_variance_outcome_type_in_revman.htm (accessed 11 August 2017).
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006; 333: 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gur TL, Kim DR, Epperson CN. Central nervous system effects of prenatal selective serotonin reuptake inhibitors: sensing the signal through the noise. Psychopharmacology (Berl) 2013; 227: 567–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muller CL, Anacker AM, Veenstra‐VanderWeele J. The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience 2016; 321: 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schain RJ, Freedman DX. Studies on 5‐hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatr 1961; 58: 315–320. [DOI] [PubMed] [Google Scholar]
- 28. Hanley HG, Stahl SM, Freedman DX. Hyperserotonemia and amine metabolites in autistic and retarded children. Arch Gen Psychiatry 1977; 34: 521–531. [DOI] [PubMed] [Google Scholar]
- 29. Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, et al Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within‐group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry 2004; 43: 491–499. [DOI] [PubMed] [Google Scholar]
- 30. Gabriele S, Sacco R, Persico AM. Blood serotonin levels in autism spectrum disorder: a systematic review and meta‐analysis. Eur Neuropsychopharmacol 2014; 24: 919–929. [DOI] [PubMed] [Google Scholar]


