Abstract
Aims
To evaluate if rivaroxaban, an oral factor Xa (FXa) inhibitor, could modify the expression in vitro of inflammatory and oxidative stress biomarkers in abdominal aortic aneurysmal (AAA) sites showing intraluminal thrombus.
Methods
AAA sites with intraluminal mural thrombus were obtained from six patients undergoing elective AAA repair. In addition, control abdominal aortic samples were obtained from six organ donors. AAA sites were incubated in the presence and absence of 50 nmol l–1 rivaroxaban.
Results
AAA sites showing thrombus demonstrated higher content of FXa than control. Interleukin‐6 levels released from AAA [Control: median: 23.45 (interquartile range: 16.17–37.15) vs. AAA: median: 153.07 (interquartile range: 100.80–210.69) pg ml–1 mg tissue–1, P < 0.05] and the expression levels of nitric oxide synthase 2 were significantly higher in AAA than in control. The protein expression level of NADPH oxidase subunits gp67‐and gp91‐phox, but did not gp47‐phox, were also significantly higher in the AAA sites than in control. Addition of rivaroxaban to AAA sites explants significantly reduced the release of interleukin‐6 [median: 51.61 (interquartile range: 30.87–74.03) pg ml–1 mg tissue–1, P < 0.05 with respect to AAA alone] and the content of nitric oxide synthase 2, gp67 and gp91‐phox NADPH subunits. The content of matrix metallopeptidase 9 was significantly higher in the AAA sites as compared to control. Rivaroxaban also reduced matrix metallopeptidase 9 content in AAA sites to similar levels to control.
Conclusions
FXa inhibition by rivaroxaban exerted anti‐inflammatory and antioxidative stress properties in human AAA sites, suggesting a role of FXa in these mechanisms associated with the pathogenesis of AAA.
Keywords: factor Xa, human abdominal aortic aneurysms, inflammation, NADPH oxidase, oxidative stress, thrombus
What is Already Known about this Subject
Factor Xa (FXa), a procoagulant agent, exerts additional effects on the vascular wall such as alter energetic metabolism and oxidative stress.
Abdominal aortic aneurysm (AAA) is important cause of cardiovascular morbidity and mortality, which is closely associated with degradation of extracellular matrix, inflammation and oxidative stress.
What this Study Adds
Rivaroxaban, an FXa inhibitor, prevented proinflammatory and oxidative effects of FXa at the AAA site. In addition, rivaroxaban reduced the protein expression of matrix metallopeptidase 9 at the AAA site.
Our findings suggest that FXa seems to be involved in different pathogenic mechanisms associated with AAA.
Introduction
Abdominal aortic aneurysm (AAA) is important cause of cardiovascular morbidity and mortality 1. However, the pathogenesis of AAA is still not fully understood.
Degradation of extracellular matrix within aortic wall is considered one of the main mechanisms involved in AAA growth 2, 3. In this regard, matrix metallopeptidase 9 (MMP9) has attracted particular interest since its expression was observed enhanced in plasma from patients with AAA and increased MMP9 expression in AAA was associated with AAA progression better than to AAA size 4, 5, 6.
In addition to MMP9, other mechanisms, such as those associated with inflammation and oxidative stress, play an important role in the genesis and progression of AAA 7, 8. In this regard, a number of both proinflammatory and anti‐inflammatory cytokines have been implicated in AAA 9, 10. In fact, increased circulating levels of the proinflammatory cytokine interleukin (IL)‐6 have been identified in patients with AAA, suggesting the existence of an inflammatory process associated with AAA 9. Moreover, studies in murine models of AAA have suggested that NADPH oxidases and even nitric oxide synthase isotype 2 (NOS2) are important sources for free radical production in aortic aneurysms 11.
A high percentage of human AAA show intraluminal thrombus 12, 13. In this regard, increased levels of coagulating‐related factors have been also found in blood of patients with AAA 14. Moreover, autopsy studies conducted on patients who died from ruptured aneurysms have demonstrated that a majority of ruptures are located beneath the intraluminal thrombus, suggesting that thrombus may participate in the growth and weakening of the arterial wall 15. In fact, thrombus and clot have been associated with aortic wall destructive remodelling, decreasing tensile strength and elasticity in the vascular wall which might increase risk of ruptures 16, 17. Moreover, it has been speculated that at the AAA site both the geometric modification of the vascular wall and the presence of the intraluminal thrombus are involved in creating a local hypoxic environment even modifying the energetic metabolic pathways to produce ATP, which may also contribute to the aortic wall weakening 13, 18.
Rivaroxaban is an oral inhibitor of factor Xa (FXa), a serine protease that catalyzes the proteolytic conversion of prothrombin to active thrombin 19. Activated platelets are source of FXa 20 and, therefore, the platelets contained into the thrombus may contribute to increase local FXa concentrations at the aneurysmal site 21.
Although the role of FXa in clotting formation is well recognized, different studies have suggested additional effects of FXa on the vascular wall 22, 23. In this regard, it was demonstrated that FXa increased oxidative stress in human vascular smooth muscle cells from saphenous vein 24. Moreover, in femoral arteries from diabetic patients, FXa modified the expression level of proteins associated with oxidative stress and energetic metabolism, effects that were prevented by rivaroxaban 25. However, to our knowledge, possible effects of endogenous FXa have not been examined on proteins associated with inflammation and/or oxidative stress in AAA.
Therefore, the aim of the present study was to in vitro evaluate if rivaroxaban could modify the level of expression of proteins associated with inflammation and oxidative stress in human AAA sites showing intraluminal thrombus. Moreover, the possible effect of rivaroxaban on MMP9 expression was analysed in the aneurysmal site.
Methods
Collections of aortic samples
Aneurysmal sites were obtained from six patients undergoing elective AAA surgery repair. AAA samples were obtained from the more dilated portion of the infrarenal aorta (>5 cm). An inclusion criterion was that anticoagulant and/or antiplatelet treatments had to be removed from 12 days before surgery. Patients with Marfan syndrome, systemic inflammatory, infectious or oncological diseases, and/or subjected to any surgical procedure within the last 6 months were excluded.
The presence of intraluminal thrombus in the AAA was assessed by computed tomography. Computed tomography was also used to discard thoracic aortic dilatation and to determine the abdominal aortic diameter.
Control samples were collected from six organ donors who died due to major head trauma and the aortic sample was obtained during organs extraction. The values of the different parameters obtained from the control abdominal aortas were used as indicator of normal values. The aortic diameter was determined before aortic extraction using a surgical caliper. None of the control abdominal aortic samples showed thrombi.
An inclusion criterion was that patients signed the informed consent; in the case of organ donors, aortic control samples were obtained with family consent. The work was performed according to principles outlined in the Declaration of Helsinki and the institutional Ethics Committee approved the study (code C.I.14/509‐E). In the text, drug/molecular target nomenclature was conformed to BJP's Concise Guide to Pharmacology 2015/16: Enzymes 27.
Experimental design
Aortic samples were carefully and immediately washed with isotonic saline. In the case of the AAA sites, the thrombi were removed from them and then aortic samples cleaned of blood and fat were cut into portions (approximately 5 mm each of them). Each AAA portion (explant) was then incubated for 24 h in the presence and in the absence of rivaroxaban (Bay 59–7939, diluted in dimethyl sulfoxide, 1% final concentration) in RPMI medium containing 1% fetal calf serum, 5 mmol l–1 glutamine, 0.01 mmol l–1 L‐arginine, 2 × 10–5 μg l–1 streptomycin and 2 × 10–5 U l–1 penicillin for 24 h at 37°C in 5% CO2. An equal amount of dimethyl sulfoxide (1%) was also added to both control and AAA samples incubated in the absence of rivaroxaban.
All the procedures were performed under sterile conditions. At the end of the incubation period, supernatants and aortic explants were separately recovered. Aortic explants were immediately frozen at –80°C until the molecular determinations were performed. Supernatants were centrifuged at 10 500 × g, 4°C and the resulting supernatants were also frozen at –80°C until cytokine determinations were performed.
Western blot analysis
The abdominal aortic samples were homogenized with an Ultra‐Turrax T8 (IKA‐Werke; GmbH & Co, Staufen, Germany) in a buffer containing 8 mol l–1 urea, 2% CHAPS w/v, 40 mmol l–1 dithiothreitol as previously we reported 18. The homogenates of aortic samples were then centrifuged at 10 500 × g for 10 min and the supernatants recovered. Protein quantitation was done using a bicinchonic acid kit (Pierce, Rockford, IL, USA).
As previously we have reported 26, protein electrophoresis were performed loading equal amount of proteins (40 μg/lane) from each aortic explant onto denaturing SDS‐PAGE 10% (w/v) polyacrylamide gels. After the electrophoresis, gels were blotted onto nitrocellulose membranes, which were then incubated with 5% (w/v) bovine serum albumin. After that, nitrocellulose membranes were incubated with monoclonal antibodies against the cytosolic NADPH oxidase subunits, gp47‐ phox (Sc‐14 015, dilution 1:1500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and gp67‐phox (Sc‐7663, dilution 1:1500; Santa Cruz Biotechnology), the mitochondrial NADPH oxidase subunit gp91‐phox (Sc‐5827, dilution 1:1500; Santa Cruz Biotechnology), and NOS2 (Sc‐8310, dilution 1:1000; Santa Cruz Biotechnology).
Polyclonal antibodies against FXa (12255–05021, dilution 1:1000; AssayPro, St Charles, MO, USA) and MMP9 (Sc‐6840, dilution 1:1000; Santa Cruz Biotechnology) were also used. Nitrocellulose was also incubated with a monoclonal anti‐β‐actin antibody (A‐5441, dilution 1:1500; Sigma–Aldrich, St Louis, MO, USA) used as loading control.
Nitrocellulose membranes were revealed using peroxidase‐conjugated anti‐rabbit IgG (1:2.500 for gp47‐phox and for FXa), peroxidase‐conjugated anti‐goat IgG antibody (1:2.000 for gp91‐phox and gp67‐phox NADPH oxidase isotypes and MMP9) and peroxidase‐conjugated anti‐mouse IgG (1:7500 for β‐actin). The expression level for each protein was obtained with chemiluminiscence reagents (ECL; GE Healthcare, Little Chalfont, Buckinghamshire, UK). Two replicates were performed for each Western blot and they were densitometrically analysed using a transilluminator (Gel Logic 440 imaging system; Kodak, Rochester, NY, USA).
Release of IL‐6 and IL‐10 and thrombin content in aortic explants
IL‐6 and IL‐10 were measured in the supernatants of the aortic samples using commercial enzyme‐linked immunosorbent assay (ELISA) kits. ELISA kits for IL‐6 (Quantikine ELISA Human IL‐6. D6050; R and D Systems, Abingdon, Oxfordshire, UK) and IL‐10 (Quantikine ELISA Human IL‐10. D1000B; R and D Systems) were performed following manufacturer's instructions. The sensitivity of the assays of IL‐6 and IL‐10 ELISA kits were 0.7 pg ml−1 and 3.9 pg ml−1, respectively. The intra‐ and interassay variation coefficients were 1.7–4.4% and 2.0–3.7% for IL‐6 and 2.5–6.6% and 5.6–7.6% for IL‐10. Thrombin content in aortic explants was determined as measurement of FXa activity. Thrombin content in aortic explants was determined using a commercial ELISA kit (Human thrombin ELISA kit (Factor II), ab108909; Abcam, Cambridge, UK). The sensitivity of the assay was 3 ng ml–1 and the intra‐ and interassay variation coefficients were 4.7 and 7.2%, respectively.
All samples were measured within the same ELISA kit.
Statistical analysis
Results of clinical characteristics and the dose–response curve of rivaroxaban are expressed as mean ± standard error of the mean. Values of expression of proteins were not normally distributed and then are represented as medians and 25th and 75th percentiles. The expression of proteins in the abdominal aorta aneurysm were compared with control (aortic samples without AAA) using the nonparametric Mann–Whitney test. Wilcoxon's test was used to compare AAA samples incubated in the presence and in the absence of rivaroxaban. To control the influence of age when aortic samples from donors and AAA were compared, a linear regression analysis was performed. The dependent variable was the different proteins, the independent variable was the aortic state (AAA and controls) and age was used as covariant. Rho–Spearman correlation analysis was used to determine associations between the expression level of the different proteins and MMP9 expression at the AAA site.
To perform the statistical analysis, the SPSS 22.0 software was used. A P value <0.05 was considered statistically significant.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 27, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 28.
Results
Clinical features of the included patients and donors are shown in Table 1. As expected, patients with AAA were older than control donors (Table 1). All the included AAA showed intraluminal mural thrombus that it was not observed in the aortas obtained from the organ donors. Unfortunately, it was not possible to analyse if some of the control donors were under pharmacological treatment because the donor process is completely anonymous and all clinical data are under protection. However, as mentioned above, it should be noted that the molecular data from the aortic samples of the donors were only used as approximation of normal values.
Table 1.
Clinical and treatment features of abdominal aortic aneurysm patients and organ donors form who samples were obtained
| Parameters | Control (n = 6) | AAA (n = 6) | P value |
|---|---|---|---|
| Age (years) | 43.50 ± 2.43 | 70.00 ± 1.97 | 0.004 |
| Male/female | 6/0 | 6/0 | |
| AAA size (mm) | 16.83 ± 0.48 | 61.00 ± 7.42 | 0.004 |
| Intraluminal mural thrombus | 0/6 | 6/6 | |
| Risk factors | |||
| Smoking history | 2/6 | ||
| Hypertension | 6/6 | ||
| Dyslipemia | 6/6 | ||
| Diabetes Mellitus | 1/6 | ||
| Drug treatment | |||
| ASA | 3/6 | ||
| ARBs | 3/6 | ||
| ACE Inhibitor | 1/6 | ||
| Beta‐blockers | 2/6 | ||
| Statins | 5/6 |
Age and AAA size are represented as means ± standard error of the mean. AAA: abdominal aortic aneurysm. ASA: acetylsalicylic acid. ARBs: Angiotensin II receptor blockers. ACE: angiotensin‐converting enzyme.
Initially, the content of FXa was analysed in the AAA sites. As Figure 1 shows, at AAA site the level of expression of FXa was significantly higher than in the abdominal aortic explants from controls. Then, to analyse the effects of rivaroxaban, the other molecular determinations were performed on AAA showing thrombus. In this regard, to determine the concentration of rivaroxaban used in the in vitro experiments, a rivaroxaban concentration–response curve was performed. In this regard, it was analyzed whether increasing rivaroxaban concentrations could inhibit thrombin content in AAA. As Figure 1 shows, 5 × 10–10 mol l–1 rivaroxaban significantly reduced thrombin content in AAA. Moreover, 5 × 10–8 mol l–1 rivaroxaban completely inhibited thrombin content in AAA (Figure 1). Accordingly, in previous studies, 50 nmol l–1 rivaroxaban was also demonstrated to inhibit FXa activity fully 29. Therefore, 50 nmol l–1 rivaroxaban was chosen for the rest of experiments.
Figure 1.
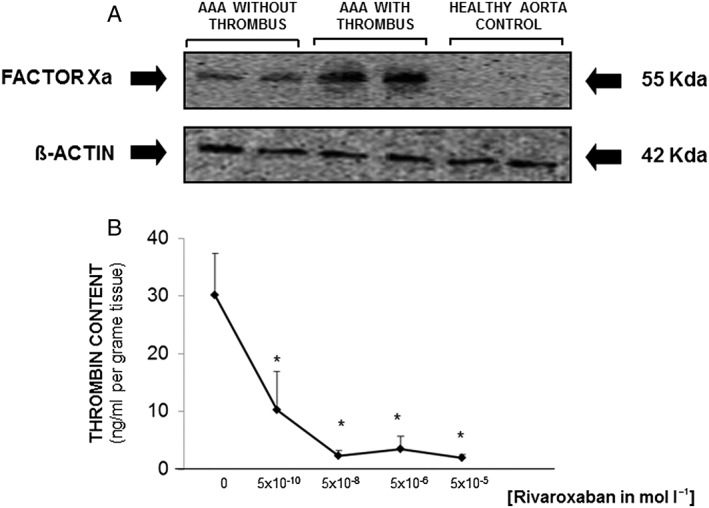
(A) Representative western blot showing FXa expression in two control aortic samples and in two aortic abdominal aneurysmal sites (AAA) with and two without intraluminal thrombus. The expression of β‐actin was used as loading control. (B) Dose–response curve of rivaroxaban based on thrombin content in three different aortic explants. Results are expressed as mean + standard error of the mean. *P<0.05 with respect to AAA without rivaroxaban
Inflammatory‐related biomarkers and rivaroxaban‐incubated AAA sites
In the supernatants from AAA sites, IL‐6 levels were found to be significantly increased with respect to those in aortic samples from organ donors used as control (Table 2). This significant difference was maintained after being used age as covariant IL‐6 and IL‐10 as response variable and aortic state (AAA and control) as independent variable.
Table 2.
Levels of expression of different markers involved in inflammatory response, oxidative stress and extracellular matrix degradation in the different experimental groups
| Healthy aorta control (n = 6) | AAA without rivaroxaban (n = 6) | AAA with rivaroxaban (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | 25th percentile | 75th percentile | Median | 25th percentile | 75th percentile | Median | 25th percentile | 75th percentile | |
| IL‐6 (pg ml –1 per mg tissue) | 23.45 | 16.17 | 37.15 | 135.07a | 100.80 | 210.69 | 51.61b | 30.87 | 74.03 |
| IL‐10 (pg ml –1 per mg tissue) | 18.29 | 10.94 | 22.85 | 19.91 | 16.07 | 24.47 | 116.87a, b | 69.17 | 138.83 |
| IL6/IL10 ratio | 1.51 | 0.94 | 2.93 | 7.20a | 4.74 | 10.35 | 0.52a, b | 0.33 | 0.68 |
| NOS2 (AU) | 1060.00 | 601.00 | 1873.50 | 3842.00a | 2604.00 | 7014.00 | 2140.50b | 1473.25 | 2690.25 |
| Gp91‐phox NADPH (AU) | 1493.00 | 1281.00 | 2073.00 | 3971.50a | 2957.25 | 6708.00 | 2022.50b | 1348.50 | 2831.25 |
| Gp67‐phox NADPH (AU) | 2358.50 | 518.75 | 4911.00 | 8002.00a | 5700.00 | 22 766.00 | 2296.50b | 525.75 | 4142.25 |
| Gp47‐phox NADPH (AU) | 1904.00 | 1483.00 | 3191.25 | 3114.00 | 2132.50 | 4032.00 | 2763.50 | 2080.50 | 3395.25 |
| MMP9 (AU) | 2581.00 | 583.25 | 3492.00 | 6487.50a | 5981.25 | 7099.00 | 3278.00b | 2184.20 | 4385.20 |
Results are expressed as medians and 25th and 75th percentiles.
P<0.05 with respect to healthy aorta control.
P<0.05 with respect to AAA without rivaroxaban.
AAA, aortic abdominal aneurysmal site; IL, interleukin; NOS2, nitric oxide synthase 2; AU, densitometric arbitrary units
Rivaroxaban incubation of AAA sites significantly reduced IL‐6 release from AAA sites, although remained slightly higher than in control (Table 2).
IL‐10 levels were similar between supernatants from AAA sites and control (Table 2). However, surprisingly, rivaroxaban‐incubated AAA sites showed a marked increase of IL‐10 release (Table 2). The index rate IL‐6/IL‐10 was significantly higher in AAA site than in control (Table 2). Rivaroxaban incubation of AAA significantly reduced IL‐6/IL‐10 index with respect to both AAA sites incubated alone and to controls (Table 2; P = 0.004 and P = 0.016 respectively).
The protein expression level of the inducible form of NOS, the NOS2 isoform, was evaluated by western blotting. As Figure 2A and Table 2 show, NOS2 expression was significantly higher in AAA sites with respect to controls. It remained significantly different using age as covariant.
Figure 2.
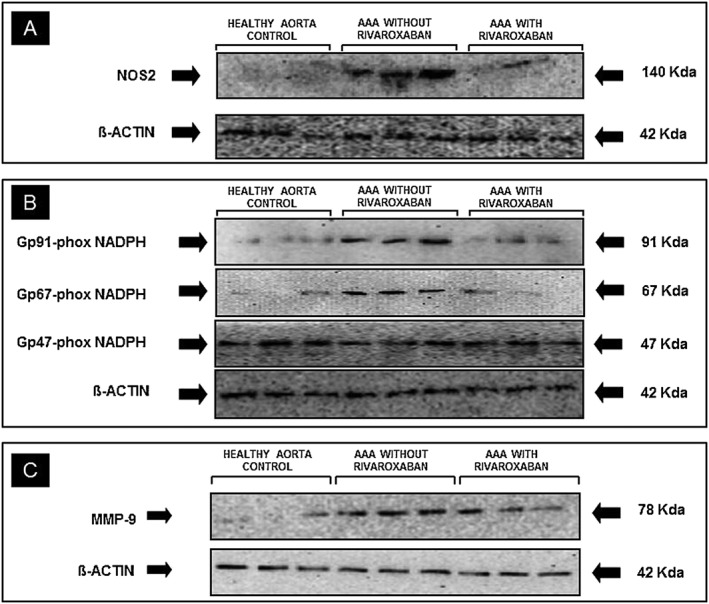
Representative western blot to analyze the protein expression of (A): nitric oxide synthase 2 (NOS2); (B): NADPH oxidase subunits, gp91‐, gp67‐ and gp47‐phox and (C): matrix metallopeptidase 9 (MMP9) in healthy aortic segments and aortic abdominal aneurysmal sites (AAA) incubated in the presence and in the absence of the FXa inhibitor, rivaroxaban (50 nmol l–1). The expression of β‐actin was used as loading control
NOS2 expression level was significantly reduced in rivaroxaban‐incubated AAA as compared with that in AAA sites incubated alone. Indeed, the level of NOS2 expression in rivaroxaban‐incubated AAA was similar to that observed in control aortas (Figure 2A and Table 2).
Expression of NADPH oxidase subunits in AAA sites
Western blot analysis showed that the expression level of the cytosolic NADPH oxidase subunit, gp67‐phox and the mitochondrial NADPH oxidase subunit gp91‐phox were significantly higher in AAA sites than in controls (Figure 2B and Table 2). These differences were also observed after using age as covariant, aortic state (control and AAA) as independent variable and gp91 and gp67‐phox as response variables (P<0.05).
In the AAA sites, the presence of rivaroxaban significantly reduced the expression level of gp67‐phox and gp91‐phox NADPH oxidase to similar levels as those observed in controls (Figure 2B and Table 2).
The expression level of another cytosolic NADPH oxidase subunit, gp47‐phox was not different between the three experimental groups (Figure 2B and Table 2).
MMP9 content in AAA sites and its association with inflammatory and oxidative‐stress related proteins
As was previously reported 4, AAA expressed higher levels of MMP9 than abdominal aortas from control (P = 0.004; Figure 2C and Table 2). The statistical significance remained after using age as covariant, aortic state as independent covariable and MMP9 as response variable. Addition of rivaroxaban to AAA significantly reduced MMP9 expression in AAA (P = 0.0006) to similar levels found in control aortas (P = 0.584 pNS; Figure 2C and Table 2).
Spearman's correlation analysis revealed that the expression levels of MMP9 in the aorta explants were positively associated with the expression level of NOS2 and gp91‐phox subunit (Table 3). Moreover, MMP9 expression levels were also positively associated with IL‐6 levels and with the ratio IL‐6/IL‐10 in the supernatants of artic explants (Table 3). The association between pg67‐phox NADPH subunit and MMP9 did not reach statistical significance although it was probably related to the sample size since P was 0.061 (Table 3).
Table 3.
Correlation between matrix metallopeptidase 9 (MMP9) expression and inflammatory and oxidative‐stress parameters
| MMP9 expression | ||
|---|---|---|
| Variables | Rho spearman coefficient | P value |
| IL‐6 concentration | 0.794 | < 0.001 |
| IL‐10 concentration | –0.049 | 0.852 |
| IL‐6 / IL‐10 ratio | 0.642 | 0.005 |
| NOS2 expression | 0.642 | 0.005 |
| Gp91‐phox NADPH expression | 0.679 | 0.003 |
| Gp67‐phox NADPH expression | 0.463 | 0.061 |
IL, interleukin; NOS2, nitric oxide synthase 2
There was no association between MMP9 expression levels in the aortic explants and IL‐10 levels in their supernatants (Table 3).
Discussion
The present study shows for the first time the possible relationship between the expression of proteins‐related to inflammation and oxidative stress in the AAA site with the presence of endogenous FXa. In fact, the data suggest that rivaroxaban, a specific inhibitor of FXa activity, reduced the expression level of two NAPDH oxidase isotypes in addition to reduction the release of the proinflammatory cytokine IL‐6 and the expression of NOS2 protein. Moreover, in the aortic wall the levels of all these proteins were positively associated with the expression level of MMP9.
In the majority of the AAA patients, the vessel wall is covered by an intraluminal thrombus that generally does not occlude the blood flow 13. In this regard, an activated coagulation state has been observed in patients with AAA 30. Although the role of the intraluminal thrombus in the AAA site remains to be clarified, the presence of the intraluminal thrombus has been considered a risk factor for AAA growth and rupture 31, 32.
In the present study, the AAA sites showed greater expression of FXa as compared to that observed in the abdominal aortic explants from the donors used as control. In addition, in the AAA sites, FXa content was significantly greater in AAA showing intraluminal thrombus than in those without it.
As mentioned above, inflammation has been associated with the pathogenesis of AAA 33, 34, 35. Several studies have reported elevated proinflammatory cytokines in AAA 36, 37, 38 leading the recruitment of immune cells and it was suggested that this promotes death of the vascular cells and weakening of the arterial wall 39. In fact, IL‐6 has been specifically implicated in the pathogenesis of aneurysms 9, 40.
Our results show that AAA released a greater amount of IL‐6 than controls. Moreover, NOS2 protein, enzyme whose upexpression has been also associated with an inflammatory state, was found to be increased in the AAA sites as compared with control. Accordingly, Zhang et al. have previously reported that NOS2 expression was increased in AAA 41. Taken together, these observations are in accordance with previous reports suggesting an inflammatory state at the AAA site.
In the AAA sites, the FXa inhibitor, rivaroxaban, decreased both the release of IL‐6 and the level of NOS2 protein. Previous work has suggested a relationship between FXa and inflammation. In this regard, it was reported proinflammatory effects of FXa in human atrial tissue, fibroblasts and cultured macrophages 42, 43, 44. Moreover, similar to our results, Hara et al. recently reported protective effects of rivaroxaban on atherosclerotic lesions by inhibiting the proinflammatory activation of macrophages 45.
The results also showed that the anti‐inflammatory cytokine IL‐10 was not significantly modified in AAA sites with respect to control. This observation may suggest a different regulation of IL‐6 and IL‐10 in the AAA site and, therefore, the increase in the release of the proinflammatory cytokine IL‐6 seems to be not accompanied by increase in the release of IL‐10 to counterbalance the proinflammatory state in the AAA site. In this regard, other authors have also observed different levels of expression of IL‐6 and IL‐10 in inflammatory‐related pathological situations. As example, Kim et al. showed differences in IL‐10 and IL‐6 levels in the postburn time course 46. Moreover, in human macrophages, different sensitivities of IL‐6 and IL‐10 signalling towards the feed‐back inhibitor suppressor of cytokine signalling 3 (SOCS3) was reported, suggesting the possibility of different regulation of IL‐6 and IL‐10 production and, therefore, showing possible differences in the frame of the inflammatory response 47.
A paradoxical observation was that rivaroxaban increased IL‐10 release in AAA in both healthy nonaneurysmal aortas and AAA incubated without rivaroxaban. Accordingly, IL‐6/IL‐10 ratio was markedly higher in AAA with respect to control and significantly lower in rivaroxaban‐incubated AAA with respect to both control and AAA incubated alone.
The fact that in supernatants from AAA explants, IL‐10 production was not modified with respect to control while in rivaroxaban‐incubated AAA was significantly increased might suggest an independent effect of rivaroxaban on endogenous FXa. In this regard, in a previous study performed in femoral arteries of diabetic patients, we have also observed an effect of rivaroxaban probably unrelated to FXa on the expression of long mitochondrial fatty acid transporters 25. As we mentioned in that report, it is known the existence of rivaroxaban‐derived metabolites and, therefore, we cannot rule out that some of the apparently FXa‐independent effects of rivaroxaban could be attributed to such metabolites 48. However, at present the analysis of this hypothesis is out of the scope of the present study and, therefore, additional specific studies are needed to clarify it.
Effects of rivaroxaban on the expression levels of oxidative stress‐associated proteins in human AAA sites
Several studies have identified oxidative stress as one of the main source of AAA progression 49, 50. Both inflammatory infiltrated cells and vascular cells are capable of forming radical oxygen species, and particularly superoxide anion, in the AAA 8. In this regard, an increased local production of superoxide anion was demonstrated in human aneurysms and it was further suggested that the major source of this oxygen free radical is the NADPH oxidases activities 8, 51.
In the present study, both the cytosolic NADPH oxidase subunit gp67‐phox and the mitochondrial gp91‐phox NADPH oxidase subunits were significantly increased in the human AAA sites. Accordingly, Guzik et al. also suggested that AAA was associated with increased NADPH oxidases activities, since higher superoxide production may promote the activation of metalloproteinases associated with AAA development 50. Furthermore, higher superoxide production by AAA was associated with high overall mortality risk 50.
At the AAA site, the FXa inhibitor, rivaroxaban, significantly reduced the expression level of the gp67‐ and 91‐phox NADPH oxidase isotypes, suggesting the involvement of FXa in such increased expression. Accordingly, in femoral arteries obtained from diabetic patients we have also demonstrated that rivaroxaban reduced the expression of oxidative stress‐related proteins 25 . Interestingly, it was suggested that NOS2, whose expression was significantly reduced by rivaroxaban in the AAA site, might be link between inflammation and oxidative stress in AAA since NOS2 was also demonstrated as primary sources of superoxide anion in AAA 50. Accordingly, in the study the effects of rivaroxaban on the expression of proteins related to inflammation and oxidative stress were discussed independently manner. However, inflammation, and oxidative stress are strongly linked between them.
The fact that the protein expression level of gp91‐ and gp67‐phox NADPH isotypes were modified by rivaroxaban but did not the NADPH isotype gp47‐phox may be related to the specificity of the observed changes.
Association between MMP9 expression and the changes in the inflammatory and oxidative‐stress‐related proteins
Several studies have reported reduction in the progression of experimental AAA by inhibitory MMPs. In the study we focused on MMP9 because, among all MMPs, this has been reported to have the greatest involvement in the pathogenesis of progression of AAA in both humans and animal models.
The results demonstrate that at the AAA site, rivaroxaban reduced the expression level of MMP9. Moreover, in the aortic wall, MMP9 levels were positively associated with the expression of NOS2 and gp91‐phox NADPH oxidase isotypes. In addition, IL‐6 release and the ratio IL‐6/IL‐10 was also positively associated with MMP9. Taken together these data suggest and support the hypothesis that all these mechanisms may be strongly associated with AAA progression. In this regard, experimental studies have demonstrated that in the aortic tissue loss of NADPH oxidases and NOS2 reduced the expression of MMP9, preventing AAA development 11. Similarly, it was also demonstrated that proinflammatory cytokines enhance MMP9 production in the vascular cells contributing to AAA growth 52. Therefore, taken together and as speculation, inhibition of FXa by rivaroxaban probably through the reduction of inflammation and oxidative stress in the aortic aneurysm may alter MMP9 expression.
Comments and study limitations
There are several limitations in our study, although probably the most relevant is the small sample size. However, the observed results were very consistent. Moreover, the current study only included AAA samples showing intraluminal thrombus. It was thought that, under these conditions, the role of FXa may be more evident as it could be inferred from the observation that these aneurysms contain more FXa than those without intraluminal thrombus. However, further comparative studies are warranted in AAA samples without thrombus.
Another previously mentioned study limitation was that, due to having to maintain the anonymity of organ donors, it was not possible to analyse the impact of the possible pharmacological treatment in the observed differences between control and AAAs. However, as we also mentioned, it is important to note that the main aim of the present study was to study the role of endogenous in AAA with thrombus in the expression of proteins associated with inflammation and oxidative stress.
The concentration of rivaroxaban used in the study was within the plasma concentration ranges after therapeutic doses of rivaroxaban 53. Indeed, 50 nmol l–1 rivaroxaban is equivalent to approximately 200 μg l–1 rivaroxaban, concentration which is reached in patients treated with 20 mg once daily 53 It is important to note that the present results are only related to rivaroxaban and further experiments are needed to assess whether other FXa inhibitors, and even other anticoagulant drugs, might exert similar effects to rivaroxaban on the expression of inflammatory and oxidative stress‐related proteins in AAA.
In conclusion, rivaroxaban has showed ability to reduce expression of proteins involved in the pathogenesis of AAA such as proteins related to oxidative stress as well as to promote an antioxidant state in human AAA sites. These findings suggest the involvement of FXa in such mechanisms. The better knowledge of the possible effects of FXa in AAA may open new clinical targets for FXa inhibition therapy.
Competing Interests
There are no competing interests to declare.
This work was supported by Fondo Investigaciones de la Seguridad Social [Redes Temáticas de Investigación Cooperativa (RETICs) RD12/0042/0040], Fondo Europeo de Desarrollo Regional (Fondos FEDER) and Bayer Pharmaceuticals.
We thank Begoña Larrea for editorial assistance.
Contributors
G.M. conceived the study and participated in its design, analysed and interpreted the data, and participated in drafting of the manuscript. J.J.Z.‐L. participated in the obtaining data and statistical analysis, participated in the analysis and interpretation of data as well as in drafting of the manuscript. P.M. participated in the obtaining data and statistical analysis. B.S. participated in the obtaining data. J.M.G.‐G. reviewed the draft for important intellectual content and approved the final version to be published. G.L.d.K. participated in the analysis and interpretation of data and reviewed the draft for important intellectual content. B.C.‐R. participated in the obtaining data and analysis and interpretation of data. M.A.G.‐F. participated in the analysis and interpretation of data. J.S. participated in the analysis and interpretation of data and reviewed the draft for important intellectual content. A.J.L.‐F. conceived the study and participated in its design, analysed and interpreted the data and drafted the manuscript.
All authors have read and approved the final manuscript.
Moñux, G. , Zamorano‐León, J. J. , Marqués, P. , Sopeña, B. , García‐García, J. M. , Laich de Koller, G. , Calvo‐Rico, B. , García‐Fernandez, M. A. , Serrano, J. , and López‐Farré, A. (2017) FXa inhibition by rivaroxaban modifies mechanisms associated with the pathogenesis of human abdominal aortic aneurysms. Br J Clin Pharmacol, 83: 2661–2670. doi: 10.1111/bcp.13383.
References
- 1. Weintraub NL. Understanding abdominal aortic aneurysm. N Engl J Med 2009; 361: 1114–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol 2006; 26: 2605–2613. [DOI] [PubMed] [Google Scholar]
- 3. Annabi B, Shédid D, Ghosn P, Kenigsberg RL, Desrosiers RR, Bojanowski MW, et al Differential regulation of matrix metalloproteinase activities in abdominal aortic aneurysms. J Vasc Surg 2002; 35: 539–546. [DOI] [PubMed] [Google Scholar]
- 4. Yamashita A, Noma T, Nakazawa A, Saito S, Fujioka K, Zempo N, et al Enhanced expression of matrix matrix metallopeptidase 9 in abdominal aortic aneurysms. World J Surg 2001; 25: 259–265. [DOI] [PubMed] [Google Scholar]
- 5. McMillan WD, Pearce WH. Increased plasma levels of metalloproteinase‐9 are associated with abdominal aortic aneurysms. J Vasc Surg 1999; 29: 122–127. [DOI] [PubMed] [Google Scholar]
- 6. McMillan WD, Tamarina NA, Cipollone M, Johnson DA, Parker MA, Pearce WH. Size matters: the relationship between MMP9 expression and aortic diameter. Circulation 1997; 96: 2228–2232. [DOI] [PubMed] [Google Scholar]
- 7. Walker DI, Bloor K, Williams G, Gillie I. Inflammatory aneurysms of the abdominal aorta. Br J Surg 1972; 59: 609–614. [DOI] [PubMed] [Google Scholar]
- 8. Miller FJ Jr, Sharp WJ, Fang X, Oberley LW, Oberley TD, Weintraub NL. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol 2002; 22: 560–565. [DOI] [PubMed] [Google Scholar]
- 9. Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjälä H, et al Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 1997; 17: 2843–2847. [DOI] [PubMed] [Google Scholar]
- 10. Treska V, Kocova J, Boudova L, Neprasova P, Topolcan O, Pecen L, et al Inflammation in the wall of abdominal aortic aneurysm and its role in the symptomatology of aneurysm. Cytokines Cell Mol Ther 2002; 7: 91–97. [DOI] [PubMed] [Google Scholar]
- 11. Xiong W, Mactaggart J, Knispel R, Worth J, Zhu Z, Li Y, et al Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis 2009; 202: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parr A, McCann M, Bradshaw B, Shahzad A, Buttner P, Golledge J. Thrombus volume is associated with cardiovascular events and aneurysm growth in patients who have abdominal aortic aneurysms. J Vasc Surg 2011; 53: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vorp DA, Lee PC, Wang DH, Makaroun MS, Nemoto EM, Ogawa S, et al Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg 2001; 34: 291–299. [DOI] [PubMed] [Google Scholar]
- 14. Kotschy M, Witkiewicz W, Grendziak R, Dubis J, Zapotoczny N, Kotschy D. Selected clotting factors in blood of patients with abdominal aortic aneurysms. Kardiol Pol 2012; 70: 574–579. [PubMed] [Google Scholar]
- 15. Koole D, Zandvoort HJ, Schoneveld A, Vink A, Vos JA, van den Hoogen LL, et al Intraluminal abdominal aortic aneurysm thrombus is associated with disruption of wall integrity. J Vasc Surg 2013; 5: 77–83. [DOI] [PubMed] [Google Scholar]
- 16. Kazi M, Thyberg J, Religa P, Roy J, Eriksson P, Hedin U, et al Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg 2003; 38: 1283–1292. [DOI] [PubMed] [Google Scholar]
- 17. Vorp DA, Vande Geest JP. Biomechanical determinants of abdominal aortic aneurysm rupture. Arterioscler Thromb Vasc Biol 2005; 25: 1558–1566. [DOI] [PubMed] [Google Scholar]
- 18. Modrego J, López‐Farré AJ, Martínez‐López I, Muela M, Macaya C, Serrano J, et al Expression of cytoskeleton and energetic metabolism‐related proteins at human abdominal aortic aneurysm sites. J Vasc Surg 2012; 55: 1124–1133. [DOI] [PubMed] [Google Scholar]
- 19. Thomas TF, Ganetsky V, Spinler SA. Rivaroxaban: an oral factor Xa inhibitor. Clin Ther 2013; 35: 4–27. [DOI] [PubMed] [Google Scholar]
- 20. Ahmad SS, Scandura JM, Walsh PN. Structural and functional characterization of platelet receptor‐mediated factor VIII binding. J Biol Chem 2000; 275: 13071–13081. [DOI] [PubMed] [Google Scholar]
- 21. Lupu C, Calb M, Ionescu M, Lupu F. Enhanced prothrombin and intrinsic factor X activation on blood platelets from diabetic patients. Thromb Haemost 1993; 70: 579–583. [PubMed] [Google Scholar]
- 22. McLean K, Schirm S, Johns A, Morser J, Light DR. FXa‐induced responses in vascular wall cells are PAR‐mediated and inhibited by ZK‐807834. Thromb Res 2001; 103: 281–297. [DOI] [PubMed] [Google Scholar]
- 23. Steinberg SF. The cardiovascular actions of protease‐activated receptors. Mol Pharmacol 2005; 67: 2–11. [DOI] [PubMed] [Google Scholar]
- 24. Jobi K, Rauch BH, Dangwal S, Freidel K, Doller A, Eberhardt W, et al Redox regulation of human protease‐activated receptor‐2 by activated factor X. Free Radic Biol Med 2011; 51: 1758–1764. [DOI] [PubMed] [Google Scholar]
- 25. López‐Farré AJ, Rodriguez‐Sierra P, Modrego J, Segura A, Martín‐Palacios N, Saiz AM, et al Effects of factor Xa on the expression of proteins in femoral arteries from type 2 diabetic patients. Br J Clin Pharmacol 2014; 78: 1366–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. López‐Farré AJ, Zamorano‐León JJ, Segura A, Mateos‐Cáceres PJ, Modrego J, Rodríguez‐Sierra P, et al Plasma desmoplakin I biomarker of vascular recurrence after ischemic stroke. J Neurochem 2012; 121: 314–325. [DOI] [PubMed] [Google Scholar]
- 27. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: Towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44: D1054–D1D68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perzborn E, Strassburger J, Wilmen A, Pohlmann J, Roehrig S, Schlemmer KH, et al In vitro and in vivo studies of the novel antithrombotic agent BAY 59‐7939‐an oral, direct Factor Xa inhibitor. J Thromb Haemost 2005; 3: 514–521. [DOI] [PubMed] [Google Scholar]
- 30. Yamazumi K, Ojiro M, Okumura H, Aikou T. An activated state of blood coagulation and fibrinolysis in patients with abdominal aortic aneurysm. Am J Surg 1998; 175: 297–301. [DOI] [PubMed] [Google Scholar]
- 31. Hans SS, Jareunpoon O, Balasubramaniam M, Zelenock GB. Size and location of thrombus in intact and ruptured abdominal aortic aneurysms. J Vasc Surg 2005; 41: 584–588. [DOI] [PubMed] [Google Scholar]
- 32. Satta J, Laara E, Juvonen T. Intraluminal thrombus predicts rupture of an abdominal aortic aneurysm. J Vasc Surg 1996; 23: 737–739. [DOI] [PubMed] [Google Scholar]
- 33. Hendel A, Ang LS, Granville DJ. Inflammaging and proteases in abdominal aortic aneurysm. Curr Vasc Pharmacol 2015; 13: 95–110. [DOI] [PubMed] [Google Scholar]
- 34. Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2006; 26: 987–994. [DOI] [PubMed] [Google Scholar]
- 35. Brophy CM, Reilly JM, Smith GJ, Tilson MD. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Ann Vasc Surg 1991; 5: 229–233. [DOI] [PubMed] [Google Scholar]
- 36. Newman KM, Jean‐Claude J, Li H, Ramey WG, Tilson MD. Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation 1994; 90: 11224–11227. [PubMed] [Google Scholar]
- 37. Stather PW, Sidloff DA, Dattani N, Gokani VJ, Choke E, Sayers RD, et al Meta‐analysis and meta‐regression analysis of biomarkers for abdominal aortic aneurysm. Br J Surg 2014; 101: 1358–1372. [DOI] [PubMed] [Google Scholar]
- 38. Eagleton MJ. Inflammation in abdominal aortic aneurysms: cellular infiltrate and cytokine profiles. Vascular 2012; 20: 278–283. [DOI] [PubMed] [Google Scholar]
- 39. Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation 1999; 99: 96–104. [DOI] [PubMed] [Google Scholar]
- 40. Dawson J, Cockerill G, Choke E, Loftus I, Thompson MM. Aortic aneurysms as a source of circulating interleukin‐6. Ann N Y Acad Sci 2006; 1085: 320–323. [DOI] [PubMed] [Google Scholar]
- 41. Zhang J, Schmidt J, Ryschich E, Mueller‐Schilling M, Schumacher H, Allenber JR. Inducible nitric oxide synthase is present in human abdominal aortic aneurysm and promotes oxidative vascular injury. J Vasc Surg 2003; 38: 360–367. [DOI] [PubMed] [Google Scholar]
- 42. Bukowska A, Zacharias I, Weinert S, Skopp K, Hartmann C, Huth C, et al Coagulation factor Xa induces an inflammatory signalling by activation of protease‐activated receptors in human atrial tissue. Eur J Pharmacol 2013; 718: 114–123. [DOI] [PubMed] [Google Scholar]
- 43. Bachli EB, Pech CM, Johnson KM, Johnson DJ, Tuddenham EG, McVey JH. Factor Xa and thrombin, but not factor VIIa, elicit specific cellular responses in dermal fibroblasts. J Thromb Haemost 2003; 1: 1935–1944. [DOI] [PubMed] [Google Scholar]
- 44. Zuo P, Zuo Z, Wang X, Chen L, Zheng Y, Ma G, et al Factor Xa induces pro‐inflammatory cytokine expression in RAW 264.7 macrophages via protease‐activated receptor‐2 activation. Am J Transl Res 2015; 7: 2326–2334. [PMC free article] [PubMed] [Google Scholar]
- 45. Hara T, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Nishimoto S, et al Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE‐deficient mice. Atherosclerosis 2015; 242: 639–646. [DOI] [PubMed] [Google Scholar]
- 46. Kim HS, Kim JH, Yim H, Kim D. Changes in the levels of interleukins 6, 8, and 10, tumor necrosis factor alpha, and granulocyte‐colony stimulating factor in Korean burn patients: relation to burn size and postburn time. Ann Lab Med 2012; 32: 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, et al Activation of STAT3 by IL‐6 and IL‐10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol 2003; 170: 3263–3272. [DOI] [PubMed] [Google Scholar]
- 48. Weinz C, Schwarz T, Kubitza D, Mueck W, Lang D. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos 2009; 37: 1056–1064. [DOI] [PubMed] [Google Scholar]
- 49. McCormick ML, Gavrila D, Weintraub NL. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2007; 27: 461–469. [DOI] [PubMed] [Google Scholar]
- 50. Guzik B, Sagan A, Ludew D, Mrowiecki W, Chwała M, Bujak‐Gizycka B, et al Mechanisms of oxidative stress in human aortic aneurysms‐association with clinical risk factors for atherosclerosis and disease severity. Int J Cardiol 2013; 168: 2389–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miller FJ Jr. Aortic aneurysms: It's all about the stress. Arterioscler Thromb Vasc Biol 2002; 22: 1948–1949. [DOI] [PubMed] [Google Scholar]
- 52. Galis ZS, Muszynski M, Sukhova GK, Simon‐Morrissey E, Unemori EN, Lark MW, et al Cytokine‐stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ Res 1994; 75: 181–189. [DOI] [PubMed] [Google Scholar]
- 53. Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet 2014; 53: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


