Abstract
Aims
The nicotinic acid analogue acipimox is an antilipolytic agent, which acutely inhibits lipolysis and suppresses systemic levels of free fatty acids (FFA) and improves insulin sensitivity in obese patients. These effects of acipimox are transient due to a counter‐regulatory increase in growth hormone levels that reverse the antilipolytic effect of acipimox. Hypopituitary patients constitute a viable model to study the growth hormone‐independent effects of acipimox and the impact of isolated changes in FFA concentrations and insulin sensitivity on parasympathetic nervous activity. The aim of the present study was to investigate if pharmacological antilipolysis with acipimox acutely affects autonomic tone.
Methods
We studied heart rate variability as a measure of autonomic tone in eight hypopituitary men with and without acipimox treatment. The standard deviation of normal‐to‐normal intervals, root mean square of successive differences and high frequency were measured as heart rate variability parameters. The patients were studied in the basal and insulin‐stimulated state with clamped plasma glucose on two occasions in a randomized, double‐blind and placebo‐controlled crossover study.
Results
Plasma glucose (4.7 vs. 4.9 mmol l–1, P = 0.02) and serum FFA (0.05 vs. 0.41 mmol l–1, P < 0.001) were significantly decreased during acipimox treatment. Acipimox had an inhibitory effect on standard deviation of normal‐to‐normal intervals (41.3 vs. 45.3 ms, P = 0.01), root mean square of successive differences (23.2 vs. 11 ms, P = 0.03) and high frequency (3.79 vs 3.60 ln (ms2), P = 0.02) and these effects were reversed during clamping.
Conclusions
Short‐term inhibition of lipolysis by acipimox treatment lowered circulating FFA levels, improved insulin sensitivity, and was accompanied by reduced parasympathetic tone. The effect of acipimox on the parasympathetic modulation was reversed by hyperinsulinaemia.
Keywords: acipimox, heart rate variability, lipolysis
What is Already Known about this Subject
Short‐term acipimox treatment improves insulin sensitivity.
Long‐term acipimox treatment induces insulin resistance.
The tone of the sympathetic and the parasympathetic nervous system can be measured by heart rate variability.
What this Study Adds
Short‐term acipimox treatment reduced parasympathetic tone as measured by heart rate variability.
The reduced tone of the parasympathetic nervous system during acipimox treatment is suggested to be part of the mechanism by which continuous acipimox treatment results in adverse metabolic effects.
Introduction
Free fatty acids (FFAs) are generated from adipose tissue and released into the blood stream for use in β‐oxidation and ATP production in target tissues. High serum levels of FFAs induce insulin resistance in the liver and in skeletal muscle 1, 2, 3 and eventually lead to type 2 diabetes (T2D) 4. Adipose tissue metabolism is subject to both hormonal and nervous control and the parasympathetic nervous system (PNS), in particular, acutely regulates substrate metabolism and insulin sensitivity 5.
PNS imbalance correlates closely with impaired insulin sensitivity and is present in T2D 6. The balance in the autonomic nervous system can be measured by analysing beat‐to‐beat fluctuations in heart rate (heart rate variability, HRV). This method, which is noninvasive and extensively validated 7, 8, 9, 10, makes it possible to measure the degree of autonomic tone 11.
It remains uncertain if the aberrations in PNS activity observed in patients with insulin resistance are causally linked to metabolic cues such as fatty acid metabolism or secondary to other features.
The nicotinic acid analogue acipimox is an antilipolytic agent that acutely inhibits lipolysis and suppresses systemic levels of FFAs and improves insulin sensitivity in obese patients both with and without diabetes 12.
We have previously demonstrated that these effects of acipimox, which are transient due to a counter‐regulatory increase in growth hormone (GH) levels, remain present in hypopituitary patients substituted with GH 13. This may therefore also constitute a viable model to study the impact of isolated changes in FFA metabolism and insulin sensitivity on PNS activity in human subjects in vivo.
The aim of the present study was to investigate if pharmacological antilipolysis with acipimox acutely affects autonomic tone. We investigated HRV in hypopituitary patients on stable replacement therapy with GH and hydrocortisone with and without acipimox treatment. The patients were studied in the basal and insulin‐stimulated state on 2 occasions in a randomized, double blind and placebo controlled crossover study.
Materials and methods
The study was conducted in accordance with the Helsinki Declaration and all subjects gave their oral and written informed consent to participate. The Ethics Committee of the central region in Denmark (approval number M‐20100157) approved the study protocol. The patients also participated in a metabolic study and data on metabolism have been previously been reported 14. In the original study, each subject underwent four randomized interventions on different study days: (i) ghrelin infusion and placebo capsules; (ii) saline infusion and placebo capsules; (iii) ghrelin infusion and acipimox capsules; and (iv) saline infusion and acipimox capsules. The aim of that study was to investigate the FFA‐independent metabolic effects of ghrelin exposure.
Subjects
Eight hypopituitary men on stable replacement therapy with GH and hydrocortisone for >3 months participated in the study. GH deficiency was documented by a GH stimulation test [peak GH level = 0.57 ± 0.21 (range 0–1.61) μg l–1]. HbA1c at screening was 37 ± 1 (range 30–41) mmol mol–1 corresponding to 5.5 ± 0.1% (range 4.9–5.9%). None of the patients had diabetes or any other concomitant chronic disease. The participants were aged 53 ± 5 years and had a BMI of 30.3 ± 4.6 kg m–2.
Study protocol
All participants were examined on two occasions separated by a minimum of 2 weeks. The studies were performed in a quiet, thermo‐neutral indoor environment. The subjects fasted 12 hours before the first HRV recording and fasted during the trials, but were allowed oral water intake.
In a double blind and placebo controlled crossover study each subject underwent two randomized interventions with capsules containing acipimox 250 mg and placebo, respectively. The capsules were administered at 2000 and 2300 h the evening before and at 0600 and 1000 h on the day of the study. The studies were performed from 0800 to 1300 h (0–300 min) in the postabsorptive state. One i.v. cannula was inserted into the antecubital region for infusion, and one i.v. cannula was positioned in a dorsal hand vein for blood sampling. The latter was placed in a heat pad for sampling of arterialized blood. Heated hand technique arterializes venous blood and the plasma glucose in the arterialized blood is a reasonable estimate of the arterial value 15. At t = 0 isotonic saline, 50 ml h–1 i.v., was commenced. The subjects were studied in the basal postabsorptive state (referred to as basal) for 120 min followed by a hyperinsulinaemic/euglycaemic clamp (referred to as clamp) for 180 min, where they received a constant infusion of insulin (0.6 mU kg–1min–1; Actrapid, Novo Nordisk, Gentofte, Denmark). During the insulin infusion, plasma glucose was clamped at 5.0 mmol l–1 by adjusting the rate of infusion of 20% glucose according to plasma glucose measurements every 10 min. Other blood samples were drawn every 30 min and analysed for insulin and FFAs. Serum leptin was measured at baseline, at 120 min, and at 300 min.
HRV
HRV was recorded before the study day (referred to as pre), at 60 and 120 min in the basal period, and in the clamp at 180, 240 and at 300 min. HRV recordings immediately before the first blood samples in the morning are referred to as pre. HRV recordings during the basal period, where blood samples were obtained and saline was infused are referred to as basal, and overall recordings during the pre and basal periods are referred to as pre + basal. Each recording lasted for 300 s and was obtained by the handheld portable apparatus Vagus (Medicus Engineering, Aarhus, Denmark).
HRV was analysed in both time and frequency domains. In the time domain, the standard deviation of normal‐to‐normal intervals (SDNN) and root mean square of successive differences (RMSSD) were used. The HRV parameter SDNN is a measure of combined sympathetic and parasympathetic activity 16 whereas the RMSSD reflects the power of the variation or the short‐term components of HRV and is presumed to reflect parasympathetic activity 7. Parasympathetic activity is reduced, when RMSSD is low. To measure the frequency‐specific fluctuations of heart rate we used an autoregressive algorithm 7 with model order 25 to estimate the power spectrum. The low frequency (LF) 0.04–0.15 Hz and high frequency (HF) 0.15–0.4 components were analysed 7. The LF component is influenced by sympathetic, parasympathetic and baroreflex sensitivity. The HF band from 0.15–0.4 Hz is influenced by parasympathetic activity 17. Reduced values of the LF and HF measurements are related to lower activity in the respective nervous systems.
Insulin sensitivity
Insulin sensitivity was estimated by the level of glucose infusion rate (GIR) during the terminal 30 min of the hyperinsulinaemic, euglycaemic clamp.
Biochemical analyses
Plasma glucose was analysed using the bedside glucose oxidase method (YSI 2300 STAT Plus; YSI Life Sciences, Yellow Springs, OH, USA). Serum samples were frozen and stored at –20°C. Serum FFAs were analysed using a commercial kit (Wako Chemicals, Neuss, Germany). Serum leptin was analysed by a commercial enzyme immunoassay (EIA) kit (catalogue no. A05174, Bertin Pharma, Montigny le Bretonneux, France). Serum insulin was analysed using time‐resolved fluoroimmunoassay assay (AutoDELFIA Insulin kit, catalogue no. B080–101; PerkinElmer, Turku, Finland).
Statistical analysis
Complete data sets were obtained for all eight subjects. For each type of treatment (acipimox or placebo) the measurements during pre + basal and during clamp were pooled to reduce variability. HRV parameters in the frequency domain were non‐normally distributed and therefore log transformed. All parameters were investigated using qq‐plot to ensure that a parametric test could be used. The two periods pre + basal and clamp were respectively compared during acipimox and placebo using paired sample t tests. Correlations were calculated by using Pearson's linear regression coefficient or Spearman's rank correlation coefficient. Leptin was analysed by comparing mean values from acipimox and placebo study days with a paired t test. Data are presented as mean ± standard error of the mean and as 95% confidence interval (CI) on the major endpoints. A P value <0.05 was considered statistically significant.
Results
Metabolites, leptin and insulin sensitivity
Data on metabolites and insulin sensitivity were reported previously 14. In summary, plasma glucose levels (mmol l–1) were significantly decreased in pre + basal during acipimox treatment as compared with placebo (mean levels): 4.7 ± 0.1 (acipimox) vs. 4.9 ± 0.1 (placebo; CI 0.5;0.05, P = 0.02). During the terminal 30 min of the clamp, plasma glucose was clamped at similar levels (in mmol l–1): 5.0 ± 0.0 (acipimox) vs. 5.0 ± 0.1 (placebo), P = 0.90.
Serum FFA levels (mmol l–1) were suppressed during acipimox treatment in both pre + basal (mean levels): 0.05 ± 0.01 (acipimox) vs. 0.41 ± 0.02 (placebo; CI 0.44;0.30, P < 0.001), and during the clamp: 0.03 ± 0.01 (acipimox) vs. 0.14 ± 0.01 (placebo; CI 0.07;0.04, P < 0.001).
Serum leptin levels (ng ml–1) were similar during acipimox and placebo treatment: 10.6 ± 2.0 (acipimox) vs. 9.0 ± 1.2 (placebo), P = 0.24.
Acipimox improved insulin sensitivity [mg glucose uptake per kg bodyweight per min: 4.91 ± 0.71 (acipimox) vs. 3.88 ± 0.56 (control)], P = 0.005, as assessed by the glucose infusion rate during the clamp.
Heart rate, pulse pressure and HRV
Measurements of heart rate and pulse pressure are presented in Table 1 and Figure 1. Heart rate increased, as compared with baseline levels, during the study days for both acipimox and placebo, but did not differ statistically between treatments. Pulse pressure was also similar between treatments. Table 1 and Figure 2 shows the HRV response to acipimox and placebo. Acipimox had an inhibitory effect on SDNN (CI 13.12;1.92, P = 0.01), RMSSD (CI 14.64;3.06, P = 0.03), and HF (CI 1.10;0.24, P = 0.02), during the pre + basal recordings, whereas the effects of acipimox were equalized during the clamp. Acipimox did not impact on LF during either the pre + basal or the clamp periods.
Table 1.
Heart rate, pulse and heart rate variability (HRV) responses to acipimox and placebo treatment
| Acipimox | Placebo | |||
|---|---|---|---|---|
| Pre + basal | Clamp | Pre + basal | Clamp | |
| Heart rate (beats min –1 ) | 62.0 (6.0) | 65.5 (8.0) | 60.8 (7.0) | 64.8 (8.0) |
| Pulse pressure (mmHg) | 49.9 (10.0) | 51.3 (11.0) | 47.2 (9.0) | 51.1 (6.0) |
| HF ln(ms 2 ) | 3.79 (1.2)* | 3.60 (1.4) | 4.45 (1.2) | 3.62 (1.2) |
| LF ln(ms 2 ) | 4.95 (1.0) | 5.07 (1.3) | 5.27 (1.0) | 4.86 (0.9) |
| RMSSD (ms) | 23.2 (11.0)* | 23.1 (13.0) | 32.1 (13.0) | 22.1 (9.0) |
| cSDNN (ms) | 22.3 (10.0)* | 23.1 (13.0) | 30.5 (13.0) | 22.0 (9.0) |
The table displays the HRV measures standard deviation of normal‐to‐normal intervals (SDNN), root mean square of successive differences (RMSSD), low frequency (LF) 0.04–0.15 Hz, and high frequency (HF) 0.15–0.4 components. Heart rate and pulse pressure were similar during acipimox and placebo treatment. The HRV parameters HF, RMSSD, and cSDNN were significantly decreased by acipimox, whereas the HRV parameter LF was similar during acipimox and placebo. The asterisk indicates a significant difference (P < 0.05) between acipimox and placebo effects during the pre + basal measurements.
Figure 1.
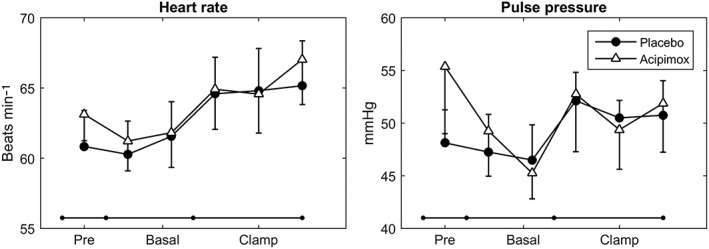
Heart rate and pulse pressure response to acipimox and placebo treatment. Acipimox did not impact on heart rate or pulse pressure
Figure 2.
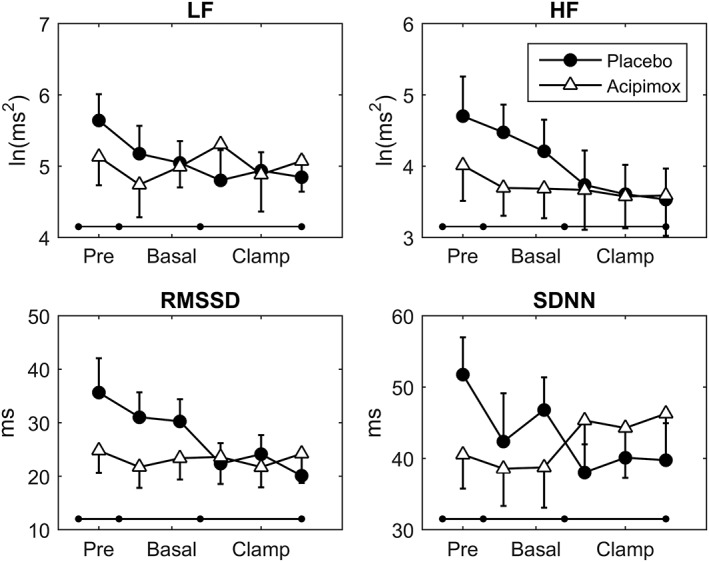
Heart rate variability. Acipimox did not impact on the low frequency component (LF) during either the pre + basal or the clamp period, but acipimox had an inhibitory effect on high frequency (HF), root mean square of successive differences (RMSSD), and standard deviation of normal‐to‐normal intervals (SDNN) components during the pre + basal recordings, whereas the effects of acipimox were equalized during the clamp
Correlations
LF and plasma glucose correlated inversely (R = –0.62, P = 0.01), whereas no correlation was revealed between plasma glucose and HF (R = –0.11, P = 0.68), SDNN (R = –0.27, P = 0.30), or RMSSD (R = –0.04, P = 0.88). HRV was not associated with FFA: HF (R = 0.34, P = 0.20), LF (R = 0.03, P = 0.90), SDNN (R = 0.18, P = 0.52), and RMSSD (R = 0.37, P = 0.15) or with leptin: HF (R = 0.50, P = 0.05), LF (R = 0.22, P = 0.41), SDNN (R = 0.29, P = 0.28), and RMSDD (R = 0.43, P = 0.09).
Discussion
In this report, we demonstrated that short‐term inhibition of lipolysis by acipimox treatment, which lowered circulating FFA levels and improved insulin sensitivity, was accompanied by reduced parasympathetic tone. The effect of acipimox on the parasympathetic modulation was reversed by hyperinsulinaemia.
The autonomic nervous system plays a major role in substrate release (sympathetic system) and vegetative and restorative processes (parasympathetic system) 5. These systems are thought to work in concert and abundant evidence suggests that imbalance in the autonomic nervous system is associated with various pathological conditions 18. The balance in the autonomic nervous system was measured noninvasively with HRV. Decreased HRV, a shift towards sympathetic domains, is an independent predictor of mortality in post–myocardial infarction patients 19, and in both high‐ and low‐risk patients with diabetes in the Atherosclerosis Risk in Communities (ARIC) study, decreased vagal activity, as measured by HRV, predicted mortality 20.
Many target tissues are innervated by both the sympathetic and parasympathetic nerve fibres (e.g. the heart and the gastrointestinal tract), whereas only sympathetic fibres innervate the adrenal glands resulting in a release of adrenaline into the bloodstream which leads to lipolysis. Furthermore, only sympathetic fibres innervate the adipose tissue 21, 22.
In this study, we observed an inhibitory effect of acipimox on parasympathetic modulation, measured with HRV in the basal period in hypopituitary patients on stable replacement with GH and hydrocortisone, and this suggests that acipimox treatment induces a relative increase in the sympathetic tone and subsequently adverse metabolic effects.
Acipimox is a well‐known antilipolytic agent that potently suppresses systemic FFAs and, thereby, increases insulin sensitivity 12. As expected, plasma glucose decreased in the basal period during acipimox treatment and was normalized to placebo levels in the clamp period by hypertonic glucose infusion. Simultaneously, the HRV parameters HF, RMSSD and SDNN were decreased in the pre + basal period and normalized during the clamp, where plasma glucose increased and was comparable to the levels during placebo treatment. This finding suggests that the metabolic aberrations during acipimox treatment (i.e. inhibited lipolysis and slightly decreased plasma glucose levels) induce an autonomic nervous system response that strive to maintain metabolic homeostasis. This is similar to the physiological response to overt hypoglycaemia in patients with type 1 diabetes and healthy controls subject to experimental hypoglycaemia 23.
Acipimox did not directly impact on the HRV parameter LF, but LF correlated inversely with plasma glucose concentrations, which supports the existence of a connection between glycaemia and autonomic modulation. The LF component is influenced by sympathetic, parasympathetic and baroreflex sensitivity 24, and is reduced during hypoglycaemia 25.
Leptin stimulates lipolysis in rodents and the mechanism seems to involve increased activity of the HSL enzyme 26. Therefore, blockade of HSL‐mediated lipolysis could lead to a feedback stimulation of leptin secretion. Leptin is also known to cross the blood–brain‐barrier and stimulate sympathetic nervous system activity 27. However, we did not record an effect of acipimox on systemic leptin concentrations, which seems to rule out leptin as a key player in the metabolic response to acipimox.
The metabolic rebound effects that have been reported after several days of acipimox treatment 28, 29, were not observed in our short‐term study. Acipimox treatment induces complex metabolic effects; acipimox treatment: induces a counter‐regulatory increase in GH levels 13; increases FFAs and skeletal muscle lipid content; and reduces insulin sensitivity and glucose disposal 29. Therefore, it could be hypothesised that autonomic nervous system aberrations are also associated with the long‐term dysmetabolic effects of acipimox, but this needs to be investigated further. Figure 3 depicts our current knowledge on the short‐ and long‐term metabolic, hormonal and parasympathetic effects of acipimox treatment.
Figure 3.
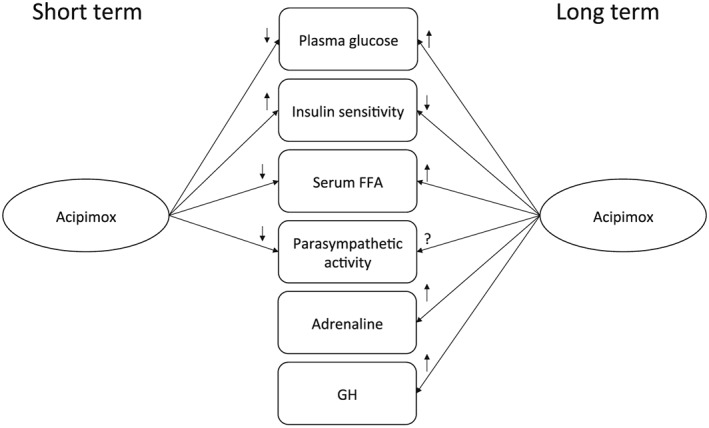
A schematic diagram showing the short‐ and long‐term effects of acipimox treatment on selected hormones, metabolites and the parasympathetic nervous system
There were a number of strengths and limitations to our study. The study was a double‐blind placebo controlled crossover study. Half of the patients were investigated during placebo then acipimox treatment and the other half vice versa separated by a minimum of 2 weeks to account for carry‐over effects. This is a powerful design because it eliminates interindividual variation. Studying hypopituitary patients rendered it possible to study the GH‐ and cortisol‐independent effect of acipimox on HRV, which is not feasible in healthy control subjects 30. Studying hypopituitary patients was, however, also a weakness because our data may not extrapolate to healthy subjects. It was also a limitation to our study that we did not do a basal HRV recording immediately prior to the intervention, which was initiated the evening before the study day. This also precluded measurements of the time–treatment response of acipimox on HRV. Theoretically, the observed effects of acipimox on HRV could be a direct drug effect or indirectly caused by the reported metabolic changes. Our study does not unravel the underlying mechanisms, but normalization of both plasma glucose and HRV during the clamp suggests that the effects of acipimox on HRV are indirectly mediated by metabolic changes. The low number of patients in our study is also recognized as a limitation to our study. Finally, the activity of the autonomic nervous system was not measured directly, but by measuring HRV, which is a proxy variable.
Perspectives
Future studies should be performed to investigate the role of the autonomic nervous system in substrate metabolism in more details, for example to determine if the autonomic nervous system impacts on insulin sensitivity directly or indirectly by modulation of lipolysis and secretion of epinephrine.
Conclusion
The results of this study indicate that acipimox‐induced decrease in circulating FFAs and plasma glucose in hypopituitary patients reduces parasympathetic modulation.
Competing Interests
J.F. is a stock owner and consultant for Medicus Engineering. No other potential conflicts of interest relevant to this article were reported. The other authors have nothing to disclose.
Mrs. E. Hornemann and Mrs. L. Buus are acknowledged for excellent technical assistance. The study was supported by a postdoctoral research fellow grant (11–105 283) from the Danish Council for Independent Research (Medical Sciences) and grants from the Riisfort Fonden and the A.P. Moller Foundation.
Contributors
E.T.V. conceptualized study, wrote protocol, screened patients, performed the clinical study, collected data, analysed data, discussed and interpreted results, wrote first manuscript draft, edited and revised manuscript, created the table, literature research, approved the final manuscript. S.L.C. collected data, analysed data, discussed and interpreted results, edited and revised manuscript, created figures and the table, literature research, approved the final manuscript. N.M. discussed and interpreted results, edited and revised manuscript, approved the final manuscript. J.O.L.J. discussed and interpreted results, edited and revised manuscript, approved the final manuscript. J.F. conceptualized study, study design, wrote protocol, analysed data, discussed and interpreted results, edited and revised manuscript, literature research, approved the final manuscript.
Vestergaard, E. T. , Cichosz, S. L. , Møller, N. , Jørgensen, J. O. L. , and Fleischer, J. (2017) Short‐term acipimox treatment is associated with decreased cardiac parasympathetic modulation. Br J Clin Pharmacol, 83: 2671–2677. doi: 10.1111/bcp.13384.
Clinical trials registration number: Clinicaltrials.gov NCT01209416 date of registration September 24, 2010.
References
- 1. Belfort R, Mandarino L, Kashyap S, Wirfel K, Pratipanawatr T, Berria R, et al Dose‐response effect of elevated plasma free fatty acid on insulin signaling. Diabetes 2005; 54: 1640–1648. [DOI] [PubMed] [Google Scholar]
- 2. Gormsen LC, Jessen N, Gjedsted J, Gjedde S, Norrelund H, Lund S, et al Dose‐response effects of free fatty acids on glucose and lipid metabolism during somatostatin blockade of growth hormone and insulin in humans. J Clin Endocrinol Metab 2007; 92: 1834–1842. [DOI] [PubMed] [Google Scholar]
- 3. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty‐acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963; 281: 785–789. [DOI] [PubMed] [Google Scholar]
- 4. Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YDI. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 1988; 37: 1020–1024. [DOI] [PubMed] [Google Scholar]
- 5. Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, et al Selective parasympathetic innervation of subcutaneous and intra‐abdominal fat – functional implications. J Clin Invest 2002; 110: 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pop‐Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care 2010; 33: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J 1996; 17: 354–381. [PubMed] [Google Scholar]
- 8. Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle‐aged and elderly men: the Zutphen study. Am J Epidemiol 1997; 145: 899–908. [DOI] [PubMed] [Google Scholar]
- 9. Engel G, Beckerman JG, Froelicher VF, Yamazaki T, Chen HA, Richardson K, et al Electrocardiographic arrhythmia risk testing. Curr Probl Cardiol 2004; 29: 365–432. [DOI] [PubMed] [Google Scholar]
- 10. DeBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of point events particularly for heart rate variability data. IEEE Trans Biomed Eng 1984; 31: 384–387. [DOI] [PubMed] [Google Scholar]
- 11. Freeman R. Assessment of cardiovascular autonomic function. Clin Neurophysiol 2006; 117: 716–730. [DOI] [PubMed] [Google Scholar]
- 12. Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, et al Overnight lowering of free fatty acids with acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 1999; 48: 1836–1841. [DOI] [PubMed] [Google Scholar]
- 13. Nielsen S, Møller N, Pedersen SB, Christiansen JS, Jørgensen JO. The effect of long‐term pharmacological antilipolysis on substrate metabolism in growth hormone (GH)‐substituted GH‐deficient adults. J Clin Endocrinol Metab 2002; 87: 3274–3278. [DOI] [PubMed] [Google Scholar]
- 14. Vestergaard ET, Jessen N, Møller N, Jørgensen JOL. Acyl ghrelin induces insulin resistance independently of GH, cortisol, and free fatty acids. Sci Rep 2017; 7: 42706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu D, Moberg E, Kollind M, Lins PE, Adamson U, Macdonald IA. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia 1992; 35: 287–290. [DOI] [PubMed] [Google Scholar]
- 16. Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, et al Low heart rate variability in a 2‐minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC study. Atherosclerosis risk in communities. Circulation 2000; 102: 1239–1244. [DOI] [PubMed] [Google Scholar]
- 17. Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat‐to‐beat cardiovascular control. Science 1981; 213: 220–222. [DOI] [PubMed] [Google Scholar]
- 18. Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 2010; 141: 122–131. [DOI] [PubMed] [Google Scholar]
- 19. Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987; 59: 256–262. [DOI] [PubMed] [Google Scholar]
- 20. Liao D, Carnethon M, Evans GW, Cascio WE, Heiss G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes 2002; 51: 3524–3531. [DOI] [PubMed] [Google Scholar]
- 21. Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol 2010; 318: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol 2014; 35: 473–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lucidi P, Rossetti P, Porcellati F, Pampanelli S, Candeloro P, Andreoli AM, et al Mechanisms of insulin resistance after insulin‐induced hypoglycemia in humans: the role of lipolysis. Diabetes 2010; 59: 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takalo R, Korhonen I, Majahalme S, Tuomisto M, Turjanmaa V. Circadian profile of low‐frequency oscillations in blood pressure and heart rate in hypertension*. Am J Hypertens 1999; 12: 874–881. [DOI] [PubMed] [Google Scholar]
- 25. Koivikko ML, Tulppo MP, Kiviniemi AM, Kallio MA, Perkiömäki JS, Salmela PI, et al Autonomic cardiac regulation during spontaneous nocturnal hypoglycemia in patients with type 1 diabetes. Diabetes Care 2012; 35: 1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steinberg GR, Bonen A, Dyck DJ. Fatty acid oxidation and triacylglycerol hydrolysis are enhanced after chronic leptin treatment in rats. Am J Physiol Endocrinol Metab 2002; 282: E593–E600. [DOI] [PubMed] [Google Scholar]
- 27. Haynes WG. Interaction between leptin and sympathetic nervous system in hypertension. Curr Hypertens Rep 2000; 2: 311–318. [DOI] [PubMed] [Google Scholar]
- 28. Saloranta C, Taskinen MR, Widén E, Härkönen M, Melander A, Groop L. Metabolic consequences of sustained suppression of free fatty acids by Acipimox in patients with NIDDM. Diabetes 1993; 42: 1559–1566. [DOI] [PubMed] [Google Scholar]
- 29. van de Weijer T, Phielix E, Bilet L, Williams EG, Ropelle ER, Bierwagen A, et al Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes 2015; 64: 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peino R, Cordido F, Peñalva A, Alvarez CV, Dieguez C, Casanueva FF. Acipimox‐mediated plasma free fatty acid depression per se stimulates growth hormone (GH) secretion in normal subjects and potentiates the response to other GH‐releasing stimuli. J Clin Endocrinol Metabol 1996; 81: 909–913. [DOI] [PubMed] [Google Scholar]


