Abstract
Aims
Oxcarbazepine is an antiepileptic drug with an activity mostly due to its monohydroxy derivative metabolite (MHD). A parent‐metabolite population pharmacokinetic model in children was developed to evaluate the consistency between the recommended paediatric doses and the reference range for trough concentration (Ctrough) of MHD (3–35 mg l–1).
Methods
A total of 279 plasma samples were obtained from 31 epileptic children (age 2–12 years) after a single dose of oxcarbazepine. Concentration‐time data were analysed with Monolix 4.3.2. The probability to obtain Ctrough between 3–35 mg l–1 was determined by Monte Carlo simulations for doses ranging from 10 to 90 mg kg–1 day–1.
Results
A parent‐metabolite model with two compartments for oxcarbazepine and one compartment for MHD best described the data. Typical values for oxcarbazepine clearance, central and peripheral distribution volume and distribution clearance were 140 l h–1 70 kg–1, 337 l 70 kg–1, 60.7 l and 62.5 l h–1, respectively. Typical values for MHD clearance and distribution volume were 4.11 l h–1 70 kg–1 and 54.8 l 70 kg–1 respectively. Clearances and distribution volumes of oxcarbazepine and MHD were related to body weight via empirical allometric models. Enzyme‐inducing antiepileptic drugs (EIAEDs) increased MHD clearance by 29.3%. Fifty‐kg children without EIAEDs may need 20–30 mg kg–1 day–1 instead of the recommended target maintenance dose (30–45 mg kg–1 day–1) to obtain Ctrough within the reference range. By contrast, 10‐kg children with EIAEDs would need 90 mg kg–1 day–1 instead of the maximum recommended dose of 60 mg kg–1 day–1.
Conclusion
This population pharmacokinetic model of oxcarbazepine supports current dose recommendations, except for 10‐kg children with concomitant EIAEDs and 50‐kg children without EIAEDs.
Keywords: childhood epilepsy, monohydroxy derivative, oxcarbazepine, population pharmacokinetics
What is Already Known about this Subject
Oxcarbazepine is an antiepileptic compound with an activity mainly due to its monohydroxy metabolite (monohydroxy derivative: MHD).
Enzyme‐inducing antiepileptic drugs increase the metabolism of both oxcarbazepine and MHD.
Younger children present a higher weight‐normalized MHD clearance than older children.
What this Study Adds
A new parent‐metabolite population model of oxcarbazepine was developed.
10‐kg children may need higher doses than recommended if they are taking concomitant enzyme inducing antiepileptic drugs.
50‐kg children not taking any inducing comedication may need lower doses than recommended.
Introduction
Oxcarbazepine (OXC) is an antiepileptic drug (AED) indicated for the treatment of partial onset seizures, with or without secondary generalization, as monotherapy or in combination, in adults and children aged from 2 or 6 years (in the USA and EU respectively). It acts by blocking voltage‐gated sodium channels in excitatory glutamatergic neurons. This stabilizes hyper‐excited neuronal membranes and inhibits repeated neuronal firing and its spread. OXC also modulates potassium and calcium activities, and reduces glutamatergic transmission 1.
Administered orally, OXC is well absorbed and rapidly and almost completely transformed in its monohydroxy derivative (MHD), by cytosolic arylcetone reductases 2. The formation of MHD is enantioselective with a predominance of the (S)‐enantiomer 3, 4. Despite this difference in exposition [the ratio of the area under the curve (AUC) values of (S)‐MHD over (R)‐MHD is 3.8 when OXC is administered orally 4], other pharmacokinetic (PK) parameters of the two enantiomers, such as the half‐lives, are similar and they both present a similar pharmacological activity 3, 4. In fact, MHD, as the sum of the two enantiomers, is mainly responsible for OXC antiepileptic action and exposure to MHD is about 15 times higher than exposure to OXC 5. MHD is principally eliminated by glucuronidation (about 45%), by renal clearance (about 28%), and minor amounts are eliminated by dihydroxylation leading to the formation of its dihydroxy derivative (DHD) 4, 6. An equilibrium between OXC and MHD is established with the back‐transformation of the metabolite in its oxidized form 4.
For 4–16‐year‐old children, it is recommended to start OXC at 8–10 mg kg–1 day–1, divided into two intakes, and to increase it by 5 mg kg–1 day–1 every 3rd day until reaching the target maintenance dose of 30–45 mg kg–1 day–1 (900 mg day–1 for 20–29 kg‐children, 1200 mg day–1 for 29.1–39‐kg children and 1800 mg day–1 for children >39 kg). For 2–4‐year‐old children, recommendations indicate to initiate the medication at 16–20 mg kg–1 day–1, divided into two intakes, achieving maintenance dose over 2–4 weeks, not to exceed 60 mg kg–1 day–1 1.
Therapeutic drug monitoring can be a tool for physicians to adapt the dose for each of their patients. In 2008, ILAE Commission on Therapeutic Strategies created guidelines for the therapeutic drug monitoring of antiepileptic drugs 7. They concluded that the reference range of MHD trough concentrations (Ctrough) should be 3–35 mg l–1, since it corresponded to Ctrough of responding patients 7. Indeed, it is well established that toxic concentrations begin between 35–40 mg l–1 8, 9, 10, and some studies have shown data of responding children with MHD Ctrough <5 mg l–1 11, 12.
Factors accounting for PK variability of OXC in children are age and association with enzyme‐inducing AEDs (EIAEDs) 13. It was demonstrated that young children (2–5 years) presented a higher MHD clearance, and thus a shorter half‐life (30% lower), and that they required a greater dose per body weight (BW) 11. Comedication with EIAEDs, such as carbamazepine, phenobarbital and phenytoin, was intensively investigated and it was established that these drugs were able to induce MHD metabolism 14, 15, 16, 17.
To date, OXC and MHD PK in children have only been studied partially by noncompartmental approaches that did not consider the continuous effect of age or BW 3, 4, 5, 14, 18, 19, 20. Some studies investigated population PK in children 15, 16, 17, 21, 22, 23, 24, modelling MHD directly from OXC administration. This method does not allow to distinguish PK changes related to OXC transformation to MHD from those related to MHD clearance. Thus, it does not permit a correct estimation of MHD PK parameters.
The aim of the present study was to develop a parent‐metabolite population PK model and to use this model to evaluate whether the recommended paediatric doses allow to obtain Ctrough of MHD within the reference range (3–35 mg l–1) for therapeutic drug monitoring.
Materials and methods
Patients
This population analysis was performed using data collected for a previously published ancillary pharmacokinetic (PK) study with a noncompartmental analysis of OXC and MHD 11. The study included paediatric patients aged 2–12 years. Because the main objective of the clinical trial was to evaluate the efficacy of OXC as add‐on medication, only children with inadequately controlled partial‐onset and/or generalized atonic, tonic or tonic–clonic seizures were included. Thus, patients were only eligible if they experienced at least one seizure per week despite being treated with one to three AEDs that remained unchanged for at least 1 month before inclusion into the study.
The exclusion criteria were: (1) contraindications to treatment with OXC, such as atrioventricular disorders, blood pressure disorders or hypersensitivity to carbamazepine or tricyclic antidepressants; (2) conditions likely to modify OXC PK, such as renal or hepatic failure, untreated known hypothyroidism, congenital metabolic diseases, abnormal BW (more than two standard deviations), concomitant medication with an enzyme‐inducing or ‐inhibiting drug (except for AEDs), alcoholism or drug abuse; (3) previous or current use of OXC; and (4) no cooperation from the patient or their family.
Study design
Children were randomized to receive a single OXC dose of 5 or 15 mg kg–1, administered as an oral suspension after an overnight fast. Blood samples of 1 ml were collected into heparinized tubes at baseline (before administration) and, approximately, 1, 2, 4, 6, 8, 12, 24, 36 and 48 h after administration. Times of dosing and sampling were recorded, as were the investigated covariates (age, BW, sex, comedications). The samples were centrifuged and the separated plasma stored at –80°C until analysis.
Ethics
The study was conducted in accordance with the Declaration of Helsinki and their protocol was approved by the ethical committee of Cochin, Saint‐Vincent de Paul and Saint‐Anne hospitals. Written informed consent was provided by a parent or legal guardian for all participating children.
Analytical method
Total MHD and OXC were assayed in plasma samples using a previously reported nonenantioselective high‐performance liquid chromatography method 25. (S) and (R) enantiomers were consequently not distinguished. Precision and inaccuracy were below 15%. The lower limits of quantification for OXC and MHD were 0.05 mg l–1 and 0.1 mg l–1, respectively.
Population pharmacokinetic model development
The population PK analysis was performed using a nonlinear mixed‐effect approach, with the Monolix software (version 4.3.2; Lixoft, Antony, France).
Model development
Population parameters for OXC and MHD were estimated using the stochastic approximation expectation maximization (SAEM) algorithm. Data below the limit of quantification (BLQ) were handled as left‐censored data, by an extended SAEM algorithm which simulate BLQ data with a right‐truncated Gaussian distribution 26. For each patient, only the first BLQ was kept in the dataset and was considered in the estimation via the CENS item in the database, corresponding to the M3 method 27.
For OXC, the structural PK models evaluated were composed by one, two or three compartments, and the absorption phases were evaluated with first‐ or zero‐order models, with or without lag time. Based on previous results evidencing a bioavailability of OXC of 0.99, this parameter was fixed to 1 4. For racemic MHD one‐ and two‐compartment models were tested. Based on previous results showing that no OXC was found unchanged in the urine 4, 28, it was assumed that all the parent was converted into MHD. Presystemic metabolite formation was investigated with a nonphysiological model where the dose enters both parent and metabolite compartments with two independent absorption rate constants, with and without dose apportionment 29. Elimination of OXC was tested with first‐ or zero‐order models. Due to the linearity of MHD PK 6, its elimination was assumed to be ruled by a first‐order process. A back‐transformation of MHD into OXC was also tested, as it was evidenced that the enantiomers can be oxidized into the parent compound 4.
Exponential models were used to describe interindividual variability (Eq. (1)):
| (1) |
where θi is the estimated value of a parameter in an individual i, θTV is the typical value of this parameter in the population and ηi is the individual deviation from this typical value, i.e. the interindividual variability, which is assumed to be normally distributed with a mean of 0 and a variance of ω2.
Additive, proportional and mixed residual error models were tested for each dependent variable.
Covariate analysis
Demographic variables (weight, age and sex) and comedication with enzyme inducing antiepileptic drugs (EIAEDs), such as carbamazepine, phenobarbital and phenytoin, were tested as potential covariates. First, variables were added one by one and were selected if their addition was able to cause a significant drop of the log‐likelihood (LL). Because the reduction in LL follows a chi‐square distribution, a decrease of 3.84 was considered significant at the 5% level (P < 0.05, one degree of freedom). Once all the covariates were tested, the significant ones were added to the model, obtaining the full model, and a backward elimination was performed. Covariates were retained if their elimination resulted in an augmentation >6.63 (P < 0.01, one degree of freedom) of the LL. After all nonsignificant covariates were removed, the final model was obtained.
The continuous covariates were included in the model using a power function equation (Eq. (2)):
| (2) |
where cov is the value of the covariate, covmedian is its median and θcov is the factor describing the relationship between the covariate and the parameter.
For BW, covmedian was fixed to the standard adult value of 70 kg and several models were tested:
θcov was empirically estimated
θcov was fixed to the theoretical values of 0.75 for clearance and to 1 for volume
two independent θcov were empirically estimated for children >6 years and children <6 years, for MHD clearance
the body weight dependent exponent (BDE) model was also tested for MHD clearance 30. In this model, the allometric exponent changes in a sigmoidal fashion with respect to BW:
| (3) |
where k0 is the value of the exponent at a theoretical BW of 0 kg, kmax is the maximum decrease of the exponent, k50 is the BW at which 50% of the maximum decrease of the exponent is attained, and γ is the Hill coefficient.
In the case of theoretical allometry, age was additionally tested as a covariate in two different ways: with (Eq. (2)) and with a maturation function (Eq. (4)):
| (4) |
where γ represents the Hill coefficient and Age50 the age at which half of the maturation is reached.
Categorical covariates (sex and EIAEDs) were incorporated using a similar model (Eq. (5)):
| (5) |
where cov is 1 or 0 in the presence or absence of the covariate.
Comparison of the tested models
The possible difference between the empirical allometry model and the theory‐based allometry model was assessed by normalized prediction distribution errors (NPDE) and prediction and variability corrected visual predictive checks (pvcVPC) against BW. These NPDE were realized with an add‐on package on R 31 using 1000 simulation of the dataset. pvcVPC were also performed using 1000 simulations with the design of the original dataset and the investigated model using Perl‐speaks for NONMEM (PsN,version 4.4.8; SourceForge) 32.
External evaluation of the tested models and comparison with previous models
To evaluate the reliability of the investigated models, the steady‐state MHD trough concentrations reported in children by Li et al. 33 were compared to the population trough concentrations predicted by the models for similar doses and BW. In their study, Li and colleagues collected blood samples from 52 children aged from 0.58 to 15 years, and provided age, weight‐normalized doses and individual MHD steady‐state trough concentrations for each child 33. Since their paper did not provide any, BW were estimated using the Advanced Paediatric Life Support manual formulae 34, which are (2 × age in years) + 8 for 1–5‐year‐old patients and (3 × age in years) + 7 for 6–12‐year‐old children. Patients without concomitant medication and whose age was not included in the 2–12 years interval were excluded from the analysis. Using these calculated BW and the corresponding doses, MHD Ctrough at steady‐state were calculated using the empirical model, the theory‐based allometry model, as well as previously published population PK models 15, 16, 17, 22. Adequacy between actual and predicted concentrations was investigated by calculating precision (RMSE) and bias (MPE) using the following formulae:
where, COBS is the observed concentration and CPRED is the predicted concentration of the subject i and n is the total number of subjects.
Evaluation of the final model
Lack of bias of the final model was investigated by visual inspection of goodness of fit curves [population prediction (PRED) vs. observed concentration, individual weighted residuals (IWRES) and NPDE vs. PRED or time after administration]. Prediction‐corrected visual predictive checks (pcVPC), stratified by the categorical covariate EIAED or not, were also performed using 500 simulations of the original dataset.
Dose evaluation
Monte Carlo simulations were performed with NONMEM 7.3 using the final model to obtain steady‐state AUCs (AUC0–12) and steady‐state Ctrough of MHD, at different daily doses in a bid regimen. One thousand children per BW, dose and cotreatment were simulated. Investigated BW were 10, 20, 30, 40 and 50 kg. Investigated doses were 10, 20, 25, 30, 40, 50, 60 and 90 mg kg–1 per day, divided into two intakes. The presence or absence of EIAEDs was also explored. Then, for each combination dose/BW/comedication, the probabilities to obtain steady‐state Ctrough within the reference range (3–35 mg l–1) and to reach the toxicity threshold (>35 mg l–1) 7 were calculated.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 35, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 36.
Results
Patient characteristics
Thirty‐one children (13 girls and 18 boys) were included in the study, having a median (range) age of 8.08 (2.25–12.5) years and a median (range) BW of 23 (12.7–56) kg. Of these children, 14 received a dose of 5 mg kg–1 and 17 received a dose of 15 mg kg–1. Six patients were cotreated with one AED, 19 with two AEDs and six with three AEDs. Concomitant AEDs are described in Table 1. Twenty‐four of these patients were comedicated with at least one EIAED. These 31 patients provided 277 and 279 sampling points for OXC and MHD, respectively. Two OXC sampling points were discarded because of analytical issues. Of these measured concentrations, 32% of OXC and 11.5% of MHD observations were below the limits of quantification. After keeping only the first BLQ of each patient, 13.7% and 6.5% of the observations remained BLQ for OXC and MHD, respectively.
Table 1.
Concomitant antiepileptic drugs
| Associated antiepileptic drug | Patients (%) |
|---|---|
| Carbamazepine | 19 (61.3%) |
| Vigabatrin | 14 (45.2%) |
| Clobazam | 8 (25.8%) |
| Phenytoin | 5 (16.1%) |
| Valproic acid | 4 (12.9%) |
| Clonazepam | 3 (9.7%) |
| Lamotrigine | 3 (9.7%) |
| Diazepam | 2 (6.5%) |
| Phenobarbital | 2 (6.5%) |
| Ethosuccimide | 1 (3.2%) |
| Progabide | 1 (3.2%) |
Population pharmacokinetic modelling
The best base model was a two‐compartment model with first‐order absorption (without lag‐time) and elimination for OXC, and a one compartment model with first‐order elimination for MHD. Taking into account the equilibrium between OXC and its metabolite MHD via a constant representing the back‐transformation of MHD into OXC improved the fit. The structural parameters for this model were the absorption rate constant of OXC (Ka), the apparent central and peripheral distribution volumes of OXC (VcOXC/F, where F is the bioavailability, and VpOXC/F), the apparent elimination and distribution clearances of OXC (CLOXC/F, QOXC/F), the apparent elimination clearance of MHD (CLMHD/F), the apparent volume of distribution of MHD (VcMHD/F), and the back‐transformation constant rate (KBT) of MHD into OXC. The fraction of OXC metabolized to MHD (Fm) was fixed to 1 and it was assumed that OXC was completely eliminated via metabolic conversion to MHD. The fraction of the dose that directly reached the metabolite compartment after oral absorption was estimated to 5.4% with the first‐pass effect model. However, this model was not retained as it did not decrease the LL significantly. Interindividual variability was estimated for all the parameters, except Ka. The residual error model used was proportional for OXC and combined for MHD.
The coadministration of EIAEDs was found to influence CLMHD/F but no influence was found on CLOXC/F. Addition of BW as a covariate on CLOXC/F, VcOXC/F, CLMHD/F and VcMHD/F via an allometric function also significantly improved the fit with models 1 and 2. For model 3, where two separate allometric exponents for CLMHD depending on the child age (> or <6 years) were estimated, two very similar estimates were obtained for both age range (0.498 for children >6 years and 0.541 for children <6 years) and both values were very similar to the value obtained for the whole population (0.549). The BDE model (model 4) on CLMHD did not provide satisfying results (the –2LL increased and the parameters of the BDE were poorly estimated). Estimated exponents (model 1) allowed a better fit of the data than the theory‐based allometric model (model 2), which was not improved by the addition of age via an allometric function or a maturation function. NPDE vs. BW and pvcVPC with BW as the independent variable were performed for both models (with empirical allometric exponents and with fixed theoretical allometric exponents; Figures 1 and 2). No significant bias was observed for each model, showing that they both described well the data across the range of BW included in the study. However, the model with estimated allometric exponents performed better on the external evaluation than the model with fixed exponents, despite a slight over prediction of the concentrations. It also performed better than formerly published models that included only MHD data (Table 2). Thus, the empirical model was chosen as the final model and was considered reliable enough to predict steady‐state exposure of MHD.
Figure 1.

Normalized prediction distribution errors (NPDE) vs. body weight (BW) of oxcarbazepine (A) and its monohydroxy derivative (MHD) (B) for the empirical model and of oxcarbazepine (C) and MHD (D) for the allometry theory based model. Upper blue area represents the simulation‐based 95% confidence interval of the 95th percentile; pink area represents the simulation‐based 95% confidence interval of the 50th percentile; lower blue area represents the simulation‐based 95% confidence interval of the 5th percentile
Figure 2.

Prediction and variability corrected visual predictive checks against body weight obtained with the empirical model for oxcarbazepine (OXC) (A) and monohydroxy derivative (MHD) (B) and obtained for the theory‐based allometry model for OXC (C) and MHD (D). Blue dots represent the predicted and variability corrected observed concentrations; upper blue area represents the simulation‐based 95% confidence interval of the 95th percentile; pink area represents the simulation‐based 95% confidence interval of the 50th percentile; lower blue area represents the simulation‐based 95% confidence interval of the 5th percentile; upper and lower blue solid lines represent the 5th and 95th empirical percentiles of the observations; red solid line represents the 50th empirical percentile of the observations
Table 2.
Comparison between monohydroxy derivative (MHD) steady‐state trough concentrations obtained in therapeutic drug monitoring by Li et al. 29 and predicted by different models
| Model | MPE | RMSE |
|---|---|---|
| Parent‐metabolite model with back‐transformation and estimated allometric coefficients | 27.7% | 7.2 |
| Parent‐metabolite model with back‐transformation and fixed allometric coefficients | 39% | 7.8 |
| MHD model developed by Peng et al. 22 | 44.1% | 14.5 |
| MHD model developed by Sallas et al. 15 | 95.6% | 14.2 |
| MHD model developed by Sugiyama et al. 16 | 69.1% | 11.4 |
| MHD model developed by Wang et al. 17 | 145.1% | 24.5 |
The final model was then:
Ka = 1.83 h–1
CLOXC/F = 140 × (WT/70)0.798 l h – 1
VcOXC/F = 337 × (WT/70)2.4 L
QOXC/F = 62.5 l h – 1
VpOXC/F = 60.7 L
CLMHD/F = 4.11 × e−0.257 × MED × (WT/70)0.549 l h – 1
VcMHD/F = 54.8 × (WT/70)1.09 L
KBT = 0.0622 h–1
where MED is 0 or 1, if enzyme‐inducing antiepileptic drugs were associated or not, and WT is the patient BW in kg.
The estimated values of the parameters of the final model and of the theory‐based allometry model and their precisions are reported on Table 3.
Table 3.
Values and precision of the parameters of the estimated allometric exponent and fixed allometric exponent models
| Parameter | Model with estimated allometric exponents | Model with fixed allometric exponents | |||
|---|---|---|---|---|---|
| Estimated value | RSE (%) | Estimated value | RSE (%) | ||
| Ka (h –1 ) | 1.83 | 4 | 1.81 | 4 | |
| CL OXC /F (l h –1 70 kg –1 ) | 140 | 24 | 116 | 8 | |
| Vc OXC /F (l 70 kg –1 ) | 337 | 41 | 55.7 | 22 | |
| Q OXC /F (l h –1 ) | 62.5 | 21 | 119/70 kg | 21 | |
| Vp OXC /F (L) | 60.7 | 25 | 235/70 kg | 23 | |
| CL MHD /F (l h –1 70 kg –1 ) | 4.11 | 14 | 5.1 | 5 | |
| Vc MHD /F (l 70 kg –1 ) | 54.8 | 16 | 47.7 | 6 | |
| K BT (h –1 ) | 0.0622 | 15 | 0.0476 | 16 | |
|
θ
|
0.798 | 26 | 0.75 | FIXED | |
|
θ
|
2.4 | 17 | 1 | FIXED | |
|
θ
|
0.75 | FIXED | |||
| WTVpoxc/F | 1 | FIXED | |||
|
θ
|
–0.257 | 42 | –0.276 | 40 | |
|
θ
|
0.549 | 21 | 0.75 | FIXED | |
|
θ
|
1.09 | 13 | 1 | FIXED | |
| ωCL OXC /F | 0.393 | 15 | 0.361 | 16 | |
| ωVc OXC /F | 0.601 | 22 | 1.07 | 16 | |
| ωQ OXC /F | 0.919 | 18 | 0.928 | 17 | |
| ωVp OXC /F | 1.26 | 15 | 1.11 | 15 | |
| ωCL MHD /F | 0.235 | 14 | 0.247 | 15 | |
| ωVc MHD /F | 0.211 | 25 | 0.209 | 23 | |
| ωK BT | 0.63 | 16 | 0.587 | 17 | |
| σ OXC | 0.32 | 7 | 0.321 | 7 | |
| σ MHD a | 0.993 | 13 | 0.972 | 13 | |
| σ MHD b | 0.0398 | 21 | 0.0406 | 20 | |
RSE, relative standard error; Ka, absorption rate constant; F, bioavailability; VcOXC, central volume of distribution of OXC; CLOXC, elimination clearance of OXC; QOXC, intercompartmental clearance of OXC; VpOXC, peripheral volume of distribution of OXC; CLMHD, elimination clearance of MHD; VcMHD, central volume of distribution of MHD; KBT, back‐transformation constant; θ, factor describing the relationship between the covariate and the parameter; WT, body weight; nEIAEDs, absence of enzyme‐inducing antiepileptic drug; ω, interindividual variability; σ, residual error; OXC, oxcarbazepine; MHD, monohydroxy derivative
Additive
Proportional
No significant bias was observed on the plot of observed vs. population prediction for OXC and MHD (data not shown). For OXC, IWRES vs. time or PRED did not present any bias (data not shown), whereas a small bias was seen in NPDE vs. time graph for time = 48 h (Figure 3). Since almost all observations at this time were BLQ data, and the drug intake is usually twice‐a‐day, it was considered that this bias would not penalize the prediction of PK profiles, for a bid regimen, with the model. No bias was observed for all the goodness of fit curves for MHD (Figure 3). pcVPC revealed no bias as the observed concentrations were homogeneously distributed around the 50th percentile of simulated concentrations (Figure 4). When stratified by the covariate EIAED, no bias was observed as well (data no shown).
Figure 3.

Normalized prediction distribution errors (NPDE) vs. time (top) and population predictions (below) of oxcarbazepine (left) and its monohydroxy derivative (MHD) (right). Blue dots are observed data; red dots are BLQ data (sampled from the conditional distribution , where and are the estimated individual and population parameters, respectively); upper blue area represents the simulation‐based 90% confidence interval of the 90th percentile; pink area represents the simulation‐based 90% confidence interval of the 50th percentile; lower blue area represents the simulation‐based 90% confidence interval of the 10th percentile
Figure 4.

Prediction‐corrected visual predictive checks obtained with the final model. Oxcarbazepine (OXC) on top, monohydrody derivative (MHD) below. Blue dots represent the observed concentrations; red dots represent the BLQ data (sampled from the conditional distribution); green lines represent 5th, 50th and 95th empirical percentiles of the observations; upper blue area represents the simulation‐based 95% confidence interval of the 95th percentile; pink area represents the simulation‐based 95% confidence interval of the 50th percentile; lower blue area represents the simulation‐based 95% confidence interval of the 5th percentile; red area represents outliers
Dose evaluation
For a same dose, steady‐state AUC0–12 of MHD increased with increasing BW, and was lower in patients taking EIAEDs than in those without concomitant EIAEDs (Table 4). Similarly, MHD Ctrough also increased with BW and dose, and its value was also lower in patients cotreated with EIAEDs (Table 5).
Table 4.
Median and nonparametric 95% confidence interval (95CI) of simulated steady‐state AUC0–12 of monohydrody derivative according to the daily dose of oxcarbazepine administered as a bid regimen
| 10 mg kg–1 day–1 | 20 mg kg–1 day–1 | 40 mg kg–1 day–1 | 60 mg kg–1 day–1 | 90 mg kg–1 day–1 | ||
|---|---|---|---|---|---|---|
| Body weight (kg) | Cotreatment | Median [95CI] | Median [95CI] | Median [95CI] | Median [95CI] | Median [95CI] |
| 10 | Without EIAEDs | 46.5 [31.0–72.3] | 92.9 [61.9–144.7] | 185.8 [123.8–289.3] | 278.8 [185.7–434.0] | 412.1 [267.1–662.6] |
| 20 | 62.2 [38.5–101.8] | 124.4 [77.0–203.5] | 248.7 [154.0–407.0] | 373.1 [231.0–610.4] | 561.3 [357.6–897.9] | |
| 30 | 75.5 [48.0–119.8] | 151.0 [96.1–239.6] | 302.0 [192.1–479.2] | 453.0 [288.2–718.7] | 680.0 [420.2–1110.2] | |
| 40 | 84.9 [56.3–132.6] | 169.9 [112.5–265.1] | 339.7 [225.0–530.2] | 509.6 [337.5–795.3] | 771.3 [472.3–1232.7] | |
| 50 | 95.1 [60.4–148.4] | 190.2 [120.9–296.9] | 380.4 [241.8–593.8] | 570.6 [362.7–890.6] | 861.0 [538.1–1349.3] | |
| 10 | With EIAEDs | 35.5 [21.9–57.4] | 71.1 [43.9–114.7] | 142.2 [87.7–229.4] | 213.3 [131.6–344.2] | 317.6 [206.3–503.9] |
| 20 | 48.7 [30.0–77.3] | 97.3 [60.1–154.6] | 194.6 [120.1–309.1] | 291.9 [180.2–463.7] | 432.8 [271.9–697.7] | |
| 30 | 58.3 [35.5–92.8] | 116.5 [71.0–185.6] | 233.1 [141.9–371.2] | 349.6 [212.9–556.8] | 519.9 [330.1–824.5] | |
| 40 | 66.3 [41.4–104.3] | 132.6 [82.8–208.6] | 265.2 [165.5–417.2] | 397.8 [248.3–625.8] | 602.0 [383.5–938.0] | |
| 50 | 72.3 [46.0–113.8] | 144.6 [92.0–227.5] | 289.1 [184.1–455.1] | 433.7 [276.1–682.6] | 673.2 [405.4–1033.0] |
EIAEDs, enzyme‐inducing antiepileptic drugs; 95CI, nonparametric 95% confidence interval
Table 5.
Median and nonparametric 95% confidence interval (95CI) of simulated steady‐state monohydrody derivative trough concentrations according to the daily dose of oxcarbazepine administered as a bid regimen
| 10 mg kg–1 day–1 | 20 mg kg–1 day–1 | 40 mg kg–1 day–1 | 60 mg kg–1 day–1 | 90 mg kg–1 day–1 | ||
|---|---|---|---|---|---|---|
| Body weight (kg) | Cotreatment | Median [95CI] | Median [95CI] | Median [95CI] | Median [95CI] | Median [95CI] |
| 10 | Without EIAEDs | 2.2 [0.6–4.5] | 4.4 [1.3–9.1] | 8.9 [2.5–18.1] | 13.3 [3.8–27.2] | 19.2 [5.2–41.4] |
| 20 | 3.3 [1.3–6.7] | 6.6 [2.5–13.5] | 13.2 [5.1–27.0] | 19.7 [7.6–40.4] | 29.6 [11.6–57.9] | |
| 30 | 4.5 [1.9–8.0] | 8.9 [3.8–16.0] | 17.9 [7.7–32.0] | 26.8 [11.5–48.0] | 39.5 [16.4–74.0] | |
| 40 | 5.4 [2.7–9.4] | 10.8 [5.3–18.7] | 21.7 [10.6–37.5] | 32.5 [16.0–56.2] | 48.7 [22.8–87.9] | |
| 50 | 6.3 [3.4–10.9] | 12.5 [6.7–21.9] | 25.1 [13.5–43.7] | 37.6 [20.2–65.6] | 56.9 [28.6–96.9] | |
| 10 | With EIAEDs | 1.5 [0.3–3.4] | 2.9 [0.6–6.9] | 5.9 [1.1–13.7] | 8.8 [1.7–20.6] | 12.2 [2.8–29.2] |
| 20 | 2.3 [0.7–4.7] | 4.6 [1.4–9.3] | 9.1 [2.9–18.7] | 13.7 [4.3–28.0] | 20.0 [5.8–42.2] | |
| 30 | 3.1 [1.2–5.9] | 6.1 [2.5–11.7] | 12.3 [5.0–23.5] | 18.4 [7.5–35.2] | 27.4 [10.5–53.6] | |
| 40 | 3.8 [1.6–7.1] | 7.7 [3.3–14.2] | 15.3 [6.6–28.4] | 23.0 [9.9–42.6] | 34.9 [14.5–62.9] | |
| 50 | 4.4 [2.1–7.9] | 8.8 [4.1–15.9] | 17.7 [8.3–31.8] | 26.5 [12.4–47.6] | 40.9 [18.4–72.1] |
EIAEDs, enzyme‐inducing antiepileptic drugs; 95CI, nonparametric 95% confidence Interval
For 10 kg children (i.e. children aged roughly 2 years) without EIEADs, a probability >95% to be within the 3–35 mg l–1 reference range for MHD Ctrough was obtained for 40–60 mg kg–1 daily doses (Figure 5B), which is in agreement with current recommendations.
Figure 5.

Probability of steady‐state monohydrody derivative (MHD) trough concentration to be within reference range (3–35 mg l–1) depending on dose and body weight when enzyme‐inducing antiepileptic drugs are associated (A) or not (B); probability of steady‐state MHD trough concentration to reach toxicity threshold (>35 mg l–1) depending on dose and body weight when enzyme‐inducing antiepileptic drugs are associated (C) or not (D)
For children with BW between 20 and 40 kg, and without EIAEDs, a probability >95% was obtained with daily doses between 20 and 40 mg kg–1 (Figure 5B), which is also consistent with current recommendations.
For 50 kg children without EIAEDs, a probability >95% was obtained with daily doses between 20 and 30 mg kg–1 (Figure 5B). Of note, these doses are inferior to the target recommended maintenance dose of 30–45 mg kg–1 day–1 for 4–12‐year‐old children. With such doses, around 10% of the patients would reach the 35 mg l–1 toxicity threshold (Figure 5D).
In case of combined treatment with EIAEDs, the probability of target attainment was lower, so higher doses were needed. The most important impact of EIAEDs was observed for 10 kg children, who may need doses up to 90 mg kg–1 day–1 (Figure 5A), which is 50% above the maximal recommended maintenance dose.
Discussion
This study was conducted with the aim to develop a parent‐metabolite population model of OXC and MHD to characterize the PK parameters of both compounds and the covariates associated with their interindividual variability. This model allows a better understanding of MHD PK resulting from its formation from OXC and its elimination and takes into account the back‐transformation of MHD into its parent compound. Previous population PK studies directly related OXC dose to MHD concentration, without considering OXC concentration 15, 16, 17, 21, 22, 23, 24. Such an approach can be supported by the high bioavailability of OXC and the fact that OXC is almost completely converted into MHD. However, since MHD concentration at a given time is the result of several phenomena (MHD formation from OXC, MHD elimination, and MHD back‐transformation to OXC), we believed a parent‐metabolite model would allow a better prediction of the PK profile of MHD. Based on the results of the external evaluation displayed on Table 2, it appeared indeed that such a model provided a better prediction of MHD concentration at a given time.
The present model could not take into account the presystemic transformation of OXC into MHD 3, since the first‐pass effect model investigated to describe this phenomenon 29 did not improve the fit. In fact, estimating all PK parameters could not be possible with oral data only and would also require intravenous data 29, 37. However, a previous report determined that the fraction of the administered OXC dose presystemically converted to MHD was only 6.5% (this fraction was estimated to 5.4% with our first‐pass effect model), minimizing its impact 38.
A mean time to reach the maximum concentration of around 1 h can be derived from our mean PK estimates for OXC, which is in accordance with the value provided by Flesch et al. 4. OXC mean apparent weight‐normalized clearance was 140 l h–1 70 kg–1. This value is in accordance with the reported value of 170.1 l h–1 70 kg–1 in adults after a single dose 39. Concerning MHD, apparent weight‐normalized clearances were 4.11 l h–1 70 kg–1 for children taking EIAEDs and 3.18 l h–1 70 kg–1 for children not taking these medications. Those values are in agreement with the results of Sallas et al. (3.2 l h–1 70 kg–1) 15. Flesch et al. 4 reported an absolute bioavailability of 99% after oral administration of OXC as well as a clearance of 3.5–5.5 l h–1, for racemic MHD, in healthy volunteers after intravenous administration of MHD. Considering that the bioavailability is almost total, our estimates are in accordance with these values.
MHD weight‐normalized clearance decreased with increasing age (Figure 6). This was already evidenced in the noncompartmental study from Rey et al. 10 and some population approaches 15, 16. This phenomenon is frequent in children, and was already observed for other antiepileptic drugs such as clobazam 40, carbamazepine 41, phenobarbital 42, felbamate 43 and valproic acid 44. The main elimination route of MHD is glucuronidation, and the maturation of the hepatic abundance/activity depends on the UDP‐glucuronosyltransferase isoforms considered: it can sometimes reach adult levels 2–3 months after birth, while it can be upregulated beyond age 2 years of age in other cases 45. Of note, UDP‐glucuronosyltransferase isoform(s) responsible for MHD glucuronidation have not yet been identified. Renal excretion has a minor contribution in MHD elimination (<20% of the MHD dose administered intravenously was found unchanged in the urine 4), nonetheless, as renal clearance follows the same allometric principles as metabolic clearance, it may also explain the decrease of weight‐normalized clearance with age.
Figure 6.
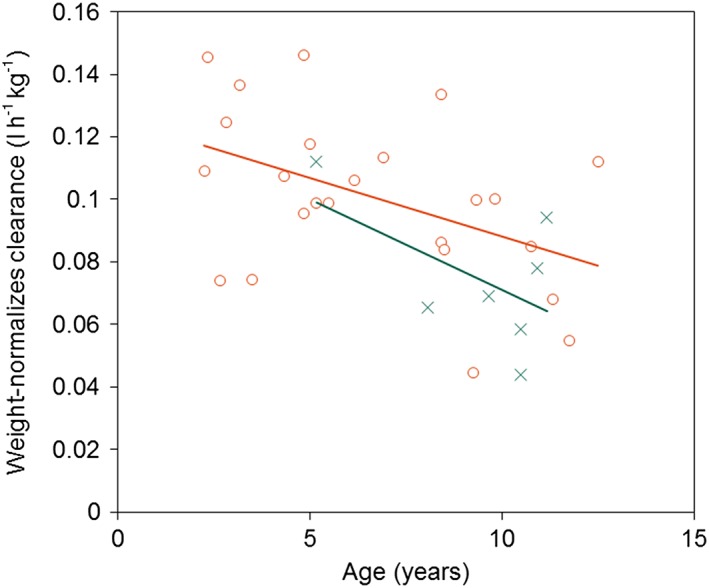
Relationship of monohydroxy derivative (MHD) weight‐normalized clearance with respect to age. Orange circles represent children taking concomitant enzyme‐inducing antiepileptic drugs; green crosses represent children not taking enzyme‐inducing antiepileptic drugs
In the present study, OXC mean apparent volume of distribution was 397.7 l 70 kg–1 (5.7 l kg–1). This value is in agreement with the adult values described in the literature which are 3.9–12.5 l kg–1 (273–875 l 70 kg–1) 6. MHD mean apparent weight‐normalized volume was 54.8 l 70 kg–1. It differed greatly from the values displayed in some former population PK studies in children (285.6 l 70 kg–1 16, 171.5 l 70 kg–1 16 and 312.9 l 70 kg–1 23). However, according to the allometric principles, weight normalized‐volume should be similar in all age groups 46. Of note, the value obtained in this study is similar to the values observed in adults, after intravenous administration of MHD: 54.7 l for (R)‐MHD and 45.9 l for (S)‐MHD 4.
A summary of a size‐standardized estimates and literature values is provided in Table 6.
Table 6.
Comparison of size‐standardized estimates and values reported in the literature
| Parameter | Estimate | Literature value | Ref. |
|---|---|---|---|
| CLOXC/F | 140 l h–1 70 kg–1 | 170,1 l h–1 70 kg–1 | 39 |
| VOXC/F | 397.7 l 70 kg–1 | 273–875 l 70 kg–1 | 6 |
| CLMHD/F | 3.18 l h–1 70 kg–1 | 3.2 l h–1 70 kg–1 | 15 |
| 3.5–5.5 l h–1 a, b | 4 | ||
| VMHD/F | 54.8 l 70 kg–1 | 54.7 L (S)‐MHDb, a | 4 |
| 45.9 L (R)‐MHDa, b | 4 |
No body weight was provided by the authors; study included 12 healthy volunteers (6 females and 6 males)
Obtained from intravenous data
In paediatric population PK studies, BW is a factor reflecting changes in body size, and is related to clearance and volume via an allometric model with theoretical exponents of 0.75 or 0.67 for clearance and 1 for the volume 47. Fixed and estimated allometric exponents were both tested. For OXC, the estimated exponents for CLOXC/F and VcOXC/F were 0.798 and 2.4, respectively. For MHD, those values were 0.549 and 1.09 for CLMHD/F and VcMHD/F, respectively. Although the obtained values were not exactly similar to the theoretical exponents (principally the 2.4 exponent related to VcOXC/F), the model with estimated exponents performed better on the external evaluation (Table 2), as evidenced by the lower mean prediction error obtained with the empirical model. The reason for this result is unclear to us. A possible explanation for the great difference between the exponent of 2.4 that was estimated for VcOXC/F and the theoretical value of 1 may result from the study design. Indeed, our PK parameters allow to calculate a distribution half‐life of 0.53 h. It is therefore possible our study design did not include enough samples during the distribution phase, which may have penalized the estimation of this allometric factor. The estimated exponent for CLMHD/F (0.549) is in accordance with the empirical allometric exponent obtained by Sugiyama et al. 16 in their population model of MHD (0.555). It was previously demonstrated that the theory‐based allometric exponent of 0.75 for CL could be inaccurate in some situations 48, 49, 50. Indeed, if this theory‐based exponent accurately predicts CL in all cases in adolescents (age 12–18 years) 51, it may not be relevant for some drugs in younger children, especially those <5 years 48, 49, 50. The fact that our population included children between 2 and 12 years and that half of them were aged <6 years may explain the difference between the empirical allometric exponent of CLMHD/F and the theoretical value of 0.75. Based on these results, we decided to use the empirical exponents to perform the dose evaluation. Nonetheless, because of the inconsistency with the allometric principles, we believe an important limitation of the present model is its inapplicability for children under 2 years.
Monte Carlo simulations were performed with the aim to evaluate the consistency between the recommended paediatric doses and the reference trough concentration of MHD (Ctrough) for therapeutic drug monitoring. Older children, represented by higher BW, achieved an AUC about 104.5% higher than younger patients (Table 4). This is consistent with the observation that weight‐normalized clearance decreased with age. For 10‐kg children, the probability for their MHD Ctrough to be within the reference range increased from 23% to 98.3% with increasing doses (10–60 mg kg–1 day–1) while, in 50‐kg children, it decreased from 98.7% to 40.6% with increasing doses (for 10 and 60 mg kg–1 day–1), as more Ctrough exceeded the limit of 35 mg l–1 and reached possibly toxic concentrations (Table S1). This confirms that older children need lower weight‐normalized doses when compared to younger children.
Association with enzyme inducing drugs is another factor accounting for OXC variability 13. Most of the patients were on concomitant enzyme‐inducing AEDs, so, MHD clearance was modelled as the clearance induced by EIAEDs and the covariate was the absence of concomitant EIAEDs. Those AEDs increased MHD clearance by 29.3%. This phenomenon is well known and has been verified in most population models 15, 16, 17, 23, 24. The drugs involved are carbamazepine, phenobarbital and phenytoin and it was demonstrated that they can reduce MHD concentration by 20–40% 14, 52, 53. With our model, patients medicated with concomitant enzyme‐inducing antiepileptic drugs had about 24% lower exposition to MHD than patients not cotreated with EIAEDs (Table 4). Therefore, it seems that children taking EIAEDs require greater weight‐normalized doses to reach similar expositions. For these patients, probabilities to be within the reference range increased with dose kg–1 and weight and it was less likely for them to reach the toxicity threshold (Table S2).
Figure 7 shows daily OXC doses allowing the attainment of a maximum probability (> 95%) for MHD Ctrough to be within the reference range with respect to BW, with and without associated enzyme‐inducing antiepileptic drugs. Recommended doses seem convenient, except for 50 kg children not comedicated with EIAEDs, who would need less than the recommended target dose of 30–45 mg kg–1 day–1, since a maximum probability of being within the reference range is attained between 20 and 30 mg kg–1 day–1, and the risk of toxicity increases with higher doses. By contrast, 10 kg children receiving concomitant EIAEDs would need more than the maximum recommendation of 60 mg kg–1 day–1 to have at least 95% chance to be within the reference range. It is not uncommon for clinicians to exceed the recommendations, as verified by Borusiak et al. in their retrospective study, where epileptic children were given OXC doses from 19 to 123 mg kg–1 day–1 9. Considering a narrower reference range of 15–35 mg l–1, as proposed by May et al. 6, the need for higher doses is, as expected, increased for 10 kg children with EIAEDs who only have, for a 90 mg kg–1 day–1 dose, a 33.8% probability to be within this reference range (Tables S1 and S2). Nonetheless, despite smaller probabilities to reach therapeutic trough concentrations, the risk of toxicity remains the same.
Figure 7.

Daily doses to obtain a maximum probability (>95%) to be within the reference range. Orange line represents doses for children taking concomitant enzyme‐inducing antiepileptic drugs; green line represents doses for children not taking concomitant enzyme‐inducing antiepileptic drugs
The present model is only applicable to 2–12‐year‐old epileptic patients and was developed based on oral suspension data. This formulation is optimal for young children (<8 years) who may have swallowing issues, but the tablet formulation is preferable for older children. In adults, bioequivalence between the oral suspension and the film‐coated tablet was evidenced 54, 55, allowing us to assume that our model is applicable to the use of tablets in children. Due to exclusion criteria, this model cannot be applied neither to patients with BW differing by >2 SDs from normal BW such as obese and malnourished children.
In conclusion, a parent‐metabolite population PK model of OXC and its monohydroxy derivative was developed in epileptic children. It identified BW and concomitant enzyme‐inducing antiepileptic drugs as important covariates explaining interindividual PK variability of these two compounds. This model also showed that the doses currently used by clinicians are appropriate to obtain MHD Ctrough within the recommended reference range 7, except for 10 kg children receiving concomitant enzyme‐inducing antiepileptic drugs who could need doses higher than recommended, and 50 kg children without concomitant enzyme inducing drugs who may need doses lower than recommended. However, as this reference concentration range is wide and the correlation between MHD plasma concentration and its antiepileptic effect has not been well established 10, only clinical responsiveness and adverse events occurrence can ultimately allow the clinicians to decide which dose their patient requires. When the dose/effect relationship is elucidated, this model could be useful to determine optimal dose regimens for children, especially the youngest ones.
Competing Interests
All authors have completed the United Competing Interest form at http://www.icmje.org/conflicts‐of‐interest/ and declare: C.R. reports personal fees from BIOCODEX, outside the submitted work; C.C. reports personal fees and nonfinancial support from BIOCODEX, personal fees from BRABANT, personal fees from UCB‐PHARMA, personal fees from BIAL, personal fees from ZOGENIX, personal fees from VIROPHARMA, outside the submitted work; E.R., O.D., E.C. and G.P. had nothing to disclose; V.J. reports grants from BIOCODEX, personal fees from ZOGENIX, outside the submitted work. A grant from CIBA‐GEIGY was obtained for the conductance of the initial PK study.
The authors would like to acknowledge the contribution of the reviewers to this article.
Supporting information
Table S1 Probability to be within trough concentration ranges for children not taking enzyme‐inducing antiepileptic drugs depending on body weight and daily dose of oxcarbazepine
Table S2 Probability to be within trough concentration ranges for children taking enzyme‐inducing antiepileptic drugs depending on body weight and daily dose of oxcarbazepine
Rodrigues, C. , Chiron, C. , Rey, E. , Dulac, O. , Comets, E. , Pons, G. , and Jullien, V. (2017) Population pharmacokinetics of oxcarbazepine and its monohydroxy derivative in epileptic children. Br J Clin Pharmacol, 83: 2695–2708. doi: 10.1111/bcp.13392.
Submitting author: Christelle Rodrigues, christelle.rodrigues@inserm.fr.
References
- 1. Full prescribing information. Trileptal. 2014.
- 2. Flesch G. Overview of the clinical pharmacokinetics of oxcarbazepine. Clin Drug Investig 2004; 24: 185–203. [DOI] [PubMed] [Google Scholar]
- 3. Volosov A, Xiaodong S, Perucca E, Yagen B, Sintov A, Bialer M. Enantioselective pharmacokinetics of 10‐hydrocarbazepine after oral administration of oxcarbazepine to healthy Chinese subjects. Clin Pharmacol Ther 1999; 66: 547–553. [DOI] [PubMed] [Google Scholar]
- 4. Flesch G, Czendlik C, Renard D, Lloyd P. Pharmacokinetics of the monohydroxy derivative of oxcarbazepine and its enantiomers after a single intravenous dose given as Racemate compared with a single oral dose of oxcarbazepine. Drug Metab Dispos 2011; 39: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 5. Kim DW, Gu N, Jang I, Chu K, Yu K, Cho JY, et al Efficacy, tolerability, and pharmacokinetics of oxcarbazepine oral loading in patients with epilepsy. Epilepsia 2012; 53: e9–12. [DOI] [PubMed] [Google Scholar]
- 6. May TW, Korn‐Merker E, Rambeck B. Clinical pharmacokinetics of oxcarbazepine. Clin Pharmacokinet 2003; 42: 1023–1042. [DOI] [PubMed] [Google Scholar]
- 7. Patsalos PN, Berry DJ, Bourgeois BFD, Cloyd JC, Glauser TA, Johannessen SI, et al Antiepileptic drugs ‐ best practice guidelines for therapeutic drug monitoring : a position paper by the subcommission on therapeutic drug monitoring. ILAE Commission on Therapeutic Strategies Epilepsia 2008; 49: 1239–1276. [DOI] [PubMed] [Google Scholar]
- 8. Striano S, Striano P, Di Nocera P, Italiano D, Fasiello C, Ruosi P, et al Relationship between serum mono‐hydroxy‐carbazepine concentrations and adverse effects in patients with epilepsy on high‐dose oxcarbazepine therapy. Epilepsy Res 2006; 69: 170–176. [DOI] [PubMed] [Google Scholar]
- 9. Borusiak P, Korn‐merker E, Holert N, Boenigk HE. A survey in treatment of childhood epilepsy: of 46 children and adolescents. J Epilepsy 1998; 11: 355–360. [Google Scholar]
- 10. Bring P, Ensom MH. Does oxcarbazepine warrant therapeutic drug monitoring? A Critical Review Clin Pharmacokinet 2008; 47: 767–778. [DOI] [PubMed] [Google Scholar]
- 11. Rey E, Bulteau C, Motte J, Tran A, Sturm Y, D'Souza J, et al Oxcarbazepine pharmacokinetics and tolerability in children with inadequately controlled epilepsy. J Clin Pharmacol 2004; 44: 1290–1300. [DOI] [PubMed] [Google Scholar]
- 12. Landmark CJ, Baftiu A, Tysse I, Valsø B, Larsson PG, Rytter E, et al Pharmacokinetic variability of four newer antiepileptic drugs, lamotrigine, levetiracetam, oxcarbazepine, and topiramate: a comparison of the impact of age and comedication. Ther Drug Monit 2012; 34: 440–445. [DOI] [PubMed] [Google Scholar]
- 13. Johannessen SI, Tomson T. Pharmacokinetic variability of newer antiepileptic drugs: when is monitoring needed? Clin Pharmacokinet 2006; 45: 1061–1075. [DOI] [PubMed] [Google Scholar]
- 14. Tartara A, Galimberti CA, Manni R, Morini R, Limido G, Gatti G, et al The pharmacokinetics of oxcarbazepine and its active metabolite 10‐hydroxy‐carbazepine in healthy subjects and in epileptic patients treated with phenobarbitone or valproic acid. Br J Clin Pharmacol 1993; 36: 366–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sallas WM, Milosavljev S, D'Souza J, Hossain M. Pharmacokinetic drug interactions in children taking oxcarbazepine. Clin Pharmacol Ther 2003; 74: 138–149. [DOI] [PubMed] [Google Scholar]
- 16. Sugiyama I, Bouillon T, Yamaguchi M, Suzuki H, Hirota T, Fink M. Population pharmacokinetic analysis for 10‐monohydroxy derivative of oxcarbazepine in pediatric epileptic patients shows no difference between Japanese and other ethnicities. Drug Metab Pharmacokinet 2015; 30 (5): 160–107. [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Zhang H, Niu C, Gao P, Chen Y, Peng J, et al Population pharmacokinetics modeling of oxcarbazepine to characterize drug interactions in Chinese children with epilepsy. Acta Pharmacol Sin 2014; 35: 1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elger C, Bialer M, Falcão A, Vaz‐da‐Silva M, Nunes T, Almeida L, et al Pharmacokinetics and tolerability of eslicarbazepine acetate and oxcarbazepine at steady state in healthy volunteers. Epilepsia 2013; 54: 1453–1461. [DOI] [PubMed] [Google Scholar]
- 19. Kristensen O, Klitgaard NA, Jonsson B, Sindrup S. Pharmacokinetics of 10‐OH‐carbazepine, the main metabolite of the antiepileptic oxcarbazepine, from serum and saliva concentrations. Acta Neurol Scand 1983; 68: 145–150. [DOI] [PubMed] [Google Scholar]
- 20. Lloyd P, Flesch G, Dieterle W. Clinical pharmacology and pharmacokinetics of oxcarbazepine. Epilepsia 1994; 35: 10–13. [DOI] [PubMed] [Google Scholar]
- 21. Yu Y, Zhang Q, Xu W, Lv C, Hao G. Population pharmacokinetic modeling of oxcarbazepine active metabolite in Chinese patients with epilepsy. Eur J Drug Metab Pharmacokinet 2016; 21: 345–351. [DOI] [PubMed] [Google Scholar]
- 22. Peng J, Zhang H, Liu Z, Xu H, Wang Y. Population pharmacokinetics of oxcarbazepine active metabolite in Chinese children with epilepsy. Int J Clin Pharmacol Ther 2014; 52: 684–692. [DOI] [PubMed] [Google Scholar]
- 23. Northam RS, Hernandez AW, Litzinger MJ, Minecan DN, Glauser TA, Mangat S, et al Oxcarbazepine in infants and young children with partial seizures. Pediatr Neurol 2005; 33: 337–344. [DOI] [PubMed] [Google Scholar]
- 24. Park K, Kim J, Joo EY, Seo DW, Hong SB, Ko J, et al Drug interaction and pharmacokinetic modeling of oxcarbazepine in Korean patients with epilepsy. Clin Neuropharmacol 2012; 35 (1): 40–44. [DOI] [PubMed] [Google Scholar]
- 25. Rouan MC, Decherf M, Le Clanche V, Lecaillon JB, Godbillon J. Automated microanalysis of oxcarbazepine and its monohydroxy ans transdiol metabolites in plasma by liquid chromatography. J Chromatogr B 1994; 658: 167–172. [DOI] [PubMed] [Google Scholar]
- 26. Samson A, Lavielle M, Mentré F. Extension of the SAEM algorithm to left‐censored data in nonlinear mixed‐effects model: application to HIV dynamics model. Comput Stat Data Anal 2006; 51: 1562–1574. [Google Scholar]
- 27. Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001; 28: 481–504. [DOI] [PubMed] [Google Scholar]
- 28. Schütz H, Feldmann KF, Faigle JW, Kriemler HP, Winkler T. The metabolism of 14C‐oxcarbazepine in man. Xenobiotica 1986; 16: 769–778. [DOI] [PubMed] [Google Scholar]
- 29. Bertrand J, Laffont CM, Mentré F, Chenel M, Comets E. Development of a complex parent‐metabolite joint population pharmacokinetic model. AAPS J 2011; 13: 390–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang C, Peeters MYM, Allegaert K, van Oud‐alblas HJB, Krekels EHJ, Tibboel D, et al A bodyweight‐dependent allometric exponent for scaling clearance across the human life‐span. Pharm Res 2012; 29: 1570–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed‐effect models: the npde add‐on package for R. Comput Methods Programs Biomed 2008; 90: 154–166. [DOI] [PubMed] [Google Scholar]
- 32. Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction‐corrected visual predictive checks for diagnosing nonlinear mixed‐effects models. AAPS J 2011; 13: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li R, Sheng X, Ma L, Yao H, Cai L, Chen C, et al Saliva and plasma monohydroxycarbamazepine concentrations in pediatric patients with epilepsy. Ther Drug Monit 2016; 38: 365–370. [DOI] [PubMed] [Google Scholar]
- 34. Samuels M, Wieteska S, eds. Advanced Paediatric Life Support: The Practical Approach. West Sussex, UK: John Wiley & Sons Ltd, 2011. [Google Scholar]
- 35. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1D68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE, et al The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 2015; 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng H, Juskot WJ. Pharmacokinetics of reversible metabolic systems. Biopharm Drug Dispos 1993; 14: 721–766. [DOI] [PubMed] [Google Scholar]
- 38. Brar S, Bhattaram A, Kumi K, Zhu H. Clinical pharmacology review. 2011. Available from: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM328328.pdf
- 39. Dickinson RG, Hooper WD, Dunstan PR, Eadie MJ. First dose and steady‐state pharmacokinetics of oxcarbazepine and its 10‐hydroxy metabolite. Eur J Clin Pharmacol 1989; 37: 69–74. [DOI] [PubMed] [Google Scholar]
- 40. Jullien V, Chhun S, Rey E, Dulac O, Tod M, Chiron C, et al Pharmacokinetics of clobazam and N‐desmethylclobazam in children with Dravet syndrome receiving concomitant stiripentol and valproic acid. Clin Pharmacokinet 2015; 54: 527–536. [DOI] [PubMed] [Google Scholar]
- 41. Delgado Iribarnegaray MF, Santo Bueldga D, Garcia Sanchez MJ, Otero MJ, Falcao A, Dominguez‐Gil A. Carbamazepine population pharmacokinetics in children: mixed‐effect models. Ther Drug Monit 1997; 19: 132–139. [DOI] [PubMed] [Google Scholar]
- 42. Yukawa E, Higuchi S, Aoyama T. Phenobarbitone population pharmacokinetics from routine clinical data: role of patient characteristics for estimating dosing regimens. J Pharm Pharmacol 1992; 44: 755–760. [DOI] [PubMed] [Google Scholar]
- 43. Banfield CR, Zhu GR, Jen JF, Jensen PK, Schumaker RC, Perhach JL, et al The effect of age on the apparent clearance of felbamate: a retrospective analysis using nonlinear mixed‐effects modeling. Ther Drug Monit 1996; 18: 19–29. [DOI] [PubMed] [Google Scholar]
- 44. Cloyd JC, Fisher JH, Kriel RL, Kraus DM. Valproic acid pharmacokinetics in children. IV. Effects of age and antiepileptic drugs on protein binding and intrinsic clearance. Clin Pharmacol Ther 1993; 53: 22–29. [DOI] [PubMed] [Google Scholar]
- 45. Strassburg CP, Strassburg A, Kneip S, Barut A, Tukey RH, Rodeck B, et al Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut 2002; 50: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holford N, Heo Y‐A, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci 2013; 102: 2941–2952. [DOI] [PubMed] [Google Scholar]
- 47. Anderson BJ, Holford NHG. Mechanism‐based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 2008; 48: 303–332. [DOI] [PubMed] [Google Scholar]
- 48. Calvier EAM, Krekels EHJ, Välitalo PAJ, Rostami‐Hodjegan A, Tibboel D, Danhof M, et al Allometric scaling of clearance in paediatric patients: when does the magic of 0.75 Fade? Clin Pharmacokinet 2017; 56: 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mahmood I. Dosing in children: a critical review of the pharmacokinetic Allometric scaling and modelling approaches in paediatric drug development and clinical settings. Clin Pharmacokinet 2014; 53: 327–346. [DOI] [PubMed] [Google Scholar]
- 50. Mahmood I. Prediction of drug clearance in children from adults: a comparison of several allometric methods. Br J Clin Pharmacol 2006; 61: 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Momper JD, Mulugeta Y, Green DJ, Karesh A, Krudys KM, Sachs HC, et al Adolescent dosing and labeling since the Food and Drug Administration amendments act of 2007. JAMA Pediatr 2013; 167: 926–932. [DOI] [PubMed] [Google Scholar]
- 52. McKee P, Blacklaw J, Forrest G, Gillham R, Walker S, Connelly D, et al A double‐blind, placebo‐controlled interaction study between oxcarbazepine and carbamazepine, sodium valproate and phenytoin in epileptic patients. Br J Clin Pharmacol 1994; 37: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Armijo J, Vega‐Gil N, Shushtarian M, Adin J, Herranz J. 10‐Hydroxycarbazepine serum concentration‐to‐oxcarbazepine dose ratio: influence of age and concomitant antiepileptic drugs. Ther Drug Monit 2005; 27: 199–204. [DOI] [PubMed] [Google Scholar]
- 54. Souppart C, Yin Q, Merz M, Hu P, Jiang J, Appel‐Dingemanse S. Bioequivalence of oxcarbazepine oral suspension vs. film‐coated tablet in healthy Chinese male subjects. Int J Clin Pharmacol Ther 2008; 46: 538–544. [DOI] [PubMed] [Google Scholar]
- 55. Flesch G, Tudor D, Denouel J, Bonner J, Camisasca R. Assessment of the bioequivalence of two oxcarbazepine oral suspensions vs. a film‐coated tablet in healthy subjects. Int J Clin Pharmacol Ther 2003; 41: 299–308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Probability to be within trough concentration ranges for children not taking enzyme‐inducing antiepileptic drugs depending on body weight and daily dose of oxcarbazepine
Table S2 Probability to be within trough concentration ranges for children taking enzyme‐inducing antiepileptic drugs depending on body weight and daily dose of oxcarbazepine


