Abstract
Reduced water quality, in particular increases in suspended sediments, has been linked to declines in fish abundance on coral reefs. Changes in gill structure induced by suspended sediments have been hypothesized to impair gill function and may provide a mechanistic basis for the observed declines; yet, evidence for this is lacking. We exposed juveniles of three reef fish species (Amphiprion melanopus, Amphiprion percula and Acanthochromis polyacanthus) to suspended sediments (0–180 mg l−1) for 7 days and examined changes in gill structure and metabolic performance (i.e. oxygen consumption). Exposure to suspended sediments led to shorter gill lamellae in A. melanopus and A. polyacanthus and reduced oxygen diffusion distances in all three species. While A. melanopus exhibited impaired oxygen uptake after suspended sediment exposure, i.e. decreased maximum and increased resting oxygen consumption rates resulting in decreased aerobic scope, the oxygen consumption rates of the other two species remained unaffected. These findings imply that species sensitive to changes in gill structure such as A. melanopus may decline in abundance as reefs become more turbid, whereas species that are able to maintain metabolic performance despite suspended sediment exposure, such as A. polyacanthus or A. percula, may be able to persist or gain a competitive advantage.
Keywords: turbidity, suspended solids, dredging, fish health, coastal development, clownfish
1. Introduction
Declining water quality is one of the leading causes of aquatic ecosystem degradation globally [1,2]. In particular, the input and resuspension of sediments are having a dramatic effect on both sessile and mobile organisms [3]. Coastal development, agriculture, overgrazing, mining, removal of riparian vegetation, dredging and shipping have increased suspended sediments in coastal waters over the past decades [1,4,5]. Continued population growth in coastal areas, especially in the tropics [6], is likely to lead to further increases in suspended sediments. Increases in suspended sediments have already led to biodiversity loss and fundamental changes in benthic and fish assemblages on coral reefs [7–11]; however, the mechanistic basis for these declines is not fully understood.
Declining water quality has been associated with reductions in abundance and/or diversity of reef fishes on coastal reefs (e.g. Great Barrier Reef [9,10], Caribbean [11] and Pacific Islands [12,13]). It is not clear, however, if these changes in fish assemblages are the result of the direct effects of suspended sediments on fish behaviour or physiology, and/or the indirect effects of suspended sediments that manifest through the degradation of benthic habitats. The negative effects of sediments on the abundance and composition of corals are well established [7,14], and numerous studies have documented declines in fish assemblages following coral loss [15,16]. However, the indirect effects of sediment-induced coral loss may be compounded by any direct impacts of suspended sediments on fishes [3,17]. For example, suspended sediments have been shown to interfere with visual acuity and olfaction in some coral reef fishes, thereby affecting larval settlement [18,19] and prey capture [20–22]. Suspended sediments, however, may also have important impacts on physiological processes, such as respiration, that could lead to negative effects at the level of performance and fitness.
Most fish species rely on their gills to extract oxygen from water [23], and suspended sediments and other pollutants have been hypothesized to interfere with this process [24]. Several studies have shown that suspended sediments and other pollutants can irritate and damage gill tissues, leading to changes in gill structure [25–28]. Specifically, exposure to suspended sediments has been shown to reduce the length of gill lamellae, thereby reducing gill surface area, and to damage the gill epithelium [29,30], which is the primary site for oxygen uptake in most fishes [31]. To repair tissue damage and to reduce the impact of pollutants, gills often secrete mucous and grow additional cell layers on the lamellae (referred to as hyperplasia), which increase the thickness of the gill epithelium and oxygen diffusion distances [32,33]. Reductions in gill surface area and increases in oxygen diffusion distances are assumed to decrease the efficiency of gas exchange and reduce the capacity of the gills for oxygen uptake [34,35]. Indeed, some species are known to undergo extensive modifications in gill surface area and oxygen diffusion distances to regulate ion- and oxygen transport across the gills in response to changes in temperature, hypoxia and air exposure [36–41]. However, little is known as to whether changes in gill structure induced by pollutants such as suspended sediments directly affect gill function and the metabolic performance of fish (but see [28]).
The capacity to perform vital aerobic activities is tied to the capacity of the gills for oxygen uptake [42,43]. For example, the maximum rate of oxygen uptake of individuals (i.e. ṀO2max) has been correlated with various activities linked to fitness and survival in fish, including locomotion and competitive ability [43,44]. Gill damage induced by suspended sediments may reduce the capacity for oxygen uptake and limit oxygen delivery to tissues, especially during activities requiring high levels of oxygen [45], thus compromising fish performance and ultimately reducing fitness and survival of fish living on turbid reefs. The potential link between gill structure, metabolic performance and individual fitness may be an important—albeit understudied—driver of the observed changes in fish assemblages with declining water quality [26,28,46]. Determining the extent to which structural gill changes induced by suspended sediments affect the metabolic performance of individual species is critical to understand current and future changes in coastal fish assemblages as water quality decreases.
The aim of this study was to investigate whether changes in gill structure resulting from exposure to suspended sediments will compromise gill function and metabolic performance of juvenile coral reef fishes, thereby providing a mechanistic link between declining water quality and fish health. To do so, we examined the effects of a range of ecologically relevant suspended sediment concentrations on the gill morphology and oxygen consumption rates of three common damselfish species using histology and intermittent-flow respirometry, respectively. The suspended sediment concentrations used reflect those currently experienced on inshore reefs of the Great Barrier Reef during resuspension events such as currents, waves and flood plumes [47,48]. These suspended sediment concentrations are likely to become more frequent in the future as population growth, coastal development and associated terrestrial run-off in tropical coastal areas rapidly increase [2,6].
2. Material and methods
(a). Experimental protocol
Larvae of Amphiprion melanopus (cinnamon clownfish), Amphiprion percula (false orange clownfish) and Acanthochromis polyacanthus (spiny chromis) were sourced from captive breeding pairs between January and May 2016 and maintained until experimentation (see electronic supplementary material for a detailed description). At 30 days after hatching, juvenile A. melanopus and A. polyacanthus were randomly assigned to one of five suspended sediment concentrations (i.e. 0, 45, 90, 135 or 180 mg l−1) corresponding to turbidity levels of 0.5 ± 0.5, 7.0 ± 2.7, 14.2 ± 3.0, 21.3 ± 3.4 and 30.1 ± 3.7 nephelometric turbidity units (NTU), respectively. Juvenile A. percula were randomly allocated to one of three suspended sediment concentrations (i.e. 0, 135 or 180 mg l−1) due to the limited number of larvae available. Water temperature was maintained at 28.5 ± 0.5°C for all fish. For each of the three species, four to six replicate aquaria were established for each of the suspended sediment concentrations, and five randomly selected individuals were placed in each aquarium. Fish were maintained in the experimental aquaria for 7 days and fed with flakes NRD 0.5–0.8 mm (Pro Aqua Pty Ltd) twice daily. Sediments were resuspended in external sumps and delivered to aquaria via submersible pumps (electronic supplementary material, figure S1 for further information). Australian bentonite, a clay with a small particle size range (less than 63 µm) and representative of sediments found in suspension on the Great Barrier Reef [49], was used as the sediment. The selected suspended sediment concentrations, turbidity levels and length of exposure to suspended sediments (i.e. 7 days) represent conditions that are currently observed on inshore reefs of the Great Barrier Reef during regular resuspension events (e.g. waves and currents) and periodic events, such as flood plumes [47,48,50].
(b). Gill histology
Following respirometry trials to measure oxygen consumption rates (see below), 12 individuals (out of 20 exposed individuals) for each species and sediment treatment were randomly selected for histological analyses. These fish were euthanized in an ice-water slurry, fixed in Bouin's solution for 24–48 h and then transferred to 70% ethanol. Fish were then serially dehydrated (Shandon Southern Duplex Processor BS5), embedded in paraffin wax blocks (Shandon Histocentre 3, Thermo Electron Corporation) and sectioned (5 µm thick) longitudinally with a microtome. Sections were stained with Mayer's haematoxylin and eosin to allow the primary gill filaments, secondary lamellae, gill epithelium and supporting pillar cell system to be distinguished visually (electronic supplementary material, figure S2). Lamellae were photographed (Olympus DP12 Microscope Digital Camera System) at 400× magnification, and 15 randomly selected lamellae per fish were analysed in ImageJ (v. 1.48, National Institute of Health, Rockville, MD, USA).
Morphological features of the lamellae (i.e. total and functional lamellar length, filament thickness and thickness of the lamellar epithelium/oxygen diffusion distance) were measured following [26] (electronic supplementary material, figure S3a–c). In brief, the total length of lamellae was measured from the tip of the lamellae to the base (including the filament epithelium), and the functional length from the tip to the edge of the filament epithelium (electronic supplementary material, figure S3a). The thickness of the filament epithelium was measured in between two lamellae (electronic supplementary material, figure S3a). The total area of the lamellae and the area of the pillar cell system of the functional lamellar length were measured, and the difference between the two was divided by twice the functional length to determine the oxygen diffusion distance (electronic supplementary material, figure S3c) [26]. Note that the oxygen diffusion distance is different from the thickness of the lamellar epithelium, as it includes non-tissue space caused by epithelial lifting (i.e. detachment of the epithelium from the pillar cell system, [51]). All filaments were analysed blindly with respect to treatments.
(c). Oxygen consumption rates
Oxygen consumption rates (ṀO2) of individuals were determined using intermittent-flow respirometry [52]. Prior to respirometry, each fish was fasted for 24 h to ensure a post-absorptive state [53]. Then, each individual fish was manually chased in a circular container (60 cm diameter, 10 cm water depth) until exhaustion [54]. Individuals were deemed exhausted when they no longer displayed burst swimming, which always occurred within 2–3 min. This method assumes that maximum oxygen uptake rates are achieved while fish recover from exhaustive anaerobic exercise [55]. On exhaustion, fish were placed immediately into their respective respirometry chambers (15.7 ml volume including tubing) and left to recover for 3 h (see electronic supplementary material for a detailed description) while oxygen levels were continuously recorded. While adult fish are usually monitored over 24 h [56], smaller fish recover much faster from exhaustive exercise and are commonly measured for 2–3 h only to minimize stress and risk of starvation (e.g. [57,58]). Flush pumps supplied each chamber with (clear) aerated, UV-filtered seawater from the surrounding water bath every 10 min for 2 min, thus preventing oxygen levels from falling below 90% air saturation. Recirculation pumps ensured homogeneous oxygen tensions throughout chambers [56]. Background microbial respiration in empty chambers was measured before and after each trial [56]. After the trial, fish were euthanized in an ice-water slurry and blotted dry, and fish standard length (to the nearest mm) and mass (to the nearest 0.001 g) were recorded. Fish length and mass (mean ± s.e.), respectively, were as follows: A. melanopus, 13.5 ± 0.3 mm and 98 ± 6 mg; A. polyacanthus, 17.6 ± 0.3 mm and 174 ± 8 mg; A. percula, 19.1 ± 0.3 mm and 153 ± 6 mg.
Oxygen consumption rates (mg O2 h−1) during closed respirometry phases (i.e. non-flushing) were calculated using linear least squares regression in Labchart v. 6.1.3 (ADinstruments, Dunedin, New Zealand). Background microbial respiration was subtracted from the total respiration in chambers to derive the oxygen consumption rates of fish [56]. The highest value of oxygen consumption rates (30 s intervals) after exercise was taken as the maximum oxygen consumption rate and usually occurred during the first measurement cycle. Resting oxygen consumption rate was estimated as the mean of the lowest 10% of all values, excluding outliers below or above 2 s.d. [56]. Aerobic scope was calculated as the difference between the maximum and resting oxygen consumption rate (see [57]).
(d). Statistical analyses
All analyses were performed in R (v. 3.3.2, R Core Team 2013). Linear and generalized linear mixed models (lme4 package, [59]) were used to analyse the effects of suspended sediment concentrations on gill morphology. Total and functional lamellar length as well as the oxygen diffusion distance were used as dependent variables, sediment treatment was used as a fixed effect and the standard length of fish was used as a covariate, allowing for interactions between sediment treatment and standard length. The standard length was mean-centred to help with the interpretation of model intercepts. Fish identity was included as a random factor to account for repeated measurements per fish. Assumptions of normality and homogeneity of residuals were visually assessed with Q–Q plots and frequency distributions. Parameters were estimated using restricted maximum-likelihood, and P-values were generated using the Kenward–Rogers approximation [60]. If residuals of models did not meet assumptions, generalized linear mixed models were used with a gamma distribution and log-link function. Residuals were assessed for homogeneity and checked for overdispersion. Parameters were estimated using the Laplace approximation, and P-values were generated using the Wald Z-test [60]. Interactions between the fixed factor and the covariate were dropped when not significant. The effects of suspended sediments on oxygen consumption rates were analysed using linear or generalized linear models as described above. Maximum and resting oxygen consumption rates and aerobic scope (in mg O2 h−1) were dependent variables, sediment treatment was a fixed effect and body mass was a covariate. Clutch identity was included as a random effect with random intercepts.
To display results visually, intercepts and standard errors produced by the model (i.e. after correcting for fish size or mass) were plotted for each treatment. Parameters on log-scales were transformed to facilitate visual interpretation.
3. Results
(a). Gill structure
Exposure to suspended sediments significantly altered the gill structure of all three species investigated, with the extent of the changes varying among species and suspended sediment concentrations. Total lamellar length (see electronic supplementary material) and functional length of the lamellae of both A. melanopus and A. polyacanthus were shorter following exposure to sediments, while no changes were observed for A. percula. For A. melanopus, the functional lamellar length was on average between 20.5% and 29.6% shorter after exposure to any of the four suspended sediment concentrations when compared with gills from control fish (45 mg l−1: t = −2.02, p = 0.0436; 90 mg l−1: t = −2.25, p = 0.0245; 135 mg l−1: t = 3.62, p = 0.0002; 180 mg l−1: t = −3.04, p = 0.002; figure 1a; electronic supplementary material, table S1). For A. polyacanthus, the functional lamellar length was on average between 21.7% and 30.6% shorter after exposure to three of the four suspended sediment concentrations when compared with control fish (45 mg l−1: t = −3.26, p = 0.0011; 135 mg l−1: t = −3.07, p = 0.0021; 180 mg l−1: t = −2.20, p = 0.027; figure 1a; electronic supplementary material, table S2).
Figure 1.
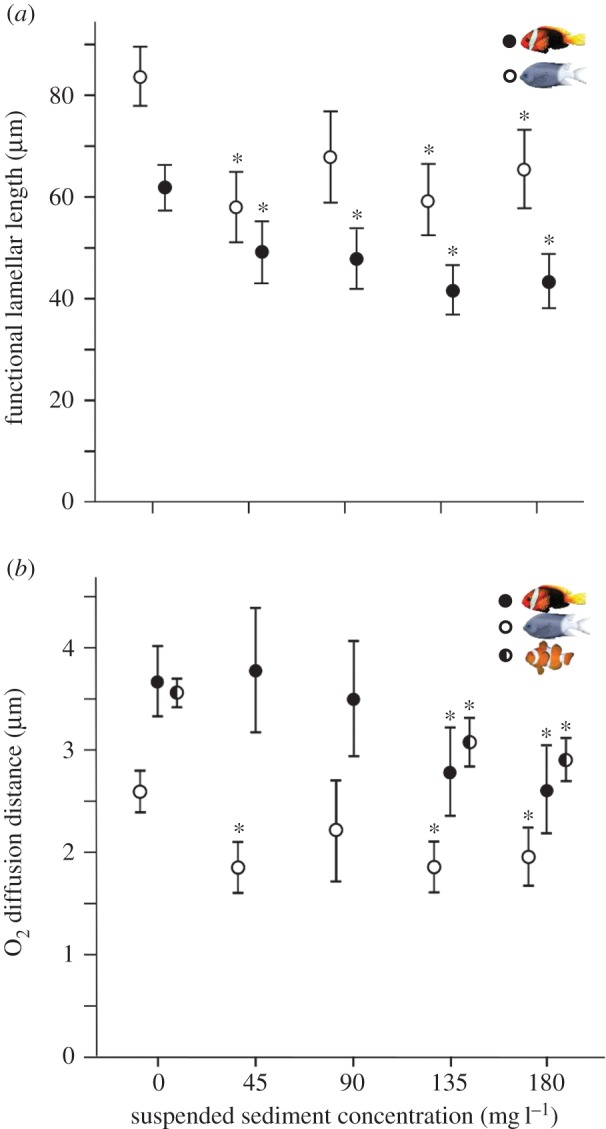
Effects of suspended sediments on (a) functional lamellar length and (b) oxygen diffusion distance in A. melanopus (filled circles), A. polyacanthus (open circles) and A. percula (half-filled circles). Circles and bars represent intercepts and standard errors, respectively, as estimated by general and linear mixed models after accounting for fish length. Asterisks indicate significant differences when compared with the control group at α < 0.05. (Online version in colour.)
All three species exhibited significant reductions in oxygen diffusion distances on suspended sediment after exposure (figure 1b; electronic supplementary material, tables S1–S3). In A. melanopus, the oxygen diffusion distance was reduced by 24.1% and 28.8%, respectively, in fish exposed to the two highest suspended sediment concentrations (135 mg l−1: t = −1.96, p = 0.049; 180 mg l−1: t = −2.28, p = 0.02; figure 1b; electronic supplementary material, table S1). Similarly, the oxygen diffusion distance was between 24.9% and 28.8% shorter in A. polyacanthus exposed to three of the four suspended sediment concentrations when compared with control fish (45 mg l−1: t = −2.68, p = 0.007; 135 mg l−1: t = −2.69, p = 0.007; 180 mg l−1: t = −2.20, p = 0.02; figure 1b; electronic supplementary material, table S2). In A. percula, reductions in oxygen diffusion distance by as much as 18.3% were evident in fish from both examined suspended sediment treatments (135 mg l−1: t = −2.12, p = 0.04; 180 mg l−1: t = −3.25, p = 0.003; figure 1b; electronic supplementary material, table S3).
(b). Oxygen consumption rates
Despite the observed changes in gill morphology in all three species of fish exposed to suspended sediments, oxygen consumption rates were only affected in one of the three species (A. melanopus). Specifically, maximum oxygen consumption rates were reduced by as much as 17.5% following exposure to the two highest suspended sediment concentrations relative to control fish (135 mg l−1: t = −2.39, p = 0.016; 180 mg l−1: t = −2.01, p = 0.044), but no changes were evident in fish exposed to 45 or 90 mg l−1 (figure 2a; electronic supplementary material, table S4). Resting oxygen consumption rates in A. melanopus were elevated on average by 36.3–64.3% for three of the four suspended sediment concentrations when compared with control fish (45 mg l−1: t = 2.92, p = 0.004; 90 mg l−1: t = 3.00, p = 0.003; 180 mg l−1: t = 2.61, p = 0.011; figure 2a; electronic supplementary material, table S4). Reflecting changes in maximum and resting oxygen consumption rates, aerobic scope was reduced by as much as 39.3% in fish on exposure to all suspended sediment concentrations when compared with control fish (45 mg l−1: t = −2.42, p = 0.015; 90 mg l−1: t = −2.60, p = 0.009; 135 mg l−1: t = −3.85, p = 0.001; 180 mg l−1: t = −2.31, p = 0.020; figure 2b; electronic supplementary material, table S4). No effects on maximum or resting oxygen consumption rates or aerobic scope were detected for A. polyacanthus or A. percula exposed to suspended sediments (electronic supplementary material, tables S5 and S6).
Figure 2.
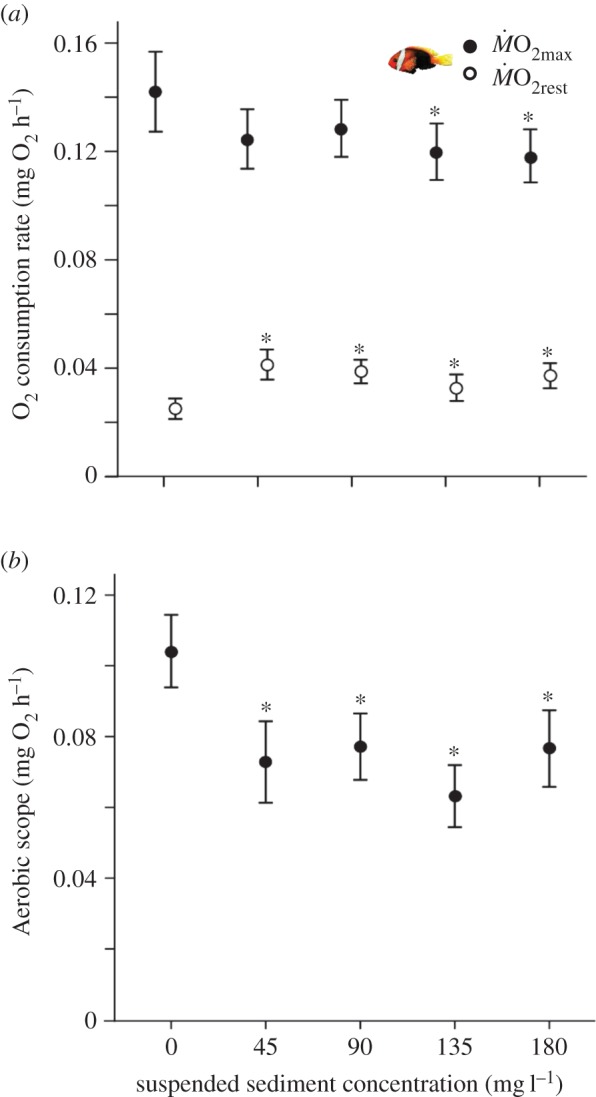
Effects of suspended sediments on oxygen consumption of A. melanopus. (a) Maximum oxygen consumption rates (ṀO2max, filled circles) and resting oxygen consumption rates (ṀO2rest, open circles), and (b) aerobic scope. Circles and bars represent intercepts and standard errors, respectively, as estimated by general and linear mixed models after accounting for fish mass. Asterisks indicate significant differences when compared with the control group at α < 0.05. (Online version in colour.)
To further explore the connection between oxygen consumption rates and changes in gill morphology, we analysed the relationship between aerobic scope and the squared mean functional lamellar length (as a proxy for gill surface area) of A. melanopus using linear regressions. Separate analyses were used for A. melanopus exposed to 0 and 180 mg l−1 suspended sediments. While there was a significant correlation between aerobic scope and squared mean functional lamellar length in A. melanopus juveniles exposed to control conditions (R2 = 0.34, F1,13 = 6.82, p = 0.02), these two variables were not significantly correlated in juveniles exposed to 180 mg l−1 suspended sediments (R2 = 0.00, F1,8 = 0.03; p = 0.85).
4. Discussion
Changes in the gill structure of fishes exposed to elevated suspended sediment concentrations have been hypothesized to reduce metabolic performance and thereby lead to declines in the diversity and abundance of fish assemblages on turbid reefs [26,28,46]. Our results confirm that short-term exposure to suspended sediments led to changes in the gill structure of all three examined species; however, these morphological changes only translated to impaired metabolic performance (i.e. oxygen consumption rates) in one species (A. melanopus). These findings imply that species that are sensitive to changes in gill structure such as A. melanopus may decline in abundance as reefs become more turbid, whereas species that are able to maintain metabolic performance despite suspended sediment exposure, such as Acanthochromis polyacanthus or A. percula, may be able to persist or gain a competitive advantage. Our results highlight that suspended sediment can have direct physiological consequences for some coral reef fish and provide further insights into how reduced water quality can shape coral reef communities.
The interspecific variation in the effect of suspended sediment exposure on oxygen uptake rates observed in this study may be related to differences in environmental tolerances among species. Following suspended sediment exposure, A. melanopus exhibited increases in resting oxygen consumption rates and reductions in both maximum oxygen consumption rates and aerobic scope. By contrast, the congener A. percula and confamilial A. polyacanthus both maintained oxygen uptake rates despite similar changes in gill structure. The widespread distribution pattern of A. polyacanthus spanning both turbid inshore and clear offshore reefs may explain the high tolerance of this species to suspended sediments [61]. The differential responses of the closely related A. percula and A. melanopus, however, are counter to expectations based on their current distributions. Although the current distribution of A. melanopus includes turbid reefs [62], this species was the most heavily affected by suspended sediments in the present study. By contrast, the species least affected by suspended sediments, A. percula, is typically rare in turbid environments [61], and the effects of suspended sediments on gills may hence not influence the distribution of this species. Understanding which species are sensitive versus those that are tolerant to changes in suspended sediment concentrations will be critical for the effective conservation of inshore coral reefs.
Our results document that juvenile A. melanopus exposed to elevated suspended sediment concentrations had a reduced capacity for oxygen uptake, as indicated by reductions in maximum oxygen consumption rates (i.e. ṀO2max). This may have been the result of gill damage, leading to the observed reductions in lamellar length and thus a reduction in gill surface area available for gas exchange. Aerobic scope was positively correlated with the squared functional lamellar length (as a proxy for gill surface area) in control fish, supporting the idea that the reduction in ṀO2max may have been caused by gill damage. Interestingly, there was no significant relationship between aerobic scope and functional lamellar length in fish after suspended sediment exposure. The reasons for this are currently not known. However, it is important to note that A. melanopus also showed reductions in oxygen diffusion distances in response to suspended sediments. This would have probably enhanced oxygen uptake efficiency, i.e. reduced oxygen diffusion distances may have partly compensated for reductions in gill surface area [35] and may have weakened the relationship between aerobic scope and functional lamellar length. An alternative hypothesis for the drivers of the observed reduction in ṀO2max could be that suspended sediment exposure may have elicited a stress response, which may have influenced ṀO2max. However, there is limited information available on the effects of stress on ṀO2max. Considering the multiple stressors that fish experience on degraded reefs, this would be an interesting avenue for further research. Regardless of the underlying mechanisms, a reduced ṀO2max suggests that oxygen delivery to tissues may be insufficient during activities with elevated oxygen demand, such as during swimming [43,63].
Individuals exposed to suspended sediments exhibited increased oxygen consumption rates at rest (i.e. ṀO2rest), which is commonly observed in fish exposed to poor water quality (e.g. [64–66]). This finding indicates that inhabiting areas with elevated suspended sediments incurs an energetic cost. The increase in ṀO2rest may have been the result of a stress response, behavioural and physiological acclimation to suspended sediments, tissue repair at the gills, an enhanced immune response and/or inefficient oxygen uptake [67,68]. Furthermore, the reduction in aerobic scope of A. melanopus exposed to suspended sediments suggests a compromised capacity to perform vital aerobic activities, such as growth, development and locomotion [69]. A reduction in aerobic scope may be especially problematic in juvenile fish, which exhibit exceptionally high growth rates [70] but low survival rates [71]. Factors that reduce growth or survival during early life-history stages can have strong effects on recruitment patterns [72,73]. While A. melanopus is common on turbid reefs today [64], our findings suggest that this species could decline in abundance as suspended sediments continue to increase in the future, or may shift to alternative/less suitable habitats (e.g. reefs further offshore). Any potential habitat shifts by A. melanopus are likely to increase competition among anemone fish species for host anemones in these habitats and (depending on the outcomes) may interfere with the other species' niche ranges.
In contrast to A. melanopus, exposing juvenile A. percula to suspended sediments had no detectable effect on the lamellar length and, hence, surface area for gas exchange, or oxygen consumption rates. This is counter to expectations, given the current distribution of A. percula. The reason why A. percula, unlike A. melanopus and A. polyacanthus, did not exhibit any reductions in lamellar length on suspended sediment exposure remains unclear. However, A. percula juveniles were on average larger in size than A. melanopus and A. polyacanthus, and may thus potentially have been more tolerant [3]. Regardless of the underlying mechanism, these findings highlight that there are interspecific variations in response to suspended sediments. Considering the limited impact of suspended sediments on the physiology of A. percula in the present study, its absence on turbid reefs is most probably driven by other factors, such as the habitat preferences of its primary host, the sea anemone Heteractis magnifica, which requires clear water [61]. However, the present study may have underestimated the effects of suspended sediments on oxygen consumption rates of fish. It is well known that suspended sediments adhere to the mucous layer on the gill epithelium [24]; it was not possible in the present study to examine whether this may have reduced oxygen uptake, because oxygen consumption rates were determined in the absence of suspended sediments. It will be important for future studies to examine whether the presence of suspended sediments due to technical limitations may affect oxygen uptake, and whether this may compound the negative effects of gill changes induced by suspended sediments.
Following exposure to elevated suspended sediments, A. polyacanthus exhibited similar changes in gill structure (i.e. reduced functional lamellar length) to A. melanopus; however, these changes did not translate to any negative effects on metabolic performance, and the reasons behind this are currently unclear. As mentioned above, it is possible that the observed reductions in oxygen diffusion distances (i.e. a thinner gill epithelium) may have compensated for declines in oxygen uptake due to reductions in surface area. Furthermore, oxygen uptake capacity is also influenced by factors other than gill structure, including haemoglobin oxygen-carrying capacity and affinity, heart rate, stroke volume and cardiac output [34,74]; some or all of these traits may have also been modified to enhance oxygen transport. It is also possible that the capacity for oxygen uptake at the gills in this species may be much higher than either the capacity to deliver oxygen to tissues or the maximum oxygen demand of tissues [40,75]. Not all species perfuse all of the available lamellae and thus do not use their gill surface area to its full potential [76,77]. This may allow them to tolerate some gill damage without adversely affecting oxygen uptake [28]. While the underlying mechanisms are not fully resolved, it is likely that the ability to tolerate or compensate for changes in gill structure may allow A. polyacanthus to persist on reefs that become increasingly more turbid, which could provide an important competitive advantage to this species.
The underlying mechanisms that led to a reduction in oxygen diffusion distances on suspended sediment exposure in all three species are not clear. The onset and intensity of gill changes in response to suspended sediments vary considerably between species, life stages, angularity of sediment particles, sediment concentration and exposure duration [3,78,79]. The reported structural changes induced by suspended sediments, however, are non-specific [24,51], and the majority of studies examining the effects of prolonged exposure to suspended sediments (greater than 3 days) have documented the growth of protective cell layers, i.e. an increase—rather than a decrease—in oxygen diffusion distances (e.g. [28,30,80]). For example, an increase in oxygen diffusion distances was observed in a previous study on settlement-stage A. percula exposed to the same sediment type, exposure duration and concentration (yet at an earlier developmental stage) as used in the present study [26]. The only study that has reported a reduction in oxygen diffusion distances in response to prolonged suspended sediment exposure (six weeks) examined this in green grouper (Epinephelus coioides), a coral reef fish living on turbid inshore reefs [25]. The observed reductions in oxygen diffusion distances in green grouper and the three coral reef fishes examined in the present study may have been caused by tissue abrasion [25]. However, when exposed to hypoxia [24,36], elevated temperatures [37], air [38,39] and even some pollutants [81], some fish species are known to actively alter oxygen diffusion distances to regulate oxygen uptake. As suggested by Au et al. [25], the observed reductions in oxygen diffusion distances may have thus been the result of a rearrangement of cell layers to enhance oxygen uptake, rather than gill damage (see also [40,41,81]). Regardless of the underlying mechanisms, reductions in oxygen diffusion distances may enhance the susceptibility of fish to parasites and pathogens and may interfere with ion- and osmoregulation [41].
5. Conclusion
Up to half of the world's coral reefs are threatened by poor water quality [82], with continued and rapid expansions of coastal human populations likely to exacerbate the inputs and resuspension of terrestrial sediments [2,6]. Identifying the mechanisms that drive changes in coral reef fish assemblages in response to human impacts is of key interest for the conservation of coral reefs [83,84]. Our findings suggest that A. melanopus, a species frequently found on turbid reefs today [61], may decline in abundance or disappear from turbid reefs as suspended sediments increase, owing to the impacts of suspended sediments on the oxygen uptake rates of juveniles. Other species, such as A. polyacanthus or A. percula, may be insensitive to short-term exposure to suspended sediments and may remain unaffected or even gain a competitive advantage under certain conditions. However, while juvenile A. polyacanthus and A. percula did not suffer any reductions in metabolic performance upon suspended sediment exposure, the observed changes in gill structure may potentially compromise their capacity to cope with other environmental factors that affect oxygen demand or oxygen availability, such as increasing water temperatures, ocean acidification or falling dissolved oxygen levels caused by climate change [85]. The combined effects of suspended sediments, elevated temperature and hypoxia on gill structure and function of reef fish will thus be important to consider in future studies. The present study shows that investigating the effects of suspended sediments on physiological processes plays an important role in the identification of winners and losers under future suspended sediment conditions and can provide crucial information for the conservation of coral reefs.
Supplementary Material
Acknowledgements
We thank Ben Lawes, Simon Wever and Andrew Thompson for their technical support and advice, Sue Reilly for guidance on histological methods, Rhondda Jones for statistical advice and two anonymous reviewers for their comments on earlier versions of this manuscript.
Ethics
This research has been conducted according to the Australian code for the care and use of animals for scientific purposes and has been approved by the Animal Ethics Committee at James Cook University (animal ethics approval no. A2218).
Data accessibility
Datasets used in this article have been archived in the Research Data Repository (Tropical Data Hub) at James Cook University, and can be accessed through the following link: http://dx.doi.org/10.4225/28/59364b00c5bce.
Author contributions
S.H., A.S.H., A.S.W. and J.L.R. conceived and designed the study. S.M. and S.H. raised fish. S.H. conducted experiments, performed respirometry and carried out the statistical analyses. L.P. analysed gill morphology. All the authors wrote the manuscript and gave their final approval.
Competing interests
The authors have no competing interests.
Funding
This research was supported by funding from an Australian Research Council (ARC) Super Science Fellowship and Early Career Discovery Award to J.L.R., an infrastructure and research allocation from the ARC Centre of Excellence for Coral Reef Studies to J.L.R., a student grant (Higher Degree Research Enhancement Scheme) allocated to S.H. by the ARC Centre of Excellence for Coral Reef studies and a JCU Postgraduate Research Scholarship awarded to S.H. by JCU.
References
- 1.Syvitski JPM, Vorosmarty CJ, Kettner AJ, Green P. 2005. Impact of humans on the flux of terrestrial sediment to the global coastal ocean. Science 308, 376–380. ( 10.1126/science.1109454) [DOI] [PubMed] [Google Scholar]
- 2.Halpern BS, et al. 2008. A global map of human impact on marine ecosystems. Science 319, 948–952. ( 10.1126/science.1149345) [DOI] [PubMed] [Google Scholar]
- 3.Wenger AS, et al. 2017. A critical analysis of the direct effects of dredging on fish. Fish and Fisheries 18, 967–985. ( 10.1111/faf.12218) [DOI] [Google Scholar]
- 4.Foley JA, et al. 2005. Global consequences of land use. Science 309, 570–574. ( 10.1126/science.1111772) [DOI] [PubMed] [Google Scholar]
- 5.Bartley R, Bainbridge ZT, Lewis SE, Kroon FJ, Wilkinson SN, Brodie JE, Silburn DM. 2014. Relating sediment impacts on coral reefs to watershed sources, processes and management: a review. Sci. Total Environ. 468, 1138–1153. ( 10.1016/j.scitotenv.2013.09.030) [DOI] [PubMed] [Google Scholar]
- 6.Lutz W, Samir KC. 2010. Dimensions of global population projections: what do we know about future population trends and structures? Phil. Trans. R. Soc. B 365, 2779–2791. ( 10.1098/rstb.2010.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabricius KE. 2005. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bull. 50, 125–146. ( 10.1016/j.marpolbul.2004.11.028) [DOI] [PubMed] [Google Scholar]
- 8.Wenger AS, Fabricius KE, Jones GP, Brodie JE. 2015. Effects of sedimentation, eutrophication and chemical pollution on coral reef fishes. In Ecology of fishes on coral reefs (ed. Mora C.), pp. 145–153. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 9.Fabricius K, De'ath G, McCook L, Turak E, Williams DM. 2005. Changes in algal, coral and fish assemblages along water quality gradients on the inshore Great Barrier Reef. Mar. Pollut. Bull. 51, 384–398. ( 10.1016/j.marpolbul.2004.10.041) [DOI] [PubMed] [Google Scholar]
- 10.Cheal AJ, Emslie M, MacNeil MA, Miller I, Sweatman H. 2013. Spatial variation in the functional characteristics of herbivorous fish communities and the resilience of coral reefs. Ecol. Appl. 23, 174–188. ( 10.1890/11-2253.1) [DOI] [PubMed] [Google Scholar]
- 11.Mallela J, Roberts C, Harrod C, Goldspink CR. 2007. Distributional patterns and community structure of Caribbean coral reef fishes within a river-impacted bay. J. Fish Biol. 70, 523–537. ( 10.1111/j.1095-8649.2007.01323.x) [DOI] [Google Scholar]
- 12.Letourneur Y, Kulbicki M, Labrosse P. 1998. Spatial structure of commercial reef fish communities along a terrestrial runoff gradient in the northern lagoon of New Caldonia. Environ. Biol. Fishes 51, 141–159. ( 10.1023/A:1007489502060) [DOI] [Google Scholar]
- 13.Beger M, Possingham HP. 2008. Environmental factors that influence the distribution of coral reef fishes: modeling occurrence data for broad-scale conservation and management. Mar. Ecol. Progress Series 361, 1–13. ( 10.3354/meps07481). [DOI] [Google Scholar]
- 14.Erftemeijer PLA, Riegl B, Hoeksema BW, Todd PA. 2012. Environmental impacts of dredging and other sediment disturbances on corals: a review. Mar. Pollut. Bull. 64, 1737–1765. ( 10.1016/j.marpolbul.2012.05.008) [DOI] [PubMed] [Google Scholar]
- 15.Wilson SK, Fisher R, Pratchett MS, Graham NAJ, Dulvy NK, Turner RA, Cakacaka A, Polunin NVC, Rushton SP. 2008. Exploitation and habitat degradation as agents of change within coral reef fish communities. Glob. Change Biol. 14, 2796–2809. ( 10.1111/j.1365-2486.2008.01696.x) [DOI] [Google Scholar]
- 16.Pratchett MS, Hoey AS, Wilson SK, Messmer V, Graham NA. 2011. Changes in biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity 3, 424–452. ( 10.3390/d3030424) [DOI] [Google Scholar]
- 17.Hamilton RJ, Almany GR, Brown CJ, Pita J, Peterson NA, Choat JH. 2017. Logging degrades nursery habitat for an iconic coral reef fish. Biol. Conserv. 210, 273–280. ( 10.1016/j.biocon.2017.04.024). [DOI] [Google Scholar]
- 18.Wenger AS, Johansen J, Jones G. 2011. Suspended sediment impairs habitat choice and chemosensory discrimination in two coral reef fishes. Coral Reefs 30, 879–887. ( 10.1007/s00338-011-0773-z) [DOI] [Google Scholar]
- 19.O'connor JJ, Lecchini D, Beck HJ, Cadiou G, Lecellier G, Booth DJ, Nakamura Y. 2016. Sediment pollution impacts sensory ability and performance of settling coral-reef fish. Oecologia 180, 11–21. ( 10.1007/s00442-015-3367-6) [DOI] [PubMed] [Google Scholar]
- 20.Wenger AS, Johansen JL, Jones GP. 2012. Increasing suspended sediment reduces foraging, growth and condition of a planktivorous damselfish. J. Exp. Mar. Biol. Ecol. 428, 43–48. ( 10.1016/j.jembe.2012.06.004) [DOI] [Google Scholar]
- 21.Wenger A, McCormick M, McLeod I, Jones G. 2013. Suspended sediment alters predator–prey interactions between two coral reef fishes. Coral Reefs 32, 369–374. ( 10.1007/s00338-012-0991-z) [DOI] [Google Scholar]
- 22.Johansen J, Jones G. 2013. Sediment-induced turbidity impairs foraging performance and prey choice of planktivorous coral reef fishes. Ecol. Appl. 23, 1504–1517. ( 10.1890/12-0704.1) [DOI] [PubMed] [Google Scholar]
- 23.Wilson JM, Laurent P. 2002. Fish gill morphology: inside out. J. Exp. Zool. Part A Ecol. Genet. Physiol. 293, 192–213. ( 10.1002/jez.10124) [DOI] [PubMed] [Google Scholar]
- 24.Heath AG. 1995. Water pollution and fish physiology. Boca Raton, FL: CRC Press. [Google Scholar]
- 25.Au D, Pollino C, Wu R, Shin P, Lau S, Tang J. 2004. Chronic effects of suspended solids on gill structure, osmoregulation, growth, and triiodothyronine in juvenile green grouper Epinephelus coioides. Mar. Ecol. Progress Series 266, 255–264. ( 10.3354/meps266255). [DOI] [Google Scholar]
- 26.Hess S, Wenger AS, Ainsworth TD, Rummer JL. 2015. Exposure of clownfish larvae to suspended sediment levels found on the Great Barrier Reef: impacts on gill structure and microbiome. Sci. Rep. 5, 10561 ( 10.1038/srep10561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe M, Morrison M, Taylor R. 2015. Harmful effects of sediment-induced turbidity on juvenile fish in estuaries. Mar. Ecol. Progress Series 539, 241–254. ( 10.3354/meps11496) [DOI] [Google Scholar]
- 28.Cumming H, Herbert N. 2016. Gill structural change in response to turbidity has no effect on the oxygen uptake of a juvenile sparid fish. Conserv. Physiol. 4, cow033 ( 10.1093/conphys/cow033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lake RG, Hinch SG. 1999. Acute effects of suspended sediment angularity on juvenile coho salmon (Oncorhynchus kisutch). Can. J. Fish. Aquat. Sci. 56, 862–867. ( 10.1139/f99-024) [DOI] [Google Scholar]
- 30.Sutherland AB, Meyer JL. 2007. Effects of increased suspended sediment on growth rate and gill condition of two southern Appalachian minnows. Environ. Biol. Fishes 80, 389–403. ( 10.1007/s10641-006-9139-8) [DOI] [Google Scholar]
- 31.Evans DH, Piermarini PM, Choe KP. 2005. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 85, 97–177. ( 10.1152/physrev.00050.2003) [DOI] [PubMed] [Google Scholar]
- 32.Herbert D, Merkens J. 1961. The effect of suspended mineral solids on the survival of trout. Int. J. Air Water Pollut. 5, 46–55. [PubMed] [Google Scholar]
- 33.Servizi JA, Martens DW. 1987. Some effects of suspended Fraser River sediments on sockeye salmon (Oncorhynchus nerka). Canadian Special Publication of Fisheries and Aquatic Sciences 96, 254–264. [Google Scholar]
- 34.Hughes G, Morgan M. 1973. The structure of fish gills in relation to their respiratory function. Biol. Rev. 48, 419–475. ( 10.1111/j.1469-185X.1973.tb01009.x) [DOI] [Google Scholar]
- 35.Lappivaara J, Nikinmaa M, Tuurala H. 1995. Arterial oxygen tension and the structure of the secondary lamellae of the gills in rainbow trout (Oncorhynchus mykiss) after acute exposure to zinc and during recovery. Aquat. Toxicol. 32, 321–331. ( 10.1016/0166-445X(94)00097-A) [DOI] [Google Scholar]
- 36.Sollid J, De Angelis P, Gundersen K, Nilsson GE. 2003. Hypoxia induces adaptive and reversible gross morphological changes in Crucian carp gills. J. Exp. Biol. 206, 3667–3673. ( 10.1242/jeb.00594) [DOI] [PubMed] [Google Scholar]
- 37.Sollid J, Nilsson GE. 2006. Plasticity of respiratory structures—adaptive remodeling of fish gills induced by ambient oxygen and temperature. Respir. Physiol. Neurobiol. 154, 241–251. ( 10.1016/j.resp.2006.02.006) [DOI] [PubMed] [Google Scholar]
- 38.LeBlanc DM, Wood CM, Fudge DS, Wright PA. 2010. A fish out of water: gill and skin remodeling promotes osmo- and ionoregulation in the mangrove killifish Kryptolebias marmoratus. Physiol. Biochem. Zool. 83, 932–949. ( 10.1086/656307) [DOI] [PubMed] [Google Scholar]
- 39.Ong K, Stevens E, Wright P. 2007. Gill morphology of the mangrove killifish (Kryptolebias marmoratus) is plastic and changes in response to terrestrial air exposure. J. Exp. Biol. 210, 1109–1115. ( 10.1242/jeb.002238) [DOI] [PubMed] [Google Scholar]
- 40.Nilsson GE. 2007. Gill remodeling in fish–a new fashion or an ancient secret? J. Exp. Biol. 210, 2403–2409. ( 10.1242/jeb.000281) [DOI] [PubMed] [Google Scholar]
- 41.Nilsson GE, Dymowska A, Stecyk JA. 2012. New insights into the plasticity of gill structure. Respir. Physiol. Neurobiol. 184, 214–222. ( 10.1016/j.resp.2012.07.012) [DOI] [PubMed] [Google Scholar]
- 42.Killen SS, Glazier DS, Rezende EL, Clark TD, Atkinson D, Willener AS, Halsey LG, Kearney M, Bronstein JL. 2016. Ecological influences and morphological correlates of resting and maximal metabolic rates across teleost fish species. Am. Nat. 187, 592–606. ( 10.1086/685893) [DOI] [PubMed] [Google Scholar]
- 43.Metcalfe N, Van Leeuwen T, Killen S. 2016. Does individual variation in metabolic phenotype predict fish behaviour and performance? J. Fish Biol. 88, 298–321. ( 10.1111/jfb.12699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norin T, Clark T. 2016. Measurement and relevance of maximum metabolic rate in fishes. J. Fish Biol. 88, 122–151. ( 10.1111/jfb.12796) [DOI] [PubMed] [Google Scholar]
- 45.Roberts RJ. 2012. Fish pathology. New York, NY: John Wiley & Sons. [Google Scholar]
- 46.Wong CK, Pak IAP, Jiang Liu X. 2013. Gill damage to juvenile orange-spotted grouper Epinephelus coioides (Hamilton, 1822) following exposure to suspended sediments. Aquaculture Res. 44, 1685–1695. ( 10.1111/j.1365-2109.2012.03173.x) [DOI] [Google Scholar]
- 47.Larcombe P, Costen A, Woolfe KJ. 2001. The hydrodynamic and sedimentary setting of nearshore coral reefs, central Great Barrier Reef shelf, Australia: Paluma Shoals, a case study. Sedimentology 48, 811–835. ( 10.1046/j.1365-3091.2001.00396.x) [DOI] [Google Scholar]
- 48.Wolanski E, Fabricius KE, Cooper TF, Humphrey C. 2008. Wet season fine sediment dynamics on the inner shelf of the Great Barrier Reef. Estuarine, Coastal and Shelf Science 77, 755–762. ( 10.1016/j.ecss.2007.10.014) [DOI] [Google Scholar]
- 49.Bainbridge ZT, Wolanski E, Álvarez-Romero JG, Lewis SE, Brodie JE. 2012. Fine sediment and nutrient dynamics related to particle size and floc formation in a Burdekin River flood plume, Australia. Mar. Pollut. Bull. 65, 236–248. ( 10.1016/j.marpolbul.2012.01.043) [DOI] [PubMed] [Google Scholar]
- 50.Wenger AS, Whinney J, Taylor B, Kroon F. 2016. The impact of individual and combined abiotic factors on daily otolith growth in a coral reef fish. Sci. Rep. 6, 28875 ( 10.1038/srep28875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mallatt J. 1985. Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can. J. Fish. Aquat. Sci. 42, 630–648. ( 10.1139/f85-083) [DOI] [Google Scholar]
- 52.Steffensen JF. 1989. Some errors in respirometry of aquatic breathers: how to avoid and correct for them. Fish Physiol. Biochem. 6, 49–59. ( 10.1007/BF02995809) [DOI] [PubMed] [Google Scholar]
- 53.Niimi A, Beamish F. 1974. Bioenergetics and growth of largemouth bass (Micropterus salmoides) in relation to body weight and temperature. Can. J. Zool. 52, 447–456. ( 10.1139/z74-056) [DOI] [PubMed] [Google Scholar]
- 54.Roche DG, Binning SA, Bosiger Y, Johansen JL, Rummer JL. 2013. Finding the best estimates of metabolic rates in a coral reef fish. J. Exp. Biol. 216, 2103–2110 ( 10.1242/jeb.082925) [DOI] [PubMed] [Google Scholar]
- 55.Reidy S, Nelson J, Tang Y, Kerr S. 1995. Post-exercise metabolic rate in Atlantic cod and its dependence upon the method of exhaustion. J. Fish Biol. 47, 377–386 ( 10.1111/j.1095-8649.1995.tb01907.x) [DOI] [Google Scholar]
- 56.Clark TD, Sandblom E, Jutfelt F. 2013. Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J. Exp. Biol. 216, 2771–2782. ( 10.1242/jeb.084251) [DOI] [PubMed] [Google Scholar]
- 57.Killen SS, Mitchell MD, Rummer JL, Chivers DP, Ferrari MC, Meekan MG, McCormick MI. 2014. Aerobic scope predicts dominance during early life in a tropical damselfish. Funct. Ecol. 28, 1367–1376. ( 10.1111/1365-2435.12296) [DOI] [Google Scholar]
- 58.McLeod IM, Rummer JL, Clark TD, Jones GP, McCormick MI, Wenger AS, Munday PL. 2013. Climate change and the performance of larval coral reef fishes: the interaction between temperature and food availability. Conserv. Physiol. 1, cot024 ( 10.1093/conphys/cot024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Statist. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 60.Bolker BM. 2008. Ecological models and data in R. Princeton, NJ: Princeton University Press. [Google Scholar]
- 61.Brolund TM, Tychsen A, Nielsen LE, Arvedlund M. 2004. An assemblage of the host anemone Heteractis magnifica in the northern Red Sea, and distribution of the resident anemonefish. J. Mar. Biol. Assoc. UK 84, 671–674. ( 10.1017/S0025315404009737h) [DOI] [Google Scholar]
- 62.Fautin DC, Allen GR. 1992. Field guide to anemonefishes and their host sea anemones Perth, Australia: Western Australian Museum. [Google Scholar]
- 63.Pörtner HO, Farrell AP. 2008. Physiology and climate change. Science 322, 690–692. ( 10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 64.Davison W, Franklin CE, Mckenzie JC, Dougan MC. 1992. The effects of acute exposure to the water soluble fraction of diesel fuel oil on survival and metabolic rate of an Antarctic fish (Pagothenia borchgrevinki). Comp. Biochem. Physiol. C: Comp. Pharmacol. 102, 185–188. ( 10.1016/0742-8413(92)90061-B) [DOI] [Google Scholar]
- 65.Reid SM, Isaac G, Metikosh S, Evans J. 2003. Physiological response of rainbow trout to sediment released during open-cut pipeline water crossing construction. Water Qual. Res. J. Can. 38, 473–481. [Google Scholar]
- 66.Baum G, Kegler P, Scholz-Böttcher B, Alfiansah Y, Abrar M, Kunzmann A. 2016. Metabolic performance of the coral reef fish Siganus guttatus exposed to combinations of water borne diesel, an anionic surfactant and elevated temperature in Indonesia. Mar. Pollut. Bull. 110, 735–746. ( 10.1016/j.marpolbul.2016.02.078) [DOI] [PubMed] [Google Scholar]
- 67.Bonga SW. 1997. The stress response in fish. Physiol. Rev. 77, 591–625. [DOI] [PubMed] [Google Scholar]
- 68.Beyers DW, Rice JA, Clements WH, Henry CJ. 1999. Estimating physiological cost of chemical exposure: integrating energetics and stress to quantify toxic effects in fish. Can. J. Fish. Aquat. Sci. 56, 814–822. ( 10.1139/f99-006) [DOI] [Google Scholar]
- 69.Claireaux G, Lefrançois C. 2007. Linking environmental variability and fish performance: integration through the concept of scope for activity. Phil. Trans. R. Soc. B 362, 2031–2041. ( 10.1098/rstb.2007.2099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suthers I. 1998. Bigger? Fatter? Or is faster growth better? Considerations on condition in larval and juvenile coral-reef fish. Austral Ecol. 23, 265–273. ( 10.1111/j.1442-9993.1998.tb00730.x) [DOI] [Google Scholar]
- 71.McCormick MI, Hoey AS. 2004. Larval growth history determines juvenile growth and survival in a tropical marine fish. Oikos 106, 225–242. ( 10.1111/j.0030-1299.2004.13131.x) [DOI] [Google Scholar]
- 72.Jones GP. 1990. The importance of recruitment to the dynamics of a coral reef fish population. Ecology 71, 1691–1698. ( 10.2307/1937578) [DOI] [Google Scholar]
- 73.Schmitt RJ, Holbrook SJ. 1999. Mortality of juvenile damselfish: implications for assessing processes that determine abundance. Ecology 80, 35–50. ( 10.1890/0012-9658(1999)080%5B0035:MOJDIF%5D2.0.CO;2) [DOI] [Google Scholar]
- 74.Farrell A. 2009. Environment, antecedents and climate change: lessons from the study of temperature physiology and river migration of salmonids. J. Exp. Biol. 212, 3771–3780 ( 10.1242/jeb.023671) [DOI] [PubMed] [Google Scholar]
- 75.Goolish EM. 1995. The metabolic consequences of body size. Biochem. Mol. Biol. Fishes 4, 335–366. ( 10.1016/S1873-0140(06)80018-4) [DOI] [Google Scholar]
- 76.Randall D, Daxboeck C. 1984. 5 Oxygen and carbon dioxide transfer across fish gills. Fish Physiol. 10, 263–314. ( 10.1016/S1546-5098(08)60321-0) [DOI] [Google Scholar]
- 77.Bowden A, Gardiner N, Couturier C, Stecyk J, Nilsson G, Munday P, Rummer J. 2014. Alterations in gill structure in tropical reef fishes as a result of elevated temperatures. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 175, 64–71. ( 10.1016/j.cbpa.2014.05.011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Newcombe CP, MacDonald DD. 1991. Effects of suspended sediments on aquatic ecosystems. North Am. J. Fish. Manag. 11, 72–82. ( 10.1577/1548-8675(1991)011%3C0072:EOSSOA%3E2.3.CO;2) [DOI] [Google Scholar]
- 79.Newcombe CP, Jensen JO. 1996. Channel suspended sediment and fisheries: a synthesis for quantitative assessment of risk and impact. North Am. J. Fisheries Manag. 16, 693–727. ( 10.1577/1548-8675(1996)016%3C0693:CSSAFA%3E2.3.CO;2) [DOI] [Google Scholar]
- 80.Humborstad O-B, Jørgensen T, Grotmol S. 2006. Exposure of cod Gadus morhua to resuspended sediment: an experimental study of the impact of bottom trawling. Mar. Ecol. Progress Series 309 247–254. ( 10.3354/309247) [DOI] [Google Scholar]
- 81.Berry KLE.2017. Effects of coal contamination on tropical marine organisms. PhD thesis, James Cook University, Australia.
- 82.Burke L, Reytar K, Spalding M, Perry A. 2011. Reefs at risk revisited. Washington, DC: World Resources Institute. [Google Scholar]
- 83.McKenzie DJ, et al. 2016. Conservation physiology of marine fishes: state of the art and prospects for policy. Conservation Physiol. 4, cow046 ( 10.1093/conphys/cow046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Illing B, Rummer JL. 2017. Physiology can contribute to better understanding, management, and conservation of coral reef fishes. Conserv. Physiol. 5, cox005 ( 10.1093/conphys/cox005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Altieri AH, Gedan KB. 2015. Climate change and dead zones. Glob. Change Biol. 21, 1395–1406. ( 10.1111/gcb.12754) [DOI] [PubMed] [Google Scholar]
- 86.Hess S, Prescott LJ, Hoey AS, McMahon SA, Wenger AS, Rummer JL. 2017. Data from: Species-specific impacts of suspended sediments on gill structure and function in coral reef fishes Dryad Digital Repository. ( 10.4225/28/59364b00c5bce) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hess S, Prescott LJ, Hoey AS, McMahon SA, Wenger AS, Rummer JL. 2017. Data from: Species-specific impacts of suspended sediments on gill structure and function in coral reef fishes Dryad Digital Repository. ( 10.4225/28/59364b00c5bce) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Datasets used in this article have been archived in the Research Data Repository (Tropical Data Hub) at James Cook University, and can be accessed through the following link: http://dx.doi.org/10.4225/28/59364b00c5bce.


