Muscle wasting is an extremely common feature of several chronic diseases associated with inflammatory activation. It can be part of a wasting syndrome known as cachexia 1, 2. The defining feature of cachexia is overt weight loss, but muscle wasting can also become prevalent without weight loss being detectable. This notion is important, because it highlights the necessity to not only measure weight, but also to assess strength as well as functional and exercise capacity in patients with advanced chronic disease, but also in healthy elderly subjects 3, 4. Relevant illnesses include, for example, chronic heart failure 5, chronic kidney disease 6, chronic obstructive pulmonary disease 7, rheumatoid arthritis 8, and cancer 9. Considering these conditions alone, it has been estimated that the number of patients at risk of developing cachexia is 17.5 million in Europe, whereas the number of patients actually being cachectic is close to 4 million 10.
Muscle wasting can be diagnosed, but it is more easily overlooked. Screening measures include the assessment of handgrip strength, the 6‐minute corridor walk test, or simple tools such as the short physical performance battery test. All of these are easily performed and take 30 s to no more than 10 min. However, the chances for the patient to actively complain about loss of strength are relatively low, and many elderly subjects consider loss of strength part of the normal ageing process rather than part of advancing disease. Measures to tackle the loss of muscle and strength and, thus, quality of life include nutritional interventions, exercise and, possibly, pharmacotherapy 11, 12, 13. The most promising drug classes in this regard that have seen research endeavour in recent years include myostatin inhibitors, ghrelin receptor agonists, selective androgen receptor modulators and anabolic steroids such as testosterone.
Testosterone was originally described by Kàroly David and colleagues in 1935 14. Its primary site of synthesis are the Leydig cells of the testis, and smaller amounts are released from the adrenal cortex and the ovaries. Therefore, plasma levels of testosterone in men are 10 times higher than those in women 15. Testosterone became available for therapeutic use as an injectable drug in the 1940s. In the 1970s, an orally available formulation was approved. Testosterone is metabolized to dihydrotestosterone and estradiol, both of which have feedback effects on luteinizing hormone secretion. This point is worth stressing, because some of the undesired effects of testosterone may be due to the formation of metabolites, rather than due to the effects of testosterone itself. Since steroid receptors just like the androgen receptor are expressed close to ubiquitously in humans, their blockade or activation can be associated with untoward effects. For example, supraphysiological testosterone levels have been associated with acne, dyslipidaemia, sleep apnoea, left ventricular hypertrophy, sodium and water retention, increases in renin‐angiotensin‐aldosterone system activity and blood pressure as well as increases in erythropoiesis 15, 16, 17. In addition, testosterone administration has been associated with increased cardiovascular mortality, prostate cancer and hepatic toxicity 15, 18, 19. On the other hand, testosterone can induce bone and muscle growth ultimately leading to increases in strength 20. Side effects of testosterone have mainly been described in patients who abuse the drug. In clinical settings, adverse effects have generally been mild and reversible 15. The mixture of desired and undesired effects is present in all steroids; however, it led to the idea of developing selective steroid receptor modulators (Figure 1) 21. The best known example in clinical use in this regard is tamoxifen, which functions as an oestrogen receptor antagonist in the breast and as an agonist in the uterus. Such selective receptor modulators have been in clinical development also as selective glucocorticoid receptor modulators, selective progesterone receptor modulators and selective androgen receptor modulators 21.
Figure 1.
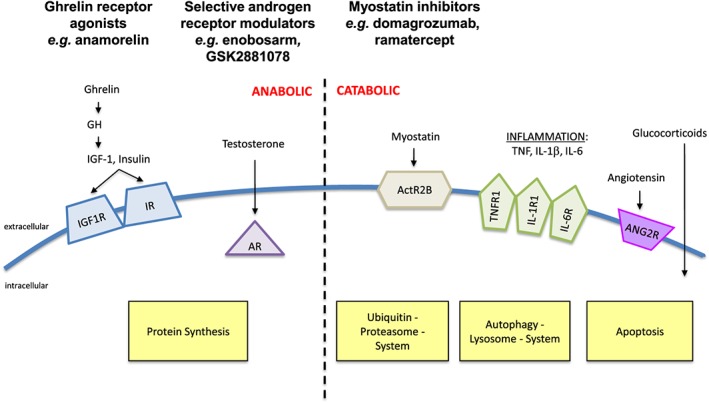
Mode of action of the most promising drugs for the treatment of muscle wasting. Ghrelin receptor agonists such as anamorelin or selective androgen receptor modulators such as enobosarm stimulate protein synthesis in the muscle. Like testosterone, the latter act via the androgen receptor. Myostatin is an anti‐anabolic substance. Myostatin inhibitors act via the ActR2B receptor and inhibit muscle breakdown
In a recent issue of BJCP, Clark and colleagues present a Phase I study of a novel selective androgen receptor modulator (SARM) named GSK2881078 22. With the European Medicines Agency's (EMA) rejection of the application for marketing authorization of anamorelin, a ghrelin receptor agonist, in May 2017 23, this class of drugs merits further scrutiny (Figure 1). Indeed, GSK2881078 adds to the list of SARMs in clinical development that include substances like DT‐100, enobosarm, ligandrol and MK‐0773 24. The only one of these to see Phase III testing is enobosarm; however, its development was unfortunately discontinued 25. Therefore, this new piece in the SARM puzzle comes as a welcome addition. On the other hand, it appears that all drugs that aim at treating muscle wasting struggle with the same problem: to improve both muscle mass and muscle function at the same time. Even though the two – muscle mass and muscle function – appear almost automatically linked, this has not been the case in the two major trials of anamorelin 26 and only partially in the two trials of enobosarm 27, 28. In fact, these trials have shown significant improvements in muscle mass, but the increase in strength or functional capacity has been less convincing.
Pharmacokinetic data of the new SARM GSK2881078 showed a rapid initial absorption and a long half‐life of more than 100 h with slightly higher values in women than men. Pharmacodynamics showed reductions in the serum levels of testosterone, dihydrotestosterone and sex‐hormone binding globulin relative to baseline. Overall safety was acceptable. In summary, no major surprises were revealed during Phase I testing, and the data may prompt testing in Phase II. Whatever endpoint the investigators may want to choose in a Phase II study, it should include testing of muscle function using easily applicable tests such as the 6‐minute walk test, the stair‐climbing power test, the short physical performance battery test or at least handgrip strength. Selecting the right patient population for such a study is another major challenge: patients with cancer cachexia, particularly in cancers of the lung or the pancreas, may simply be too sick to benefit from pharmacological treatment of muscle wasting. It may be worth selecting patients at an earlier stage such as in pre‐cachexia or sarcopenia with the aim of maintaining (rather than improving) muscle mass and function. Such questions are so important that regulators at the EMA or the Food and Drug Administration are requested to provide even more guidance in what they expect to achieve in a clinical trial of muscle wasting 29.
Competing Interests
S.v.H. has received consultant honoraria from Vifor Pharma, Chugai, Helsinn, Novartis, Pfizer and Boheringer Ingelheim.
von Haehling, S. (2017) Wasting away: How to treat cachexia and muscle wasting in chronic disease? Br J Clin Pharmacol, 83: 2599–2601. doi: 10.1111/bcp.13387.
References
- 1. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al Cachexia: a new definition. Clin Nutr 2008; 27: 793–799. [DOI] [PubMed] [Google Scholar]
- 2. von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle 2016; 7: 507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leong DP, Teo KK, Rangarajan S, Kutty VR, Lanas F, Hui C, et al Reference ranges of handgrip strength from 125,462 healthy adults in 21 countries: a prospective urban rural epidemiologic (PURE) study. J Cachexia Sarcopenia Muscle 2016; 7: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC‐F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016; 7: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol 2017; 14: 323–341. [DOI] [PubMed] [Google Scholar]
- 6. Ou SM, Chen YT, Hung SC, Shih CJ, Lin CH, Chiang CK, et al, Taiwan Geriatric Kidney Disease (TGKD) Research Group . Association of estimated glomerular filtration rate with all‐cause and cardiovascular mortality: the role of malnutrition‐inflammation‐cachexia syndrome. J Cachexia Sarcopenia Muscle 2016; 7: 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanders KJ, Kneppers AE, van de Bool C, Langen RC, Schols AM. Cachexia in chronic obstructive pulmonary disease: new insights and therapeutic perspective. J Cachexia Sarcopenia Muscle 2016; 7: 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Masuko K. Rheumatoid cachexia revisited: a metabolic co‐morbidity in rheumatoid arthritis. Front Nutr 2014; 1: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Penet MF, Bhujwalla ZM. Cancer cachexia, recent advances, and future directions. Cancer J 2015; 21: 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers – update 2014. J Cachexia Sarcopenia Muscle 2014; 5: 261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drescher C, Konishi M, Ebner N, Springer J. Loss of muscle mass: current developments in cachexia and sarcopenia focused on biomarkers and treatment. J Cachexia Sarcopenia Muscle 2015; 6: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mochamat, Cuhls H, Marinova M, Kaasa S, Stieber C, Conrad R, et al A systematic review on the role of vitamins, minerals, proteins, and other supplements for the treatment of cachexia in cancer: a European Palliative Care Research Centre cachexia project. J Cachexia Sarcopenia Muscle 2017; 8: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dev R, Wong A, Hui D, Bruera E. The evolving approach to management of cancer cachexia. Oncology (Williston Park) 2017; 31: 23–32. [PubMed] [Google Scholar]
- 14. David K, Dingemanse E, Freud J, Laqueur E. Über krystallinisches männliches Hormon aus Hoden (Testosteron) wirksamer als aus Harn oder aus Cholesterin bereitetes Androsteron. Hoppe Seylers Z Physiol Chem 1935; 233: 281. [Google Scholar]
- 15. Woerdeman J, de Ronde W. Therapeutic effects of anabolic androgenic steroids on chronic diseases associated with muscle wasting. Expert Opin Investig Drugs 2011; 20: 87–97. [DOI] [PubMed] [Google Scholar]
- 16. Quan A, Chakravarty S, Chen JK, Chen JC, Loleh S, Saini N, et al Androgens augment proximal tubule transport. Am J Physiol Renal Physiol 2004; 287: F452–F459. [DOI] [PubMed] [Google Scholar]
- 17. Bachman E, Travison TG, Basaria S, Davda MN, Guo W, Li M, et al Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 2014; 69: 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vigen R, O'Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, et al Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 2013; 310: 1829–1836. [DOI] [PubMed] [Google Scholar]
- 19. Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al Adverse events associated with testosterone administration. N Engl J Med 2010; 363: 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, et al The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 1996; 335: 1–7. [DOI] [PubMed] [Google Scholar]
- 21. Segal S, Narayanan R, Dalton JT. Therapeutic potential of the SARMs: revisiting the androgen receptor for drug discovery. Expert Opin Investig Drugs 2006; 15: 377–387. [DOI] [PubMed] [Google Scholar]
- 22. Clark RV, Walker AC, Andrews S, Turnbull P, Wald JA, Magee MH. Safety, pharmacokinetics and pharmacologic effects of the selective androgen receptor modulator, GSK2881078, in healthy men and postmenopausal women. Br J Clin Pharmacol 2017; 83: 2179–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Medicines Agency. Refusal of the marketing authorisation for Adlumiz (anamorelin hydrochloride). 19 May 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_‐_Initial_authorisation/human/003847/WC500228047.pdf (accessed 19/05/2017).
- 24. Saitoh M, Ishida J, Ebner N, Anker SD, von Haehling S. Myostatin inhibitors as pharmacological treatment for muscle wasting and muscular dystrophy. J Cachexia Sarcopenia Muscle Clin Rep 2017; in press. [Google Scholar]
- 25. Srinath R, Dobs A. Enobosarm (GTx‐024, S‐22): a potential treatment for cachexia. Future Oncol 2014; 10: 187–194. [DOI] [PubMed] [Google Scholar]
- 26. Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol 2016; 17: 519–531. [DOI] [PubMed] [Google Scholar]
- 27. Crawford J, Prado CM, Johnston MA, Gralla RJ, Taylor RP, Hancock ML, et al Study design and rationale for the phase 3 clinical development program of enobosarm, a selective androgen receptor modulator, for the prevention and treatment of muscle wasting in cancer patients (POWER trials). Curr Oncol Rep 2016; 18: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ebner N, Steinbeck L, Doehner W, Anker SD, von Haehling S. Highlights from the 7th Cachexia Conference: muscle wasting pathophysiological detection and novel treatment strategies. J Cachexia Sarcopenia Muscle 2014; 5: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fearon K, Argiles JM, Baracos VE, Bernabei R, Coats A, Crawford J, et al Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle 2015; 6: 272–274. [DOI] [PMC free article] [PubMed] [Google Scholar]


