Abstract
Our study evaluates the feasibility of compassionate exemption of Radium-223 (223Ra) treatment in metastatic hormone-sensitive high-grade prostate cancer (mHSHGPC) patients with concomitant androgen deprivation-therapy (ADT). Seven patients with mHSHGPC, were treated with six cycles of 223Ra plus ADT. All patients had undergone to 18F-NaF-PET/CT. A qualitative analyses of the 18F-NaF-PET/CT was performed in conjunction with Alkaline Phosphatase (ALP), Lactate-dehydrogenase (LDH) and Prostatic-Specific Antigen (PSA) values. The mean of SUVmax values were used as a quantitative measure of tumoral burden. Changes in PSA, ALP, LDH from baseline were evaluated, and were defined as increase or decrease of at least 30%. Clinical response was achieved if there was pain reduction using visual analogic scale. Four patients showed a significant reduction in mean SUVmax after 3 cycles of 223Ra, and one after 6 cycles. Patients who showed reductions in mean SUVmax after Ra-223 also showed reductions in PSA, ALP and LDH. Four weeks after the last cycle of 223Ra all patients had decreased total PSA, ALP and LDH values ≥ 30% also significant improvement on pain. No progress disease was documented after 14 ± 4 weeks. We found slight to moderate decreases in neutrophils and hemoglobin in two patients. We concluded that 223Ra plus ADT can be useful in mHSHGPC; the semi-quantitative 18F-NaF-PET/CT as a method effective to monitor the treatment response. Due to concomitant administration of ADT, 18F-NaF-PET/CT cannot differentiate whether the findings were due to androgen blockade or the 223Ra; nevertheless, data supporting the efficacy of 223Ra is the significant improvement on pain.
Keywords: Ra-223, 18F-NaF PET/CT, prostate cancer high-grade, hormone-sensitive
Introduction
Prostate cancer (PC) is the most common cancer in men and represents the third most common cause for death in men cancer-associated [1]. Early detection of primary disease is highly relevant in terms of prognosis and therapy management.
In Mexico PC represents the first malignant neoplasm in men with highest incidence and mortality. More than 50% of the patients who underwent an initial evaluation have metastatic disease [2,3]. These patients usually respond to androgen deprivation therapy (ADT); however, most patients do not respond to pain, despite optimal painkillers [4]. In 2015, 2 large randomized trials demonstrated the survival benefit of docetaxel in hormone sensitive prostate cancer, showing for the first time that treatment with drugs with benefit in the context of the castrate resistant disease, could be more beneficial if they were used earlier in the course of the disease [5,6].
Radium-223 (223Ra) is a bone-seeking radiopharmaceutical that emits α-particles that deposit high linear energy within a short penetration range to areas of increased bone turnover, as radioactive decay occurs, near osseous metastatic sites, it selectively kills cancer cells. 223Ra has a complex decay scheme in which 4 alfa particles resulting in high energy deposition (28.2 MeV), the high linear energy transfer of radiation results in generation of double-strand DNA breaks, and gives rise to cytotoxicity that is independent of dose rate, cell cycle growth phase, and oxygen concentration. The range of the α particles (< 100 µm) results in less hematologic toxicity than expected from β emitters [7,8].
223Ra was the first bone-targeting agent to demonstrate improved overall survival in patients with Castration Resistant Prostate Cancer (CRPC) and bone metastases, in addition to bone pain relief, improvement in quality of life (QoL), and prolonging time to skeletal-related events (SREs) [9,10].
In the present study, we evaluate the feasibility of compassionate exemption 223Ra treatment, with concomitant ADT, in metastatic hormone-sensitive high-grade prostate cancer (mHSHGPC) patients with multiple painful bone metastases.
Materials and methods
Patients
Patients with histologically confirmed, mHSHGPC, evidence of more than 10 metastatic bone sites, evaluated by bone scintigraphy at diagnosis, and bone pain as predominant symptom, were included. All the patients required hematological parameters within normal reference values (neutrophils ≥ 1.5 × 109/L, platelets ≥ 100 × 109/L, hemoglobin ≥ 10 g/dL) before the application of 223Ra which were obtained 1 ± 5 days before the application. Patients with evidence of visceral metastases, lymph nodes metastases > 3 cm in long-axis diameter, confirmed by a CT scan, or ADT initiation more than 3 months, were excluded. Other exclusion criteria included history of cancer other than prostate cancer, recent or complicated nonhealing fracture, and use of concomitant radiotherapy. Treatment with ADT was initiated after diagnosis of mHSHGPC and before compassionate exemption of 223Ra treatment, and was given continuously. ADT included bicalutamide 150 mg per day + luteinizing hormone-releasing hormone agonist (leuprolide) 11.25 mg every three months.
Imaging protocol
Patients underwent to 370 MBq (10 mCi) sodium fluoride PET/CT (18F-NaF-PET/CT) whole body scan 1 ± 5 days prior to the administration of 223Ra, as per institution protocol. Each hybrid PET/CT (mCT LSO; Siemens, Erlangen, Germany) was performed 1 hour after IV administration of the radiotracer. Low-dose helical CT transmission scan [pitch 0.8, 50 mA s, 120 kV (peak)] was performed. PET acquisition was started at a mean time of 59 ± 10.1 min after tracer injection (range, 49-70 min). PET was then performed within 2 minutes per bed position at a sufficient number of bed positions to cover the anatomic regions from the top of the head to the feet. Raw CT data were reconstructed into 5-mm thick section of transverse images, and reformatted sagittal and coronal CT images were generated. CT-based attenuation-corrected PET images were reconstructed and viewed on a high-resolution colored monitor. PET and CT images could be viewed on a continuous fusion scale from PET only to CT only images using image fusion software (E-soft; Siemens). In addition to quantifying the osteoblastic lesions, the median values of SUV were obtained to assess tumor burden.
Image interpretation
Fused PET/CT images for each scan were interpreted by 2 board-certified nuclear medicine physicians with more than 4 years of experience interpreting hybrid PET/CT studies. No reader for a particular scan was aware of the findings on the other paired scan. All interpreted pairs of PET/CT studies were again reexamined for agreement by consensus. Maximum-intensity-projection images were examined to help facilitate lesion detection. All maximum standardized uptake value (SUVmax) values of suspicious lesions (defined as foci of nonphysiological uptake above regional background activity associated with osteoblastic lesions by tomography findings) were obtained using 3D region of interest with the vendor-provided software (syngo by SIEMENS). After measurement of all metastatic lesions, mean values were obtained. A region of interest (ROI) was outlined within areas of increased uptake and measured on each slice. When the lesion was extensively heterogeneous, the ROI was set to cover all the components of the lesion. The SUVmax was recorded by using the maximum pixel activity within the ROI and measurement of all the metastatic lesions, finally obtaining a mean value for each patient.
223Ra treatment
Patients were administered intravenous 223Ra at 50 kBq/kg body weight (until May 2016, after this point the dose were calculated at 55 kBq/kg body weight after implementation of National Institute of Standards and Technology update on April 18, 2016) every 4 weeks for 6 cycles. Previous to each cycle, absolute blood count (hemoglobin, erythrocytes, leukocytes, platelets, neutrophils and lymphocytes) was evaluated. Treatment was continued as long as absolute blood count was ≥ 1.0 g/L for neutrophils and ≥ 50 g/L for platelets. Written informed consent was obtained from each patient before administration of 223Ra (Table 1).
Table 1.
Baseline characteristics of patients studied
| Patient | Age | Gleason score | PSA (ng/ml) | AP (UI/l) | LDH (UI/l) | VAS | Therapy initiated |
|---|---|---|---|---|---|---|---|
| 1 | 64 | 9 | 234 | 554 | 285 | 9 | LHRH + Bicalutamide |
| 2 | 59 | 8 | 530 | 200 | 198 | 10 | LHRH + Bicalutamide |
| 3 | 46 | 9 | 220 | 443 | 245 | 8 | LHRH + Bicalutamide |
| 4 | 64 | 9 | 36.4 | 160 | 291 | 8 | LHRH + Bicalutamide |
| 5 | 70 | 9 | 421 | 788 | 450 | 9 | LHRH + Bicalutamide |
| 6 | 66 | 8 | 199 | 245 | 209 | 8 | LHRH + Bicalutamide |
| 7 | 71 | 9 | 604 | 647 | 551 | 10 | LHRH + Bicalutamide |
PSA: Prostatic specific antigen; AP: Alkaline Phosphatase; LDH: Lactate Dehydrogenase; VAS: Visual Analog Scale for Pain; LHRH: luteinizing hormone-releasing hormone.
Other response assessments
Alkaline phosphatase (ALP), Lactate dehydrogenase (LDH), Prostatic specific-antigen (PSA) and 18F-NaF-PET/CT were acquired prior cycle 1, 4, and 4 weeks after cycle 6. The follow-up visit was 14 ± 4 weeks after the last administration of 223Ra. Changes in ALP, LDH and PSA were recorded. Clinical response was achieved if there was pain reduction using visual analogic scale. Molecular response was assessed by 18F-NaF-PET/CT whole body scan, and was defined as any measurable decrease in the sum of the osteoblastic metastatic disease of all pre-therapeutically detected tumor lesions.
Statistical analysis
A qualitative comparison of the 18F-NaF-PET/CT whole body scans was performed in conjunction to the ALP, LDH and PSA results. A semi-quantitative comparison was performed by measuring the SUVmax values in all bone metastases in each patient. The means of the lesions SUVmax measurements in each patient were used as a quantitative measure of global metastatic activity previous to receive the first cycle of 223Ra, after 3 and after 6 cycles of 223Ra. Changes in PSA, ALP, LDH from baseline were evaluated, and were defined as an increase or a decrease of at least 30% from baseline.
Results
From January 2015 to December 2016, 7 patients with mHSPC were treated with 223Ra + ADT. All patients had bone metastases without soft-tissue lesions or visceral metastases at diagnosis; they all had baseline bone scans. None of the patients were pretreated with 223Ra or another bone seeking radiopharmaceutical. Two patients had a history of localized radiotherapy (3 months before starting treatment with 223Ra) to bone, due to intense pain, at diagnosis. All patients had pain at least VAS 7, despite to receive painkillers such as NAIDS and opioids. Baseline characteristics before starting treatment with 223Ra are presented in Table 2. All patients were evaluated with 18F-NaF-PET/CT whole body scan 1 ± 1 week before starting treatment with 223Ra. Osteoblastic lesions were selected to represent both axial and appendicular sites when present, and the SUVmax calculated from a ROI of each lesion. The average SUVmax of the total lesions was then calculated for each patient at each scanning time point.
Table 2.
Baseline characteristics before starting treatment with Ra-223
| Patient | Metastatic sites | Mean SUVmax (Range) | PSA (ng/ml) | ALP (UI/L) | LDH (UI/L) | VAS | Hb (g/dl) | Plt (× 103/µL) | Neutrophils (× 103/µL) | Leukocytes (× 103/µL) | Previous RT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SUPERSCAN | 91.6 (79.1-144.1) | 3.2 | 270 | 181 | 8 | 13.3 | 286 | 4.5 | 7.2 | NO |
| 2 | 15 | 66.8 (26.9-84.2) | 34 | 196 | 189 | 8 | 12.9 | 190 | 4.1 | 5.5 | SI |
| 3 | 12 | 58.7 (22.5-76.4) | 26 | 348 | 207 | 8 | 14.8 | 323 | 5.4 | 5.1 | NO |
| 4 | > 20 | 81.7 (33.1-91.9) | 36.4 | 160 | 291 | 8 | 14.2 | 263 | 3.8 | 7.3 | NO |
| 5 | 18 | 69.1 (36.9-101.2) | 18.3 | 455 | 299 | 7 | 11.1 | 328 | 3.2 | 4.2 | NO |
| 6 | > 20 | 89.7 (53.1-101.6) | 6.7 | 211 | 189 | 7 | 13.1 | 401 | 3.7 | 4.5 | NO |
| 7 | SUPERSCAN | 96.6 (84.7-155.0) | 71 | 901 | 604 | 7 | 12.1 | 388 | 2.9 | 3.1 | SI |
RT: Radiotherapy; Hb: Hemoglobin; Plt: platelets.
Four patients had high tumor burden, two of them had a “superscan” with high mean SUVmax (81.7; 89.7; 91.2 and 96.6, respectively). Six patients had high values of ALP before to start treatment with 223Ra (where the median value was 270 UI/l [range 196-901 UI/l]); five of these associated to high values of PSA (where the median value was 26 ng/ml [range 3.2-71 ng/ml]) and LDH (where the media value was 207 UI/l [range 181-604 UI/l]).
Therapy with 223Ra was started 2.2 ± 1.1 months after initial diagnosis of metastatic prostate cancer. All patients completed all six cycles of 223Ra (mean cumulative dose 28.6 ± 3.8 MBq).
In the semi-quantitative analysis, four patients showed a significant reduction in mean SUVmax after 3 cycles of 223Ra, and one other showed a significant reduction after 6 cycles. One patient showed minimal changes after 3 and 6 cycles of 223Ra. In six patients’ partial response based in extent of disease and intensity (osteoblastic activity) were observed, although not directly measuring tumour cell activity, as might be possible with other PET radiotracers including 18F-fluorodeoxyglucose, 68Ga-prostate-specific membrane antigen, changes in 18F-NaF uptake were similar to changes in ALP, PSA, LDH after 6 cycles of 223Ra. In one patient stable disease was documented after six cycles of 223Ra. No progression of disease was documented. All patients who showed reductions in mean SUVmax after 223Ra treatment also showed reductions in PSA, ALP and LDH, including the patient with minimal changes visualized by PET/CT; the overall changes by each patient are shown in Figures 1, 2, 3 and 4. Of interest, an increase of sclerotic lesions was noted after treatment with 223Ra (Figures 5 and 6).
Figure 1.
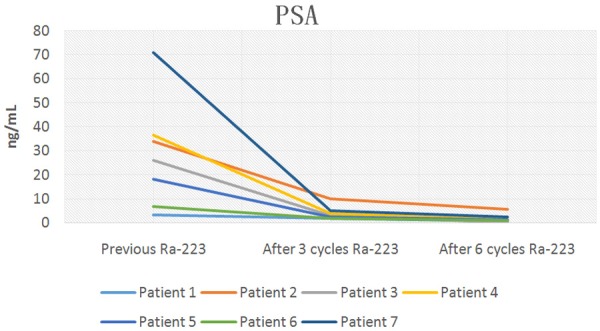
Baseline Prostate specific-antigen and subsequent levels after 3 and 6 doses of 223Ra plus ADT.
Figure 2.
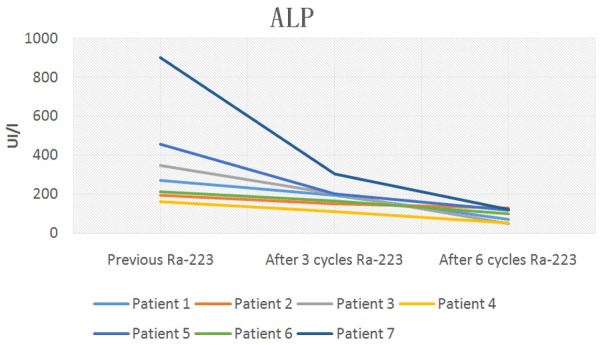
Baseline Alkaline Phosphatase and subsequent levels after 3 and 6 doses of 223Ra plus ADT.
Figure 3.
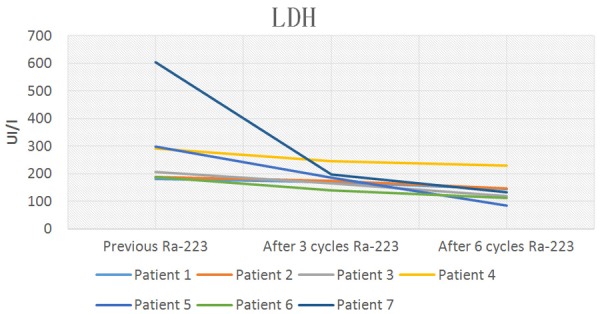
Baseline Lactate dehydrogenase and subsequent levels after 3 and 6 doses of 223Ra plus ADT.
Figure 4.
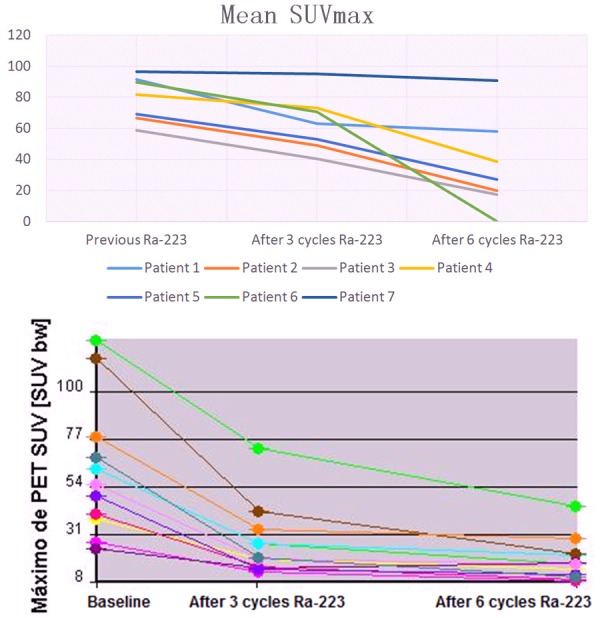
Top. Semi-quantitative analysis of Mean SUVmax metastatic lesions baseline and after 3 and 6 doses of 223Ra plus ADT. Lower. The average SUVmax of the total lesions for each patient at each scanning time point.
Figure 5.
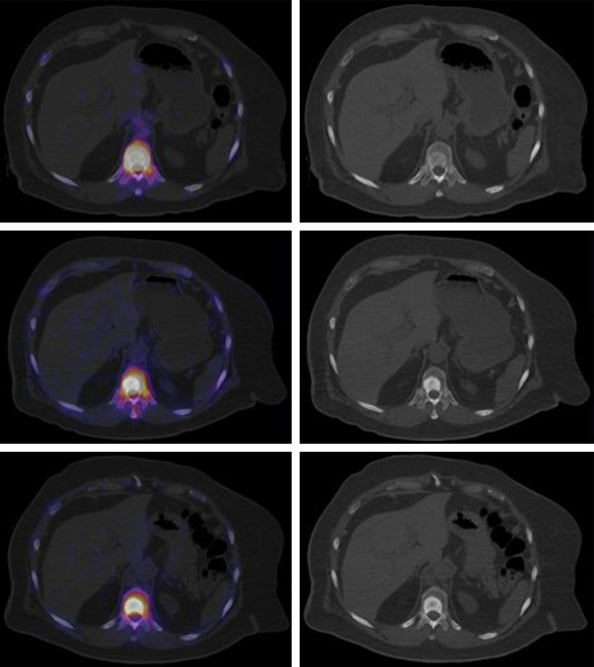
Top. Fused axial slice of baseline 18F-NaF-PET/CT and high radionuclide uptake (left), axial slice showed blastic lesion in vertebral body (right). Middle. Decrease focal uptake in blastic lesion (left), important increase of sclerotic component, after 3 cycles of 223Ra is seen. Lower. decrease focal uptake in blastic lesion (left), note the increase of sclerotic component, after 6 cycles of 223Ra plus ADT.
Figure 6.
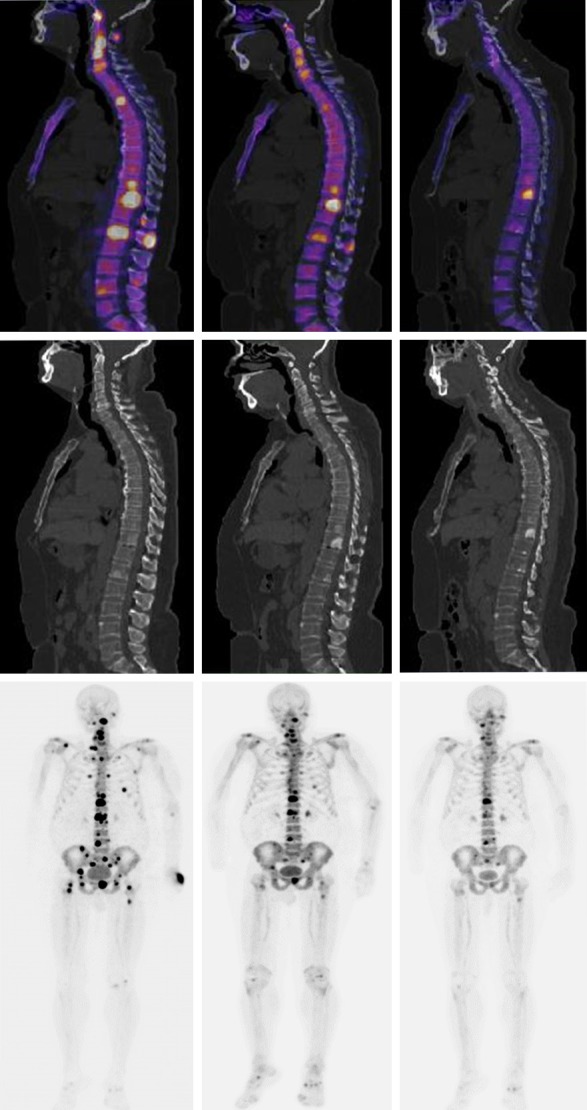
Top Left. Fused sagittal slice of baseline 18F-NaF-PET/CT and high radionuclide uptake in multiple vertebral bodies. Top middle. Fused sagittal slice 18F-NaF-PET/CT after 3 cycles of 223Ra. Top Right. Fused sagittal slice 18F-NaF-PET/CT after 6 cycles of 223Ra plus ADT, note the important osteoblastic activity. Middle. Sagittal slices corresponding only a CT baseline, CT after 3 cycles of 223Ra and CT after 6 cycles of 223Ra plus ADT, note the increased sclerotic changes. Lower Left. multiple sites of focal high radionuclide uptake that result in metastatic bone disease predominantly blastic type axial skeleton in the MIP 18F-NaF-PET. Lower middle. MIP 18F-NaF PET after 3 cycles of 223Ra. Lower Right. MIP 18F-NaF-PET after 6 cycles of 223Ra plus ADT, note the important decreased of osteoblastic activity (patient 4).
Table 3, shows tumor markers evaluated and potential tumor-volume-related determinants in serum after 6 cycles of 223Ra. Total PSA, ALP and LDH decreased significantly during 223Ra therapy. Three months after treatment initiation with 223Ra, five of the seven patients had decreased total PSA, ALP and LDH values. Four weeks after the last cycle of 223Ra all patients had decreased total PSA, ALP and LDH values greater than or equal to 30% below the baseline.
Table 3.
Characteristics of patients studied after 6 cycles with Ra-223
| Patient | Mean SUVmax (Range) | Mean SUVmax (Range) [% of baseline] After 3 cycles* | Mean SUVmax (Range) [% of baseline] After 6 cycles** | PSA (ng/ml) | ALP (UI/L) | LDH (UI/L) | VAS | Hb (g/dl) | Plt (× 103/µL) | Neutrophils (× 103/µL) | Leukocytes (× 103/µL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 91.6 (79.1-144.1) | 63.3 (45.4-99.1) [69.1%] | 58.1 (40.7-70.3) [63.4%] | 0.6 | 70 | 145 | 4 | 12.5 | 216 | 3.7 | 4.9 |
| 2 | 66.8 (26.9-84.2) | 49.3 (18.9-69.7) [73.8%] | 19.9 (14.4-49.0) [30%] | 5.7 | 129 | 148 | 2 | 13.1 | 202 | 4 | 5.5 |
| 3 | 58.7 (22.5-76.4) | 40.2 (17.2-54) [68.5%] | 17.3 (4.1-28.8) [29.5%] | 0.13 | 49 | 120 | 1 | 13.1 | 300 | 3.3 | 4.7 |
| 4 | 81.7 (33.1-91.9) | 73.0 (28.6-80.1) [89.3%] | 38.7 (14.8-66.2) [47.4%] | 1.3 | 53 | 230 | 1 | 13.1 | 277 | 3.9 | 6.3 |
| 5 | 69.1 (36.9-101.2) | 52.9 (31.0-89.7) [76.5%] | 27.3 (14.4-49.9) [39.5%] | 1.2 | 119 | 85 | 2 | 9.7 | 188 | 2.5 | 4.1 |
| 6 | 89.7 (53.1-101.6) | 70.5 (42.1-88.8) [78.6%] | 66.9 (38.7-80.9) [74.6%] | 0.9 | 101 | 113 | 2 | 9.9 | 283 | 3.4 | 3.9 |
| 7 | 96.6 (84.7-155.0) | 95.3 (88.3-141.1) [98.6%] | 90.9 (80.7-131.7) [94.1%] | 2.4 | 120 | 134 | 4 | 8.8 | 209 | 2.9 | 2.9 |
Percentage of declined Mean SUVmax after 3 cycles of Ra-223.
Percentage of declined Mean SUVmax after 6 cycles of Ra-223.
Improvement of bone pain was observed in all patients at the end of treatment compared to baseline. In three patients a significant decrease in pain was observed after the first two cycles of 223Ra; in two patients after four cycles.
Treatment-related adverse events were observed in two patients (patients with superscan); such as fatigue, diarrhea and nausea; meanwhile did not affect continuation of therapy. We found slight to moderate decreases in neutrophils and hemoglobin in two patients at the end of entire therapy. During treatment and at term, no patient required transfusion. One patient (with superscan) required prior to the last cycle administration of colony stimulating factor. None patient presented severe adverse event’s Grade III or IV according to Common Terminology Criteria for Adverse Events (CTCAE). No progress disease was documented after 14 ± 4 weeks.
Discussion
223Ra was shown to be safe and well tolerated, regardless of prior or concurrent exposure to abiraterone, enzalutamide or docetaxel [11]. However, it not yet understood if an earlier setting of abiraterone, enzalutamide and/or when resistance to castration has been established, the 223Ra would be beneficial, especially in patients with high bone tumor burden at the diagnosis of the disease as betide in many countries, including Latin-American countries [2].
This pilot study with off-label use of 223Ra in mHSHGPC patients with multiple painfully bone metastases with concomitant ADT, has shown can be safe and effective, not only in controlling the pain and decrease the need for the intake of painkillers and opioids; also to decrease biochemical markers linked to the patient’s prognosis, such as PSA, LDH and ALP.
Serum biomarkers, such as PSA and bone ALP, are commonly used as early efficacy markers in CRPC and bone metastases; several studies shown that a PSA reduction ≥ 30% from baseline has been shown to correlate more closely with survival than earlier ≥ 50%; however, in the context of mHSHGPC not fully understood [12,13].
In a recent study by Sartor et al. studied the baseline clinical variables are prognostic for overall survival (OS) in patients with CRPC; they demonstrated that total alkaline phosphatase (tALP), LDH, and PSA at baseline were associated with OS [14].
In addition it has shown that the feasibility of measuring the semi-quantitative changes of 18F-NaF-PET/CT scan using SUVs is able to measure changes not detected by qualitative visual inspection in patients with bone metastases from prostate cancer receiving systemic therapy with 223Ra. Previous reports suggest this method as a tool for measurements of blastic lesions after treatment with 223Ra [15,16].
Another study by Wenter et al. suggest that 223Ra for primary bone metastases in patients with metastatic Hormone-Sensitive PC after radical prostatectomy is feasible and alleviates pain. ALP, rather than PSA, may be a good marker for assessing treatment response. Meanwhile, bone scintigraphy was performed to assess treatment response not 18F-NaF-PET/CT. Seven of ten patients completed six cycles of 223Ra. Discontinuation was due to leukopenia and lymphopenia, progressive lymph node metastasis or newly diagnosed liver metastasis. Treatment-related adverse events occurred in three patients such as fatigue, abdominal discomfort and nausea. Overall, a median decrease of 28% in ALP and a median decrease of 83% in PSA were noted at follow-up. However, PSA progressed in five patients at follow-up [17].
In our previous study we demonstrated the usefulness and effectiveness of 223Ra in as an aiding agent that can be employed in conjunction with bicalutamide and gosereline to produce excellent results in treating patients with hormone-sensitive PC with symptomatic bone metastases, and the evaluation of response with 18F-NaF-PET/CT in conjunction with PSA and ALP [18]. PET/CT also has the inherent advantage over the conventional 99mTc-MDP bone scintigraphy of more accurate and absolute quantification of radioactive tracer concentrations, and therefore lends itself to a semi-quantitative approach [19-21]. In addition to being shown to have greater sensitivity and specificity in detecting metastatic osteoblastic disease [22,23].
Age, in addition to tumor burden, may be a factor involved in treatment efficacy; in our study, the most long-lived patient (number 7) had a high tumor burden (superscan), and no significant benefit was seen when quantitatively assessed with 18F-NaF-PET/CT, however, if there was an improvement in pain at the end of the 6 cycles. Probably one reason is the low medullary reserve associated with aging. These findings need to be confirmed with a larger cohort of patients.
The principal limitation of this study is due to concomitant administration of ADT, because imaging cannot differentiate whether the findings were due to androgen blockade or to the administration of 223Ra; nevertheless data supporting the efficacy of 223Ra is the significant improvement on pain. The mechanism of action of both ADT plus 223Ra may represent a synergistic effect; molecular imaging can be additionally value in the earlier evaluation of response of this patients [24]. Given the short time of follow-up, no information on overall survival or progression free survival could be obtained.
Despite these limitations, this study demonstrated the off-label use of 223Ra for bone metastatic disease in patients with mHSHGPC at initial diagnosis of the diseases without radical prostatectomy. However, the design of future randomized trials are needed to confirm these findings.
Conclusion
223Ra can be useful in mHSHGPC in concomitant ADT despite to RT palliative, in addition we suggest that semi-quantitative 18F-NaF-PET/CT whole body scan as a method to monitor the treatment response in bone metastases following therapy with 223Ra and ADT in conjunction with biochemical markers linked to the patient’s prognosis, such as PSA, LDH and ALP.
Acknowledgements
We thank Nuclear Medicine Department at Instituto Nacional de Cancerologia, México.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F, editors. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC cancer base No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. Available: http://globocan.iarc.fr, accessed 20/May/2017. [Google Scholar]
- 2.Erazo-Valle A, Martinez-Cedillo J, Rivera-Rivera S, et al. Reunión de panel de expertos en cáncer de próstata. Gaceta Mexicana de Oncología. 2014;13:2–17. [Google Scholar]
- 3.Sánchez-Barriga J. Mortalidad por cáncer de próstata en México. Gaceta Médica de México. 2013;149:576–85. [PubMed] [Google Scholar]
- 4.Sharifi N, Dahut WL, Steinberg SM, Figg WD, Tarassoff C, Arlen P, Gulley JL. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005;96:985–9. doi: 10.1111/j.1464-410X.2005.05798.x. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, Dreicer R, Vogelzang NJ, Picus J, Shevrin D, Hussain M, Garcia JA, DiPaola RS. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard G, de Bono J, Cross W, Jones RJ, Thalmann G, Amos C, Matheson D, Millman R, Alzouebi M, Beesley S, Birtle AJ, Brock S, Cathomas R, Chakraborti P, Chowdhury S, Cook A, Elliott T, Gale J, Gibbs S, Graham JD, Hetherington J, Hughes R, Laing R, McKinna F, McLaren DB, O’Sullivan JM, Parikh O, Peedell C, Protheroe A, Robinson AJ, Srihari N, Srinivasan R, Staffurth J, Sundar S, Tolan S, Tsang D, Wagstaff J, Parmar MK. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrasquillo JA, O’Donoghue JA, Pandit-Taskar N, Humm JL, Rathkopf DE, Slovin SF, Williamson MJ, Lacuna K, Aksnes AK, Larson SM, Scher HI, Morris MJ. Phase I pharmacokinetic and biodistribution study with escalating doses of 223Ra dichloride in men with castration resistant metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40:1384–93. doi: 10.1007/s00259-013-2427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandit-Taskar N, Larson SM, Carrasquillo JA. Bone-Seeking radiopharmaceuticals for treatment of osseous metastases, part 1: α therapy with 223Ra-Dichloride. J Nucl Med. 2014;55:268–74. doi: 10.2967/jnumed.112.112482. [DOI] [PubMed] [Google Scholar]
- 9.In national comprehensive cancer network (NCCN) clinical practice guidelines in oncology. version 2. National Comprehensive Cancer Network; 2017. Prostate cancer. [DOI] [PubMed] [Google Scholar]
- 10.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, Bottomley D, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall’Oglio M, Franzén L, Coleman R, Vogelzang NJ, O’Bryan-Tear CG, Staudacher K, Garcia-Vargas J, Shan M, Bruland ØS, Sartor O. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 11.Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Widmark A, Johannessen DC, Hoskin P, James ND, Solberg A, Syndikus I, Vogelzang NJ, O’Bryan-Tear CG, Shan M, Bruland ØS, Parker C. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15:738–46. doi: 10.1016/S1470-2045(14)70183-4. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong AJ, Garrett-Mayer E, Ou Yang YC, Carducci MA, Tannock I, de Wit R, Eisenberger M. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J. Clin. Oncol. 2007;25:3965–70. doi: 10.1200/JCO.2007.11.4769. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong A, Febbo P. Using surrogate biomarkers to predict clinical benefit in men with castration-resistant prostate cancer: an update and review of the literature. Oncologist. 2009;14:816–27. doi: 10.1634/theoncologist.2009-0043. [DOI] [PubMed] [Google Scholar]
- 14.Sartor O, Coleman RE, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Vogelzang NJ, Bruland Ø, Kobina S, Wilhelm S, Xu L, Shan M, Kattan MW, Parker C. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol. 2017;28:1090–97. doi: 10.1093/annonc/mdx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etchebehere EC, Araujo JC, Fox PS, Swanston NM, Macapinlac HA, Rohren EM. Prognostic factors in patients treated with 223Ra: the role of skeletal tumor burden on baseline 18F-fluoride PET/CT in predicting overall survival. J Nucl Med. 2015;56:1177–84. doi: 10.2967/jnumed.115.158626. [DOI] [PubMed] [Google Scholar]
- 16.Cook G, Parker C, Chua S, Johnson B, Aksnes AK, Lewington VJ. 18F-fluoride PET: changes in uptake as a method to assess response in bone metastases from castrate-resistant prostate cancer patients treated with 223Ra-chloride (Alpharadin) Eur J Nucl Med Mol Imaging Res. 2011;1:1–4. doi: 10.1186/2191-219X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenter V, Herlemann A, Fendler WP, Ilhan H, Tirichter N, Bartenstein P, Stief CG, la Fougère C, Albert NL, Rominger A, Gratzke C. Radium-223 for primary bone metastases in patients with hormone-sensitive prostate cancer after radical prostatectomy. Oncotarget. 2017;8:44131–40. doi: 10.18632/oncotarget.17311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medina-Ornelas SS, García-Pérez FO. Molecular imaging in the evaluation of 6 doses of Ra-223 in high-grade prostate cancer: case report. Clin Genitourin Cancer. 2017;15:e159–e164. doi: 10.1016/j.clgc.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high risk prostate cancer: 99mTc MDP planar bone scintigraphy, single and multi field of view SPECT, 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–97. [PubMed] [Google Scholar]
- 20.Even-Sapir E, Metser U, Flusser G, Zuriel L, Kollender Y, Lerman H, Lievshitz G, Ron I, Mishani E. Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med. 2004;45:272–8. [PubMed] [Google Scholar]
- 21.Daisne JF, Sibomana M, Bol A, Doumont T, Lonneux M, Grégoire V. Tri-dimensional automatic segmentation of PET volumes based on measured source-to-background ratios: influence of reconstruction algorithms. Radiother Oncol. 2003;69:247–50. doi: 10.1016/s0167-8140(03)00270-6. [DOI] [PubMed] [Google Scholar]
- 22.Avinash DL, Bal C, Bandopadhyaya GP, Kumar L, Kumar P, Malhotra A, Lata S. The role of 18Ffluoride PET-CT in the detection of bone metastases in patients with breast, lung and prostate carcinoma: a comparison with FDG PET/CT and 99mTc-MDP bone scan. Jpn J Radiol. 2013;31:262–9. doi: 10.1007/s11604-013-0179-7. [DOI] [PubMed] [Google Scholar]
- 23.Withofs N, Grayet B, Tancredi T, Rorive A, Mella C, Giacomelli F, Mievis F, Aerts J, Waltregny D, Jerusalem G, Hustinx R. 18F-fluoride PET/CT for assessing bone involvement in prostate and breast cancers. Nucl Med Commun. 2011;32:168–76. doi: 10.1097/MNM.0b013e3283412ef5. [DOI] [PubMed] [Google Scholar]
- 24.Saad F, Carles J, Gillessen S, Heidenreich A, Heinrich D, Gratt J, Lévy J, Miller K, Nilsson S, Petrenciuc O, Tucci M, Wirth M, Federhofer J, O’Sullivan JM. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17:1306–16. doi: 10.1016/S1470-2045(16)30173-5. [DOI] [PubMed] [Google Scholar]


