Abstract
Parsley is a medicinal plant used widely in urolithiasis. The present study aimed to evaluate the antiurolithiatic effect of parsley and its mechanism. 24 rats divided into four groups: group A (negative control), group B (positive control), group C (cystone® group) and group D (parsley group). Group B were treated with EG and Ammonium chloride (AC). Group C were treated as B plus cystone® and group D was treated as B plus parsley. The period of experiment was 15 days. Urine samples were analysis on days 0 and 15 days. Kidneys of rats from all groups were removed, and histopathologically examined. The kidnies of parsley treated group appeared mostly to be calculi-free (less CaOx) even better than the cystone treated group. CaOx crystals was significantly lower both in histological sections and in urine samples in parsley treated group. We further investigated the mechanism of parsley by adding another 6 rats. The latter treated by parsley only after adaptation period. We found significant increase in urine volume and pH in parsley treated rats compared to negative control. We concluded that parsley acts as antiurolithiatic drug through decreasing urinary calcium excretion, increasing urinary pH, dieresis, decreasing urinary protein excretion and its nephroprtective activity. We recommended to use it in pharmaceutical forms as it is safe and effective as antiurolithiasis remedy.
Keywords: Parsley-urolithiasis-mechanism-ethylene glycol, pH, CaOx
Introduction
Nephrolithiasis (renal stones, urinary stones, urolithiasis and renal calculi) affects great number of patients worldwide [1]. Urolithiasis has afflicted humankind since antiquity and can persist with serious consequences throughout patient’s lifetimes [2]. It is a recurrent disease with a relapse rate of 50% in 5-10 year and 75% in 20 year. Males are more affected than females with a higher recurrence rate (70-80% in males and 47-60% in females) [3,4]. Calcium oxalate (CaOx) comprise 80% of renal stones, 10% struvite, 9% uric acid and the remaining 1% composed of cystine or ammonium acid urate [5].
Mechanisms involved in stone formation are: supersaturated urine, change in urinary pH, change in urine composition and nucleation [6].
Treatment of urolithiasis is different ranging from surgical removal, Extracorporeal Shock Wave Lithotripsy (ESWL) to medical treatment using different kinds of synthetic drugs [7]. Phytotherapy is another alternative kind of treatment of urolithiasis including parsley [8].
Parsley, family Umbelliferae, locally known as Baqdunis, has been used medicinally for many European, Mediterranean and Asian countries [9]. It is a medicinal herb used world-wide because of antioxidant activity, anti-inflammatory, anti-edema, anti-hypertensive, anti-diabetic, anti-microbial, laxative in digestive tract, antioxidant, balance enzyme activities, increase glutathione in the kidney and reconstruct kidney tissue after nephrotoxicity [10,11].
Gumaih et al. found significant decreases in serum urea, creatinine, uric acid and electrolytes and conclude the nephroprotective effect of parsley on rats treated with EG. They also found a significant decrease in urinary calcium and proteins and conclude the antiurolithiatic effect of parsley [12].
The present study aims to investigate other effects by which parsley combat urolithiasis to complete the full picture of its mechanisms. Cystone® (a conventional herbal drug used for urolithiasis) also used for comparison.
Materials and methods
Experimental animals
Male albino rats weighing between 150 to 200 g were used in this study. The whole experiment carried out in the same environmental conditions at room temperature. Animals stay seven days prior to experiment as adaptation period and the bedding of the animal cages changed every 48 hrs.
Extract preparation
Parsley seeds were collected from local market. The dried seeds were pulverized into fine powder using a grinder and stored in airtight container. About 750 ml of 70% ethanol was added to 100 g of powder and kept on a mechanical shaker (magnetic starrier) at 55°C for 72 hrs, The content was filtered and kept in an incubator at 37°C for 36 hrs. The concentrated extract was stored in dry state at -20°C in deep freeze according to others [13].
Experimental design
Stone induction
Ethylene glycol (EG) plus ammonium chloride (AC) were used to induce urolithiasis. EG (0.75% v/v) and AC (1% w/v) in drinking water ad libitum for 15 days according to others [14].
Dose preparation
The ethanolic extract of parsley was dissolved in distilled water (D.W) at a dose 500 mg/kg body weight of rat then shacked until completely dissolved, whereas, cystone drug was dissolved in D.W at a dose 500 mg/kg body weight of rat using a stomach tube.
Twenty-four male rats were randomly divided into four groups, each of six rats. Group-A: negative control, fed normal diet. Group-B: took normal diet with EG (0.75%) and AC (1%) for 15 days and serve as a positive control. Group-C: took the same substances as group-B with 500 mg/kg of cystone for 15 days. Group-D: took the same substances as in group-B with 500 mg/kg of Parsley for 15 days.
Assessment of antiurolithiatic activity
Urine collection and analysis
All animals were fasted overnight then urine samples were collected from each rat only the first and last days before and after treatment respectively. These animals were kept in individual cages, and urine was collected in the morning without any preservative substances for crystalluria analysis. The type and number of the crystals were identified and counted using alight microscope (Leica Galen III, America). Crystalluria was scored as follows: “0” no crystals; “1” 1 to 5 crystals per high power field (×400); “2” 6 to 20 crystals/HPF; and “3”>20 crystals/HPF.
Histopathological study
At the end of the experiment the animals were killed by cervical decapitation. The abdomen was incised and opened to remove both kidneys from each animal, cleaned off extraneous tissue by ice-cold normal saline. The kidneys fixed in formalin 10% and processes through graded alcohol series and xylene. Then the specimens were embedded in paraffin, sectioned at 5 μm, and stained with the hematoxylin and eosin for histopathological examination under light microscope (Leica Galen III America). The number of crystal deposits were counted on 10 HPFs. The mean crystal number per field was taken for each kidney examined. Crystal density was scored as: “0” no crystal deposition; “1” 1-2 crystals/HPF; “2” 3-4 crystals/HPF; and “3”>4 crystals/HPF. Pictures were taken using digital camera (canon, 12.1 mega pixels, Japan).
Assessment of diuretic activity of parsley and its effect on urinary pH
Additional six rats were used to determine the effect of parsley on urine volume and pH.
Assessment of urine volume
The six rates were adopted for 7 days, housed individually in cages and allowed free access to food and drinking water. Then urine was collected over a 24 hrs and urine volume was measured. After this period, the same rats toke ethanolic extract of parsley for another seven days through which urine collected every 24 hrs and its volume was measured. Thus every animal served as control and treated animal in the same time.
Assessment of urine pH
The fresh urine samples from the six rats was measured using standard pH paper (Cybow™, DFI Co., Ltd. Korea). We measured urinary pH in the morning and the afternoon for each rat to know the effect of parsley on urine pH.
Statistical analysis
The data that obtained by the various parameters were statistically evaluated using SPSS software (Version 16). One-way analysis of variance (ANOVA) and Chi square were applied. The mean values ± SEM were calculated for each parameter.
Results
Calcium oxalate in urine of different groups
Crystal density of CaOx was significantly decreased in group D compared to group B and C. No significant decrease observed in group C compared to group B. (Table 1; Graph 1, Panel 1).
Table 1.
Calcium oxalate density in urine in different rat groups
| Group | Crystal density |
|---|---|
| A | 0.08±0.0 |
| B | 2.7±0.2a |
| C | 2.2±0.2a |
| D | 1.3±0.2b |
A= negative control, B= positive control, C= cystone treated, D= parsley treated. The values are in mean ± SEM. Significant difference between a&b (p<0.001).
Graph 1.
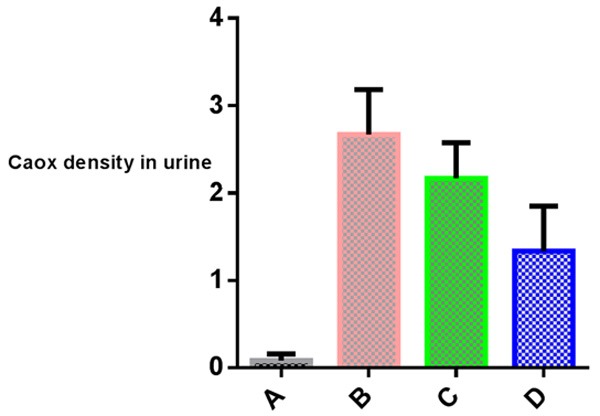
Calcium oxalate crystal density in urine of different groups.
Panel 1.
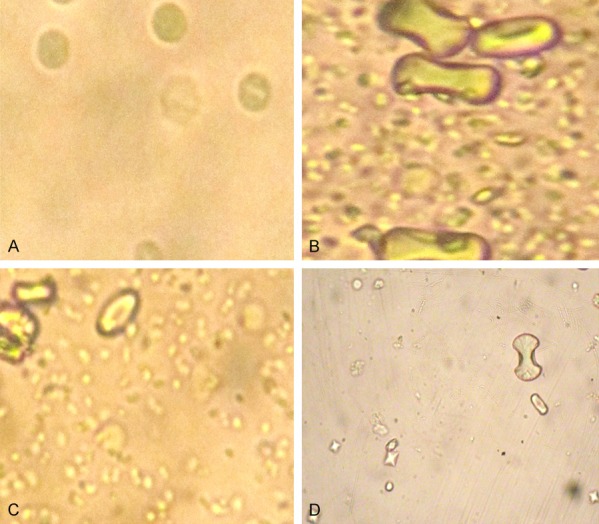
Calcium oxalate crystals in urine in different groups. They are lesser and smaller in urine of parsley group (D); numerous and of boney type in positive control group (B) and in cystone group (C). (A) negative control.
Calcium oxalate density in renal tissue of different groups
Calcium oxalate were plentiful in rats treated with EG/AC (group B), and larger in size. In addition, they were in tubules with obvious tubular injury and encroachment upon the interstitium (Panel 2B). The number and size of crystals are decreased significantly in parsley (group D) compared to either group B or C (Table 2, Graph 2). Also, the integrity of renal tissue is better in group (D) than positive control and cystone groups (Panel 2).
Panel 2.
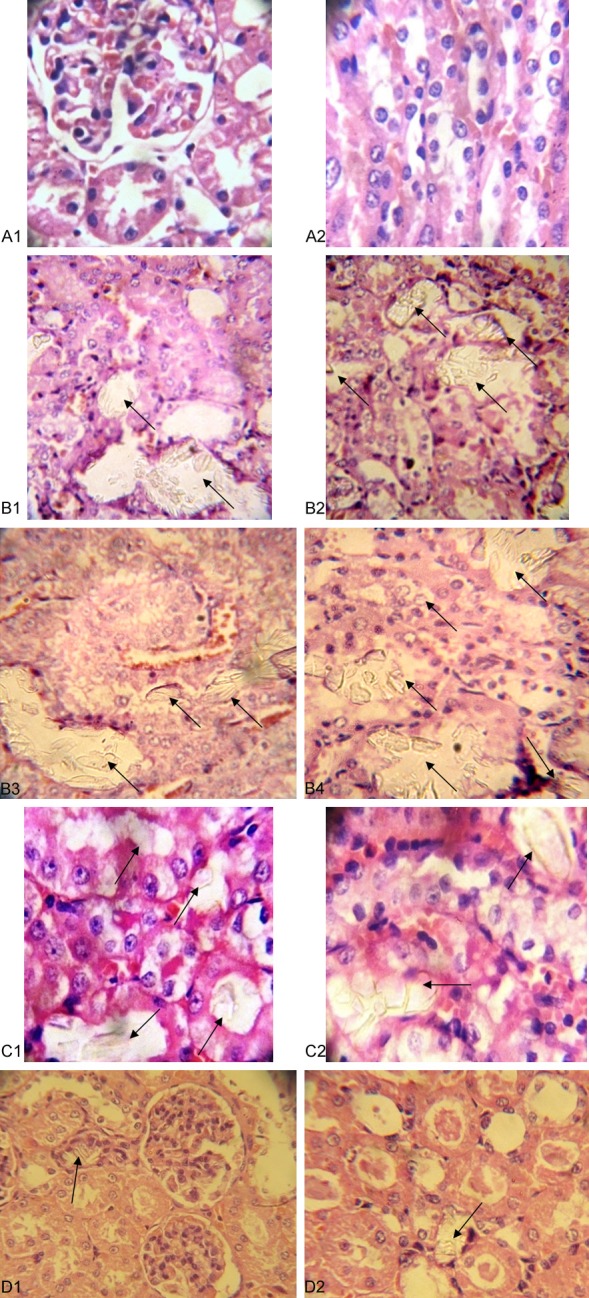
A1, A2. No crystals seen in negative control group; B1-4. Calcium oxalate crystals (arrow) were plentiful and large in size in rats treated with EG/AC (group B). The crystals are distributed in tubules with obvious tubular injury and encroachment of the crystals upon the interstitium; C1, C2. Calcium oxalate crystals in renal tissue of cystone group; D1, D2. Few crystals in parsley group.
Table 2.
The mean crystal density in renal tissue
| Group | Crystal density In renal tissue |
|---|---|
| A | 0.0±0.0 |
| B | 2.5±0.2a |
| C | 2.0±0.2a |
| D | 1.1±0.1b |
A= negative control, B= positive control, C= cystone treated, D= parsley treated. The values are in mean ± SEM. Significant difference between a&b (p<0.001).
Graph 2.
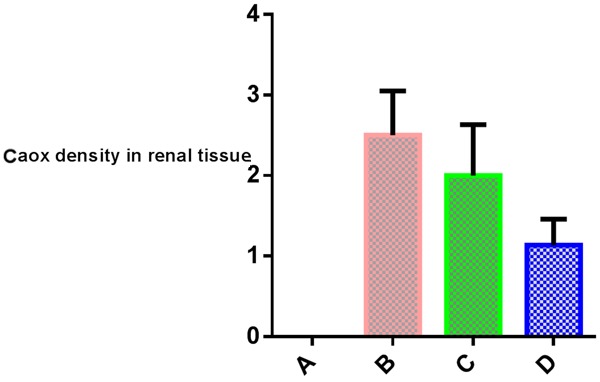
Calcium oxalate density in renal tissue.
Relation between calcium oxalate density in renal tissue and in urine
Crystal density in both renal tissue and urine have direct proportion relationship and this shown in Table 3 and illustrated in Graph 3. Significant decrease in crystal density in parsley group both in renal tissue and urine compared to groups B&C.
Table 3.
Relation between calcium oxalate density in renal tissue and urine
| Group | Crystal density | |
|---|---|---|
|
|
||
| In urine | In renal tissue | |
| A | 0.8±0.0 | 0.0±0.0 |
| B | 2.7±0.2a | 2.5±0.2a |
| C | 2.2±0.2a | 2.0±0.3a |
| D | 1.3±0.2b | 1.1±0.1b |
A= negative control, B= positive control, C= cystone treated, D= parsley treated. The values are in mean ± SEM. Significant difference between a&b.
Graph 3.
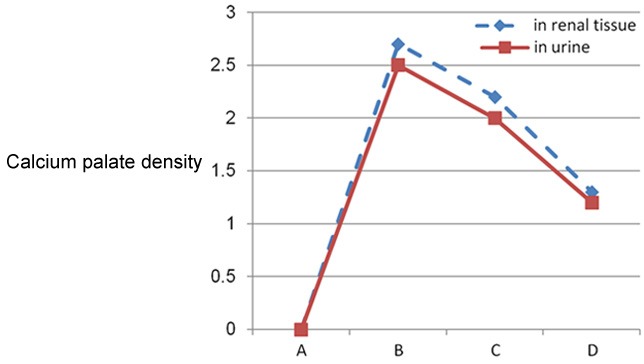
Calcium oxalate density in renal tissue and urine.
Water intake and urine volume
The mean water intake and mean urine volume are significantly increased in parsley group (P<0.0001).
Urine pH in the morning and the afternoon
There was significant increase in urinary pH in parsley-taking rats compared to no-parsley group both in morning and afternoon urine samples (P<0.001 & p<0.0003 respectively) Table 5, Graph 5.
Table 5.
Change in urine pH in parsley group compared to rats without parsley group
| Group | Morning pH | Afternoon pH |
|---|---|---|
| With out parsly | 6.7±0.3a | 5.9±0.1a |
| With Parsley | 8.1±0.2b | 7.5±0.3b |
Significant difference between a&b.
Graph 5.
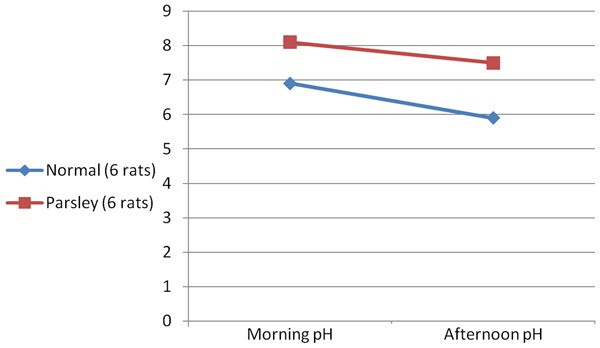
Change in urine pH in parsley rats and negative control.
Discussion
The current study was carried out for scientific authentication of parsley used traditionally for urolithiasis. Although the role of parsley in urolithiasis is well recognized and documented, very few scientific data have been published supporting its claimed mechanism.
Calcium oxalate in urine of different groups
An increased urinary calcium concentration is a factor favoring the nucleation and precipitation of CaOx or apatite (calcium phosphate) from urine and subsequent crystal growth [15].
A significant increase in serum as well as urinary calcium was found in rats subjected to EG [12]. This increase may be responsible for formation of urinary CaOx noted in our study. In addition, the significant decrease in CaOx in this study in parsley-treated rats is consistent with the decrease in urinary calcium in parsley treated rats found in the previous study [12].
Among calcium stone-formers, two of the most common urinary abnormalities are hypercalciuria and hypocitraturia [15]. Citrate is a potent inhibitor of stone formation by uniting with calcium making it unavailable to bind with oxalate or phosphate and prevents crystallization [17].
Weather parsley decreasing urinary calcium, hence CaOx through lowering serum Ca level [12] or through increasing urinary citrate a point needs further investigations, although there is a study denoted that no changes in urinary citrate [18].
Some studies reported that parsley has large amount of chlorophyll that may inhibit the growth of calcium oxalate dehydrate which considered to be a primary phase in calcium oxalate stone formation [19,20].
Calcium oxalate density in renal tissue of different groups
In our study CaOx crystals was seen in EG/AC rat kidneys in renal tubules with obvious tubular injury (cloudy swelling and irregular luminal border) and encroachment of the crystals upon the interstitium, compared to normal (negative control) kidneys. Tubular dilatation also noted but interstitial inflammation was seen focally in rare kidneys and not related to CaOx crystals. Talekar et al. stated that the presence of polymorphic irregular crystals inside the tubules causes dilatation of the proximal tubules along with interstitial inflammation [21].
We found significant decrease in CaOx density in renal tissue in rats taking parsley compared to positive control and cystone and this is consistent with others [22].
Relation between calcium oxalate density in renal tissue and in urine
The significant decrease in density (both number and size) of renal CaOx crystals was reflected in urine crystal density. This finding denotes that parsley decreases oxalate synthesis through interacting either with EG absorption in intestine or with the enzyme responsible for oxalate synthesis from EG in the liver. Serum oxalate level should be investigated in future studies although some authors said no significant difference [18]. The latter study also denoted no significant difference in serum calcium both in serum and urine, findings completely different from the previous study which shows significant difference in serum calcium and urine [12].
Previous studies found that the level of magnesium is slightly lowered in hyperoxaluric-rat urine, low levels of magnesium are also encountered in stone formers as well as stone forming rats. Moreover, magnesium can reduce the supersaturation of calcium oxalate by reacting with oxalate. Furthermore, magnesium has been found to decrease the growth and nucleation rates of calcium oxalate crystals [23,24]. Similarly, parsley contains large amount of Mg so it may reduce oxalate in intestine and in urine, decreasing its availability for Ca in renal tubules preventing CaOx formation. In addition, parsley extract has the ability to reduce the toxic effects of EG because it has high amount of vitamins (A, C, riboflavin, and niacin) and minerals (Fe, Mg, P, K, Ca, Na, and Zn) [25].
Some studies suggested that a high intake of dietary calcium inhibits the absorption of oxalate through the increase in intestinal luminal calcium complexed with oxalate, thereby reducing the pool of free oxalate available for absorption, hence reducing urinary oxalate excretion and CaOx stone formation [26].
Water intake and urine volume
Increased fluid intake correlates with a reduced saturation of calcium phosphate (brushite), calcium oxalate, and monosodium urate [27]. We found that parsley increased water intake and urine volume (Table 4, Graph 4). The increase in water intake may be due to its high Na content [15] hence thirst. However, serum Na decreased significantly in rats receiving concomitant EG/AC and parsley, a finding explained by parsley-induced diuresis [12] as well as by increased water intake found in present study. The direct diuretic effect of parsley was attributed to inhibition of the Na/K pump that would lead to reduction in Na+ and K+ reabsorption and osmotic diuresis [15]. Other studies attributed the diuretic effect of parsley to numerous compounds such as flavoniods, saponins or organic acids [28]. The diuretic activity of flavonoids may be due to their binding with adenosine A1 receptors [29].
Table 4.
Water intake and urine volume in rats With and without Parsley
| Group | Water intake ml/24 h | Urine volume ml/24 h |
|---|---|---|
| Without parsley | 19.3±2.0a | 4.3±0.5a |
| With Parsley | 29.7±1.5b | 8.1±0.5b |
The values are in mean ± SEM. Significant difference between a&b.
Graph 4.
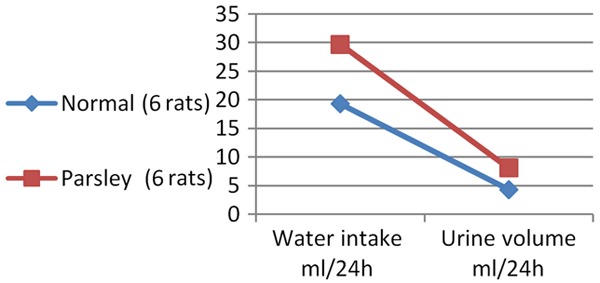
Water intake and urine volume in rats with and without parsley.
The decrease in urinary output causes supersaturation of urine by calcium, oxalate and phosphate leading to formation of stones [30]. Hence, the increased water intake and urine volume noted in our study may be responsible for the decrease in urinary calcium and CaOx crystals preventing stone formation.
There are many side effects of diuretics and hypokalemia is the most dangerous one. Parsley is rich in potassium, so its diuretic effect did not lead to potassium depletion [31] making it a safe diuretic and this is consistent with a previous study that serum K didn’t significantly decrease in parsley treated rats [12].
Urine pH in the morning and the afternoon
Alterations in urinary pH may be due to genetic variants or mutations in transport pathways, life style habits such as specific diets, or metabolic diseases and infections. Inappropriately acidic or alkaline urine affects the solubility of various metabolites and salts. Alkaline urine reduces solubility of calcium phosphate products, whereas acidic urine pH promotes formation of CaOx, uric acid and cystine-containing stones [32].
Urinary pH is a major determinant for kidney stone formation suggested that urine pH approximately near 6 in pH scale reduces the risk of kidney stone formation. The risk of calcium oxalate (CaOx) crystallization at different pH levels was determined in urine from recurrent CaOx-stone formers and normal subjects. The highest crystallization risk was observed between pH 4.5 and 5.5 [33]. In our study there was a significant increase in urinary pH in parsley treated group, indicating that the antiurolithiatic effect of parsley also related to elevation of urinary pH making it a good anti-CaOx remedy. However this action may enhance calcium phosphate stone formation.
Suarez and Youssef denoted that when urinary pH rises, renal tubular citrate reabsorption decreases, hence increases its excretion [34], another mechanisms by which parsley do its action.
We concluded that parsley act as antiurolithiatic drug through decreasing urinary calcium excretion, increasing urinary pH making urine unsuitable for CaOx crystallization, diuresis and increasing urine volume hence decreasing supersaturation of urine, decreasing nucleation through decreasing urinary protein excretion and its nephroprtective activity. We recommend to use it in pharmaceutical forms as it is safe and effective as antiurolithiasis remedy.
References
- 1.Rajat M, Anu W, Sumeet G. New frontiers on nephrolithiasis: pathoysiology and management of kidney stones. Int J Res Ayurv Pharma. 2011;2:775–786. [Google Scholar]
- 2.Patel PK, Patel MA, Saralai MG, Gandhi TR. Antiurolithiatic effects of solanum xanthocarpum fruit extract on ethylene-glycol-induced nephrolithiasis in rats. J Young Pharmacists. 2002;4:164–170. doi: 10.4103/0975-1483.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006;367:333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 4.Soundararajan P, Mahesh RT, Ramesh T, Begum VH. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol. 2006;44:981–986. [PubMed] [Google Scholar]
- 5.Devi KS, Damayanti M, Velmurugan D, Singh NR. Analysis of kidney stones by PXRD and evaluation of the antiurolithic potential of coix lacryma joba. Int J Sci Res Publ. 2015;5:1–5. [Google Scholar]
- 6.Kalpana S, Nirmaladevi R, Rai TS, Karthika P. Inhibition of calcium oxalate crystallization in vitro by extract of banana cultivar monthan. Int J Pharm Pharmace Sci. 2013;4:649–653. [Google Scholar]
- 7.Matlaga BR, Lingeman JE. Surgical management of upper urinary tract calculi. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW and Peters CA. Campbell-Walsh Urology. 2012;2:1357–1410. [Google Scholar]
- 8.Aggarwal A, singla SK, Tandon C. Urolithiasis phytotherapy as an adjunct therapy. Indian J Exp Biol. 2014;53:103–111. [PubMed] [Google Scholar]
- 9.Soliman HA, Eltablawy NA, Hamed MS. The ameliorative effect of Petroselinum crispum (parsley) on some diabetes complications. J Medicinal Plants Studies. 2015;3:92–100. [Google Scholar]
- 10.Moazedi AA, Mirzaie DN, Seyyednejad SM, Zadkarami MR, Amirzargar A. Spasmolytic effect of Petroselinum crispum (parsley)on Rat,s ileum at different calcium chloride concentrations. Pak J Biol Sci. 2007;10:4036–4042. doi: 10.3923/pjbs.2007.4036.4042. [DOI] [PubMed] [Google Scholar]
- 11.Wahba NM, Ahmed AS, Ebraheim ZZ. Antimicrobial effects of pepper, parsley, and dill and their roles in the microbiological quality enhancement of traditional egyptian kareish cheese. Foodborne Pathog Dis. 2010;7:411–8. doi: 10.1089/fpd.2009.0412. [DOI] [PubMed] [Google Scholar]
- 12.Gumaih H, Al-Yousofy F, Ibrahim H, Ali S, Alasbahy A. Evaluation of ethanolic seed extract of parsley on ethylene glycol induced calcium oxalate, experimental model. Int J Sci Res. 2017;6:1683–1688. [Google Scholar]
- 13.Jassim AM. Protective Effect of Petroselinum crispum (parsley) extract on histopathological changes in liver, kidney and pancreas induced by sodium valproate-in male rats. Kufa J Veter Med Sci. 2013;4:20–27. [Google Scholar]
- 14.Fan J, Glass MA, Chandhoke PS. Impact of ammonium chloride administration on a rat ethylene glycol urolithiasis model. Sca Microsc Int. 1999;13:299–306. [Google Scholar]
- 15.Kreydiyyeh SI, Usta J. Diuretic effect and mechanism of action of parsley. J Ethnopharma. 2002;79:353–357. doi: 10.1016/s0378-8741(01)00408-1. [DOI] [PubMed] [Google Scholar]
- 16.Eisner BH, Sheth S, Dretler SP, Herrick B, Pais VM. Abnormalities of 24-hour urine composition in first-time and recurrent stone-formers. Urology. 2012;80:776–779. doi: 10.1016/j.urology.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Soni H, Thakkar T, Patel R, Patel G. Evaluation of acute toxicity and anti-urolithiatic activity of ural capsule. Int J Phytophar. 2014;5:15–20. [Google Scholar]
- 18.Alyami FA, Rabah DM. Effect of drinking parsley leaf tea on urinary composition and urinary stones risk factors. Saudi J Kidney Dis. 2011;22:511–514. [PubMed] [Google Scholar]
- 19.Kuzma P, Druzynska B, Obiedzinski M. Optimization of extraction condition of some polyphenolic compounds from parsley leaves (Petroselinum crispum) Acta Sci Pol. 2014;13:145–154. doi: 10.17306/j.afs.2014.2.4. [DOI] [PubMed] [Google Scholar]
- 20.İnanç AL. Chlorophyll structural properties, health benefits and its occurrence in virgin olive oils. Acad Food J. 2011;9:26–32. [Google Scholar]
- 21.Talekar YP, Gund KA, Kale SD, Apte KG, Parab PB. Antiurolithic activity of corn silk extract in rats. Int J Univers Pharm Bio Sci. 2013;2:65–77. [Google Scholar]
- 22.Jafar S, Mehri L, Hadi B, Jamshid M. The antiurolithiasic and hepatocurative activities of aqueous extracts of petroselinum sativum on ethylene glycol-induced kidney calculi in rats. Acade J. 2012;7:1577–1583. [Google Scholar]
- 23.Riley JM, Kim H, Averch TD, Kim HJ. Effect of magnesium on calcium and oxalate ion binding. J Endouro. 2013;27:1487–1492. doi: 10.1089/end.2013.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liebman M, Costa G. Effects of calcium and magnesium on urinary oxalate excretion after oxalate loads. J Urol. 2000;163:1565–9. [PubMed] [Google Scholar]
- 25.Barbary MI, Mehrim AI. Protective effect of antioxidant medicinal herbs, rosemary and parsley, on subacute aflatoxicosis in oreochromis niloticus. J Fisheries Aquatic Sci. 2009;4:178–190. [Google Scholar]
- 26.Ruml LA, Pearle MS, Pak YC. Medical therapy calcium oxalate urolithiasis. Urol Clin Nor Am. 1997;24:117–133. doi: 10.1016/s0094-0143(05)70358-x. [DOI] [PubMed] [Google Scholar]
- 27.Pak CY, Sakhaee K, Crowther C, Brinkley L. Evidence justifying a high fluid intake in treatment of nephrolithiasis. Ann Intern Med. 1980;93:36–9. doi: 10.7326/0003-4819-93-1-36. [DOI] [PubMed] [Google Scholar]
- 28.Patel U, Kulkarni M, Undale V, Bhosale A. Evaluation of diuretic activity of aqueous and methanol extracts of lepidium sativum garden cress (cruciferae) in rats. Trop J Pharm Res. 2009;8:215–219. [Google Scholar]
- 29.Yuliana ND, Khatib A, Struensee AM, Ijzerman AP, Zakaria FR, Choi YH, Verpoorte R. Adenosine A1 receptor binding activity of methoxy flavonoids from orthosiphon stamineus. Planta Med. 2009;75:132–136. doi: 10.1055/s-0028-1088379. [DOI] [PubMed] [Google Scholar]
- 30.Michell AR. Urolithiasis-historical, comparative and pathophysiological aspects: a review. J Roy Soc Med. 1989;82:669–672. doi: 10.1177/014107688908201112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Senthilkumar GP, Selvan TV, Mazumder UK, Gupta M, Ray SK. Study on diuretic activity and electrolytes excretion of methanol extract of Lippia nodiflora (Verbenaceae) in rats. Ori Pharm Exper Medi. 2008;8:39–46. [Google Scholar]
- 32.Wagner CA, Mohebbi N. Urinary pH and stone formation. J Nephrol. 2010;23:165–169. [PubMed] [Google Scholar]
- 33.Berg C, Tiselius HG. The effect of pH on the risk of calcium oxalate crystallization in urine. Eur Urol. 1986;12:59–61. doi: 10.1159/000472578. [DOI] [PubMed] [Google Scholar]
- 34.Suarez M, Youssef RF. Potassium citrate: treatment and prevention of recurrent calcium nephrolithiasis. J Clin Nephro Res. 2015;2:1015–1011. [Google Scholar]


