Abstract
Aims
To determine the disposition and effects of caffeine after administration using a new dosage form (AeroShot) that delivers caffeine by inspiration of a fine powder into the oral cavity and compare it to an equivalent dose of an oral solution (energy drink) as the reference standard.
Methods
Healthy human subjects (n = 17) inspired a 100 mg caffeine dose using the AeroShot device or consumed an energy drink on separate study days. Heart rate, blood pressure and subject assessments of effects were measured over an 8‐h period. Plasma concentrations of caffeine and its major metabolites were determined by liquid chromatography–mass spectrometry. Pharmacokinetic, cardiovascular and perceived stimulant effects were compared between AeroShot and energy drink phases using a paired t test and standard bioequivalency analysis.
Results
Caffeine disposition was similar after caffeine administration by the AeroShot device and energy drink: peak plasma concentration 1790 and 1939 ng ml–1, and area under the concentration–time curve (AUC) 15 579 and 17 569 ng ml–1 × h, respectively, but they were not bioequivalent: AeroShot AUC of 80.3% (confidence interval 71.2–104.7%) and peak plasma concentration of 86.3% (confidence interval 62.8–102.8%) compared to the energy drink. Female subjects did have a significantly larger AUC compared to males after consumption of the energy drink. The heart rate and blood pressure were not significantly affected by the 100 mg caffeine dose, and there were no consistently perceived stimulant effects by the subjects using visual analogue scales.
Conclusion
Inspiration of caffeine as a fine powder using the AeroShot device produces a similar caffeine profile and effects compared to administration of an oral solution (energy drink).
Keywords: AeroShot, caffeine, energy drink, pharmacodynamics, pharmacokinetics
What is Already Known about this Subject
Caffeine abuse is a growing problem that has been exacerbated by the marketing of energy drinks and novel dosage forms designed to maximize stimulant effects.
Consumption of caffeine with alcohol is associated with increased alcohol consumption.
The co‐abuse of caffeine and alcohol increases the risk of alcohol‐related toxicity, traumatic injury and sexual assault.
What this Study Adds
The first pharmacokinetic/pharmacodynamic human study of a new caffeine dosage form (inspired powder).
A comparative analysis of caffeine delivery of an inspired caffeine powder using an oral solution (energy drink) as the reference standard.
Introduction
The introduction of energy drinks (e.g. Red Bull) in Austria in 1987 and the USA in 1997 quickly lead to the widespread consumption of high caffeine‐content beverages with worldwide sales of the top 15 selling brands of $22.17 billion in 2015 (www.caffeineinformer.com). The primary target of drink manufacturer advertising is young adults, and this group represents the majority of energy drink consumers 1. This is also the age group with the highest risk of drug abuse 2, which has led to a growing concern of the potential increase in the abuse of caffeine alone and in combination with alcohol. In one survey of college students, 51% reported consuming an energy drink within the last month, and 54% of energy drink users consumed energy drinks with alcohol 3.
The consumption of energy drinks is associated with an increase in alcohol consumption and consequent alcohol‐related problems such as severe alcohol intoxication, violence, sexual assault, drunk driving and traumatic injuries 4, 5. This association is consistent with the finding that the combined consumption of energy drinks and alcohol does not counteract alcohol impairment, but potentially enables greater alcohol consumption 6. In 2011, a new dosage form of caffeine was introduced in the USA known as AeroShot (Breathable Foods, Inc., Cambridge, MA, USA), which delivers 100 mg of caffeine as an inspired fine powder. How efficiently caffeine is absorbed by this method of administration is unknown, because it is marketed as a nutritional supplement, which does not require studies of human systemic exposure. Concerns have been expressed by the Food and Drug Administration about its safety and potential for abuse including the possibility that this product may be used as a method to administer caffeine in combination with alcohol (Food and Drug Administration Warning Letter, 3 March 2012, Breathable Foods, Inc).
AeroShot is not an inhaled form of caffeine as the particle size of the powder is >10 μm, which is too large to penetrate into the lungs. Previous studies with caffeine containing gums have reported rapid caffeine absorption suggesting that mucosal absorption could play a role in addition to oral absorption of swallowed powder 7, 8. A better understanding of the disposition of caffeine following caffeine administration by the AeroShot device is an important initial step in assessing the potential abuse and toxicity of this method of caffeine administration.
The objective of this human study is to compare the pharmacokinetic disposition, cardiovascular effects and subject perceived stimulant effects of a 100 mg dose of caffeine administered as an inspired powder using the AeroShot device with the consumption of an equivalent dose by oral administration of an energy drink.
Methods
Study subjects
Healthy human subjects between the ages of 18 and 45 years who were moderate consumers of caffeine (≤300 mg of caffeine per day) were recruited to participate in this study. Both male and female nonsmokers of any ethnicity were eligible to participate. To establish health status, subjects underwent screening that included evaluation of past medical history, medications, a basic metabolic panel (electrolytes, glucose, calcium, blood urea nitrogen and creatinine), urinalysis (pH, protein, glucose, specific gravity), electrocardiograph (ECG) and physical examination. Only subjects without any chronic disease and not taking any chronic medications were enrolled into the study. Female subjects were excluded if they were pregnant or seeking to become pregnant, and were required to be on an effective contraceptive method other than oral contraceptives during the study.
Study design
All study procedures were conducted at the University of Tennessee Clinical Research Center at Methodist University Hospital in Memphis, TN, and the human study protocol and consent form were approved by the University of Tennessee Institutional Review Board (13–02648‐FB). This was a single‐centre, two‐phase, crossover study supported by the National Institutes of Health (National Institute on Drug Abuse Grant R03DA035347). Each subject received 100 mg of caffeine by oral administration of an energy drink (Guru Lite: caffeine in the form of guarana seed extract, and sweetened with stevia leaf extract) or inspiration of a fine powder into the oral cavity (AeroShot: 100 mg of caffeine with 20 mg of vitamin B3, 2 mg of vitamin B6 and 6 μg of vitamin B12) on separate study days with at least a 1‐week washout between phases. The order of administration was randomly assigned. Subjects were asked to refrain from consuming caffeine for 24 h prior to their arrival at the Clinical Research Center in the morning. Following a light breakfast of buttered toast and 2% milk an indwelling catheter was placed in a forearm vein and a baseline blood sample drawn, heart rate and blood pressure measured, and subject self‐assessments of relaxation, alertness, jitteriness, tiredness, tension, and overall mood completed. Subjects then consumed 100 mg of caffeine by oral administration (248 ml of Guru Light) or oral inspiration (complete inspiration of the contents of a single AeroShot container) and blood samples drawn (5 ml per sample), blood pressure and heart measured, and self‐assessments of caffeine effects obtained at 1, 2, 5, 10, 15, 20, 30, 40 and 60 min, and 2, 3, 4, 6 and 8 h after the start of caffeine consumption. Subjects were given a maximum of 5 min to consume the 100‐mg caffeine dose.
Cardiovascular and subject self‐assessment of caffeine effects
The heart rate and blood pressure were monitored using a Welch Allyn Vital Signs Monitor 300 series device (Skaneateles Falls, NY, USA). Heart rate and blood pressure were recorded at baseline and at each blood collection time point over the 8‐h sampling period. The subject self‐assessments of caffeine effects were recorded using analogue scales as previously described 9. Briefly, at baseline and when each blood sample was collected, the subject completed self‐assessment visual analogue scales (VAS) for relaxation, alertness, jitteriness, tiredness, tenseness and overall mood. Subjects marked on a 100 mm line between not at all on the left and extremely on the right (for overall mood very good was on the left and very bad on the right) to indicate how they felt at the time of the blood sampling. The distance from the start of the scale on the left to the point the subject marked on the line was measured in mm. The effects of the 100 mg caffeine dose administered by energy drink consumption or AeroShot inspiration on heart rate, diastolic blood pressure, systolic blood pressure and the subject self‐assessments of caffeine's effects were determined by comparing the effect at the time the peak concertation occurred (Tmax) to the baseline effect for each subject.
Caffeine assay
Details and validation of the assay used in this study are described in our previous publication 10. Briefly, an Agilent 1100 series liquid chromatography system (Waldbronn, Germany) was coupled to an AB SCIEX 3000 triple quadrupole mass spectrometer (Toronto, Canada). The chromatographic separation was achieved on a 3.5‐μm Waters Symmetry C18 column (75 × 4.6 mm i.d.). Mobile phase consisted of water for phase A and methanol for phase B, both containing 25 mmol l–1 formic acid. Separation was optimized using a gradient method with mobile phase A/B set to 95%/5% from 0.00 to 0.10 min and 60%/40% from 0.11 to 2.50 min and then back to 95%/5% from 2.51 to 6.00 min. The mass spectrometer was operated in the positive ion mode. The nebulizer gas, curtain gas, ionspray voltage and source temperature were set at 12 psi (82.7 kPa), 15 psi (103.4 kPa), 5500 V, and 550°C, respectively. The precursor‐product ion pairs used for multiple reaction monitoring of caffeine, paraxanthine, theophylline, theobromine, caffeine‐d9 and paraxanthine‐d3 were m/z 194.9 → 137.8, 181.0 → 124.2, 181.1 → 124.2, 181.1 → 137.8, 204.2 → 144.0 and 184.1 → 124.2, respectively. The liquid chromatography eluent was introduced into the electrospray source at a flow rate of 700 μl min–1 with flow splitting (split ratio 1:1) throughout the gradient programme. Weighted linear regression method was used to calculate the plasma concentrations of caffeine and its metabolites.
Pharmacokinetic analysis
The amount of the caffeine dose for each of the formulations was estimated by determining the caffeine content from five Guru Lite energy drinks and five AeroShot containers. Pharmacokinetic parameters were determined by standard noncompartmental methods. The peak caffeine plasma concentration (Cmax) and Tmax were obtained directly from the concentration–time curves. The terminal elimination rate constant (k) was calculated from the linear regression of the last four time points. The total area under the concentration–time curve (AUC) was estimated by the trapezoidal method with the area from the last time point to infinity estimated by dividing the final plasma concentration by k. If the baseline plasma sample had a quantifiable plasma concentration the zero‐time caffeine concentration was subtracted from all subsequent caffeine concentrations prior to pharmacokinetic analysis. The noncompartmental calculations including the linear regression were performed using Microsoft Excel 2016 (version 16.0.7329.1051). A standard average bioequivalence assessment for AUC and Cmax was performed using Phoenix WinNonlin Version 6.4 (Certara, Princeton, NJ, USA), as initially described in the July 1992 FDA guidance on Statistical Procedures for Bioequivalence Studies Using a Standard Two‐Treatment Crossover Design, and further refined in the US FDA Guidance for Industry (August 1999) entitled Average, Population and Individual Approaches to Establishing Bioequivalence. The model used the typical mixed effects ANOVA model for the natural log‐transformed values of either AUC or Cmax with sequence, period and formulation as fixed effects and subject by sequence as random effect. The energy drink was designated as reference and AeroShot as the test formulation.
Statistical analysis
The energy drink was considered the reference standard and the disposition of caffeine after administration using the AeroShot device was evaluated by comparing the difference in the Tmax, Cmax and AUC between the energy drink and AeroShot phases using a paired t‐test. Mean plasma concentrations between the energy drink and AeroShot phases were compared using a paired t‐test with an adjustment for multiple comparisons using the Benjamini‐Hochberg procedure 11. All data are reported as the mean ± standard deviation (SD). The level of significance was P < 0.05.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 12, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 13.
Results
A total of 24 subjects were enrolled in the study and 17 subjects (nine male and eight female) completed both phases of the study. Five subjects were excluded during the initial assessment due to abnormal laboratory values and ECG findings, and two subjects completed only a single phase of the study. Only the subjects completing both phases are included in the analysis of study results. The average and standard deviation of the age and weight for the males (n = 9) were 28.7 ± 5.4 years and 87.2 ± 10.1 kg, and for the females (n = 8) were 26.3 ± 3.0 years and 62.9 ± 12.2 kg, respectively.
In both phases of the study the subjects consumed 100 mg of caffeine, which was confirmed by assaying the caffeine content of five Guru Lite energy drinks and AeroShot devices. The mean ± SD of the caffeine content was 96.6 ± 3.6 and 97.7 ± 1.0 mg for the energy drink and AeroShot, respectively. The plasma concentration–time profiles for caffeine, paraxanthine, theobromine and theophylline after the energy drink and AeroShot phases are shown in Figure 1.
Figure 1.
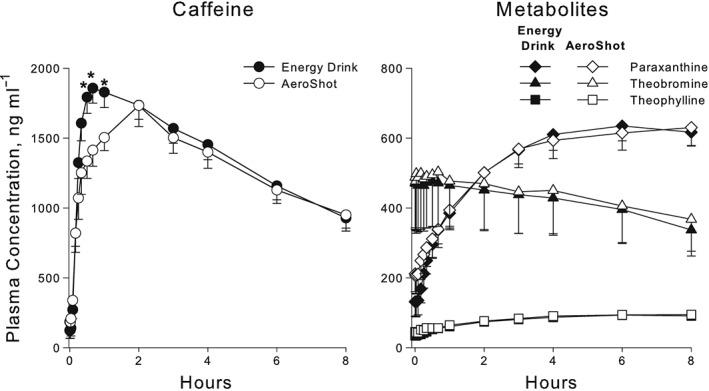
Mean ± standard error of the mean for caffeine and metabolite plasma concentrations after 100 mg caffeine dose administered by oral inspiration (AeroShot) compared to oral administration (energy drink). Caffeine concentrations were higher after energy drink administration at the 30‐, 40‐ and 60‐min sampling times (* indicates P < 0.05 with adjustment for multiple comparisons using the Benjamini–Hochberg procedure)
Only the disposition of caffeine was compared between the energy drink and AeroShot phases. The peak caffeine plasma concentrations (Cmax) and AUC for caffeine did not differ between the AeroShot and energy drink phases (Table 1). Thus, the total systemic exposure and Cmax achieved after the 100 mg dose administered by the AeroShot and energy drink were similar. The absorption rate as indicated by the time to reach the Tmax was longer after AeroShot administration, and this is reflected in Figure 1, which shows the mean caffeine plasma concentrations at 30, 40 and 60 min were higher after the energy drink than after AeroShot administration. The AeroShot was not bioequivalent to the energy drink with an AUC of 80.3% [confidence interval (CI) 71.2–104.7%] and Cmax of 86.3% (CI 62.8–102.8%) compared to the energy drink (Table 1).
Table 1.
Bioequivalence analysis: a 100 mg dose of caffeine was administered by oral administration (energy drink, Guru Lite) or inspiration (AeroShot). Subjects (n = 17) received both dosage forms separated by at least 1 week
| Energy drink | AeroShot | Ratio | 90% CI | |||
|---|---|---|---|---|---|---|
| Pharmacokinetic parameter | Mean ± SD | Adjusted gMean (gCV [%]) | Mean ± SD | Adjusted gMean (gCV [%]) | Adjusted gMean [%] (gSE) | |
| C max (ng ml –1 ) | 1939 ± 341 | 1909 (18.8) | 1790 ± 686 | 1648 (49.1) | 86.3 (1.12) | 62.8–102.8% |
| AUC (ng ml –1 × h) | 17 569 ± 6869 | 16 414 (40.3) | 15 579 ± 8387 | 13 188 (75.5) | 80.3 (1.15) | 71.2–104.7% |
| T max range (h) | 0.33–2.0 | 0.25–3.0 | ||||
| Half‐life (h) | 6.0 | 5.3 | ||||
| CL/F (l h –1 ) | 6.5 ± 2.5 | 10.1 ± 9.5 | ||||
| V/F (l) | 53.9 ± 13.1 | 64.5 ± 32.2 | ||||
CI, confidence interval; SD, standard deviation; Cmax, peak caffeine plasma concentration; AUC, area under the concentration–time curve; Tmax, time of peak caffeine plasma concentration; CL/F, clearance/bioavailability; V/F, volume of distribution/bioavailability
The female subjects achieved a higher caffeine AUC and Cmax than the male subjects, but when these parameters were normalized to body weight there were no differences (Table 2). Caffeine administration by consumption of the energy drink and AeroShot administration had no significant effects on heart rate, systolic or diastolic blood pressure (Table 3 and Figure 2). The VAS assessing caffeine effects perceived by the subjects following caffeine administration did not produce any significant changes whether the dose was administered by energy drink or AeroShot (Figure 3).
Table 2.
Comparison of area under the concentration–time curve (AUC) and peak caffeine plasma concentration (Cmax) by sex
| AUC (ng ml–1 × h) | Cmax (ng ml–1) | |||
|---|---|---|---|---|
| Actual | Normalized | Actual | Normalized | |
| Male (n = 9) | 14 029 ± 4416 | 12430 ± 4690 | 1758 ± 341 | 1526 ± 306 |
| Female (n = 8) | 21 551 ± 7163 | 13 699 ± 6186 | 2142 ± 208 | 1322 ± 176 |
| P value | 0.018 | 0.638 | 0.014 | 0.118 |
Table 3.
Mean ± SD of the cardiovascular and subject perceived effects (visual analogue scales) of 100 mg caffeine dose at baseline (BL) and at the time of the peak caffeine plasma concentration (Tmax)
| Energy Drink | AeroShot | |||||
|---|---|---|---|---|---|---|
| Cardiovascular | BL | Tmax | Tmax – BL | BL | Tmax | Tmax – BL |
| Heart rate (beats/min) | 71 ± 10 | 64 ± 10 | −6.6 ± 5.9 | 70 ± 16 | 69 ± 24 | 1.2 ± 24.4 |
| Systolic blood pressure (mmHg) | 118 ± 12 | 118 ± 12 | 0.7 ± 10.9 | 117 ± 15 | 114 ± 18 | −2.8 ± 19.3 |
| Diastolic blood pressure (mmHg) | 73 ± 7 | 70 ± 9 | −3.5 ± 6.8 | 73 ± 10 | 73 ± 10 | 0.3 ± 6.2 |
| Visual analogue scales | ||||||
| Alert (mm) | 74 ± 26 | 78 ± 19 | 3.5 ± 15.8 | 74 ± 23 | 78 ± 21 | 3.8 ± 10.5 |
| Tense (mm) | 7 ± 13 | 7 ± 10 | −0.5 ± 9.7 | 9 ± 13 | 6 ± 12 | −3.5 ± 7.8 |
| Jittery (mm) | 6 ± 14 | 8 ± 11 | 2.0 ± 12.9 | 15 ± 28 | 6 ± 13 | −9.1 ± 28.7 |
| Mood (mm) | 10 ± 12 | 11 ± 16 | 0.3 ± 13.9 | 11 ± 13 | 10 ± 10 | −1.3 ± 8.4 |
| Tired (mm) | 34 ± 26 | 24 ± 20 | −10.0 ± 32.8 | 37 ± 26 | 24 ± 17 | −12.7 ± 25.8 |
| Relaxed (mm) | 80 ± 25 | 83 ± 15 | 3.1 ± 26.9 | 81 ± 16 | 87 ± 14 | 5.7 ± 17.6 |
Figure 2.
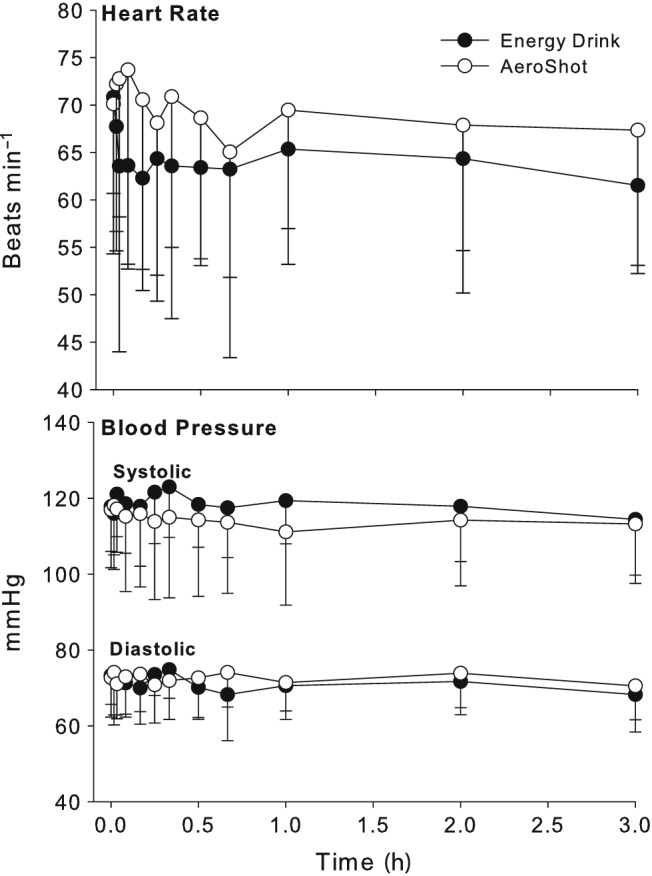
Mean ± standard deviation for heart rate and blood pressure after 100 mg caffeine dose administered by oral inspiration (AeroShot) and oral administration (energy drink)
Figure 3.
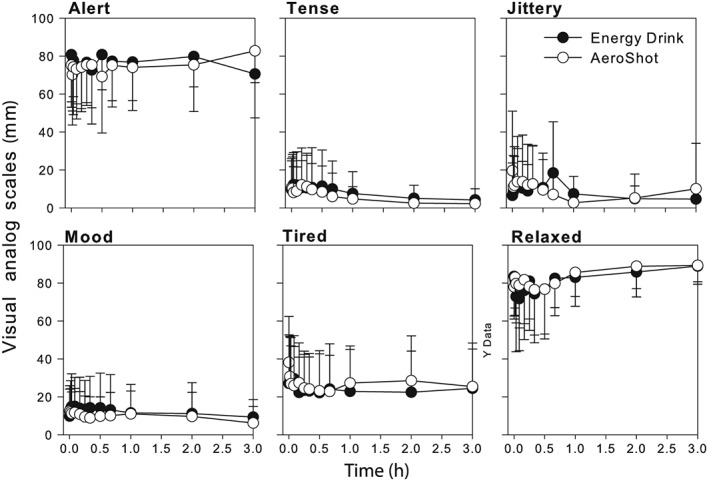
Mean ± standard deviation of subjects' self‐assessment of the effects of 100 mg caffeine dose administered by oral inspiration (AeroShot) and oral administration (energy drink) using visual analogue scales
Discussion
The major finding of this study is that administration of caffeine using the AeroShot device does not result in any increase in the absorption rate of caffeine into the systemic circulation when compared to oral administration of a caffeine solution. In fact, the time to reach the peak caffeine concentration was longer (1.34 vs. 0.88 h) and the caffeine concentrations generally lower after administration using the AeroShot device especially in the initial absorption phase (Figure 1). The two methods of caffeine administration were not bioequivalent with an AeroShot mean AUC and Cmax of 80.3% and 86.3%, respectively, compared to the energy drink phase. However, two subjects with very low AUCs after AeroShot of 12.9 and 36.2% compared to the energy drink phase is highly suggestive of poor administration technique, and if removed, the two administration methods would be bioequivalent with a mean AeroShot AUC and Cmax of 95.4% (CI 85.8–106.1%) and 95.6% (CI 81.7–111.8%), respectively, compared to the energy drink phase. Given the consistent caffeine content (energy drink = 96.6 ± 3.6 mg and AeroShot = 97.7 ± 1.0 mg) of the dosage forms and the complete oral bioavailability of caffeine 14, it is concluded that administration of caffeine using the AeroShot device is comparable to administration by oral consumption of an energy drink. We speculate that demonstrated bioequivalence after dropping the two outliers indicates that caffeine absorption following administration by inspiration using the AeroShot device is primarily via oral absorption occurring after the powder is swallowed.
A previous study comparing the disposition of caffeine concentrations after administration in a capsule vs. chewing gum concluded that caffeine absorption was faster after the gum suggesting that absorption via the mucosa was potentially faster than oral absorption 7. Our data do not provide support for this inference as the mean Tmax value after administration of the gum was longer than the value we found for the energy drink, a finding that is also further supported by the shorter Tmax values that have been recently reported by White et al. for caffeine administered in the form of an energy drink and hot coffee 15. A comparison of caffeine disposition after administration by capsule, gum, coffee, energy drink and AeroShot in Table 4 demonstrates the similarity in caffeine disposition irrespective of the dosage form, with the only differences being the Tmax, which is energy drink = coffee < gum < AeroShot < capsule. Collectively, we would speculate from these results that caffeine is absorbed after swallowing and absorption through the mucosa does not contribute significantly to systemic absorption after administration by AeroShot or gum. The longer Tmax values for the capsule and AeroShot are most likely to be due to the lag in the absorption after administration of the capsule and the fact that subjects using the AeroShot device were not allowed to drink water within 30 min after dosing slowing the transit of caffeine‐containing powder to the stomach.
Table 4.
Comparison of caffeine pharmacokinetics after administration of 100 mg of caffeine between different formulations
|
Cmax
(ng ml–1) |
Tmax
(h) |
AUC (ng ml–1 × h) |
V/F/kg (l kg–1) |
CL/F/kg (l h–1 kg–1) |
t½ (h) |
Study | |
|---|---|---|---|---|---|---|---|
| Capsule | 1840 ± 700 | 1.56 ± 0.76 | 14 700 ± 7260 | 0.77 ± 0.38 | 0.116 ± 0.070 | 4.3 | Kamimori 7 |
| Gum | 1200 ± 510 | 1.25 ± 0.77 | 10 900 ± 7000 | 1.17 ± 0.17 | 0.171 ± 0.100 | 4.6 | Kamimori 7 |
| ED | 1939 ± 341 | 0.88 ± 0.57 | 17 569 ± 6869 | 0.71 ± 0.09 | 0.089 ± 0.035 | 6.0 | Laizure |
| AeroShot | 1790 ± 686 | 1.34 ± 0.83 | 15 579 ± 8387 | 0.87 ± 0.43 | 0.135 ± 0.128 | 5.3 | Laizure |
| ED | 1963* | 1.17 ± 0.73 | 21948* | 0.71 ± 0.41 | 0.090 ± 0.048 | 6.7 | White 15 |
| Coffee (hot) | 2338* | 0.98 ± 0.45 | 24083* | 0.62 ± 0.13 | 0.072 ± 0.036 | 6.8 | White 15 |
Caffeine dose 160 mg. Cmax and AUC normalized to 100 mg caffeine dose.
Cmax, peak caffeine plasma concentration; AUC, area under the concentration–time curve; Tmax, time of peak caffeine plasma concentration; CL/F, clearance/bioavailability; V/F, volume of distribution/bioavailability
As observed in other studies the disposition of caffeine was different between male and female subjects (Table 2) with female subjects having a higher Cmax and AUC compared to males, but there was no difference in these parameters after normalizing for the subjects' body weight. A previous study reported similar results though a significant difference between males and females in the Cmax and AUC was maintained even after normalized for body weight 15. The primary metabolic pathway of caffeine is conversion to paraxanthine by CYP1A2, and lower CYP1A2 activity in women vs. men has been reported in Caucasian, African‐American and Chinese subjects 16, which would be consistent with greater systemic exposure to caffeine in females given an equivalent dose as males. Although the reported differences are modest and have not been shown to result in differences in the physiological responses, investigations including this one have been confined to single‐dose studies. Consumption of repeated doses over a short time interval, which would be facilitated by a device such as the AeroShot used in this study would result in much greater caffeine exposure in females consuming equivalent caffeine doses as males.
The cardiovascular response and subject self‐assessments failed to demonstrate any effect of the 100 mg caffeine dose whether administered as an energy drink or using the AeroShot device (Figure 2 and 3). This is consistent with previous findings that caffeine doses in excess of 200 mg are generally required to produce clinically meaningful changes in cardiovascular parameters 17, 18, 19, 20. However, some studies have reported significant effects on heart rate or blood pressure at doses as low as 80 mg 21, 22, 23. This may be due to other ingredients contained in the tested dosage forms such as glucose and taurine both common ingredient in energy drinks. In this study Guru Lite, which does not contain sugar or taurine, and AeroShot, which contains caffeine and B vitamin supplements only were used.
In summary, administration of caffeine as a fine powder inspired into the oral cavity using the AeroShot device produces a caffeine pharmacokinetic profile comparable to administration by an oral solution in the form of an energy drink. These data suggest that the abuse potential for AeroShot alone or in combination with alcohol is likely to be of equivalent risk to an energy drink. However, the risk profile of the AeroShot device may increase substantially with rapid repeated doses, because administration of the powder is not impeded by the large volume load that would be required to achieve an equivalent dose from energy drink consumption.
Contributors
S.C.L.: Principal investigator for the human study, pharmacokinetic data analysis, primary author of paper. B.M.: Performed the bioequivalence analysis. K.N.: Assisted in performing the human study, data analysis. F.C.: Performed assay of caffeine and metabolite plasma concentrations. Z.H.: Performed assay of caffeine and metabolite plasma concentrations. R.P.: Assisted in the design and performance of the human study and the writing of the paper.
Competing Interests
There are no competing interests to declare.
This work was supported by NIH National Institute on Drug Abuse grant number R03DA035347.
Laizure, S. C. , Meibohm, B. , Nelson, K. , Chen, F. , Hu, Z.‐Y. , and Parker, R. B. (2017) Comparison of caffeine disposition following administration by oral solution (energy drink) and inspired powder (AeroShot) in human subjects. Br J Clin Pharmacol, 83: 2687–2694. doi: 10.1111/bcp.13389.
References
- 1. Heckman MA, Sherry K, de Mejia G. Enery drinks: an assessment of their market size, comsumer demographics, ingredient profile, funtionality, and regulations in the United States. Comp Rev Food Sci Safe 2010; 9: 303–317. [DOI] [PubMed] [Google Scholar]
- 2. Center for Behavioral Health Statistics and Quality . (2015). Behavioral health trends in the United States: Results from 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15‐4927, NSDUH Series H‐50). Retrieved from http://www.samhsa.gov/data [Google Scholar]
- 3. Malinauskas BM, Aeby VG, Overton RF, Carpenter‐Aeby T, Barber‐Heidal K. A survey of energy drink consumption patterns among college students. Nutr J 2007; 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks – a growing problem. Drug Alcohol Depend 2009; 99: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brache K, Stockwell T. Drinking patterns and risk behaviors associated with combined alcohol and energy drink consumption in college drinkers. Addict Behav 2011; 36: 1133–1140. [DOI] [PubMed] [Google Scholar]
- 6. Attwood AS, Rogers PJ, Ataya AF, Adams S, Munafo MR. Effects of caffeine on alcohol‐related changes in behavioural control and perceived intoxication in light caffeine consumers. Psychopharmacology (Berl) 2012; 221: 551–560. [DOI] [PubMed] [Google Scholar]
- 7. Kamimori GH, Karyekar CS, Otterstetter R, Cox DS, Balkin TJ, Belenky GL, et al The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. Int J Pharm 2002; 234: 159–167. [DOI] [PubMed] [Google Scholar]
- 8. Syed SA, Kamimori GH, Kelly W, Eddington ND. Multiple dose pharmacokinetics of caffeine administered in chewing gum to normal healthy volunteers. Biopharm Drug Dispos 2005; 26: 403–409. [DOI] [PubMed] [Google Scholar]
- 9. Rogers PJ, Martin J, Smith C, Heatherley SV, Smit HJ. Absence of reinforcing, mood and psychomotor performance effects of caffeine in habitual non‐consumers of caffeine. Psychopharmacology (Berl) 2003; 167: 54–62. [DOI] [PubMed] [Google Scholar]
- 10. Chen F, Hu ZY, Parker RB, Laizure SC. Measurement of caffeine and its three primary metabolites in human plasma by HPLC‐ESI‐MS/MS and clinical application. Biomed Chromatogr 2017; 31: e3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statistical Soc 1995; 57: 289–300. [Google Scholar]
- 12. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blanchard J, Sawers SJ. The absolute bioavailability of caffeine in man. Eur J Clin Pharmacol 1983; 24: 93–98. [DOI] [PubMed] [Google Scholar]
- 15. White JR Jr, Padowski JM, Zhong Y, Chen G, Luo S, Lazarus P, et al Pharmacokinetic analysis and comparison of caffeine administered rapidly or slowly in coffee chilled or hot versus chilled energy drink in healthy young adults. Clin Toxicol (Phila) 2016; 54: 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meibohm B, Beierle I, Derendorf H. How important are gender differences in pharmacokinetics? Clin Pharmacokinet 2002; 41: 329–342. [DOI] [PubMed] [Google Scholar]
- 17. Phan JK, Shah SA. Effect of caffeinated versus noncaffeinated energy drinks on central blood pressures. Pharmacotherapy 2014; 34: 555–560. [DOI] [PubMed] [Google Scholar]
- 18. Lane JD, Williams RB Jr. Cardiovascular effects of caffeine and stress in regular coffee drinkers. Psychophysiology 1987; 24: 157–164. [DOI] [PubMed] [Google Scholar]
- 19. Teng CL, Lim WY, Chua CZ, Teo RS, Lin KT, Yeo JC. Does a single cup of caffeinated drink significantly increase blood pressure in young adults? A randomised controlled trial. Aust Fam Physician 2016; 45: 65–68. [PubMed] [Google Scholar]
- 20. Shah SA, Occiano A, Nguyen TA, Chan A, Sky JC, Bhattacharyya M, et al Electrocardiographic and blood pressure effects of energy drinks and Panax ginseng in healthy volunteers: a randomized clinical trial. Int J Cardiol 2016; 218: 318–323. [DOI] [PubMed] [Google Scholar]
- 21. Hajsadeghi S, Mohammadpour F, Manteghi MJ, Kordshakeri K, Tokazebani M, Rahmani E, et al Effects of energy drinks on blood pressure, heart rate, and electrocardiographic parameters: an experimental study on healthy young adults. Anatol J Cardiol 2016; 16: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McLellan TM, Caldwell JA, Lieberman HR. A review of caffeine's effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev 2016; 71: 294–312. [DOI] [PubMed] [Google Scholar]
- 23. Peveler WW, Sanders G, Marczinski C, Holmer B. Effects of energy drinks on economy and cardiovascular measures. J Strength Cond Res 2016; 31: 882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]


