Abstract
The formal (3+2) cycloaddition between terminal allenes and aryl or styryl gold(I) carbenes generated by a retro‐Buchner reaction of 7‐substituted 1,3,5‐cycloheptatrienes led to indenes and cyclopentadienes, respectively. These cycloaddition processes have been applied to the construction of the carbon skeleton of the cycloaurenones and the dysiherbols as well as to the total synthesis of (±)‐laurokamurene B.
Keywords: cycloaddition, cyclopentadienes, gold catalysis, indenes, total synthesis
We previously found that cationic gold(I) complexes promote the retro‐Buchner reaction of 7‐substituted 1,3,5‐cycloheptatrienes, such as 1 and 2, via their norcaradiene tautomers, leading to reactive metal carbenes1 [LAu=CHR]+ 3 and 4 (Scheme 1), which react with alkenes to give cyclopropanes2, 3 or undergo intramolecular Friedel–Crafts‐type reactions.4 Cyclopentenes were obtained by the formal (4+1) cycloaddition of gold(I) carbenes 3 with cyclobutenes or methylenecyclopropanes.5 We have now discovered that aryl and styryl gold(I) carbenes 3 and 4 react with allenes 5 to give highly substituted indenes6, 7 and cyclopentadienes,8, 9 respectively, by a formal (3+2) cycloaddition.
Scheme 1.
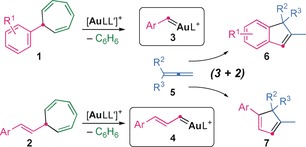
Formal (3+2) cycloaddition between allenes and both aryl and styryl gold(I) carbenes generated by a retro‐Buchner reaction.
Indenes are important motifs present in many biologically relevant natural products,10 and are building blocks in organic synthesis, organometallic chemistry, and in the field of materials science.11 Similarly, cyclopentadienes are important substrates, mainly as reactive diene components in the Diels–Alder reaction and as ligands in organometallic chemistry.12 To illustrate the application of the new gold(I)‐catalyzed (3+2) cycloaddition reactions in the context of natural product synthesis, we have developed a route for the construction of the tetracyclic carbon skeleton common to the cycloaurenones (8 a–c)13 and the dysiherbols (9 a–c),14 and a total synthesis of (±)‐laurokamurene B (10)15 (Figure 1).
Figure 1.
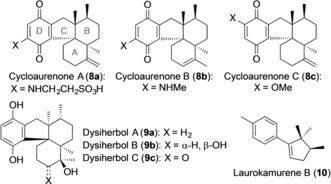
Cycloaurenones (8 a–c), dysiherbols (9 a–c), and laurokamurene B (10).
We first studied the reaction of 7‐(1‐napthyl)‐1,3,5‐cycloheptatriene (1 a) with allene 5 a with different gold(I) catalysts (Table 1). The reaction of 1 a with 5 a (2.0 equiv) in the presence of the gold(I) complex [(JohnPhos)Au(MeCN)]SbF6 (A; 5 mol %) in 1,2‐dichloroethane at 120 °C for 8 h gave indene 6 a in 66 % yield for the isolated product (Table 1, entry 1). Other gold(I) complexes B–F could also be used in the reaction (Table 1, entries 2–6), although none of them outperformed catalyst A. Complex G bearing a phosphite ligand failed to promote this transformation.
Table 1.
Catalyst screening for the reaction of 1 a with allene 5 a.[a]

| Entry | Catalyst | 6 a Yield [%][b] |
|---|---|---|
| 1 | A | 74 (66)[c] |
| 2 | B | 55 |
| 3 | C | 61 |
| 4 | D | 55 |
| 5 | E | 31 |
| 6 | F | 57 |
| 7 | G | –[d] |
[a] Reaction conditions: 1 a (0.1 m in DCE), 5 a (2.0 equiv), catalyst (5 mol %), 120 °C, 8 h. [b] The yield was determined by 1H NMR spectroscopy with 1,3,5‐trimethoxybenzene as an internal standard. [c] The yield of the isolated product is given in parentheses. [d] Not detected. Cy=cyclohexyl, DCE=1,2‐dichloroethane, Tf=trifluoromethanesulfonyl. 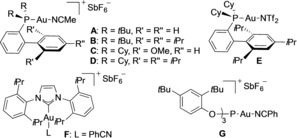
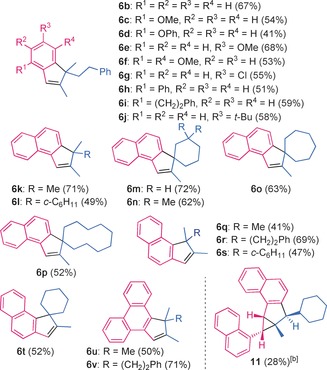
Indenes 6 b–v were obtained under the standard conditions in 41–72 % yield by the reaction of 1,1‐disubstituted allenes 5 a–g (see the Supporting Information for structures) with cycloheptatrienes with an unsubstituted, ortho‐ or para‐substituted, or ortho,meta‐disubstituted aryl group (substrates 1 b–j), a 1‐ or 2‐naphthyl group (substrates 1 a and 1 k), or a 9‐phenanthryl group (substrate 1 l; Table 2). This reaction is perfectly suited for the preparation of spiro compounds, such as 6 m–p and 6 t. The structure of indenes 6 d, 6 t, and 6 u was confirmed by single‐crystal X‐ray diffraction.16 Although the resulting indenes have a reactive double bond, 2:1 adducts were only observed as very minor products in the crude reaction mixtures. In contrast, a 2:1 adduct was obtained in the reaction of 1‐cyclohexylallene 5 h, a monosubstituted allene, with 1 a to form 11, whose relative configuration was determined by X‐ray diffraction.16
Table 2.
Scope of the gold(I)‐catalyzed synthesis of indenes.[a]
|
[a] Reaction conditions: 1 (0.1 m in 1,2‐dichloroethane), allene 5 (2.0 equiv), catalyst A (5 mol %), 120 °C, 8 h. Yields are for the isolated product. [b] Reaction time: 16 h.
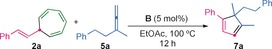
Styryl cycloheptatriene 2 a reacted with allene 5 a to give cyclopentadiene 7 a in 50 % yield in the presence of gold(I) complex B (5 mol %) in EtOAc at 100 °C (Table 3). The less bulky catalyst A performed similarly (Table 3, entry 2), whereas NHC gold(I) complexes, such as F, were less reactive (Table 3, entry 4).
Table 3.
Optimization of the synthesis of cyclopentadienes.[a]
| Entry | Deviation from above[a] | Yield [%][b] |
|---|---|---|
| 1 | none | 52 (50)[c] |
| 2 | catalyst A instead of B | 45 |
| 3 | catalyst D instead of B | –[d] |
| 4 | catalyst F instead of B | 17 |
| 5 | DCE instead of EtOAc | 25 |
| 6 | 90 °C | 33 |
| 7 | 120 °C | 40 |
[a] Reaction conditions: 2 a (0.1 m in EtOAc), 5 a (1.5 equiv), catalyst B (5 mol %), 100 °C, 12 h. [b] The yield was determined by GC–FID with diphenylmethane as an internal standard. [c] The yield of the isolated product is given in parentheses. [d] Not detected. FID=flame ionization detection.
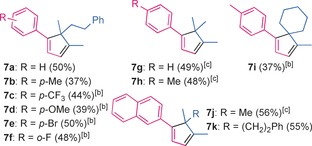
A range of styryl cycloheptatrienes gave rise to 1,1,2,5‐tetrasubstituted cyclopentadienes bearing both electron‐rich (products 7 b, 7 d) and electron‐poor substituents (products 7 c, 7 e, 7 f) in the aryl moiety in 37–56 % yield (Table 4).
Table 4.
Scope of the synthesis of cyclopentadienes.[a]
|
[a] Reaction conditions: 2 (0.1 m in EtOAc), allene 5 (1.5 equiv), catalyst B (5 mol %), 100 °C, 12 h. Yields are for the isolated product. [b] The reaction was carried out with 2.0 equivalents of the allene. [c] The reaction was carried out with 3.0 equivalents of the allene.
A Diels–Alder reaction between 7 g and maleic anhydride led to the crystalline endo adduct 12 in excellent yield (Scheme 2). The X‐ray diffraction structure of 12 16 allowed confirmation of the structures assigned to 7 a–k (Table 4).
Scheme 2.
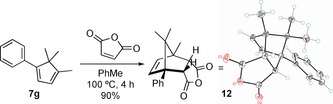
Diels–Alder reaction of 7 g with maleic anhydride.
Laurokamurene B (10), isolated from the red algae Laurencia okamurae, is a member of a small family of natural compounds displaying antifungal and cytotoxic activity.15 (±)‐Laurokamurene B (10) was readily synthesized in good yield by simple hydrogenation of cyclopentadiene 7 h in the presence of the Wilkinson catalyst (Scheme 3). Considering that (E)‐7‐(4‐methylstyryl)cyclohepta‐1,3,5‐triene (2 b), the cycloheptatriene required for the preparation of 7 h, can be readily obtained by the treatement of potassium (E)‐(4‐methylstyryl)trifluoroborate with tropylium tetrafluoroborate in almost quantitative yield,3 this total synthesis requires just three steps and provides (±)‐10 in 39 % overall yield from commercially available starting materials, which compares favorably with previous syntheses of (±)‐10.17
Scheme 3.
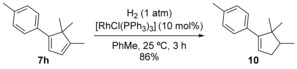
Total synthesis of (±)‐laurokamurene B (10).
Cycloaurenones A–C (8 a–c) feature a cis‐decalin moiety, whereas the dysiherbols (9 a–c) show trans fusion of the A/B rings (Figure 1). They also differ in their absolute configuration. These compounds are biogenetically related to other natural products isolated from sponges, such as (+)‐smenoqualone,18 ilimaquinone,19 and smenospongin.20 Many of these natural products display antimicrobial, anti‐HIV, anti‐inflamatory, antiproliferative, and antisecretory activities and have attracted the interest of synthetic chemists.21 However, no approach towards the synthesis of the cycloaurenones and the dysiherbols has been reported. As a first approach to the synthesis of these natural products, we considered applying the (3+2) cycloaddition together with an intramolecular radical cyclization to build up the carbocyclic core structure of 8 a–c and 9 a–c (Scheme 4).
Scheme 4.
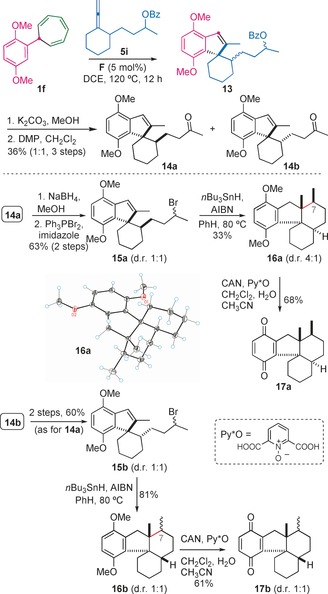
Assembly of the tetracyclic carbon skeleton of the cycloaurenones (structure 17 a) and dysiherbols (structure 16 b). AIBN= azobisisobutyronitrile, Bz=benzoyl.
The synthesis of allene 5 i was performed on a gram scale in seven steps from cyclohexanone, starting with the Michael addition of its cyclohexylimine to acrylonitrile.22, 23 The gold(I)‐catalyzed reaction between 5 i and cycloheptatriene 1 f was most efficient with [(IPr)Au(PhCN)]SbF6 (F) as the catalyst and gave spiroindene 13 as a mixture of four stereoisomers (Scheme 4).23 Cleavage of the benzoate and oxidation of the secondary alcohols with Dess–Martin periodinane (DMP) delivered ketones 14 a and 14 b in a 1:1 ratio and 36 % yield over three steps, after only one chromatographic purification. Ketone 14 a was reduced to the corresponding alcohol and then treated with triphenylphosphine dibromide to give alkyl bromide 15 a in 63 % yield over two steps. Treatment of 15 a with nBu3SnH in the presence of AIBN triggered a radical cyclization, which delivered the corresponding tetracyclic product 16 a in 33 % yield (4:1 mixture of epimers at C7). The major isomer displayed the configuration of cycloaurenones, as shown by X‐ray diffraction.16 Finally, oxidative deprotection of the methoxy groups with cerium ammonium nitrate (CAN) and pyridine‐2,6‐dicarboxylic acid N‐oxide (Py*O)24 led to quinone 17 a. By the same sequence of reactions, 16 b, corresponding to the tetracyclic carbon skeleton of the dysiherbols, was obtained as a 1:1 mixture of epimers through a remarkably efficient radical cyclization of 15 b (81 % yield).23 Oxidation of 16 b as before provided 17 b.
Both (3+2) cycloaddition reactions start with the gold(I)‐promoted retro‐Buchner reaction of 1 or 2 to release benzene and generate the gold(I) carbene 3 or 4, respectively, which undergoes electrophilic attack at the central carbon atom of allenes 5 to give an allyl cationic species Ia or Ib (Scheme 5). In the first case, intramolecular electrophilic aromatic substitution gives intermediate IIa, which can undergo rearomatization and protonolysis of the Au−C bond to form IIIa, which can undergo isomerization to give indene 6. Alternatively, protonation at the exocyclic double bond of IIa with concomitant deauration would directly furnish 6. For the cyclopentadiene synthesis, the cyclization of Ib would give IIb, which is converted into 7 by isomerization. Indeed, monitoring of the reaction by GC–MS allowed observation of the rapid formation of IIb (as a mixture of anti‐ and syn‐IIb when R2≠R3), which slowly underwent isomerization to 7. Intermediates IIb were also observed by 1H NMR spectroscopy, and their isomerization to 7 was found to be catalyzed by gold(I).23
Scheme 5.
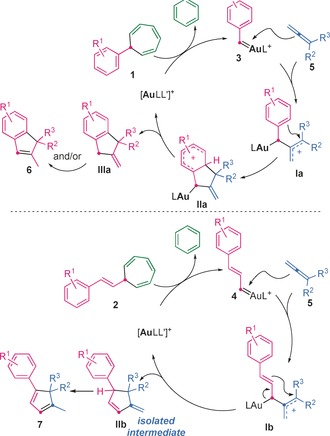
Proposed mechanisms for the formal (3+2) cycloaddition between allenes and aryl or styryl gold(I) carbenes.
In summary, two new gold(I)‐catalyzed formal (3+2) cycloaddition reactions have been developed between allenes and aryl or styryl gold(I) carbenes generated by a retro‐Buchner reaction of 7‐substituted cycloheptatrienes, thus leading to highly substituted indenes and cyclopentadienes, respectively. The usefulness of these new methods has been demonstrated by the shortest total synthesis of laurokamurene B reported to date and by the ready construction of the carbon skeleton of the cycloaurenones and the dysiherbols. Efforts directed towards the synthesis of these natural products are under way.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank the European Research Council (Advanced Grant No. 321066), MINECO/FEDER, UE (CTQ2016‐75960‐P), MINECO‐Severo Ochoa Excellence Accreditation 2014–2018, SEV‐2013‐0319), and the CERCA Program/Generalitat de Catalunya for financial support. We also thank the ICIQ X‐ray diffraction unit, CELLEX‐ICIQ HTE laboratory, and Dr. Fedor Miloserdov for helpful discussions.
X. Yin, M. Mato, A. M. Echavarren, Angew. Chem. Int. Ed. 2017, 56, 14591.
References
- 1.Review on new methods for the generation of metal carbenes: Jia M., Ma S., Angew. Chem. Int. Ed. 2016, 55, 9134–9166; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 9280–9313. [Google Scholar]
- 2.
- 2a. Solorio-Alvarado C. R., Echavarren A. M., J. Am. Chem. Soc. 2010, 132, 11881–11883; [DOI] [PubMed] [Google Scholar]
- 2b. Solorio-Alvarado C. R., Wang Y., Echavarren A. M., J. Am. Chem. Soc. 2011, 133, 11952–11955. [DOI] [PubMed] [Google Scholar]
- 3. Herlé B., Holstein P. M., Echavarren A. M., ACS Catal. 2017, 7, 3668–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.
- 4a. Wang Y., McGonigal P. R., Herlé B., Besora M., Echavarren A. M., J. Am. Chem. Soc. 2014, 136, 801–809; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4b. Lebœuf D., Gaydou M., Wang Y., Echavarren A. M., Org. Chem. Front. 2014, 1, 759–764. [Google Scholar]
- 5. Wang Y., Muratore M. E., Rong Z., Echavarren A. M., Angew. Chem. Int. Ed. 2014, 53, 14022–14026; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 14246–14250. [Google Scholar]
- 6.For the synthesis of indenes, see:
- 6a. Sue D., Kawabata T., Sasamori T., Tokitoh N., Tsubaki K., Org. Lett. 2010, 12, 256–258; [DOI] [PubMed] [Google Scholar]
- 6b. Martínez A., García-García P., Fernández-Rodríguez M. A., Rodríguez F., Sanz R., Angew. Chem. Int. Ed. 2010, 49, 4633–4637; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 4737–4741; [Google Scholar]
- 6c. Zeng X., Ilies L., Nakamura E., J. Am. Chem. Soc. 2011, 133, 17638–17640; [DOI] [PubMed] [Google Scholar]
- 6d. Dethe D. H., Murhade G. M., Ghosh S., J. Org. Chem. 2015, 80, 8367–8376; [DOI] [PubMed] [Google Scholar]
- 6e. Das B. G., Chirila A., Tromp M., Reek J. N. H., de Bruin B., J. Am. Chem. Soc. 2016, 138, 8968–8975; [DOI] [PubMed] [Google Scholar]
- 6f. Borie C., Vanthuyne N., Bertrand M. P., Siri D., Nechab M., ACS Catal. 2016, 6, 1559–1564; [Google Scholar]
- 6g. Ma B., Wu Z., Huang B., Liu L., Zhang J., Chem. Commun. 2016, 52, 9351–9354; [DOI] [PubMed] [Google Scholar]
- 6h. Gabriele B., Mancuso R., Veltri L., Chem. Eur. J. 2016, 22, 5056–5094; [DOI] [PubMed] [Google Scholar]
- 6i. Borie C., Ackermann L., Nechab M., Chem. Soc. Rev. 2016, 45, 1368–1386; [DOI] [PubMed] [Google Scholar]
- 6j. Barroso R., Paraja M., Cabal M.-P., Valdés C., Org. Lett. 2017, 19, 4086–4089. [DOI] [PubMed] [Google Scholar]
- 7.For the gold(I)-catalyzed (3+2) cycloaddition of α-aryl diazonitriles with allenamides to give 1-amino-1H-indenes, see: Singh R. R., Pawar S. K., Huang M.-J., Liu R.-S., Chem. Commun. 2016, 52, 11434–11437. [DOI] [PubMed] [Google Scholar]
- 8.For the synthesis of cyclopentadienes, see:
- 8a. Xi Z., Song Q., Chen J., Guan H., Li P., Angew. Chem. Int. Ed. 2001, 40, 1913–1916; [PubMed] [Google Scholar]; Angew. Chem. 2001, 113, 1967–1970; [Google Scholar]
- 8b. Datta S., Odedra A., Liu R.-S., J. Am. Chem. Soc. 2005, 127, 11606–11607; [DOI] [PubMed] [Google Scholar]
- 8c. Zhou S., Yan B., Liu Y., J. Org. Chem. 2005, 70, 4006–4012; [DOI] [PubMed] [Google Scholar]
- 8d. Zhang W., Luo S., Fang F., Chen Q., Hu H., Jia X., Zhai H., J. Am. Chem. Soc. 2005, 127, 18–19; [DOI] [PubMed] [Google Scholar]
- 8e. Lee J. H., Toste F. D., Angew. Chem. Int. Ed. 2007, 46, 912–914; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 930–932; [Google Scholar]
- 8f. Lv Y., Yan X., Yan L., Wang Z., Chen J., Deng H., Shao M., Zhang H., Cao W., Tetrahedron 2013, 69, 4205–4210; [Google Scholar]
- 8g. Cheng X., Zhu L., Lin M., Chen J., Huang X., Chem. Commun. 2017, 53, 3745–3748; [DOI] [PubMed] [Google Scholar]
- 8h. Schmidt E. Y., Bidusenko I. A., Ushakov I. A., Vashchenko A. V., Trofimov B. A., Org. Lett. 2017, 19, 3127–3130. [DOI] [PubMed] [Google Scholar]
- 9.For the gold(I)-catalyzed (3+2) cycloaddition of stabilized vinyldiazo compounds with allenes to give substituted 4-methylenecyclopent-1-enes, see: López E., Lonzi G., González J., López L. A., Chem. Commun. 2016, 52, 9398–9401. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Clegg N. J., Paruthiyil S., Leitman D. C., Scanlan T. S., J. Med. Chem. 2005, 48, 5989–6003; [DOI] [PubMed] [Google Scholar]
- 10b. Voets M., Antes I., Scherer C., Müller-Vieira U., Biemel K., Marchais-Oberwinkler S., Hartmann R. W., J. Med. Chem. 2006, 49, 2222–2231; [DOI] [PubMed] [Google Scholar]
- 10c. Majetich G., Shimkus J. M., J. Nat. Prod. 2010, 73, 284–298; [DOI] [PubMed] [Google Scholar]
- 10d. Norrgård M. A., Mannervik B., J. Mol. Biol. 2011, 412, 111–120; [DOI] [PubMed] [Google Scholar]
- 10e. Chanda D., Saikia D., Kumar J. K., Thakur J. P., Agarwal J., Chanotiya C. S., Shanker K., Negi A. S., Bioorg. Med. Chem. Lett. 2011, 21, 3966–3969; [DOI] [PubMed] [Google Scholar]
- 10f. Seltzman H. H., Shiner C., Hirt E. E., Gilliam A. F., Thomas B. F., Maitra R., Snyder R., Black S. L., Patel P. R., Mulpuri Y., Spigelman I., J. Med. Chem. 2016, 59, 7525–7543; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10g. Herdman C. A., Strecker T. E., Tanpure R. P., Chen Z., Winters A., Gerberich J., Liu L., Hamel E., Mason R. P., Chaplin D. J., Trawick M. L., Pinney K. G., MedChemComm 2016, 7, 2418–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.
- 11a. O'Connor J. M., Casey C. P., Chem. Rev. 1987, 87, 307–318; [Google Scholar]
- 11b. Chen Z., Halterman R. L., J. Am. Chem. Soc. 1992, 114, 2276–2277; [Google Scholar]
- 11c. He Y., Zhao G., Peng B., Li Y., Adv. Funct. Mater. 2010, 20, 3383–3389; [Google Scholar]
- 11d. He Y., Chen H.-Y., Hou J., Li Y., J. Am. Chem. Soc. 2010, 132, 1377–1382; [DOI] [PubMed] [Google Scholar]
- 11e. He Y., Li Y., Phys. Chem. Chem. Phys. 2011, 13, 1970–1983; [DOI] [PubMed] [Google Scholar]
- 11f. Diesendruck C. E., Steinberg B. D., Sugai N., Silberstein M. N., Sottos N. R., White S. R., Braun P. V., Moore J. S., J. Am. Chem. Soc. 2012, 134, 12446–12449; [DOI] [PubMed] [Google Scholar]
- 11g. Dang J.-S., Wang W.-W., Zhao X., Nagase S., Org. Lett. 2014, 16, 170–173; [DOI] [PubMed] [Google Scholar]
- 11h. Trost B. M., Ryan M. C., Angew. Chem. Int. Ed. 2017, 56, 2862–2879; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 2906–2924. [Google Scholar]
- 12.
- 12a. Lauher J. W., Hoffmann R., J. Am. Chem. Soc. 1976, 98, 1729–1742; [Google Scholar]
- 12b. Metallocenes (Eds.: A. Togni, R. L. Halterman), Wiley-VCH, New York, 1998; [Google Scholar]
- 12c. Halterman R. L., Chem. Rev. 1992, 92, 965–994; [Google Scholar]
- 12d. Encyclopedia of Polymer Science and Technology, Wiley, Hoboken, 2007. pp. 313–314; [Google Scholar]
- 12e. Winterfeldt E., Chem. Rev. 1993, 93, 827–843; [Google Scholar]
- 12f. McKnight A. L., Waymouth R. M., Chem. Rev. 1998, 98, 2587–2598; [DOI] [PubMed] [Google Scholar]
- 12g. Resconi L., Cavallo L., Fait A., Piemontesi F., Chem. Rev. 2000, 100, 1253–1346; [DOI] [PubMed] [Google Scholar]
- 12h. Encyclopedia of Polymer Science and Technology, Wiley, Hoboken, 2007. pp. 313–314; [Google Scholar]
- 12i. Ye B., Cramer N., Science 2012, 338, 504–506. [DOI] [PubMed] [Google Scholar]
- 13. Kim C.-K., Woo J.-K., Kim S.-H., Cho E., Lee Y.-J., Lee H.-S., Sim C. J., Oh D.-C., Oh K.-B., Shin J., J. Nat. Prod. 2015, 78, 2814–2821. [DOI] [PubMed] [Google Scholar]
- 14. Jiao W.-H., Shi G.-H., Xu T.-T., Chen G.-D., Gu B.-B., Wang Z., Peng S., Wang S.-P., Li J., Han B.-N., Zhang W., Lin H.-W., J. Nat. Prod. 2016, 79, 406–411. [DOI] [PubMed] [Google Scholar]
- 15.
- 15a. Yu X.-Q., He W.-F., Liu D.-Q., Feng M.-T., Fang Y., Wang B., Feng L.-H., Guo Y.-W., Mao S.-C., Phytochemistry 2014, 103, 162–170; [DOI] [PubMed] [Google Scholar]
- 15b. Angawi R. F., Alarif W. M., Hamza R. I., Badria F. A., Ayyad S.-E. N., Helv. Chim. Acta 2014, 97, 1388–1395; [Google Scholar]
- 15c. Gribble G. W., Mar. Drugs 2015, 13, 4044–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CCDC 1571036, 1571032, 1571033, 1571035, 1571037, and 1571034 (6 d, 6 t, 6 u, 11, 12, and 16 a) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 17.
- 17a. Srikrishna A., Khan I. A., Babu R. R., Sajjanshetty A., Tetrahedron 2007, 63, 12616–12620; [Google Scholar]
- 17b. Srikrishna A., Beeraiah B., Babu R. R., Tetrahedron: Asymmetry 2008, 19, 624–627; [Google Scholar]
- 17c. Tallineau J., Bashiardes G., Coustard J.-M., Lecornué F., Synlett 2009, 2761–2764. [Google Scholar]
- 18. Bourguet-Kondracki M.-L., Martin M.-T., Guyot M., Tetrahedron Lett. 1992, 33, 8079–8080. [Google Scholar]
- 19. Capon R. J., MacLeod J. K., J. Org. Chem. 1987, 52, 5059–5060. [Google Scholar]
- 20. Kazlauskas R., Murphy P. T., Warren R. G., Wells R. J., Blount J. F., Aust. J. Chem. 1978, 31, 2685–2697. [Google Scholar]
- 21.
- 21a. Bruner S. D., Radeke H. S., Tallarico J. A., Snapper M. L., J. Org. Chem. 1995, 60, 1114–1115; [Google Scholar]
- 21b. Radeke H. S., Digits C. A., Bruner S. D., Snapper M. L., J. Org. Chem. 1997, 62, 2823–2831; [DOI] [PubMed] [Google Scholar]
- 21c. Poigny S., Guyot M., Samadi M., J. Org. Chem. 1998, 63, 5890–5894; [DOI] [PubMed] [Google Scholar]
- 21d. Ling T., Poupon E., Rueden E. J., Kim S. H., Theodorokis E. A., J. Am. Chem. Soc. 2002, 124, 12261–12267; [DOI] [PubMed] [Google Scholar]
- 21e. Ling T., Poupon E., Rueden E. J., Theodorokis E. A., Org. Lett. 2002, 4, 819–822; [DOI] [PubMed] [Google Scholar]
- 21f. Marcos I. S., Conde A., Moro R. F., Basabe P., Díez D., Urones J. G., Tetrahedron 2010, 66, 8280–8290; [Google Scholar]
- 21g. Speck K., Magauer T., Chem. Eur. J. 2017, 23, 1157–1165; [DOI] [PubMed] [Google Scholar]
- 21h. Speck K., Wildermuth R., Magauer T., Angew. Chem. Int. Ed. 2016, 55, 14131–14135; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 14337–14341; [Google Scholar]
- 21i. Katoh T., Atsumi S., Saito R., Narita K., Katoh T., Eur. J. Org. Chem. 2017, 3837–3849. [Google Scholar]
- 22. Woodmansee D. H., Muller M.-A., Neuburger M., Pfaltz A., Chem. Sci. 2010, 1, 72–78. [Google Scholar]
- 23.See the Supporting Information for details.
- 24.
- 24a. Syper L., Kloc K., Mlochowski J., Szulc Z., Synthesis 1979, 521–522; [Google Scholar]
- 24b. Kawamata Y., Yan M., Liu Z., Bao D.-H., Chen J., Starr J. T., Baran P. S., J. Am. Chem. Soc. 2017, 139, 7448–7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


