Abstract
Recombination promotes genomic integrity among cells and tissues through double-strand break repair, and is critical for gamete formation and fertility through a strict regulation of the molecular mechanisms associated with proper chromosomal disjunction. In humans, congenital defects and recurrent structural abnormalities can be attributed to aberrant meiotic recombination. Moreover, mutations affecting genes involved in recombination pathways are directly linked to pathologies including infertility and cancer. Recombination is among the most prominent mechanism shaping genome variation, and is associated with not only the structuring of genomic variability, but is also tightly linked with the purging of deleterious mutations from populations. Together, these observations highlight the multiple roles of recombination in human genetics: its ability to act as a major force of evolution, its molecular potential to maintain genome repair and integrity in cell division and its mutagenic cost impacting disease evolution.
This article is part of the themed issue ‘Evolutionary causes and consequences of recombination rate variation in sexual organisms’.
Keywords: recombination, mutation load, cancer, disease, gene conversion, PRDM9
1. Introduction
Major advances in human population genetics in the past decade include the characterization of the primary genomic forces generating and shaping human variation: mutation [1–4] and recombination [5–11]. Recombination is a key mechanism shaping mutational variation across genomes and its impact is critical in evolutionary biology and human disease. Recombination can be simply defined as the process by which chromosomes exchange genetic material. In meiosis, new combinations of the parental genetic material are created to be transmitted to offspring. In mitosis, recombination-related processes ensure a conservative repair of double-strand breaks (DSBs) to minimize altered transmission of DNA to daughter cells.
From the early 1930s until the end of the 1970s, theoretical research [12–17] focused on models identifying conditions necessary for recombination (sexual reproduction) to evolve. In the 1980s, recombination was discovered to be critical in stabilizing homologous chromosomes and ensuring accurate chromosomal disjunction during meiosis in eukaryotes, as abnormal crossover frequencies were found to correlate with aneuploidy frequencies [18–20]. Additionally, recombination was found to be involved in the repair machinery of damaged DNA, which otherwise would accumulate lesions inflicted spontaneously or induced by the surrounding cell environment [21,22]. More recently, advances in statistical methods coupled with large collections of genomic variation have resulted in a better understanding of the distribution of recombination rates along the human genome and in the discovery that recombination frequencies are driven by genetic and epigenetic factors [5–11].
In this review, we focus on the impact of recombination on mutation accumulation and disease in humans by describing the interplay between molecular and evolutionary mechanisms associated with the localization and regulation of meiotic and mitotic recombination. Specifically, we concentrate on the mechanisms underlying chromosome pairing and crossover establishment as well as on downstream pathways associated with DNA repair known to result in genomic disorders in the event of their disruption. We start by describing the association between recombination hotspots and its major regulator, PRDM9, as well as recent evidence for the role of PRDM9 in infertility and speciation. We also discuss the impact of dysregulated recombination-related pathways on fertility and how aberrant recombination affects structural genetic abnormalities, congenital defects and disease. We then examine the role of mitotic recombination on disease, particularly in cancer, by reviewing current evidence for the implication of homologous recombination mechanisms in DNA repair and tumorigenesis and finally, the potential of mitotic recombination as a mutagenic agent. To conclude, we present the current evidence for the impact of chromosomal linkage and GC-biased gene conversion on the accrual of deleterious mutations in human populations.
2. Meiotic recombination and infertility
Meiotic recombination is essential for accurate chromosomal disjunction and maintenance of genomic stability during meiosis in eukaryotes. During meiosis, the recombination process is initiated by the introduction of DSBs at specific locations across the genome, and their successful positioning, repair and resolution into crossovers depend upon many molecular processes that are essential to ensure genome integrity. Substantial variation in the rate and distribution of crossovers has been found within and among species, genders, populations and individuals. Within genomes, recombination rates and locations vary among chromosomes, at megabase and kilobase scales. In mammals, the distribution of crossovers along the genome is known to vary, and substantial regions of DNA with unusually low recombination are observed, known as coldspots, while highly localized peaks of recombination, known as hotspots, are also seen. In this section, we describe the evolutionary and molecular mechanisms of such variation in recombination rate and their implications for fertility in mammals.
(a). Meiotic recombination hotspots, PRDM9 and infertility
The first insights on the distribution of meiotic crossover events in humans emerged from the analysis of patterns of genetic inheritance among families focusing on a few specific regions of the genome [23,24]. Later, with genome-wide markers genotyped in families, genetic maps were constructed first at the megabase scale [25,26] and later at higher resolution [4,7,8,27]. High-resolution recombination maps revealed the presence of heterogeneous rates of recombination across the genome and sex-specific genetic map lengths [27,28], whereas molecular characterization of recombination events through single and pooled sperm genotyping led to the identification and characterization of individual hotspots [29–31]. With sperm genotyping, such areas were shown to correspond to clusters of recombination breakpoints spanning 1–2 kb [29].
Linkage disequilibrium (LD), the non-random association of alleles among different loci, is mainly driven by local rates of recombination: the smaller the rate, the higher the covariation of alleles between loci. As distance between pairs of polymorphic sites increases, the probability of a recombination event occurring between them increases, and covariation is reduced. Across the human genome, LD can be mainly described by large blocks of close associations that are intermittently broken [30,32]. Population genetic-based statistical methods [7,33–37] exploiting distributions of associations allow for the characterization of recombination rate variation at relatively fine resolution. For instance, LD-based analyses confirmed that meiotic crossovers are non-randomly distributed in the human genome, with the largest number of them occurring only in 10% of the genome and preferentially not within genes [7]. Furthermore, LD-based methods led to the identification of thousands of recombination hotspots genome-wide, whose location is associated with the distribution of a degenerate 13-mer sequence motif [8,38] crucial in recruiting crossover activity in at least 40% of the human recombination hotspots, regardless of the population ancestry background and sex, and associated with genomic instability and disease-causing breakpoints [38]. The 13-mer motif was inferred to be the binding site of a zinc-finger (ZnF) protein [38]—the PR domain-containing 9 (PRDM9), a histone methyltransferase [9–11]. Molecular experiments and bioinformatics analyses confirmed the binding affinity of multiple PRDM9 ZnF alleles to specific motifs including the previously identified degenerate hotspot motif [9]. Polymorphisms in the ZnF domain of PRDM9 were shown to be associated with alternative sequence motifs and underlie differences in the location of hotspots in human populations [39]. The epigenetic modification H3K4me3 (the tri-methylation of histone H3 on lysine 4) has been reported to mark the activity of meiotic recombination hotspots [40], and recent studies in mice [41] and human cell lines [42] have also associated recombination initiation sites with the H3K36me3 (the tri-methylation of histone H3 on lysine 36) mark. Interestingly, PRDM9 was found to catalyse both of these epigenetic marks [41–43]. Together, these findings indicate that the binding sites of PRDM9 specify the genome-wide location of hotspots and control the distribution of recombination events, probably by promoting recombination initiation through DSBs [44].
In chimpanzees, the PRDM9 homologue does not recognize the human PRDM9 13-mer sequence binding motif, while appearing to bind to different sequence motifs [10]. Furthermore, the PRDM9 ZnF array appears to have diverged more from the human counterpart than any other homologous ZnF protein [10]. These observations are consistent with the hypothesis that recombination hotspot locations are evolving at a rapid rate [8,45,46], explaining the differences in recombination hotspot distributions between humans and chimpanzees [47–50] and differences between current (sperm genotyping) and historical (LD-based) hotspot locations [29,45,51]. The rapid evolution of PRDM9 may be driven by the quick turnover of DNA motifs at sites of recombination [10]. During DSB repair at heterozygous sites, the homologous chromosome containing the non-recombinogenic allele will be used to repair the broken DNA sequence, a mechanism known as gene conversion, and will drive the extinction of the PRDM9 binding motif [51,52]. As the extinction of hotspots challenges requirements for proper chromosomal segregation in meiosis, new PRDM9 variants may emerge that are able to recognize new motifs and thus ensure the proper functioning of recombination. PRDM9 includes a ZnF minisatellite-like structure vulnerable to sequence rearrangements during DNA replication and likely to explain high levels of polymorphism of the PRDM9 ZnF array [10]. Additionally, single-nucleotide polymorphisms within PRDM9 concentrate at the DNA-binding amino acids of the array not only in humans but also in primates and rodents [53]. Together, the evidence suggests that high mutation rate and positive selection may jointly contribute to fast rates of evolution at PRDM9. A ‘Red-Queen’ dynamic has been proposed to model the motif erosion, via biased gene conversion, and the fast generation of new forms of PRDM9 to recognize new binding sites and thus maintain chromosomal crossover rates has been suggested to follow [46,54–57]. Under such a model of evolution, the 13-mer sequence motif, the current target of the most common human allele, might be condemned to extinction in the next 3 million years [55]. Interestingly, while PRDM9 plays a unique role in rapidly changing the location of recombination hotspots in primates and rodents, taxonomic groups lacking PRDM9 homologues appear to have conserved stable recombination landscapes [46,58,59].
Importantly, PRDM9 has been implicated in infertility due to abnormal placement of meiotic DSBs and early pachytene arrest and is the first (and only) ‘speciation’ gene to be described in vertebrates. Although PRDM9 knockouts in mice cause sterility in both sexes [43], allelic incompatibility between Mus musculus musculus and Mus musculus domesticus [60] causes hybrid sterility only in males. Recent findings have established the connection between hybrid male sterility, allelic variation at PRDM9 and chromosomal asynapsis during meiosis [61–63]. A compelling model for the mechanistic basis of hybrid sterility and chromosomal asynapsis was proposed, based on analyses of DSB maps in transgenic mice [63]. The erosion of PRDM9 motifs leads to asymmetric PRDM9 binding in hybrids, which is associated with high asynapsis rates in pachytene and downregulation of autosomal genes [64], potentially leading to major meiotic defects and sterility. It is therefore plausible that these newly discovered mechanisms affect levels of hybrid fertility in other mammalian species with PRDM9, and possibly play a broader role in speciation.
(b). Meiosis, recombination and infertility
Meiosis is a complex developmental process of two cell divisions, transforming one diploid cell into four haploid cells. The first division (meiosis I) is characterized by an extended prophase, which includes steps governing the movement and organization of meiotic chromosomes. Our understanding of the genetic control of meiosis comes from different experimental systems [65], with genomic and functional information defining a ‘core meiotic recombination machinery’ that exhibits strong conservation across eukaryotes [66]. Successful meiosis completion depends upon proper positioning of crossover events between paired homologues that provide temporary connections between homologues, called chiasmata. Chiasma formation is well described by the Szostak model [67], which predicts that the central intermediate of crossover formation is a four-way DNA junction structure, known as double Holliday junction, that physically connects the two recombining DNA molecules and allows them to orient and segregate towards opposite poles of the spindle in metaphase I [68]. The synaptonemal complex spans the gap between paired chromosomes during meiosis and may regulate chromosome-wide crossover distribution [69]. Errors in meiotic recombination are often a source of harmful mutations, aberrant chromosomes and defective gametes, with important clinical consequences.
Severe genetic defects in prophase I key players generally lead to infertility owing to gametocyte apoptosis. For instance, in mice, null alleles in genes involved in chromosome synapsis (e.g. SMC1, REC8, SYCP2 and SYCP3) and DSB repair result in elevated aneuploidy rates by meiotic arrest, highly reduced fertility or even infertility [70–74]. Also in mice, sensitivity to meiotic disruption is often sexually dimorphic. Some genetic defects affecting prophase I progression will lead to sterile males as a result of apoptosis of spermatocytes, whereas females remain fully fertile or subfertile (e.g. FKBP6, PRDM9). In other cases, meiotic progression stops at different stages in females and males, revealing distinct molecular functions of key meiotic players, or altered checkpoints on recombination-linked phenotypes in the two sexes (SPO11, RAD51C) [75,76]. Whether the sexually dimorphic nature of meiotic genes involved in both recombination and infertility is recapitulated in humans is unclear, but important sex differences in recombination rates are widely established [27,28,77–79]. For instance, genome-wide crossover rates in humans correlate with polymorphisms in RNF212 [80], with haplotypes increasing recombination rate in one sex associated with reduced recombination rate in the other [81].
In humans, infertility is a relatively common problem but infertility-causing mutations in meiotic genes have remained largely elusive, with the exception of SPO11, SYCP3, PRDM9 and CDK2 mutations [82–86]. Even when novel associations are reported, the identification of causative polymorphisms and mechanisms remains problematic. However, a CRISPR/Cas9 genome editing strategy has been successful in modelling putatively deleterious variants in mouse orthologues of human fertility genes [85]. Alternatively, generating primordial germ cells using induced pluripotent stem cells from infertile patients is likely to provide valuable in vitro genetic models to improve our understanding of meiotic mechanisms causing infertility in humans [87].
3. Meiotic and mitotic genomic disorders
(a). Aberrant recombination promotes genomic instability
In humans, altered meiotic recombination is associated with large structural rearrangements, aneuploidies and infertility. These instabilities are mostly caused by disturbances at different steps of the molecular process as briefly summarized in table 1. Indeed, altered meiotic recombination is the first correlate associated with abnormal chromosome segregation occurring in at least 5% of clinically recognized human pregnancies, making aneuploidy the leading cause of pregnancy loss [88]. More than 20% of human oocytes are estimated to be aneuploid, compared to only 2% of spermatocytes [88] even though human males have lower recombination rates, highlighting a dramatic difference between female and male regulation of chromosome segregation. The molecular factors of this sexually dimorphic error-prone process remain largely unknown, except for disturbances in crossover pathways, which are associated with non-disjunction. In humans, a significant reduction in the number of crossover events is a feature of all trisomies studied [88] and suboptimally positioned chiasmata are frequently observed, such that exchanges occurring too close to the centromere, as well as too far, are risk factors for non-disjunction [104–108].
Table 1.
Diseases associated with dysfunctional recombination mechanisms.
| recombination mechanism implicated | type of disorder |
|---|---|
| chromosome synapsis and recombination initiation | infertility [71,72,74] |
| maintenance of physical connections between chromosomes Holliday junction resolution |
aneuploidies [88]: trisomy 13, 15, 16, 18, 21 mosaic variegated aneuploidy |
| non-allelic homologous recombination | genomic disorders [89]: (e.g. Charcot–Marie–Tooth disease type 1A, neurofibromatosis type 1, Williams–Beuren syndrome, Smith–Magenis syndrome, hereditary neuropathy with liability to pressure palsies, DiGeorge syndrome, Prader–Willi syndrome, childhood spinal muscular atrophy, 17q21.31 microdel syndrome, etc.) |
| mitotic non-allelic homologous recombination | autism, 8p23.1 deletion, 16p11.2 deletion, 17q11.2 deletion [90], neurofibromin-1 [91] |
| Holliday junction resolution | Fanconi anaemia [92], squamous cell carcinomas [93] |
| illegitimate immunoglobulin recombination | severe combined immunodeficiencies [94] |
| recognition of double-strand breaks | ataxia telangiectasia [95] |
| Nijmegen's breakage syndrome [96] | |
| excessive homologous recombination | Bloom syndrome [97] |
| DNA repair by homologous recombination acquired chromosomal translocations |
cancers [98] |
| chromoplexis, chromothripsis | cancers, congenital disorders [97,99,100,101] |
| acquired chromosomal translocations illegitimate immunoglobulin recombination |
leukaemias [102] and lymphomas [103] |
Furthermore, aneuploidy rates increase with age in females. This ‘maternal age effect’ is particularly pronounced: under the age of 25, a woman has a 2% chance of having a trisomic pregnancy, but over the age of 40, this chance rises to 35%. This effect is thought to be due to age-related insults to the meiotic system at each stage of the oocyte development [109]. A number of studies have analysed different cohorts to determine if there is a similar age-related relationship with recombination [110–114], with either positive, negative or no relationship of chiasma number and age being observed. Nonetheless, the effect sizes and variance explained by age on chiasma frequency are either small or insignificant genome-wide, and recombination alone is unlikely to be responsible for maternal age effects on aneuploidies.
Aberrant gametogenesis leading to recurrent structural genetic abnormalities is a major cause of congenital birth defects. DSBs at sites of recombination will sometimes be aberrantly repaired with non-homologous loci, in a process called non-allelic homologous recombination (NAHR, table 1), which results in structural rearrangements. In most cases, rearrangements are flanked by low copy repeats that typically share sequence similarity greater than 98%. Generally, repeated DNA sequences play an important role in mediating disease-causing recombination errors. Pairing and homologous recombination between misaligned repetitive elements have been observed at rearrangement breakpoints related to disease and are thought to be the main mechanism of NAHR [89]. NAHR can result in chromosomal inversions and translocations or in local duplications and deletions. These rearrangements are likely to dramatically disrupt genes, possibly creating fusion genes, and are for the most part deleterious. Genomic disorders associated with NAHR include: Charcot–Marie–Tooth disease type 1A, neurofibromatosis type 1, Williams–Beuren syndrome, Smith–Magenis syndrome, hereditary neuropathy with liability to pressure palsies, DiGeorge syndrome, Prader–Willi syndrome, childhood spinal muscular atrophy and the 17q21.31 microdeletion syndrome (table 1). Many of them result from megabase-scale duplications, as in Charcot–Marie–Tooth disease [115], or deletions, as in Smith–Magenis, Williams–Beuren, DiGeorge and Prader–Willi syndromes. Disease-causing and other NAHR breakpoints are not distributed evenly along the low copy repeats and cluster in narrow hotspots [116] that are often found at strikingly similar positions to those of hotspots resulting from allelic recombination [117,118]. Furthermore, NAHR hotspots and recombination hotspots share similar properties of distribution of strand exchange [118], suggesting that they are functionally related. Many lines of evidence also suggested that PRDM9 variation correlates with instability in minisatellite repeats [38] and with recurrent pathological rearrangements, such as 17p11.2 deletions/duplication events [119] and 7q11.23 microdeletions [120]. Recurrent duplications or deletions at 17p11.2 are implicated in Charcot–Marie–Tooth disease and hereditary neuropathy with liability to pressure palsies, whereas 7q11.23 microdeletions cause Williams–Beuren syndrome. PRDM9 thus appears to be involved in meiotic instabilities leading to genomic disorders.
There appears to be a sex-dependent component to some rearrangements, which do not arise at the same frequencies in paternal and maternal meioses. For example, the duplication or deletion at 17p11.2, associated with Charcot–Marie–Tooth disease or hereditary neuropathy with liability to pressure palsies, respectively, arises from two distinct sex-dependent mechanisms [121]. Most de novo rearrangements are from paternal origin and arise by NAHR between the two chromosome 17 homologues, whereas the rare rearrangements of maternal origin result from an intra-chromosomal process. Interestingly, this region of chromosome 17 appears to have higher recombination rates in females than in males, suggesting that oogenesis may afford greater protection from misalignment during synapsis, or that male-specific factors may operate during spermatogenesis to help stabilize the rearrangements. Alternatively, sex-specific differences might reflect different selection bias against the rearranged alleles in male and female germ lines. Differences in NAHR frequency between male and female were also found at other loci, with childhood spinal muscular atrophy deletions originating mainly in spermatogenesis [122], whereas 80% of de novo neurofibromatosis type 1 deletions are of maternal origin [123].
(b). Mitotic homologous recombination and disease
Parallels appear to exist between meiotic recombination and tumorigenesis in somatic cells: in addition to the role of homologous recombination in promoting genomic stability by repairing DSBs in cells undergoing mitosis [124,125] and the overlapping molecular machinery involved [98,126,127], aberrant expression of proteins exclusively expressed in healthy adult testis, and associated with meiosis-specific functions, has recently been observed in tumours originating from non-germline tissues [128]. Moreover, PRDM9 and the inter-sister chromatid cohesion protein RAD21 L were found to be expressed in some cancer cell lines [129]. Owing to the meiotic recombination-specific functions of these proteins, it has been hypothesized that they might interfere with mitotic genome regulation [128].
Genome integrity in mitotic cells greatly relies on recombination, required for accurate repair of DSBs incurred either by exogenous (e.g. ultraviolet light) or endogenous processes (e.g. damage incurred during replication) during the life of the cell [130]. Owing to the essential role of mitotic recombination in genome integrity, the dysregulation of molecular mechanisms involved can often lead to diseases, including cancer (table 1). Research on the effect of mitotic recombination on the progression of tumorigenesis has largely focused on understanding the impact of loss of function mutations in tumour suppressor genes that are part of the homologous recombination repair (HRR) pathway, such as those included in the recognition of the DSB by the MRE11A-NBS1-RAD50 complex, the DNA resection guided by BRCA1 or the location of the recombinase RAD51 by BRCA2 [98,131,132]. The loss of function mutations in genes involved in the HRR pathway often lead to its inactivation, rendering DSB repair entirely dependent on the alternative non-homologous end-joining pathway. The non-homologous end-joining pathway does not involve homologous sequences as a template for repair, resulting in small insertions and deletions at the breakpoint locations [133] and therefore leaves a distinct mutational signature, characterized by increased genomic rearrangements and small indels, in tumours with HRR-pathway inactivation [134]. Capturing the genomic signature underlying the inactivation of the HRR pathway is important in cancer research, given the therapeutic success of poly(ADP-ribose) polymerase inhibitors that target HRR-pathway-deficient tumours [135,136]. By preventing genomic rearrangements through the accurate repair of highly damaging DSBs, mitotic recombination processes are essential for ensuring genomic stability, although recombination processes may also be mutagenic when they go awry.
Mutagenic mitotic recombination events can mainly be identified through: (i) structural variation generated by NAHR or (ii) loss of heterozygosity (LOH) driven by biased gene conversion (figure 1). Structural variation detection algorithms can be used naively to detect NAHR events [141]. However, because NAHR often involves repetitive loci sharing a high homology, structural variation algorithms identify NAHR events with high error rates. Parks et al. [141] developed a Bayesian probabilistic model improving the detection of NAHR from sequencing data by focusing on regions prone to NAHR through their repetitive nature, which complements structural variation detection algorithms by enhancing their detection. Chromosomal microarray analysis of a cohort of 25 144 disease patients has recently catalogued NAHR-mediated copy number variants among numerous diseases (table 1) [90]. Furthermore, mitotic NAHR, found in a mosaic pattern across cells, appears to be associated with diseases involving genes with a large number of repeats such as the neurofibromin-1 loci [91] (table 1), further involving the mutagenicity of mitotic recombination to disease.
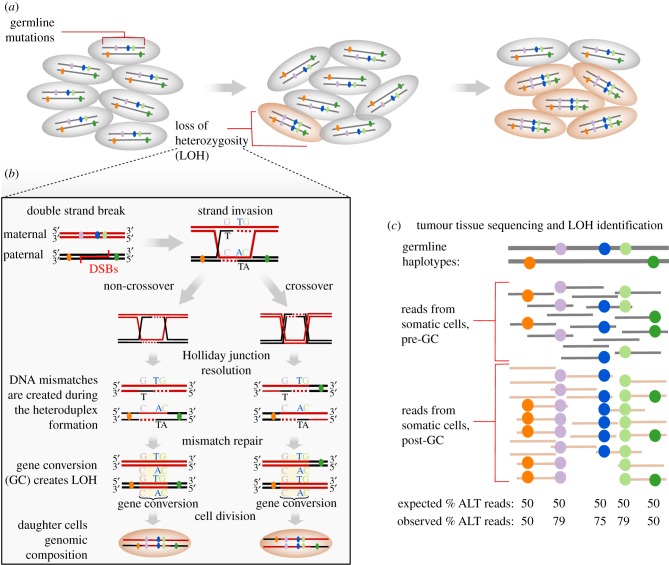
Figure 1.
Gene conversion leading to LOH events in tumour cells. (a) A potential tumour cell that has incurred a LOH will increase in frequency as a subpopulation within a tissue or tumour. (b) A cell repairing a DSB mediated via a crossover or non-crossover, giving rise to two daughter cells exhibiting LOH. The examples shown correspond to a model in which the DSB repair pathway is followed by the resolution of a double Holliday junction, although other mechanisms may lead to gene conversion (as described in [137–139]). Mismatched bases may originate via DNA synthesis when the sister chromatid is used as a template for repair, resulting in a non-reciprocal exchange between both DNA strands [140]. LOH occurs during gene conversion when germline variants are heterozygous (as shown). If homologous recombination occurs during a two-homologue chromatid invasion, as is more often the case, only a non-crossover model leads to LOH, whereas with a four chromatid invasion, crossovers and non-crossovers lead to LOH only when recombinant chromatids segregate to the same daughter cell. (c) LOH events can be captured by counting the number of sequencing reads from tumour samples carrying the alternative alleles at heterozygous sites identified in germline (or healthy tissue). Nevertheless, challenges are associated with the detection of LOH events from sequencing data. Owing to cell mixture in the tumour, the signal for LOH events can be lost, and may not distinguishable from sequencing errors and mapping bias effects.
Mitotic-biased gene conversion is generally thought to be associated with tumorigenesis, as local LOH events are frequent among tumour genomes [142,143]. Notably, LOH of the wild-type allele of tumour suppressors is frequently and recurrently observed. The detection of LOH is possible through sequencing and identification of heterozygous loci in the germline genome that are homozygous in the tumour genome (figure 1c) [137]. Traditionally, LOH events were captured by detecting karyotypic changes [144], while newer methods leverage read counts from high throughput sequences [145] and SNP array information [143]. Mitotic-biased gene conversion events leading to LOH are identified once they have reached fixation in the tumour population due to their high selective advantage on tumour growth, whereas passenger alleles generated by mitotic-biased gene conversion with neutral effects will be much less likely to be found at high frequency across tumour cells. Some LOH events likely originated owing to general genome instability, making it challenging to distinguish which LOH events are associated with causing genomic instability, and which are a consequence of it. Nonetheless, LOH inference has been used to identify candidate cancer drivers, as it is likely that the loss of an allele throughout cancer clonal populations confers a selective advantage for cancer progression, such as the loss of function of wild-type alleles of MLH1/MSH2 [146].
Finally, some genomic regions are prone to mitotic recombination events, including Alu transposable elements, which are additionally found neighbouring leukaemia translocation events [147], suggesting a link of the mutagenic effect of mitotic recombination to leukaemogenesis [103].
4. Evolution and functional impact of sex and recombination
(a). Evolutionary advantages of recombination
Fisher [12] and Muller [13] proposed that sexual reproduction and recombination are evolutionarily advantageous as they accelerate the rate of fixation of beneficial mutations and thereby the rate of adaptation by bringing beneficial alleles that arise initially on different genomic backgrounds together on the same chromosome. Later, when assuming that most mutations are deleterious, Muller proposed that in the absence of recombination, disadvantageous mutations accumulate in an irreversible manner such that a mutation-free state can never be recovered (reverse mutations are rare), the so-called Muller's ratchet effect [14]. These initial theoretical models were extended by the introduction of additional assumptions on the role of random drift, selection and linkage. Such developments led to the emergence of concepts like Hill–Robertson (HR) interference, which postulates that in the presence of drift and linkage, linked loci subject to selection will interfere with each other's allelic frequency trajectories over time [15,17]. Allele frequencies at one site will not only depend on drift and their evolutionary fitness but will also depend on the fitness of linked genotypes. The persistence of associations between loci generated by HR interference depends on the recombination rate between them: the smaller the recombination rate, the longer these associations will last.
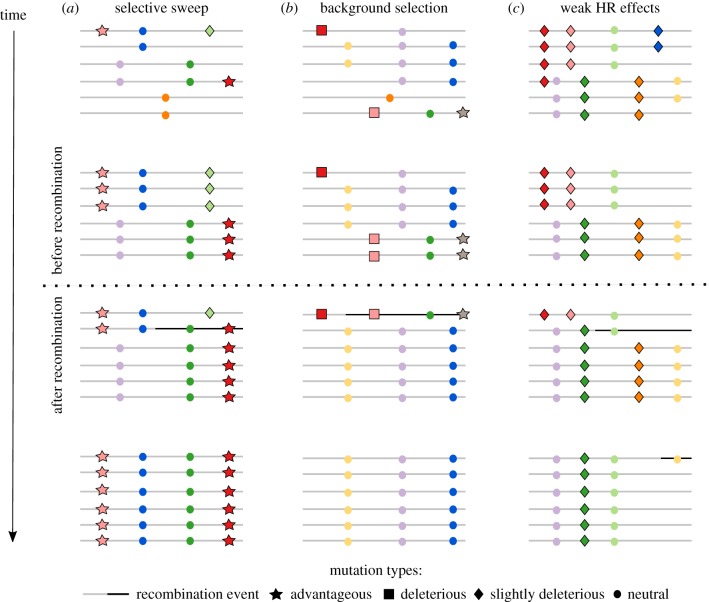
Selection on beneficial mutations drives target mutations to fixation, increasing LD and decreasing neutral diversity in linked surrounding regions (figure 2a), commonly referred to as selective sweeps. Positive selection was thought to be responsible for genetic diversity troughs and elevated population differentiation in or close to genic regions [149] as well as in regions of low recombination [150]. However, complete selective sweep signatures across the genome were found to be scarce [151] and instead, low levels of diversity in regions of low recombination are more likely to have been generated by background selection against deleterious mutations [152–154] (figure 2b). Under a scenario of positive selection and in the presence of HR interference [17,148,155], slightly deleterious mutations may reach high frequencies when neighbouring the target of positive selection (green diamond, figure 2a). In the case of background selection with HR interference, advantageous mutations arising on a chromosome carrying several deleterious mutations will have a lower, if not null, chance of spreading in the population (grey star, figure 2b). Moreover, in the presence of HR interference, selection against slightly deleterious linked mutations will interfere with the elimination of neighbouring harmful alleles of small effect onto alternative haplotypes [156]. The impact of HR interference on the effectiveness of selection can be described as a process associated with a reduction in effective population size at a locus under selection and surrounding loci, reducing variation and selection efficacy. Recombination has the potential to elevate effective population size locally by recombining selected alleles onto other backgrounds, making natural selection more efficient.
Figure 2.
Schematic of HR interference under alternative selection regimes: (a) advantageous mutations arise on different backgrounds (haplotypes), interfere with each other and prevent each other's fixation. Linked neutral and slightly deleterious variants will increase in frequency until recombination generates new haplotypes, which drive beneficial mutations (now in the same haplotype) quickly to fixation while purging slightly deleterious alleles. (b) Deleterious alleles enter the population on different haplotypes. Owing to drift and/or to interference with selective advantageous mutations, they remain at low frequencies in the population until recombination generates a new haplotype resulting from the combination of the two deleterious alleles. Selection will remove this new haplotype more efficiently. However, an advantageous mutation will be lost, given that there was not enough time for recombination to break its association with a deleterious background. (c) Negative selection on multiple linked slightly deleterious mutations (referred to as a weak HR effect in Charlesworth et al. [148])—owing to the limited burden carried by such mutations, slightly deleterious variants tend to remain and accumulate in populations. Haplotypes that carry a larger mutational burden can be successively removed from the population. Interference occurs when there is little to no recombination, and selection at other loci on different haplotypes reduces the effective population size, impacting the rate at which they are lost from the population by making it more difficult to remove haplotypes that carry these deleterious mutations. Recombination combines chromosomes to create haplotypes that are free of or are loaded with deleterious mutations, increasing the efficacy of selection. Examples of mutations along the chromosomes (grey lines) are represented by different colours.
(b). Impact of recombination on genomic diversity and mutation load in humans
Exome variation among human populations has revealed that humans carry a surprisingly large number of potentially damaging or disease-causing mutations [157–159]. Understanding why mutational burden persists requires understanding the role of population demographic and recombination history in the accrual of deleterious mutations. Several studies have shown that smaller human populations harbour relatively more damaging functional variation, relative to the number of neutral variants, when compared with their larger progenitor populations [157,160,161]. Elevated mutational burden in small populations may be caused by either inbreeding or decreased effectiveness of selection in removing potentially damaging mutations. While studies disagree with respect to the existence of significant differences in total mutation load among populations with different demographic histories [162,163], none of the above studies evaluated the impact of genomic heterogeneity in recombination frequencies on mutation load. More recently, by comparing patterns of accumulation of putative damaging mutations across regions with low (coldspots) and high (hotspots) rates of recombination, Hussin et al. [164] showed that coding regions with low rates of crossing over harbour relatively larger amounts of potentially damaging mutations than highly recombining regions, consistent with reduced efficacy of purifying selection in purging harmful variation in coldspots. Furthermore, the efficiency of purifying selection was found to lessen as the number of alleles being selected against on the same haplotype increases, consistent with HR interference, with this effect being amplified as the effective population size becomes smaller (figure 2c).
While recombination rates have been found to shape diversity along the human genome mainly by increasing the efficacy of natural selection [164–166], evidence exists for local impact of recombination on sequence evolution via GC-biased gene conversion [46,138,167,168]. GC-biased gene conversion results in the non-reciprocal transmission of genomic content during the recombination process and increases the transmission probability of GC alleles over AT. The biased transmission of GC alleles may ultimately cause the local fixation of GC alleles in hotspots [169], likely contributing to the human mutation load [138,170]. Although genome-wide GC-biased gene conversion is a relatively weak evolutionary force [166,171], this process may lead to an increase in disease burden when recessive derived alleles have a higher chance of transmission due to GC-biased gene conversion [171]. In addition, it has been suggested that GC-biased gene conversion has evolved to compensate for the mutational burden directly associated with high mutation rates caused by the deamination of methylated cytosines at recombination hotspots [172].
5. Conclusion and future perspectives
In the past decade, our understanding of meiosis and the molecular regulation of recombination has greatly improved [68,172]. We have learned that the genomic location of DSBs, which promote meiotic recombination initiation, is non-randomly distributed and controlled by genetic and epigenetic factors such as PRDM9 [7,8,10,43,173–175] and potential interactors [46,176,177]. While the processes catalysed by PRDM9 binding are important, it is equally critical that we characterize the molecular factors contributing to the initial recruitment of PRDM9 to its binding sites (see the review by Tiemann-Boege et al. in this issue [46]). Differences in the intensity of DSB hotspots are only partially explained by genetic variation at PRDM9 binding sites [44]. This suggests that not all potential PRDM9 binding sites will incur a DSB and initiate the process of recombination, with recent evidence for additional levels of regulation implicating KRAB-ZNF genes in meiotic recombination suppression [178]. Local chromatin state influences the binding of PRDM9, but it remains to be investigated how both wider sequence context and chromatin accessibility are associated with differences in the intensity of DSB hotspots in individuals carrying the same PRDM9 alleles. An intriguing hypothesis is that fertility may be influenced by the specific allele one carries, if it positively affects the expression of important meiotic genes, such as CTCFL [178]. Furthermore, while some missense PRDM9 mutations have been associated with infertility in human males [84,86], a loss of function variant of PRDM9 in a fertile human female has recently been observed [179], raising the possible existence of sexual dimorphism in recombination-associated infertility in our species. Together this suggests that in humans, PRDM9 might not be imperative for the correct functioning of recombination processes, as compensatory factors may exist, at least in human females.
Despite such advances, key steps of the mammalian meiotic programme are weakly understood, because meiosis remains challenging to study due to the lack of appropriate in vitro models. For example, cytological techniques require fetal ovarian tissue or testicular biopsies, but more importantly, these methods cannot be used for high-resolution analyses of DSBs and crossovers (see [180] for exception). On the other hand, sperm genotyping assays, which rely on PCR amplification of DNA from single-sperm and pooled-sperm, can examine thousands of meioses from a single individual at resolutions of less than 0.5 kb [181], the trade-off being that it remains technically challenging to study genomic regions larger than 300 kb at high resolution.
More recently, novel techniques have been developed to facilitate genome-wide identification of epigenetic marks, sites of recombination and nucleosome organization in meiosis [41,46,181–184]. These approaches generally map DSB sites directly and while not directly mapping crossovers, DSB formation is the prelude to recombination. One of these assays, using chromatin immunoprecipitation followed by sequencing (ChIP-seq) and sensitive detection of single-stranded DNA, revealed that PRDM9 is not required for DSBs to occur in mice, but rather, moves them away from H3K4me3-marked promoter sites [185]. Furthermore, H3K36me3 and H3K4me3 ChIP-seq data in spermatocytes show that PRDM9 is able to place the two epigenetic marks on the same histone molecule in vivo, a signature that is exclusive to recombination hotspots [41]. Finally, a newly developed nucleotide-resolution technique, which sequences short oligonucleotides covalently bound to SPO11, provides detailed description of DSB hotspots, locating them among methylated nucleosomes, and has highlighted the importance of the ATM kinase in shaping sex-chromosome and the autosomal DSB landscape [186]. Application of these new techniques has so far been limited to studying male recombination, but new approaches are emerging to study female meiosis directly, and to provide precise information about meiosis in human oocytes. For example, it is now possible to generate genome-wide maps of crossovers and chromosome segregation patterns by recovering all three products of a single female meiosis, namely the two polar bodies and the activated oocyte, allowing the analysis of human tetrads [185,187,188]. Similarly, genomic analyses of single human oocytes using the polar bodies and recovering the female pronucleus from zygotes can be performed with multiple annealing and looping-based amplification cycle-based sequencing technology [189]. These technologies supported the generation of genome-wide oocytes' crossover maps and offer improved detection of chromosome abnormalities.
Finally, our understanding of the impact of GC-biased gene conversion, meiotic drive and recombination-related mutagenicity, beyond large-scale chromosomal rearrangements, on individual mutational burden may benefit considerably from single-cell sequencing by allowing the measurement of de novo mutations in the germline [189–191]. Single-cell sequencing of the gamete transcriptomes would specifically allow a better understanding of the repeat instability occurring in the PRDM9 ZnF coding sequence [192], and the rate at which new alleles of PRDM9 are generated. As the costs of single-cell sequencing technologies decrease, we will be able to dissect complex and heterogeneous gametocyte populations, which will shed light on the extent to which individual-specific hotspots differ from the expected hotspot distribution and how these exceptions impact human health.
Data accessibility
This article has no additional data.
Competing interests
We have no competing interests.
Funding
A.A.H. is supported by an Ontario Graduate Scholarship. J.G.H. is an EPAC Junior Research Fellow at Linacre College, Oxford. I.A. and P.A. are supported by a Ministry of Research and Innovation (Ontario) Senior Investigator Award (Awadalla).
References
- 1.Roach JC, et al. 2010. Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science 328, 636–639. ( 10.1126/science.1186802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awadalla P, et al. 2010. Direct measure of the de novo mutation rate in autism and schizophrenia cohorts. Am. J. Hum. Genet. 87, 316–324. ( 10.1016/j.ajhg.2010.07.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conrad DF, et al. 2011. Variation in genome-wide mutation rates within and between human families. Nat. Genet. 43, 712–714. ( 10.1038/ng.862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong A, et al. 2012. Rate of de novo mutations and the importance of father's age to disease risk. Nature 488, 471–475. ( 10.1038/nature11396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The International HapMap Consortium. 2005. A haplotype map of the human genome. Nature 437, 1299–1320. ( 10.1038/nature04226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frazer KA, et al. 2007. A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861. ( 10.1038/nature06258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McVean G, Myers SRR, Hunt S, Deloukas P, Bentley DRR, Donnelly P. 2004. The fine-scale structure of recombination rate variation in the human genome. Science 304, 581–584. ( 10.1126/science.1092500) [DOI] [PubMed] [Google Scholar]
- 8.Myers S, Bottolo L, Freeman C, McVean G, Donnelly P. 2005. A fine-scale map of recombination rates and hotspots across the human genome. Science 310, 321–324. ( 10.1126/science.1117196) [DOI] [PubMed] [Google Scholar]
- 9.Baudat F, Buard J, Grey C, Fledel-Alon A, Ober C, Przeworski M, Coop G, de Massy B. 2010. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science 327, 836–840. ( 10.1126/science.1183439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers S, Bowden R, Tumian A, Bontrop REE, Freeman C, MacFie TSS, McVean G, Donnelly P. 2010. Drive against hotspot motifs in primates implicates the PRDM9 gene in meiotic recombination. Science 327, 876–879. ( 10.1126/science.1182363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parvanov EDD, Petkov PMM, Paigen K. 2010. Prdm9 controls activation of mammalian recombination hotspots. Science 327, 835 ( 10.1126/science.1181495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher RA. 1930. The genetical theory of natural selection: a complete variorum edition. Oxford, UK: Oxford University Press. [Google Scholar]
- 13.Muller HHJ. 1932. Some genetic aspects of sex. Am. Nat. 66, 118–138. ( 10.1086/280418) [DOI] [Google Scholar]
- 14.Muller HJ. 1964. The relation of recombination to mutational advance. Mutat. Res. Mol. Mech. Mutagen. 1, 2–9. ( 10.1016/0027-5107(64)90047-8) [DOI] [PubMed] [Google Scholar]
- 15.Hill WG, Robertson A. 1966. The effect of linkage on limits to artificial selection. Genet. Res. 8, 269–294. ( 10.1017/S0016672300010156) [DOI] [PubMed] [Google Scholar]
- 16.Hill WG, Robertson A. 1968. Linkage disequilibrium in finite populations. Theor. Appl. Genet. 38, 226–231. ( 10.1007/BF01245622) [DOI] [PubMed] [Google Scholar]
- 17.Felsenstein J. 1974. The evolutionary advantage of recombination. Genetics 78, 737–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker BS, Carpenter ATC, Esposito MS, Esposito RE, Sandler L. 1976. The genetic control of meiosis. Annu. Rev. Genet. 10, 53–134. ( 10.1146/annurev.ge.10.120176.000413) [DOI] [PubMed] [Google Scholar]
- 19.Koehler KE, Hawley RS, Sherman S, Hassold T. 1996. Recombination and nondisjunction in humans and flies. Hum. Mol. Genet. 5(Suppl. 1), 1495–1504. ( 10.1093/hmg/5.Supplement_1.1495) [DOI] [PubMed] [Google Scholar]
- 20.Baudat F, de Massy B. 2007. Regulating double-stranded DNA break repair towards crossover or non-crossover during mammalian meiosis. Chromosom. Res. 15, 565–577. ( 10.1007/s10577-007-1140-3) [DOI] [PubMed] [Google Scholar]
- 21.Bernstein H, Byerly HC, Hopf FA, Michod RE. 1985. Genetic damage, mutation, and the evolution of sex. Science 229, 1277–1281. ( 10.1126/science.3898363) [DOI] [PubMed] [Google Scholar]
- 22.Bernstein H, Byers GS, Michod RE. 1981. Evolution of sexual reproduction: importance of DNA repair, complementation, and variation. Am. Nat. 117, 537–549. ( 10.1086/283734) [DOI] [Google Scholar]
- 23.Chakravarti A, Buetow KH, Antonarakis SE, Waber PG, Boehm CD, Kazazian HH. 1984. Nonuniform recombination within the human beta-globin gene cluster. Am. J. Hum. Genet. 36, 1239–1258. [PMC free article] [PubMed] [Google Scholar]
- 24.Chakravarti A, Elbein SC, Permutt MA. 1986. Evidence for increased recombination near the human insulin gene: implication for disease association studies. Proc. Natl Acad. Sci. USA 83, 1045–1049. ( 10.1073/pnas.83.4.1045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dib C, et al. 1996. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380, 152–154. ( 10.1038/380152a0) [DOI] [PubMed] [Google Scholar]
- 26.Murray JC, et al. 1994. A comprehensive human linkage map with centimorgan density. Cooperative Human Linkage Center (CHLC). Science 265, 2049–2054. ( 10.1126/science.8091227) [DOI] [PubMed] [Google Scholar]
- 27.Broman KWW, Murray JCC, Sheffield VCC, White RLL, Weber JLL. 1998. Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am. J. Hum. Genet. 63, 861–869. ( 10.1086/302011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong A, et al. 2002. A high-resolution recombination map of the human genome. Nat. Genet. 31, 241–247. ( 10.1038/ng917) [DOI] [PubMed] [Google Scholar]
- 29.Jeffreys AJ, Ritchie A, Neumann R. 2000. High resolution analysis of haplotype diversity and meiotic crossover in the human TAP2 recombination hotspot. Hum. Mol. Genet. 9, 725–733. ( 10.1093/hmg/9.5.725) [DOI] [PubMed] [Google Scholar]
- 30.Jeffreys AJ, Kauppi L, Neumann R. 2001. Intensely punctate meiotic recombination in the class II region of the major histocompatibility complex. Nat. Genet. 29, 217–222. ( 10.1038/ng1001-217) [DOI] [PubMed] [Google Scholar]
- 31.May CA, Shone AC, Kalaydjieva L, Sajantila A, Jeffreys AJ. 2002. Crossover clustering and rapid decay of linkage disequilibrium in the Xp/Yp pseudoautosomal gene SHOX. Nat. Genet. 31, 272–275. ( 10.1038/ng918) [DOI] [PubMed] [Google Scholar]
- 32.Daly MJ, Rioux JD, Schaffner SF, Hudson TJ, Lander ES. 2001. High-resolution haplotype structure in the human genome. Nat. Genet. 29, 229–232. ( 10.1038/ng1001-229) [DOI] [PubMed] [Google Scholar]
- 33.Wall JD. 2000. A comparison of estimators of the population recombination rate. Mol. Biol. Evol. 17, 156–163. ( 10.1093/oxfordjournals.molbev.a026228) [DOI] [PubMed] [Google Scholar]
- 34.Fearnhead P, Donnelly P. 2001. Estimating recombination rates from population genetic data. Genetics 159, 1299–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McVean G, Awadalla P, Fearnhead P. 2002. A coalescent-based method for detecting and estimating recombination from gene sequences. Genetics 160, 1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auton A, McVean G. 2007. Recombination rate estimation in the presence of hotspots. Genome Res. 17, 1219–1227. ( 10.1101/gr.6386707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li N, Stephens M. 2003. Modeling linkage disequilibrium and identifying recombination hotspots using single-nucleotide polymorphism data. Genetics 165, 2213–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers S, Freeman C, Auton A, Donnelly P, McVean G. 2008. A common sequence motif associated with recombination hot spots and genome instability in humans. Nat. Genet. 40, 1124–1129. ( 10.1038/ng.213) [DOI] [PubMed] [Google Scholar]
- 39.Berg IL, Neumann R, Sarbajna S, Odenthal-hesse L, Butler NJ, Jeffreys AJ. 2011. Variants of the protein PRDM9 differentially regulate a set of human meiotic recombination hotspots highly active in African populations. Proc. Natl Acad. Sci. USA 108, 12 378–12 383. ( 10.1073/pnas.1109531108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buard J, Barthès P, Grey C, de Massy B. 2009. Distinct histone modifications define initiation and repair of meiotic recombination in the mouse. EMBO J. 28, 2616–2624. ( 10.1038/emboj.2009.207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powers NR, Parvanov ED, Baker CL, Walker M, Petkov PM, Paigen K. 2016. The meiotic recombination activator PRDM9 trimethylates both H3K36 and H3K4 at recombination hotspots in vivo. PLoS Genet. 12, e1006146 ( 10.1371/journal.pgen.1006146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eram MS, et al. 2014. Trimethylation of histone H3 lysine 36 by human methyltransferase PRDM9 protein. J. Biol. Chem. 289, 12 177–12 188. ( 10.1074/jbc.M113.523183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashi K, Yoshida K, Matsui Y. 2005. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature 438, 374–378. ( 10.1038/nature04112) [DOI] [PubMed] [Google Scholar]
- 44.Pratto F, Brick K, Khil P, Smagulova F, Petukhova GV, Camerini-Otero RD. 2014. Recombination initiation maps of individual human genomes. Science 346, 1256442 ( 10.1126/science.1256442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeffreys AJ, Neumann R, Panayi M, Myers S, Donnelly P. 2005. Human recombination hot spots hidden in regions of strong marker association. Nat. Genet. 37, 601–606. ( 10.1038/ng1565) [DOI] [PubMed] [Google Scholar]
- 46.Tiemann-Boege I, Schwarz T, Striedner Y, Heissl A. 2017. The consequences of sequence erosion in the evolution of recombination hotspots. Phil. Trans. R. Soc. B 372, 20160462 ( 10.1098/rstb.2016.0462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ptak SE, Hinds DA, Koehler K, Nickel B, Patil N, Ballinger DG, Przeworski M, Frazer KA, Pääbo S. 2005. Fine-scale recombination patterns differ between chimpanzees and humans. Nat. Genet. 37, 429–434. ( 10.1038/ng1529) [DOI] [PubMed] [Google Scholar]
- 48.Wall JD, Frisse LA, Hudson RR, Di Rienzo A. 2003. Comparative linkage-disequilibrium analysis of the β-Globin hotspot in primates. Am. J. Hum. Genet. 73, 1330–1340. ( 10.1086/380311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winckler W, et al. 2005. Comparison of fine-scale recombination rates in humans and chimpanzees. Science 308, 107–111. ( 10.1126/science.1105322) [DOI] [PubMed] [Google Scholar]
- 50.Auton A, et al. 2012. A fine-scale chimpanzee genetic map from population sequencing. Science 336, 193–198. ( 10.1126/science.1216872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeffreys AJ, Neumann R. 2002. Reciprocal crossover asymmetry and meiotic drive in a human recombination hot spot. Nat. Genet. 31, 267–271. ( 10.1038/ng910) [DOI] [PubMed] [Google Scholar]
- 52.Coop G, Myers SR. 2007. Live hot, die young: transmission distortion in recombination hotspots. PLoS Genet. 3, e35 ( 10.1371/journal.pgen.0030035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliver PLL, et al. 2009. Accelerated evolution of the Prdm9 speciation gene across diverse metazoan taxa. PLoS Genet. 5, e1000753 ( 10.1371/journal.pgen.1000753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boulton A, Myers RS, Redfield RJ. 1997. The hotspot conversion paradox and the evolution of meiotic recombination. Proc. Natl Acad. Sci. USA 94, 8058–8063. ( 10.1073/pnas.94.15.8058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lesecque Y, Glémin S, Lartillot N, Mouchiroud D, Duret L. 2014. The red queen model of recombination hotspots evolution in the light of archaic and modern human genomes. PLoS Genet. 10, e1004790 ( 10.1371/journal.pgen.1004790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Úbeda F, Wilkins JF. 2011. The Red Queen theory of recombination hotspots. J. Evol. Biol. 24, 541–553. ( 10.1111/j.1420-9101.2010.02187.x) [DOI] [PubMed] [Google Scholar]
- 57.Baker CLL, Kajita S, Walker M, Saxl RLL, Raghupathy N, Choi K, Petkov PM, Paigen K. 2015. PRDM9 drives evolutionary erosion of hotspots in Mus musculus through haplotype-specific initiation of meiotic recombination. PLoS Genet. 11, e1004916 ( 10.1371/journal.pgen.1004916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singhal S, et al. 2015. Stable recombination hotspots in birds. Science 350, 928–932. ( 10.1126/science.aad0843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Auton A, et al. 2013. Genetic recombination is targeted towards gene promoter regions in dogs. PLoS Genet. 9, e1003984 ( 10.1371/journal.pgen.1003984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mihola O, Trachtulec Z, Vlcek C, Schimenti JCJC, Forejt J. 2009. A mouse speciation gene encodes a meiotic histone H3 methyltransferase. Science 323, 373–375. ( 10.1126/science.1163601) [DOI] [PubMed] [Google Scholar]
- 61.Flachs P, et al. 2012. Interallelic and intergenic incompatibilities of the Prdm9 (Hst1) gene in mouse hybrid sterility. PLoS Genet. 8, e1003044 ( 10.1371/journal.pgen.1003044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flachs P, Bhattacharyya T, Mihola O, Piálek J, Forejt J, Trachtulec Z. 2014. Prdm9 incompatibility controls oligospermia and delayed fertility but no selfish transmission in mouse intersubspecific hybrids. PLoS ONE 9, e95806 ( 10.1371/journal.pone.0095806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davies B, et al. 2016. Re-engineering the zinc fingers of PRDM9 reverses hybrid sterility in mice. Nature 530, 171–176. ( 10.1038/nature16931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larson EL, Keeble S, Vanderpool D, Dean MD, Good JM. 2017. The composite regulatory basis of the large x-effect in mouse speciation. Mol. Biol. Evol. 34, 282–295. ( 10.1093/molbev/msw243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Handel MA, Schimenti JC. 2010. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat. Rev. Genet. 11, 124–136. ( 10.1038/nrg2723) [DOI] [PubMed] [Google Scholar]
- 66.Villeneuve AM, Hillers KJ. 2001. Whence meiosis? Cell 106, 647–650. ( 10.1016/S0092-8674(01)00500-1) [DOI] [PubMed] [Google Scholar]
- 67.Szostak JW, Orr-Weaver TL, Rothstein RJ, Stahl FW. 1983. The double-strand-break repair model for recombination. Cell 33, 25–35. ( 10.1016/0092-8674(83)90331-8) [DOI] [PubMed] [Google Scholar]
- 68.Zickler D, Kleckner N. 1999. Meiotic chromosomes: integrating structure and function. Annu. Rev. Genet. 33, 603–754. ( 10.1146/annurev.genet.33.1.603) [DOI] [PubMed] [Google Scholar]
- 69.Rog O, Kohler S, Dernburg AF. 2017. The synaptonemal complex has liquid crystalline properties and spatially regulates meiotic recombination factors. Elife 6, e21455 ( 10.7554/eLife.21455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roeder GS, Bailis JM. 2000. The pachytene checkpoint. Trends Genet. 16, 395–403. ( 10.1016/S0168-9525(00)02080-1) [DOI] [PubMed] [Google Scholar]
- 71.Revenkova E, Eijpe M, Heyting C, Hodges CA, Hunt PA, Liebe B, Scherthan H, Jessberger R. 2004. Cohesin SMC1β is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat. Cell Biol. 6, 555–562. ( 10.1038/ncb1135) [DOI] [PubMed] [Google Scholar]
- 72.Xu H, Beasley MD, Warren WD, van der Horst GTJ, McKay MJ. 2005. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev. Cell 8, 949–961. ( 10.1016/j.devcel.2005.03.018) [DOI] [PubMed] [Google Scholar]
- 73.Yuan L, Liu JG, Zhao J, Brundell E, Daneholt B, Höög C. 2000. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol. Cell 5, 73–83. ( 10.1016/S1097-2765(00)80404-9) [DOI] [PubMed] [Google Scholar]
- 74.Yang F, De La Fuente R, Leu NA, Baumann C, McLaughlin KJ, Wang PJ. 2006. Mouse SYCP2 is required for synaptonemal complex assembly and chromosomal synapsis during male meiosis. J. Cell Biol. 173, 497–507. ( 10.1083/jcb.200603063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bellani MA, Boateng KA, McLeod D, Camerini-Otero RD. 2010. The expression profile of the major mouse SPO11 isoforms indicates that SPO11β introduces double strand breaks and suggests that SPO11α has an additional role in prophase in both spermatocytes and oocytes. Mol. Cell. Biol. 30, 4391–4403. ( 10.1128/MCB.00002-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuznetsov S, et al. 2007. RAD51C deficiency in mice results in early prophase I arrest in males and sister chromatid separation at metaphase II in females. J. Cell Biol. 176, 581–592. ( 10.1083/jcb.200608130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coop G, Przeworski M. 2007. An evolutionary view of human recombination. Nat. Rev. Genet. 8, 23–34. ( 10.1038/nrg1947) [DOI] [PubMed] [Google Scholar]
- 78.Donis-Keller H, et al. 1987. A genetic linkage map of the human genome. Cell 51, 319–337. ( 10.1016/0092-8674(87)90158-9) [DOI] [PubMed] [Google Scholar]
- 79.Kong A, et al. 2010. Fine-scale recombination rate differences between sexes, populations and individuals. Nature 467, 1099–1103. ( 10.1038/nature09525) [DOI] [PubMed] [Google Scholar]
- 80.Kong A, et al. 2008. Sequence variants in the RNF212 gene associate with genome-wide recombination rate. Science 319, 1398–1401. ( 10.1126/science.1152422) [DOI] [PubMed] [Google Scholar]
- 81.Kong A, et al. 2014. Common and low-frequency variants associated with genome-wide recombination rate. Nat. Genet. 46, 11–16. ( 10.1038/ng.2833) [DOI] [PubMed] [Google Scholar]
- 82.Miyamoto T, Hasuike S, Yogev L, Maduro MR, Ishikawa M, Westphal H, Lamb DJ. 2003. Azoospermia in patients heterozygous for a mutation in SYCP3. Lancet 362, 1714–1719. ( 10.1016/S0140-6736(03)14845-3) [DOI] [PubMed] [Google Scholar]
- 83.Christensen GL, Ivanov IP, Atkins JF, Mielnik A, Schlegel PN, Carrell DT. 2005. Screening the SPO11 and EIF5A2 genes in a population of infertile men. Fertil. Steril. 84, 758–760. ( 10.1016/j.fertnstert.2005.03.053) [DOI] [PubMed] [Google Scholar]
- 84.Irie S, Tsujimura A, Miyagawa Y, Ueda T, Matsuoka Y, Matsui Y, Okuyama A, Nishimune Y, Tanaka H. 2009. Single-nucleotide polymorphisms of the PRDM9 (MEISETZ) gene in patients with nonobstructive azoospermia. J. Androl. 30, 426–431. ( 10.2164/jandrol.108.006262) [DOI] [PubMed] [Google Scholar]
- 85.Singh P, Schimenti JC. 2015. The genetics of human infertility by functional interrogation of SNPs in mice. Proc. Natl Acad. Sci. USA 112, 10 431–10 436. ( 10.1073/pnas.1506974112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyamoto T, Koh E, Sakugawa N, Sato H, Hayashi H, Namiki M, Sengoku K. 2008. Two single nucleotide polymorphisms in PRDM9 (MEISETZ) gene may be a genetic risk factor for Japanese patients with azoospermia by meiotic arrest. J. Assist. Reprod. Genet. 25, 553–557. ( 10.1007/s10815-008-9270-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mouka A, et al. 2017. Induced pluripotent stem cell generation from a man carrying a complex chromosomal rearrangement as a genetic model for infertility studies. Sci. Rep. 7, 39760 ( 10.1038/srep39760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hassold T, Hunt P. 2001. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2, 280–291. ( 10.1038/35066065) [DOI] [PubMed] [Google Scholar]
- 89.Purandare SM, Patel PI. 1997. Recombination hot spots and human disease. Genome Res. 7, 773–786. ( 10.1101/gr.7.8.773) [DOI] [PubMed] [Google Scholar]
- 90.Dittwald P, et al. 2013. NAHR-mediated copy-number variants in a clinical population: mechanistic insights into both genomic disorders and Mendelizing traits. Genome Res. 23, 1395–1409. ( 10.1101/gr.152454.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roehl AC, et al. 2010. Intrachromosomal mitotic nonallelic homologous recombination is the major molecular mechanism underlying type-2 NF1 deletions. Hum. Mutat. 31, 1163–1173. ( 10.1002/humu.21340) [DOI] [PubMed] [Google Scholar]
- 92.Gari K, Décaillet C, Stasiak AZ, Stasiak A, Constantinou A. 2008. The fanconi anemia protein FANCM can promote branch migration of Holliday junctions and replication forks. Mol. Cell. 29, 141–148. ( 10.1016/j.molcel.2007.11.032) [DOI] [PubMed] [Google Scholar]
- 93.Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, Goberdhan A, Shah JP, Singh B. 2003. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch. Otolaryngol. Neck Surg. 129, 106–112. ( 10.1001/archotol.129.1.106) [DOI] [PubMed] [Google Scholar]
- 94.Lieber MR, Hesse JE, Lewis S, Bosma GC, Rosenberg N, Mizuuchi K, Bosma MJ, Gellert M. 1988. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell 55, 7–16. ( 10.1016/0092-8674(88)90004-9) [DOI] [PubMed] [Google Scholar]
- 95.Cornsforth MN, Bedford JS. 1985. On the nature of a defect in cells from individuals with ataxia-telangiectasia. Science 227, 1589–1592. ( 10.1126/science.3975628) [DOI] [PubMed] [Google Scholar]
- 96.Carney JP, Maser RS, Olivares H, Davis EM, Le Beau M, Yates JR III, Hays L, Morgan WF, Petrini JHJ. 1998. The hMre11/hRad50 protein complex and nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell 93, 477–486. ( 10.1016/S0092-8674(00)81175-7) [DOI] [PubMed] [Google Scholar]
- 97.Wu L, Hickson ID.. 2003. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature 426, 870–874. ( 10.1038/nature02253) [DOI] [PubMed] [Google Scholar]
- 98.Moynahan ME, Jasin M. 2010. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat. Rev. Mol. Cell Biol. 11, 196–207. ( 10.1038/nrm2851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haffner MC, et al. 2010. Androgen-induced TOP2B-mediated double strand breaks and prostate cancer gene rearrangements. Nat. Genet. 42, 668–675. ( 10.1038/ng.613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kloosterman WP, et al. 2012. Constitutional chromothripsis rearrangements involve clustered double-stranded DNA breaks and nonhomologous repair mechanisms. Cell Rep. 1, 648–655. ( 10.1016/j.celrep.2012.05.009) [DOI] [PubMed] [Google Scholar]
- 101.Zhang C-Z, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, Meyerson M, Pellman D. 2015. Chromothripsis from DNA damage in micronuclei. Nature 522, 179–184. ( 10.1038/nature14493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hussin J, et al. 2013. Rare allelic forms of PRDM9 associated with childhood leukemogenesis. Genome Res. 23, 419–430. ( 10.1101/gr.144188.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rodić N, Zampella JG, Cornish TC, Wheelan SJ, Burns KH. 2013. Translocation junctions in TCF3-PBX1 acute lymphoblastic leukemia/lymphoma cluster near transposable elements. Mob. DNA 4, 22 ( 10.1186/1759-8753-4-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hassold T, Merrill M, Adkins K, Freeman S, Sherman S. 1995. Recombination and maternal age-dependent nondisjunction: molecular studies of trisomy 16. Am. J. Hum. Genet. 57, 867–874. [PMC free article] [PubMed] [Google Scholar]
- 105.Lamb NE, et al. 1997. Characterization of susceptible chiasma configurations that increase the risk for maternal nondisjunction of chromosome 21. Hum. Mol. Genet. 6, 1391–1399. ( 10.1093/hmg/6.9.1391) [DOI] [PubMed] [Google Scholar]
- 106.Thomas NS, Ennis S, Sharp AJ, Durkie M, Hassold TJ, Collins AR, Jacobs PA. 2001. Maternal sex chromosome non-disjunction: evidence for X chromosome-specific risk factors. Hum. Mol. Genet. 10, 243–250. ( 10.1093/hmg/10.3.243) [DOI] [PubMed] [Google Scholar]
- 107.Bugge M, et al. 1998. Non-disjunction of chromosome 18. Hum. Mol. Genet. 7, 661–669. ( 10.1093/hmg/7.4.661) [DOI] [PubMed] [Google Scholar]
- 108.Robinson WP, et al. 1998. Maternal meiosis I non-disjunction of chromosome 15: dependence of the maternal age effect on level of recombination. Hum. Mol. Genet. 7, 1011–1019. ( 10.1093/hmg/7.6.1011) [DOI] [PubMed] [Google Scholar]
- 109.Hassold T, Hunt P. 2009. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr. Opin. Pediatr. 21, 703–708. ( 10.1097/MOP.0b013e328332c6ab) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hussin J, Roy-Gagnon M-HH, Gendron R, Andelfinger G, Awadalla P. 2011. Age-dependent recombination rates in human pedigrees. PLoS Genet. 7, e1002251 ( 10.1371/journal.pgen.1002251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kong A, et al. 2004. Recombination rate and reproductive success in humans. Nat. Genet. 36, 1203–1206. ( 10.1038/ng1445) [DOI] [PubMed] [Google Scholar]
- 112.Bleazard T, Ju YS, Sung J, Seo JS. 2013. Fine-scale mapping of meiotic recombination in Asians. BMC Genet. 14, 19 ( 10.1186/1471-2156-14-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin HC, et al. 2015. Multicohort analysis of the maternal age effect on recombination. Nat. Commun. 6, 7846 ( 10.1038/ncomms8846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Campbell CL, Furlotte NA, Eriksson N, Hinds D, Auton A.. 2015. Escape from crossover interference increases with maternal age. Nat. Commun. 6, 6260 ( 10.1038/ncomms7260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lupski JR, et al. 1991. DNA duplication associated with Charcot–Marie–Tooth disease type 1A. Cell 66, 219–232. ( 10.1016/0092-8674(91)90613-4) [DOI] [PubMed] [Google Scholar]
- 116.Lupski JR. 2004. Hotspots of homologous recombination in the human genome: not all homologous sequences are equal. Genome Biol. 5, 242 ( 10.1186/gb-2004-5-10-242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Raedt TD, et al. 2006. Conservation of hotspots for recombination in low-copy repeats associated with the NF1 microdeletion. Nat. Genet. 38, 1419–1423. ( 10.1038/ng1920) [DOI] [PubMed] [Google Scholar]
- 118.Lindsay SJ, Khajavi M, Lupski JR, Hurles ME. 2006. A chromosomal rearrangement hotspot can be identified from population genetic variation and is coincident with a hotspot for allelic recombination. Am. J. Hum. Genet. 79, 890–902. ( 10.1086/508709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Berg IL, Neumann R, Lam K-WWG, Sarbajna S, Odenthal-Hesse L, May CA, Jeffreys AJ. 2010. PRDM9 variation strongly influences recombination hot-spot activity and meiotic instability in humans. Nat. Genet. 42, 859–863. ( 10.1038/ng.658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Borel C, Cheung F, Stewart H, Koolen DA, Phillips C, Thomas NS, Jacobs PA, Eliez S, Sharp AJ. 2012. Evaluation of PRDM9 variation as a risk factor for recurrent genomic disorders and chromosomal non-disjunction. Hum. Genet. 131, 1519–1524. ( 10.1007/s00439-012-1180-4) [DOI] [PubMed] [Google Scholar]
- 121.Lopes J, et al. 1997. Sex-dependent rearrangements resulting in CMT1A and HNPP. Nat. Genet. 17, 136–137. ( 10.1038/ng1097-136) [DOI] [PubMed] [Google Scholar]
- 122.Wirth B, Schmidt T, Hahnen E, Rudnik-Schoneborn S, Krawczak M, Muller-Myhsok B, Schönling J, Zerres K. 1997. De novo rearrangements found in 2% of index patients with spinal muscular atrophy: mutational mechanisms, parental origin, mutation rate, and implications for genetic counseling. Am. J. Hum. Genet. 61, 1102–1111. ( 10.1086/301608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lazaro C, Gaona A, Ainsworth P, Tenconi R, Vidaud D, Kruyer H, Ars E, Volpini V, Estivill X. 1996. Sex differences in mutational rate and mutational mechanism in the NF1 gene in neurofibromatosis type 1 patients. Hum. Genet. 98, 696–699. ( 10.1007/s004390050287) [DOI] [PubMed] [Google Scholar]
- 124.Symington LS, Rothstein R, Lisby M. 2014. Mechanisms and regulation of mitotic recombination in Saccharomyces cerevisiae. Genetics 198, 795–835. ( 10.1534/genetics.114.166140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lisby M, Rothstein R. 2016. Cell biology of mitotic recombination. Cold Spring Harb. Perspect. Biol. 7, 1–22. ( 10.1101/cshperspect.a016535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shinohara A, Gasior S, Ogawa T, Kleckner N, Bishop DK. 1997. Saccharomyces cerevisiae recA homologues RAD51 and DMC1 have both distinct and overlapping roles in meiotic recombination. Genes Cells 2, 615–629. ( 10.1046/j.1365-2443.1997.1480347.x) [DOI] [PubMed] [Google Scholar]
- 127.Klein HL. 1997. RDH54, a RAD54 homologue in Saccharomyces cerevisiae, is required for mitotic diploid-specific recombination and repair and for meiosis. Genetics 147, 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sammut SJ, Feichtinger J, Stuart N, Wakeman JA, Larcombe L, McFarlane RJ. 2014. A novel cohort of cancer-testis biomarker genes revealed through meta-analysis of clinical data sets. Oncoscience 1, 349–359. ( 10.18632/oncoscience.37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Feichtinger J, et al. 2012. Meta-analysis of clinical data using human meiotic genes identifies a novel cohort of highly restricted cancer-specific marker genes. Oncotarget 3, 843–853. ( 10.18632/oncotarget.580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mehta A, Haber JE.. 2014. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harb. Perspect. Biol. 6, a016428 ( 10.1101/cshperspect.a016428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Krejci L, Altmannova V, Spirek M, Zhao X. 2012. Homologous recombination and its regulation. Nucleic Acids Res. 40, gks270 ( 10.1093/nar/gks270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lord CJ, Ashworth A. 2016. BRCAness revisited. Nat. Rev. Cancer 16, 110–120. ( 10.1038/nrc.2015.21) [DOI] [PubMed] [Google Scholar]
- 133.Jackson SPP. 2002. Sensing and repairing DNA double-strand breaks. Carcinogenesis 23, 687–696. ( 10.1093/carcin/23.5.687) [DOI] [PubMed] [Google Scholar]
- 134.Alexandrov LB, et al. 2013. Signatures of mutational processes in human cancer. Nature 500, 415–421. ( 10.1038/nature12477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ledermann JA, Drew Y, Kristeleit RS. 2016. Homologous recombination deficiency and ovarian cancer. Eur. J. Cancer 60, 49–58. ( 10.1016/j.ejca.2016.03.005) [DOI] [PubMed] [Google Scholar]
- 136.Brown JS, Kaye SB, Yap TA. 2016. PARP inhibitors: the race is on. Br. J. Cancer 114, 713–715. ( 10.1038/bjc.2016.67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen J-M, Cooper DN, Chuzhanova N, Férec C, Patrinos GP. 2007. Gene conversion: mechanisms, evolution and human disease. Nat. Rev. Genet. 8, 762–775. ( 10.1038/nrg2193) [DOI] [PubMed] [Google Scholar]
- 138.Galtier N, Duret L, Glémin S, Ranwez V. 2009. GC-biased gene conversion promotes the fixation of deleterious amino acid changes in primates. Trends Genet. 25, 1–5. ( 10.1016/j.tig.2008.10.011) [DOI] [PubMed] [Google Scholar]
- 139.Marais G. 2003. Biased gene conversion: implications for genome and sex evolution. Trends Genet 19, 330–338. ( 10.1016/S0168-9525(03)00116-1) [DOI] [PubMed] [Google Scholar]
- 140.Spies M, Fishel R. 2015. Mismatch repair during homologous and homeologous recombination. Cold Spring Harb Perspect Biol. 7, a022657 ( 10.1101/cshperspect.a022657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parks MM, Lawrence CE, Raphael BJ. 2015. Detecting non-allelic homologous recombination from high-throughput sequencing data. Genome Biol. 16, 72 ( 10.1186/s13059-015-0633-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cavenee WK, Dryja TP, Phillips RA, Benedict WF, Godbout R, Gallie BL, Murphree AL, Strong LC, White RL. 1983. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. Nature 305, 779–784. ( 10.1038/305779a0) [DOI] [PubMed] [Google Scholar]
- 143.Ryland GL, et al. 2015. Loss of heterozygosity: what is it good for? BMC Med. Genomics 8, 45 ( 10.1186/s12920-015-0123-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thiagalingam S, Laken S, Willson JKV, Markowitz SD, Kinzler KW, Vogelstein B, Lengauer C. 2001. Mechanisms underlying losses of heterozygosity in human colorectal cancers. Proc. Natl Acad. Sci. USA 98, 2698–2702. ( 10.1073/pnas.051625398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li H, Yang B, Xing K, Yuan N, Wang B, Chen Z, He W, Zhou J. 2014. A preliminary study of the relationship between breast cancer metastasis and loss of heterozygosity by using exome sequencing. Sci. Rep. 4, 5460 ( 10.1038/srep05460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang J, Lindroos A, Ollila S, Russell A, Marra G, Mueller H, Peltomaki P, Plasilova M, Heinimann K. 2006. Gene conversion is a frequent mechanism of inactivation of the wild-type allele in cancers from MLH1/MSH2 deletion carriers. Cancer Res. 66, 659–664. ( 10.1158/0008-5472.CAN-05-4043) [DOI] [PubMed] [Google Scholar]
- 147.Morales ME, et al. 2015. The contribution of Alu Elements to mutagenic DNA double-strand break repair. PLoS Genet. 11, e1005016 ( 10.1371/journal.pgen.1005016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Charlesworth B, Betancourt AJ, Kaiser VB, Gordo I. 2009. Genetic recombination and molecular evolution. Cold Spring Harb. Symp. Quant. Biol. 74, 177–186. ( 10.1101/sqb.2009.74.015) [DOI] [PubMed] [Google Scholar]
- 149.Barreiro LB, Laval G, Quach H, Patin E, Quintana-Murci L. 2008. Natural selection has driven population differentiation in modern humans. Nat. Genet. 40, 340–345. ( 10.1038/ng.78) [DOI] [PubMed] [Google Scholar]
- 150.Keinan A, Reich D. 2010. Human population differentiation is strongly correlated with local recombination rate. PLoS Genet. 6, e1000886 ( 10.1371/journal.pgen.1000886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hernandez RD, Kelley JL, Elyashiv E, Melton SC, Auton A, McVean G, Sella G, Przeworski M. 2011. Classic selective sweeps were rare in recent human evolution. Science 331, 920–924. ( 10.1126/science.1198878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Charlesworth B, Morgan MT, Charlesworth D. 1993. The effect of deleterious mutations on neutral molecular variation. Genetics 134, 1289–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu X-S, He F. 2005. Background selection and population differentiation. J. Theor. Biol. 235, 207–219. ( 10.1016/j.jtbi.2005.01.004) [DOI] [PubMed] [Google Scholar]
- 154.Charlesworth B. 2012. The effects of deleterious mutations on evolution at linked sites. Genetics 190, 5–22. ( 10.1534/genetics.111.134288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hartfield M, Keightley PD. 2012. Current hypotheses for the evolution of sex and recombination. Integr. Zool. 7, 192–209. ( 10.1111/j.1749-4877.2012.00284.x) [DOI] [PubMed] [Google Scholar]
- 156.Keightley PD, Otto SP. 2006. Interference among deleterious mutations favours sex and recombination in finite populations. Nature 443, 89–92. ( 10.1038/nature05049) [DOI] [PubMed] [Google Scholar]
- 157.Fu W, et al. 2013. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature 493, 216–220. ( 10.1038/nature11690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tennessen JA, et al. 2012. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science 337, 64–69. ( 10.1126/science.1219240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tabor HK, et al. 2014. Pathogenic variants for Mendelian and complex traits in exomes of 6,517 European and African Americans: implications for the return of incidental results. Am. J. Hum. Genet. 95, 183–193. ( 10.1016/j.ajhg.2014.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Casals F, et al. 2013. Whole-exome sequencing reveals a rapid change in the frequency of rare functional variants in a founding population of humans. PLoS Genet. 9, e1003815 ( 10.1371/journal.pgen.1003815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lohmueller KE, et al. 2008. Proportionally more deleterious genetic variation in European than in African populations. Nature 451, 994–997. ( 10.1038/nature06611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Do R, Balick D, Li H, Adzhubei I, Sunyaev S, Reich D. 2015. No evidence that selection has been less effective at removing deleterious mutations in Europeans than in Africans. Nat. Genet. 47, 126–131. ( 10.1038/ng.3186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Simons YB, Turchin MC, Pritchard JK, Sella G. 2014. The deleterious mutation load is insensitive to recent population history. Nat. Genet. 46, 220–224. ( 10.1038/ng.2896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hussin JG, Hodgkinson A, Idaghdour Y, Grenier J-C, Goulet J-P, Gbeha E, Hip-Ki E, Awadalla P. 2015. Recombination affects accumulation of damaging and disease-associated mutations in human populations. Nat. Genet. 47, 400–404. ( 10.1038/ng.3216) [DOI] [PubMed] [Google Scholar]
- 165.Nachman MW. 2001. Single nucleotide polymorphisms and recombination rate in humans. Trends Genet. 17, 481–485. ( 10.1016/S0168-9525(01)02409-X) [DOI] [PubMed] [Google Scholar]
- 166.Yu A, et al. 2001. Comparison of human genetic and sequence-based physical maps. Nature 409, 951–953. ( 10.1038/35057185) [DOI] [PubMed] [Google Scholar]
- 167.Galtier N, Piganeau G, Mouchiroud D, Duret L. 2001. GC-content evolution in mammalian genomes: the biased gene conversion hypothesis. Genetics 159, 907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Glémin S, Arndt PF, Messer PW, Petrov D, Galtier N, Duret L. 2015. Quantification of GC-biased gene conversion in the human genome. Genome Res. 25, 1215–1228. ( 10.1101/gr.185488.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Duret L, Arndt PF. 2008. The impact of recombination on nucleotide substitutions in the human genome. PLoS Genet. 4, e1000071 ( 10.1371/journal.pgen.1000071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Webster MT, Hurst LD. 2012. Direct and indirect consequences of meiotic recombination: implications for genome evolution. Trends Genet. 28, 101–109. ( 10.1016/j.tig.2011.11.002) [DOI] [PubMed] [Google Scholar]
- 171.Lachance J, Tishkoff SA. 2014. Biased gene conversion skews allele frequencies in human populations, increasing the disease burden of recessive alleles. Am. J. Hum. Genet. 95, 408–420. ( 10.1016/j.ajhg.2014.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Arbeithuber B, Betancourt AJ, Ebner T, Tiemann-Boege I. 2015. Crossovers are associated with mutation and biased gene conversion at recombination hotspots. Proc. Natl Acad. Sci. USA 112, 2109–2114. ( 10.1073/pnas.1416622112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Baudat F, Imai Y, de Massy B.. 2013. Meiotic recombination in mammals: localization and regulation. Nat. Rev. Genet. 14, 794–806. ( 10.1038/nrg3573) [DOI] [PubMed] [Google Scholar]
- 174.Billings T, Parvanov EDED, Baker CLCL, Walker M, Paigen K, Petkov PMPM. 2013. DNA binding specificities of the long zinc-finger recombination protein PRDM9. Genome Biol. 14, R35 ( 10.1186/gb-2013-14-4-r35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Baker CLCL, Petkova P, Walker M, Flachs P, Mihola O, Trachtulec Z, Petkov PM, Paigen K. 2015. Multimer formation explains allelic suppression of PRDM9 recombination hotspots. PLoS Genet. 11, e1005512 ( 10.1371/journal.pgen.1005512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Striedner Y, Schwarz T, Welte T, Futschik A, Rant U, Tiemann-Boege I. 2017. The long zinc finger domain of PRDM9 forms a highly stable and long-lived complex with its DNA recognition sequence. Chromosome Res. 25, 155–172. ( 10.1007/s10577-017-9552-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Parvanov ED, Tian H, Billings T, Saxl RL, Spruce C, Aithal R, Krejci L, Paigen K, Petkov PM. 2016. PRDM9 interactions with other proteins provide a link between recombination hotspots and the chromosomal axis in meiosis. Mol. Biol. Cell 28, 488–499. ( 10.1091/mbc.E16-09-0686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Altemose N, Noor N, Bitoun E, Tumian A, Imbeault M, Chapman JR, Aricescu AR, Myers SR. 2017. Human PRDM9 can bind and activate promoters, and other zinc-finger proteins associate with reduced recombination in cis. bioRxiv 144295 ( 10.1101/144295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Narasimhan VM, et al. 2016. Health and population effects of rare gene knockouts in adult humans with related parents. Science 352, 474–477. ( 10.1126/science.aac8624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Prakash K, et al. 2015. Superresolution imaging reveals structurally distinct periodic patterns of chromatin along pachytene chromosomes. Proc. Natl Acad. Sci. USA 112, 14 635–14 640. ( 10.1073/pnas.1516928112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kauppi L, Jeffreys AJ, Keeney S. 2004. Where the crossovers are: recombination distributions in mammals. Nat. Rev. Genet. 5, 413–424. ( 10.1038/nrg1346) [DOI] [PubMed] [Google Scholar]
- 182.Khil PP, Smagulova F, Brick KM, Camerini-Otero RD, Petukhova GV. 2012. Sensitive mapping of recombination hotspots using sequencing-based detection of ssDNA. Genome Res. 22, 957–965. ( 10.1101/gr.130583.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Baker CL, Walker M, Kajita S, Petkov PM, Paigen K. 2014. PRDM9 binding organizes hotspot nucleosomes and limits Holliday junction migration. Genome Res. 24, 724–732. ( 10.1101/gr.170167.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Walker M, et al. 2015. Affinity-seq detects genome-wide PRDM9 binding sites and reveals the impact of prior chromatin modifications on mammalian recombination hotspot usage. Epigenet. Chromatin 8, 31 ( 10.1186/s13072-015-0024-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Brick K, Smagulova F, Khil P, Camerini-Otero RD, Petukhova GV. 2012. Genetic recombination is directed away from functional genomic elements in mice. Nature 485, 642–645. ( 10.1038/nature11089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lange J, Yamada S, Tischfield SE, Pan J, Kim S, Zhu X, Socci ND, Jasin M, Keeney S. 2016. The landscape of mouse meiotic double-strand break formation, processing, and repair. Cell 167, 695–708.e16. ( 10.1016/j.cell.2016.09.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Handyside AH, Harton GL, Mariani B, Thornhill AR, Affara N, Shaw M-A, Griffin DK. 2010. Karyomapping: a universal method for genome wide analysis of genetic disease based on mapping crossovers between parental haplotypes. J. Med. Genet. 47, 651–658. ( 10.1136/jmg.2009.069971) [DOI] [PubMed] [Google Scholar]
- 188.Ottolini CS, et al. 2014. Genome-wide maps of recombination and chromosome segregation in human oocytes and embryos show selection for maternal recombination rates. Nat. Genet. 47, 727–735. ( 10.1038/ng.3306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hou Y, et al. 2013. Genome analyses of single human oocytes. Cell 155, 1492–1506. ( 10.1016/j.cell.2013.11.040) [DOI] [PubMed] [Google Scholar]
- 190.Ottolini CS, Capalbo A, Newnham L, Cimadomo D, Natesan SA, Hoffmann ER, Ubaldi FM, Rienzi L, Handyside AH. 2016. Generation of meiomaps of genome-wide recombination and chromosome segregation in human oocytes. Nat. Protoc. 11, 1229–1243. ( 10.1038/nprot.2016.075) [DOI] [PubMed] [Google Scholar]
- 191.Wang J, Fan HC, Behr B, Quake SR. 2012. Genome-wide single-cell analysis of recombination activity and de novo mutation rates in human sperm. Cell 150, 402–412. ( 10.1016/j.cell.2012.06.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jeffreys AJ, Cotton VE, Neumann R, Lam KG. 2013. Recombination regulator PRDM9 influences the instability of its own coding sequence in humans. Proc. Natl Acad. Sci. USA 110, 600–605. ( 10.1073/pnas.1220813110) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.