Abstract
Background
Poor adherence to topical therapy in psoriasis remains an issue; it is associated with poor clinical outcomes, reduced quality of life and increased costs. Treatment‐related factors leading to poor adherence include lack of efficacy, excessive time applying medication and poor cosmetic characteristics (e.g. slow absorption, greasiness).
Objective
To assess the topical treatment attributes that influence patient preference for fixed combination calcipotriol 50 μg/g (Cal) and betamethasone 0.5 mg/g as dipropionate (BD) foam vs. gel, as well as in comparison with the latest topical treatment (LTT) a patient received.
Methods
PSO‐INSIGHTFUL was a Phase IIIb, prospective, multicentre (Canada/Germany), open‐label, randomized, two‐arm crossover study in patients aged ≥18 years with mild‐to‐severe psoriasis (NCT02310646). Following a washout period of up to 4 weeks, patients were randomized 1 : 1 to once‐daily Cal/BD foam for 1 week, followed by Cal/BD gel for 1 week, or vice versa. Patients completed six questionnaires evaluating patient preferences.
Results
A total of 213 patients were randomized; 118 had received a topical treatment in the previous 3 months. Based on the Subject's Preference Assessment, 50% of patients preferred Cal/BD foam and 50% preferred Cal/BD gel. Based on the Topical Product Usability Questionnaire (TPUQ), overall mean scores were high for both Cal/BD foam and gel, and were often significantly in favour of both products compared with LTT. Greater differences between Cal/BD foam and gel vs. LTT occurred when the previous treatment was an ointment or cream. Cal/BD foam was generally preferred by younger patients (aged 18–39 years), whereas Cal/BD gel tended to be preferred by older patients (aged ≥40 years). Results from other questionnaires were aligned with the TPUQ.
Conclusions
Patients with psoriasis have diverse needs and different preferences for topical treatment. This knowledge may help prescribers to choose the right formulation for the right patient, potentially leading to improved adherence and better treatment outcomes.
Introduction
Topical therapies are the mainstay of psoriasis treatment, either as first‐line therapy for mild‐to‐moderate psoriasis or in combination with ultraviolet or systemic therapies in more severe disease.1, 2, 3 However, poor adherence to topical therapies remains a significant problem; indeed, some studies have reported adherence rates of just 40%–70%.4, 5, 6 Poor adherence leads to a number of additional issues, such as decreased clinical outcomes, reduced quality of life and increased costs.7, 8, 9 Adherence is influenced by multiple factors, which can be patient, disease or treatment related.10 Treatment‐related reasons include lack of efficacy, excessive time applying the medication and poor cosmetic characteristics.4 The vehicle used can therefore also impact adherence,11 as patients generally prefer a vehicle that is simple to apply, quickly absorbed and not greasy.12 However, patient preferences for topical treatments are different and may vary over time.13
The efficacy and safety of fixed combination calcipotriol 50 μg/g (Cal) and betamethasone 0.5 mg/g as dipropionate (BD) have been confirmed in a number of studies.14, 15, 16, 17, 18, 19, 20, 21 The ointment and gel formulations of this fixed combination are established first‐line treatments for psoriasis.22 An aerosol foam formulation, which was developed to enhance adherence and increase the therapeutic options available to patients, has been shown to be effective and well tolerated.17, 23 The Phase IIIb PSO‐INSIGHTFUL study was designed to assess the patient‐reported factors that influence preference following once‐daily topical treatment with Cal/BD foam and gel, whilst minimizing the impact of efficacy.
Methods
Patients
Eligible patients were aged ≥18 years with mild‐to‐severe psoriasis vulgaris of ≥6 months’ duration, involving 2%–30% body surface area (BSA; trunk and/or limbs) and with a modified (excluding the head, which was not treated) Psoriasis Area and Severity Index (mPASI) of ≥2. All patients were naïve to both Cal/BD foam and gel. Patients on stable doses of systemic antipsoriatic therapies, including biologic agents, who otherwise met inclusion criteria could be enrolled. Patients were not eligible if they had received any topical antipsoriatic treatment on the trunk and/or limbs within 2 weeks, psoralen plus UVA therapy within 4 weeks, or UVB therapy within 2 weeks before randomization. Other exclusion criteria included the following: planned excessive exposure of the treated area to sunlight; current diagnosis of guttate, erythrodermic, exfoliative or pustular psoriasis or other inflammatory skin disorders; disorders of calcium metabolism associated with hypercalcaemia; or hypersensitivity to any component of the investigational products. All patients provided written informed consent.
Study design
PSO‐INSIGHTFUL was a prospective, multicentre, Phase IIIb, open‐label, randomized, two‐arm crossover study (NCT02310646) (Fig. 1). Following a 4‐week washout period (if needed due to previous treatment), patients were randomized 1 : 1 (stratified by site, via a centralized randomization service and in accordance with a preplanned computer‐generated randomization schedule) to once‐daily Cal/BD foam for 1 week, followed by once‐daily Cal/BD gel for 1 week (group 1) or vice versa (group 2). A 1‐week treatment cycle was considered sufficient to assess the usability of each product while limiting the impact of treatment efficacy on preference. Patients were allowed to treat plaques on the trunk and/or limbs and were instructed not to apply Cal/BD foam and gel on the face, scalp, genitals or skin folds. The institutional review board or independent ethics committee of all investigational sites approved the protocol. The study was performed in accordance with the Declaration of Helsinki and good clinical practice.
Figure 1.
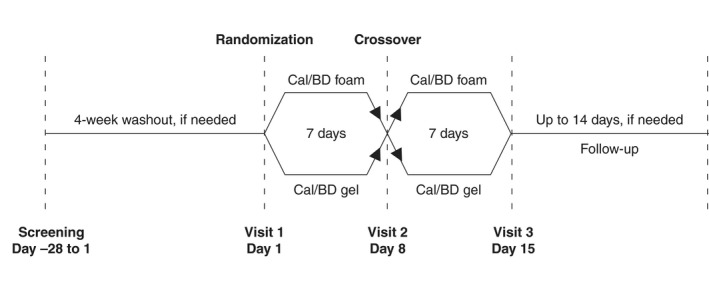
Study design.
Objectives and assessments
The overall objective was to assess the topical treatment attributes that influence patient preference for Cal/BD foam vs. gel and in both products vs. the latest topical treatment (LTT) received; to allow clear recall, the LTT should have been used within 3 months before baseline. Efficacy evaluation was not included as an endpoint.
Questionnaires
Patients completed six questionnaires. Two were in‐licensed (Dermatology Life Quality Index24 [DLQI] and Vehicle Preference Measure25 [VPM]), while four were developed by LEO Pharma in collaboration with an external research agency (Topical Product Usability Questionnaire [TPUQ], Comparison to Latest Topical Treatment [CLTT], Subject's Preference Assessment [SPA] and Subject's Assessment of Behaviour and Attitudes [SABA]). For the latter four questionnaires, items relevant for patient preference were selected based on a literature search of product attributes impacting usability. These items were then sorted into domains identified from in‐house knowledge at LEO Pharma and a review of relevant literature. Patient interviews were conducted to ensure that the questionnaires assessed relevant, clinically important issues, that the chosen domains were logical and that the questions and instructions for use were understandable; each questionnaire was also tested by nine selected patients. Finally, focus group interviews were conducted to evaluate the relevance and practicality of the questions and domains, ease of completion and if the length of the questionnaires was appropriate. The SABA aimed to gain insight into: (i) how patients’ lives were impacted by psoriasis; and (ii) patients’ attitudes to psoriasis and psoriasis treatments. The TPUQ, CLTT and SPA assessed how product attributes impacted usability. The literature search identified 25 items that were organized into four domains: ‘application’ (n = 9), ‘formulation’ (n = 9), ‘container’ (n = 4) and ‘satisfaction’ (n = 3). An additional item assessed overall experience/satisfaction/preference with Cal/BD foam and gel or LTT. All items were assessed in each of the questionnaires (except for ‘satisfaction’, which was not included in SPA).
Following randomization, patients completed the following:
SABA: Comprised 13 questions: six on the ‘impact of psoriasis’ (e.g. work/social life, sexual/social relationships, physical/mental wellbeing) whereby patients responded ‘yes’ or ‘no’, and seven on ‘attitudes to psoriasis and treatments’ using a five‐point scale ranging from −2, ‘strongly disagree’, to +2, ‘strongly agree’.
DLQI: Comprised 10 questions, each scored between 0 and 3; the final DLQI was calculated by summing the score of each question. A score of 0 or 1 (range 0–30) indicates no impact of psoriasis on the patient's life.
TPUQ: At baseline, the TPUQ assessed the LTT. Each patient assessed the extent to which they agreed with each of the 26 items using a five‐point scale ranging from −2, ‘strongly disagree’, to +2, ‘strongly agree’.
During visits to the clinic at the end of weeks 1 and 2, before the investigator assessments, patients completed three questionnaires based on their treatment experience during the previous 7 days:
TPUQ.
VPM: Comprised seven items that were assessed on a seven‐point scale ranging from −3, ‘extremely unappealing’, to +3, ‘extremely appealing’.
CLTT: Patients stated whether they preferred their LTT or Cal/BD foam/gel, or had no preference.
At the end of week 2 visit, patients also completed the following:
SPA: Comprised two parts: the patient indicated (i) if they preferred Cal/BD foam or gel based on the previous 14 days; and (ii) how much each of the 22 application‐related, formulation‐related and container‐related items contributed to their overall preference using a four‐point scale ranging from ‘very important’ to ‘not at all important’.
The TPUQ at baseline and CLTT were only completed if the patient had used topical treatment within 3 months before baseline.
Safety assessments
Safety was assessed by the evaluation of standard adverse events (AEs) and adverse drug reactions. AEs were coded based on Medical Dictionary for Regulatory Activities version 15.1.
Statistical analysis
For sample size calculations, the proportion of patients preferring Cal/BD foam over gel was assumed to be 0.70. With a sample size of 200, a two‐sided 95% confidence interval for the proportion of patients preferring Cal/BD foam would extend 0.064 from the observed proportion (normal approximation). With 100 patients in each group, Fisher's exact test with a two‐sided 5% significance level had 94% power to detect a difference between 50% in group 1 and 75% in group 2 preferring Cal/BD foam (nQuery Advisor® version 7.0).
For the SPA, individual baseline characteristics were examined in a two‐factor logistic regression model including treatment sequence and various baseline characteristics (gender, age, disease severity, psoriasis distribution, plaque size, plaque thickness and psoriasis onset age) as factors. For the TPUQ, the Wilcoxon signed rank test assessed the period differences for group 1 vs. group 2, as well as within‐subject differences to LTT. Summary scores were calculated by summing numeric scores for items under each domain. Within‐subject differences between Cal/BD foam and gel (TPUQ sum scores and overall satisfaction) and overall preferences (SPA) were analysed in exploratory analyses of variance using stepwise forward selection (entry significance level 5%); these analyses tested various baseline characteristics. Post hoc, TPUQ sum scores and overall satisfaction were analysed separately for Cal/BD foam and gel using exploratory analyses of variance with stepwise forward selection (entry significance level 1%). Missing values were not imputed, and summary scores (TPUQ and VPM) were not calculated for patients with any missing items. DLQI scores were not calculated for patients with more than one missing item.
The full analysis set (FAS) comprised all randomized patients who completed an on‐study questionnaire. The safety analysis set comprised all patients who received at least one dose of study medication and for whom postbaseline safety data were available. The LTT analysis set comprised all randomized patients who had used topical treatment within 3 months before baseline.
Results
Patients
Overall, 219 patients were enrolled in Canada and Germany between 10 February 2015 and 16 June 2015; six were screen failures (Fig. 2). The remaining 213 patients were randomized and all received at least one application of Cal/BD foam or gel. Two patients discontinued, one of whom was excluded from the FAS. Of the randomized patients, 118 had received a topical treatment in the previous 3 months. Most patients (57.5%) had psoriasis of moderate severity based on PGA, and 84% had the disease for >5 years (Table 1).
Figure 2.
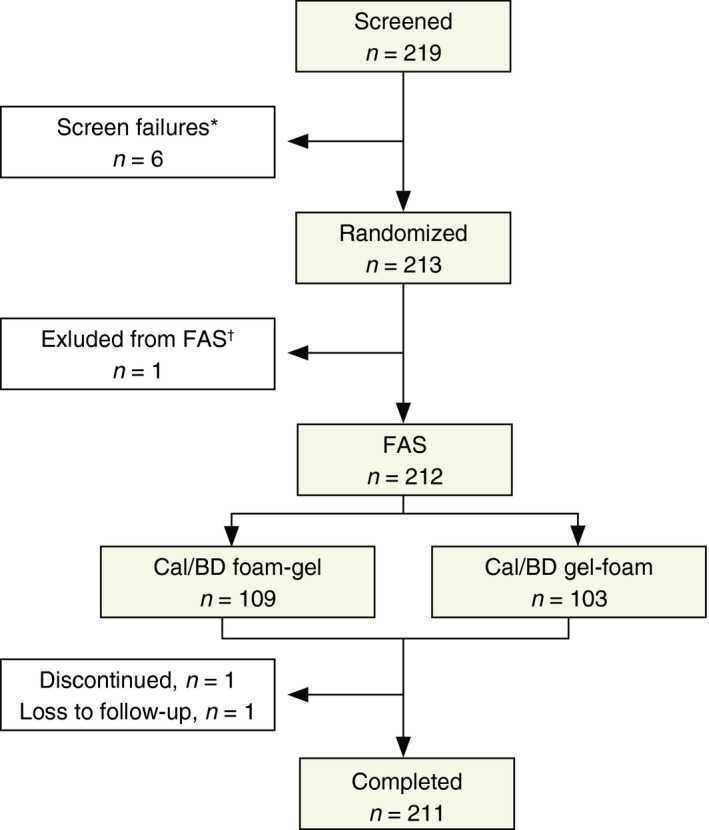
Patient disposition. *Four patients had previously received Cal/BD gel; one patient had an inflammatory skin disorder; one patient was unable to communicate with the investigator and understand and comply with the study requirements; †Patient did not complete any of the on‐treatment questionnaires.
Table 1.
Patient demographics and baseline characteristics (FAS)
| All patients (n = 212) | |
|---|---|
| Age category, n (%) | |
| 18–39 years | 48 (22.6) |
| 40–59 years | 92 (43.4) |
| ≥60 years | 72 (34.0) |
| Male : female, n (%) | 133 : 79 (63 : 37) |
| BMI, n (%) | |
| <25 kg/m2 | 37 (17.5) |
| 25–30 kg/m2 | 73 (34.4) |
| >30 kg/m2 | 102 (48.1) |
| PGA, n (%) | |
| Mild | 61 (28.8) |
| Moderate | 122 (57.5) |
| Severe | 29 (13.7) |
| Duration of psoriasis, n (%) | |
| <2 years | 4 (1.9) |
| 2–5 years | 30 (14.2) |
| >5 years | 178 (84.0) |
| BSA, n (%) | |
| <4% | 93 (43.9) |
| 4%–6% | 56 (26.4) |
| 6%–11% | 38 (17.9) |
| 11%–15% | 11 (5.2) |
| ≥15% | 14 (6.6) |
| mPASI, n (%) | |
| 2–5 | 86 (40.6) |
| 5.1–10 | 91 (42.9) |
| >10 | 35 (16.5) |
| Mean DLQI | 7.8 |
| Localized: widespread distribution of psoriasis, % | 62 : 38 |
BMI, body mass index; BSA, body surface area; DLQI, dermatology life quality index; FAS, full analysis set; mPASI, modified psoriasis and severity index; PGA, physician's global assessment of disease severity.
SABA: baseline behaviour and attitudes
Approximately 50% of patients reported that psoriasis impacts their ‘self‐confidence’ (59.0%), ‘emotional wellbeing’ (53.1%) and ‘social life’ (49.5%) (Table S1). Most patients were ‘keen to try the newest treatments available’ (90.0%) and reported that ‘being able to apply treatment quickly is very important to me’ (90.1%) (Table S1).
SPA: overall patient preferences
Based on the SPA, patient preference for Cal/BD foam and gel was 50 : 50 (Table 2); the treatment sequence did not significantly impact the responses. Logistic regression analyses demonstrated a significant age effect (P = 0.001), whereby younger patients preferred Cal/BD foam and older patients preferred gel (Table 2); there were no significant findings for other baseline characteristics. The reasons for the stated preferences were not very discriminatory. However, for Cal/BD foam, size of application area and items related to feelings of relief and soothing seemed to be of importance (Fig. S1). For Cal/BD gel, precision of application impacted patient preference (Fig. S2).
Table 2.
Overall patient preferences, by age, for Cal/BD foam or gel
| Cal/BD foam, % | Cal/BD gel, % | |
|---|---|---|
| All patients (n = 208) | 49.5 | 50.5 |
| Aged 18–39 years (n = 48) | 72.9 | 27.1 |
| Aged 40–59 years (n = 90) | 44.4 | 55.6 |
| Aged ≥60 years (n = 70) | 40.0 | 60.0 |
TPUQ: evaluation of study treatments
Mean application, container and satisfaction domain scores were high for both Cal/BD foam and gel (Table 3). There were significant differences in favour of Cal/BD foam for some formulation items (e.g. ‘immediate feeling of relief’ and ‘felt soothing’), and in favour of Cal/BD gel for some application (e.g. ‘ease of application’ and ‘ease of spreading’), container (‘accurately dispense wanted amount’) and formulation (e.g. ‘odourless’ and ‘not greasy’) items (Table 3). Satisfaction scores were similar between treatment groups.
Table 3.
Mean TPUQ scores, by domain, for Cal/BD foam and gel
| Cal/BD foam (n = 212) | Cal/BD gel (n = 212) | P value | |
|---|---|---|---|
| Application domain scores | |||
| Ease of application | 1.1 | 1.5 | ** |
| Ease of application on lesion only | 0.9 | 1.4 | *** |
| Ease of spreading | 1.5 | 1.7 | ** |
| Lack of mess | 0.8 | 1.0 | NS |
| Good for use on small areas | 1.0 | 1.4 | *** |
| Good for use on large areas | 1.4 | 1.5 | NS |
| Quick to apply | 1.4 | 1.4 | NS |
| Total time spent acceptable | 1.5 | 1.5 | NS |
| Easily incorporated into daily routine | 1.4 | 1.5 | NS |
| Formulation domain scores | |||
| Quickly absorbed | 0.7 | 0.7 | NS |
| Dried quickly | 0.5 | 0.5 | NS |
| Immediate feeling of relief | 1.0 | 0.7 | ** |
| Felt soothing | 1.2 | 1.0 | ** |
| Appealing to touch | 0.9 | 0.9 | NS |
| Felt moisturizing | 1.1 | 1.2 | NS |
| Not greasy | 0 | 0.3 | * |
| Odourless | 1.3 | 1.6 | *** |
| No staining | 1.0 | 1.0 | NS |
| Container domain scores | |||
| Easy to get medication out of container | 1.1 | 1.3 | NS |
| Easy to use | 1.1 | 1.4 | *** |
| Easy to keep clean | 1.2 | 1.4 | * |
| Accurately dispense wanted amount | 0.9 | 1.5 | *** |
| Satisfaction domain scores | |||
| Confidence in using | 1.2 | 1.2 | NS |
| Would use regularly | 1.3 | 1.3 | NS |
| Would recommend | 1.2 | 1.1 | NS |
| Overall satisfaction | 1.1 | 1.2 | NS |
*P < 0.05; **P < 0.01; ***P < 0.001.
Range: −2, ‘strongly disagree’ to +2, ‘strongly agree’.
NS, not significant; TPUQ, topical product usability questionnaire.
Multiple regression analyses for TPUQ score differences demonstrated that patients aged 18–39 years preferred Cal/BD foam, while patients aged 40–59 years and ≥60 years preferred Cal/BD gel (Table S2). Interestingly, a significant age effect was observed for Cal/BD gel (Table S3), but not for Cal/BD foam. The forward selection procedure also identified psoriasis distribution as a significant factor; there was a trend towards more favourable scores for Cal/BD foam in patients with localized distribution and in favour of gel for patients with widespread distribution (Table S4).
TPUQ: comparison with latest topical treatment
Mean TPUQ domain scores were often significantly in favour of both Cal/BD foam and gel compared with LTT (Table 4). Scores for Cal/BD gel were generally higher than for LTT. Most scores for Cal/BD foam were higher, although some related to ease of application and container items were comparable to LTT (Table 4). Greater differences between Cal/BD foam and gel vs. LTT occurred when the previous treatment was an ointment; smaller differences occurred when the LTT was ‘other’ (including solution, liquid, lotion or other; n = 12) (Table S5).
Table 4.
Mean TPUQ scores compared with LTT, by domain, for Cal/BD foam and gel
| LTT (n = 118) | Cal/BD foam (n = 116) | Cal/BD gel (n = 115) | |
|---|---|---|---|
| Application domain scores | |||
| Ease of application | 1.4 | 1.2 | 1.5 |
| Ease of application on lesion only | 1.3 | 0.9* | 1.4 |
| Ease of spreading | 1.5 | 1.5 | 1.7* |
| Lack of mess | 0.6 | 0.9 | 1.0** |
| Good for use on small areas | 1.1 | 1.0 | 1.4* |
| Good for use on large areas | 0.9 | 1.4*** | 1.5*** |
| Quick to apply | 1.2 | 1.5** | 1.3 |
| Total time spent acceptable | 1.1 | 1.6*** | 1.4** |
| Easily incorporated into daily routine | 1.0 | 1.5*** | 1.4*** |
| Formulation domain scores | |||
| Quickly absorbed | 0.2 | 0.7** | 0.6** |
| Dried quickly | 0 | 0.5** | 0.4** |
| Immediate feeling of relief | 0.1 | 1.1*** | 0.7*** |
| Felt soothing | 0.6 | 1.3*** | 1.0** |
| Appealing to touch | 0.2 | 1.0*** | 0.9*** |
| Felt moisturizing | 0.6 | 1.3*** | 1.2*** |
| Not greasy | −0.5 | 0.2*** | 0.2*** |
| Odourless | 1.2 | 1.3 | 1.5** |
| No staining | 0.4 | 0.9** | 0.9*** |
| Container domain scores | |||
| Easy to get medication out of container | 1.3 | 1.2 | 1.3 |
| Easy to use | 1.3 | 1.2 | 1.4 |
| Easy to keep clean | 1.1 | 1.3 | 1.3 |
| Accurately dispense wanted amount | 1.0 | 0.9 | 1.5*** |
| Satisfaction domain scores | |||
| Confidence in using | 0.6 | 1.3*** | 1.2*** |
| Would use regularly | 0.9 | 1.4** | 1.3* |
| Would recommend | 0.4 | 1.3*** | 1.0*** |
| Overall satisfaction | 0.3 | 1.2*** | 1.1*** |
*P < 0.05; **P < 0.01; ***P < 0.001 vs. LTT.
LTT included various corticosteroids (of different potencies) and combination products, with similar types of products in all categories (ointment, cream, ‘other’).
Range: −2, ‘strongly disagree’ to +2, ‘strongly agree’.
LTT, latest topical treatment; TPUQ, topical product usability questionnaire.
CLTT: patient preferences vs. latest topical treatment
Overall, the results from the CLTT were in line with the TPUQ. Patient preference was for Cal/BD foam and gel over LTT for most application and formulation items, and for all container and satisfaction items (Table S6). The highest preference rates for Cal/BD foam were for ‘immediate feeling of relief’ (71.6%), ‘felt soothing’ (70.7%), ‘would recommend’ (72.2%) and ‘overall experience’ (76.5%); for Cal/BD gel, they were ‘quick to apply’ (67.2%), ‘quickly absorbed’ (60.9%) and ‘overall experience’ (70.2%). The preference rates for Cal/BD foam and gel were usually higher when the LTT was ointment or cream (Table S7).
VPM: patient preferences for the vehicle
Overall, the results from the VPM were in line with the TPUQ. Mean VPM scores were generally high for both Cal/BD foam and gel (Table S8). The highest mean scores for foam were for ‘time it takes to apply’ and ‘how it feels on the skin’; for gel, they were ‘time it takes to apply’, ‘ease of application’ and ‘how it smells’. There were no major differences in VPM scores between Cal/BD foam and gel. The only significant difference was in favour of Cal/BD gel vs. foam for ‘how it smells’ (mean of 1.9 vs. 1.6; P = 0.003). Younger patients tended to give higher scores for Cal/BD foam, while older subjects tended to give higher scores for gel (Table S8). VPM scores tended to be higher for both Cal/BD foam and gel in patients with more severe disease (Table S9) and thicker lesions (Table S10).
Safety
Twenty‐one patients (9.9%) experienced a total of 21 AEs. Nasopharyngitis was the only AE experienced by >1 patient (n = 4). No serious AEs, severe AEs or AEs leading to withdrawal were observed. Two patients had investigator‐assessed treatment‐related AEs: one patient experienced mild folliculitis 3 days after starting treatment with Cal/BD foam, which had a duration of 7 days; one patient experienced moderate contact dermatitis on the same day that the Cal/BD gel treatment was initiated, which had a duration of 1 day.
Discussion
The PSO‐INSIGHTFUL study demonstrated that overall patient preference was similar between Cal/BD foam and gel, with both products scoring well on patient‐reported assessments of usability and satisfaction. This highlights that patients have different preferences for the topical treatment of psoriasis, suggesting that individualized approaches are important. This is in agreement with a previous preference study, which concluded that there is no ‘one size fits all’ approach in psoriasis and that patient needs should be considered when deciding treatment regimens.13 The current study was deliberately designed to minimize the impact of efficacy; however, as efficacy is an important factor in treatment adherence,4, 26 the results should be viewed in the context of the known efficacy profiles of Cal/BD foam17, 23 and gel.19, 21
The main drivers for patients preferring Cal/BD foam over gel were items related to sensation (i.e. ‘immediate feeling of relief’ and ‘felt soothing to my skin’), whereas Cal/BD gel scored significantly higher than foam for items related to ease of application, the ability to apply the medication on specific psoriatic lesions, and the user friendliness of the container. Cal/BD gel also scored significantly higher than foam for the formulation‐related items ‘not greasy’ and ‘odourless’. These findings agree with a previous analysis, in which patients rated the feel on the skin, the ease and speed of application, and messiness as important characteristics of a topical therapy.25 It is interesting to note that both Cal/BD foam and gel were preferred to the LTT that patients received, particularly when the LTT was an ointment or cream. This is consistent with previous studies showing lower patient preference scores for ointments than for foam, gel and solution vehicles, primarily owing to the stickiness and messiness of ointment formulations.25, 27 However, there is a potential selection bias in the comparison with LTT, as patients participating in the study may already have been dissatisfied with their last treatment options before enrolment.
Cal/BD gel was preferred by those with widespread distribution of psoriasis, whereas a trend for preference was noted for foam in patients with localized distribution. Combined with the preference data related to ease of application described above, this may suggest that application of Cal/BD gel is most suitable for patients with small lesions distributed widely over the body, whereas patients with larger lesions may prefer application of Cal/BD foam. Cal/BD foam was generally preferred by younger patients (aged 18–39 years), whereas Cal/BD gel tended to be preferred by older patients (aged ≥40 years). Although we can only speculate, older patients may prefer the gel formulation as they are more familiar with it and find it easier to apply than the newer foam formulation, while younger patients may be more open to using a newer formulation such as foam.
The results of the PSO‐INSIGHTFUL study should be interpreted with caution because of a lack of experience with the questionnaires used. Of the six questionnaires that were used to assess patient preferences, two were validated (the DLQI24 and VPM25); however, the remaining four questionnaires (SABA, TPUQ, CLTT and SPA) were developed by LEO Pharma based on work by Zschocke et al.,28 and were used for the first time in this study. Patient and focus group interviews were conducted to assess the construct and content validity of these four questionnaires to ensure that they assessed clinically important, relevant issues, that the domains were logical and that the questionnaires were understood by patients. The observed results are consistent across the validated and LEO‐generated questionnaires, suggesting that the data obtained from the latter are robust. In addition, the fact that there were many similar questions related to the application and formulation items may contribute to a high weighting of these questions on the sum scores that were used in the regression analyses. On a similar note, the specific reasons for patient preferences are not ranked, and it is difficult to discriminate between the factors driving preference; this may be related to a lack of sensitivity within the questionnaires. Finally, as the statistical analyses were not adjusted for multiple testing, all P values should be evaluated with this in mind.
In conclusion, the PSO‐INSIGHTFUL study demonstrates that patients with psoriasis have diverse needs and different preferences for topical treatment, which are influenced by several factors such as age, psoriasis distribution and LTT. This knowledge may help prescribers choose the right formulation for the right patient, which could potentially lead to improved adherence and better treatment outcomes.
Supporting information
Table S1. SABA responses at baseline (FAS)
Table S2. Difference in total TPUQ score between study treatments by age (FAS)
Table S3. Total TPUQ score by age: Cal/BD gel (FAS)
Table S4. Difference in total formulation score (TPUQ) between study treatments by psoriasis distribution phenotype (FAS)
Table S5. Total TPUQ score by LTT (LTT analysis set)
Table S6. Proportion of patients preferring Cal/BD foam or gel (CLTT) over LTT (LTT analysis set)
Table S7. Overall preference item (CLTT) by LTT (LTT analysis set)
Table S8. Mean (±SD) individual VPM scores by treatment and age category (FAS)
Table S9. Mean (±SD) individual VPM scores by treatment and baseline disease severity (FAS)
Table S10. Mean (±SD) individual VPM scores by treatment and thickness phenotype (FAS)
Fig. S1. Reasons for preference of Cal/BD foam (SPA): (a) Application; (b) Formulation; and (c) Container items (FAS)
Fig. S2. Reasons for preference of Cal/BD gel (SPA): (a) Application; (b) Formulation; (c) Container items (FAS)
Acknowledgements
Medical writing support was provided by Andrew Jones, PhD, from Mudskipper Business Limited. Kim A Papp was the international coordinating investigator for this study.
Conflicts of interest
C‐H.H has been a consultant, lecturer and advisory board member and has conducted clinical trials for LEO Pharma. K.A.P has been a consultant for LEO Pharma. K.W.L and P.S are employees of LEO Pharma. S.P has served as a consultant, speaker, investigator and advisory board member for LEO Pharma.
Funding sources
This study was sponsored by LEO Pharma. Medical writing support was funded by LEO Pharma.
References
- 1. Canadian Psoriasis Guidelines Committee . Canadian guidelines for the management of plaque psoriasis.[WWW document] 2009. http://www.dermatology.ca/wp-content/uploads/2012/01/cdnpsoriasisguidelines.pdf (Last accessed: 31 August 2017)
- 2. Committee Canadian Psoriasis Guidelines . 2016 addendum to the Canadian guidelines for the management of plaque psoriasis 2009. J Cutan Med Surg 2016; 20: 375–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nast A, Gisondi P, Ormerod AD et al European S3‐guidelines on the systemic treatment of psoriasis vulgaris–update 2015–short version–EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol 2015; 29: 2277–2294. [DOI] [PubMed] [Google Scholar]
- 4. Devaux S, Castela A, Archier E et al Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol 2012; 26(Suppl 3): 61–67. [DOI] [PubMed] [Google Scholar]
- 5. Zschocke I, Mrowietz U, Karakasili E et al Non‐adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol 2014; 28(Suppl 2): 4–9. [DOI] [PubMed] [Google Scholar]
- 6. Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol 2004; 140: 408–414. [DOI] [PubMed] [Google Scholar]
- 7. Barber N, Parsons J, Clifford S et al Patients’ problems with new medication for chronic conditions. Qual Saf Health Care 2004; 13: 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carroll CL, Feldman SR, Camacho FT et al Better medication adherence results in greater improvement in severity of psoriasis. Br J Dermatol 2004; 151: 895–897. [DOI] [PubMed] [Google Scholar]
- 9. Bewley A, Page B. Maximizing patient adherence for optimal outcomes in psoriasis. J Eur Acad Dermatol Venereol 2011; 25(Suppl 4): 9–14. [DOI] [PubMed] [Google Scholar]
- 10. Feldman SR, Horn EJ, Balkrishnan R et al Psoriasis: improving adherence to topical therapy. J Am Acad Dermatol 2008; 59: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 11. Tan X, Feldman SR, Chang J et al Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv 2012; 9: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 12. Eastman WJ, Malahias S, Delconte J et al Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis 2014; 94: 46–53. [PubMed] [Google Scholar]
- 13. Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther (Heidelb) 2016; 6: 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kragballe K, Austad J, Barnes L et al Efficacy results of a 52‐week, randomised, double‐blind, safety study of a calcipotriol/betamethasone dipropionate two‐compound product (Daivobet®/Dovobet®/Taclonex®) in the treatment of psoriasis vulgaris. Dermatology 2006; 213: 319–326. [DOI] [PubMed] [Google Scholar]
- 15. Kragballe K, Austad J, Barnes L et al A 52‐week randomized safety study of a calcipotriol/betamethasone dipropionate two‐compound product (Dovobet®/Daivobet®/Taclonex®) in the treatment of psoriasis vulgaris. Br J Dermatol 2006; 154: 1155–1160. [DOI] [PubMed] [Google Scholar]
- 16. Leonardi C, Bagel J, Yamauchi P et al Aerosol foam formulation of the fixed combination calcipotriene plus betamethasone dipropionate improves the health‐related quality of life in patients with psoriasis vulgaris: results from the randomized PSO‐FAST study. J Drugs Dermatol 2016; 15: 981–987. [PubMed] [Google Scholar]
- 17. Paul C, Stein Gold L, Cambazard F et al Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy versus gel in patients with psoriasis vulgaris: randomized, controlled PSO‐ABLE study. J Eur Acad Dermatol Venereol 2017; 31: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kragballe K, van de Kerkhof P. Pooled safety analysis of calcipotriol plus betamethasone dipropionate gel for the treatment of psoriasis on the body and scalp. J Eur Acad Dermatol Venereol 2014; 28(Suppl 2): 10–21. [DOI] [PubMed] [Google Scholar]
- 19. Lambert J, Hol CW, Vink J. Real‐life effectiveness of once‐daily calcipotriol and betamethasone dipropionate gel vs. ointment formulations in psoriasis vulgaris: final analysis of the 52‐week PRO‐long study. J Eur Acad Dermatol Venereol 2015; 29: 2349–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lebwohl M, Tyring S, Bukhalo M et al Fixed combination aerosol foam calcipotriene 0.005% (Cal) plus betamethasone dipropionate 0.064% (BD) is more efficacious than Cal or BD aerosol foam alone for psoriasis vulgaris: a randomized, double‐blind, multicenter, three‐arm, phase II study. J Clin Aesthet Dermatol 2016; 9: 34–41. [PMC free article] [PubMed] [Google Scholar]
- 21. Menter A, Stein Gold L, Bukhalo M et al Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double‐blind, vehicle‐controlled trial. J Drugs Dermatol 2013; 12: 92–98. [PubMed] [Google Scholar]
- 22. Laws PM, Young HS. Topical treatment of psoriasis. Expert Opin Pharmacother 2010; 11: 1999–2009. [DOI] [PubMed] [Google Scholar]
- 23. Leonardi C, Bagel J, Yamauchi P et al Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris ‐ a randomized phase III study (PSO‐FAST). J Drugs Dermatol 2015; 14: 1468–1477. [PubMed] [Google Scholar]
- 24. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) ‐ a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 25. Housman TS, Mellen BG, Rapp SR et al Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis 2002; 70: 327–332. [PubMed] [Google Scholar]
- 26. Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol 2005; 19(Suppl 3): 2–6. [DOI] [PubMed] [Google Scholar]
- 27. Sandoval LF, Huang KE, Harrison J et al Calcipotriene 0.005%‐betamethasone dipropionate 0.064% ointment versus topical suspension in the treatment of plaque psoriasis: a randomized pilot study of patient preference. Cutis 2014; 94: 304–309. [PubMed] [Google Scholar]
- 28. Zschocke I, Mrowietz U, Lotzin A et al Assessing adherence factors in patients under topical treatment: development of the Topical Therapy Adherence Questionnaire (TTAQ). Arch Dermatol Res 2014; 306: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. SABA responses at baseline (FAS)
Table S2. Difference in total TPUQ score between study treatments by age (FAS)
Table S3. Total TPUQ score by age: Cal/BD gel (FAS)
Table S4. Difference in total formulation score (TPUQ) between study treatments by psoriasis distribution phenotype (FAS)
Table S5. Total TPUQ score by LTT (LTT analysis set)
Table S6. Proportion of patients preferring Cal/BD foam or gel (CLTT) over LTT (LTT analysis set)
Table S7. Overall preference item (CLTT) by LTT (LTT analysis set)
Table S8. Mean (±SD) individual VPM scores by treatment and age category (FAS)
Table S9. Mean (±SD) individual VPM scores by treatment and baseline disease severity (FAS)
Table S10. Mean (±SD) individual VPM scores by treatment and thickness phenotype (FAS)
Fig. S1. Reasons for preference of Cal/BD foam (SPA): (a) Application; (b) Formulation; and (c) Container items (FAS)
Fig. S2. Reasons for preference of Cal/BD gel (SPA): (a) Application; (b) Formulation; (c) Container items (FAS)


