Summary
Aims
This open‐label prospective phase I/IIa clinical study used autologous bone marrow‐derived mesenchymal stromal cells (BM‐MSCs) followed by mesenchymal stromal cells conditioned media (MSC‐CM) for the first time to treat multiple sclerosis (MS) patients. The primary goal was to assess the safety and feasibility and the secondary was efficacy. The correlation between the MSC‐CM content and treatment outcome was investigated.
Methods
Ten MS patients who failed conventional therapy were enrolled. Adverse events were recorded to assess safety. The Expanded Disability Status Scale (EDSS) was the primary efficacy measurement, the secondary included clinical (25WFT, 9‐PHT), cognitive (MMS), ophthalmology (OCT, VEP), and radiological (MRI lesion and volume) tests. The MSCs‐CM concentration of 27 inflammatory biomarkers was investigated.
Results
The treatment protocol was well tolerated by patients. There was an overall trend of improvement in all the tests, except the lesion volume which increased significantly. A decrease of 4 and 3.5 points on the EDSS was achieved in two patients. We report a correlation between a decreased lesion number at baseline and higher IL‐6, IL‐8, and VEGF MSC‐CM content.
Conclusion
The used protocol was safe and feasible with possible efficacy. The addition of MSC‐CM could be related to the magnitude of EDSS improvement observed.
Keywords: conditioned media, expanded disability status scale, magnetic resonance imaging, mesenchymal stem cells, multiple sclerosis, optical coherence tomography, visual evoked potential
1. INTRODUCTION
Multiple sclerosis (MS) was described more than 150 years ago by the French neurologist Jean‐Martin Charcot after finding distinctive postmortem scars in the brain of a lady who had presented to him with tremor, slurred speech, and abnormal eye movement.1 Current knowledge defines MS as a demyelinating, neurodegenerative, and neuro‐inflammatory disease of the CNS.2 The disease leads to a progressive disabling course over the period of one to two decades in the majority of the young adult patients it affects.3 There were no effective treatments for this disease until the early nineties of the last century when interferons and glatiramer acetate were introduced and accepted as quite safe and moderately effective treatments that improved only the relapse rate in one‐third of patients but did not change the progressive course of the disease. During the past two decades, other treatment modalities (biological, monoclonal, and recently oral agents) have been introduced with better outcome compared to interferon. But all the available therapy for MS has some limitations related to intolerability, efficacy, or safety.4, 5, 6, 7 Stem cell therapy may be an ideal intervention; MSCs are considered to have multiple capabilities, including homing to the site of injury, halting the destructive inflammation, and regenerating the damaged areas of the CNS.8, 9, 10 MSCs have also been found to differentiate into the damaged cell types, neurons and glial cells, given the appropriate environment.11, 12
In addition, The MSC secretome found in the growth and expansion media, also called, conditioned media (MSC‐CM), has been investigated for its therapeutic potential.13, 14 For instance, MSCs secrete certain proregenerative mediators that tip the equation toward repair rather than destruction, including cytokines, chemokines, and growth factors.15, 16 MSCs also produce neuroprotective factors that promote the survival and regeneration of neurons.17, 18 Furthermore, they have been found to produce many immune‐modulatory proteins that affect the adaptive as well as the innate cells.13 In vitro, MSC‐CM from MS patients attenuated the secretion of inflammatory cytokines in a microglia and oligodendrocyte model cell lines.19 The intravenous injection of MSC‐CM without MSCs in an animal model of MS—the experimental autoimmune encephalomyelitis (EAE)—showed significant improvement indicating the importance of MSC‐CM in the repair process.20 In another in vivo study, MSC‐CM attenuated neural degeneration in a spinocerebellar ataxia mice model.21 A number of promising clinical trials using different sources, doses, and routes of MSCs injections have been conducted, none of them investigated the clinical implications of injecting MSCs‐CM.22, 23, 24, 25, 26, 27, 28 Thus examining the clinical reproducibility of the encouraging preclinical evidence of the benefits of MSC‐CM is necessary.
The aim of this study was to answer two questions: First, is the intrathecal treatment with a high target dose of 100*106 BM‐MSC followed by MSC‐CM feasible and safe in MS patients? Second, is such treatment efficacious in halting or reversing the disease in patients who did not respond to conventional medical treatment? The primary efficacy parameter used in this study was the Expanded Disability Status Scale (EDSS) while other tests including MRI, cognitive, and ophthalmology tests were secondary parameters.
2. METHODS
This clinical trial was approved by the institutional review board at Jordan University Hospital and was conducted between October 2012 and February 2016 (NIH clinical trials registry identifier: NCT01895439). This trial was funded by the Deanship of Scientific Research, The University of Jordan. It was performed in accordance with the ethical standards laid down in an appropriate version of the Declaration of Helsinki (as revised in Brazil 2013).
2.1. Patients
Patients enrolled had a clinically definite MS based on McDonald's criteria.29, 30 The inclusion criteria indicated a failure of at least one‐first‐line treatment option including the three available interferons (INF): (i) INF‐beta‐1a 44 micrograms(mcg) subcutaneous (SC) injection 3 times weekly, or 30 mcg by intramuscular (IM) injection weekly, or INF‐beta‐1b 8 million international units SQ on alternate days. Glatiramer acetate is not registered in Jordan, whereas patients using available second‐line therapy such as mitoxantrone (MXT) , cyclophosphamide (CP), or natalizumab were invited to participate in the study. Failure of treatment was defined as a definite clinical relapse lasting more than 24 hours (optic neuritis, spinal cord syndrome, brainstem syndrome, or evidence of subcortical white matter disease) associated with enhancing or new white matter lesions that can explain the clinical syndrome while on the treatment. Before enrollment, patients were screened for the presence of infectious agents including viral hepatitis B and C, HIV, and syphilis.
Of 25 patients screened, 10 patients were excluded as they did not fulfill one or more inclusion criteria. Fifteen patients were eligible and received the cellular treatment. Three patients withdrew and did not show up for the second intrathecal injection due to pain and discomfort during or postcellular injection or not feeling an improvement in their condition one month posttreatment. Two patients had severe spasticity which prevented the completion of MRI and ophthalmology tests and were thus excluded from the study analysis. The remaining 10 patients completed posttreatment follow‐up analysis (Figure 1). The studied patients were 4 females and 6 males, 8 of them had secondary progressive (SP) MS, and 2 had the relapsing remitting (RR) type (Table 1).
Figure 1.
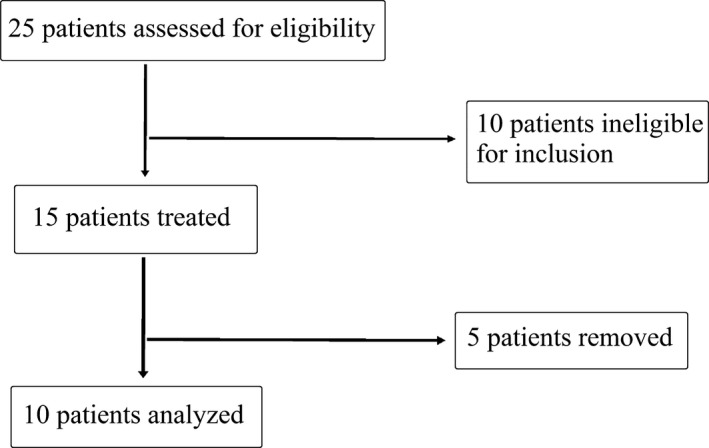
Flowchart of the study
Table 1.
Baseline characteristics of patients and cellular treatment administered (n = 10)
| Mean (±SD) | Range | |
|---|---|---|
| (A) | ||
| Age (Y) | 34.9 (±9.54) | (18; 54) |
| MS duration (Y) | 9.6 (±2.91) | (4; 15) |
| Post ‐treatments | n | % |
|---|---|---|
| Interferon b‐1 | 4 | 40 |
| Interferon MXT | 4 | 40 |
| Interferon Nat | 1 | 10 |
| Interferon &SC | 1 | 10 |
| Mean (±SD) | Range | |
|---|---|---|
| (B) | ||
| Number of MSCs injected (×106) | 110 (±23.1) | (93; 168) |
| CM volume (mL) | 18.3 (±2.79) | (13; 20) |
MXT, Mitoxantrone; Nat, Natalizumab; SC, stem cell; MSCs, mesenchymal stem cells; CM, conditioned media.
2.2. Mesenchymal stromal cells (MSCs) isolation and clinical expansion
All 15 patients underwent BM aspiration of 30‐50 mL from the iliac crest under local anesthesia. Clinical grade BM‐MSCs were generated using standard operating procedures under good manufacturing practices conditions.31 Briefly, BM mononuclear cells were isolated by density gradient centrifugation in Histopaque‐1077 (Sigma, St. Louis, MO, USA). Cells were washed with phosphate‐buffered saline (GIBCO, Gaithersburg, MD, USA), counted, and seeded at a density of 180*104 cells per cm2 in culture media. After 24 hours, nonadherent cells were removed by media exchange, and adherent cells were cultured in fresh media. The culture media used are composed of α‐MEM, 2 mmol L−1 l‐glutamine, 1% penicillin/streptomycin (all from GIBCO, USA), and 5% of human platelet lysate (hPL). The hPL was prepared by pooling platelet bags from the corresponding blood group to each patient. After reaching 70%‐80% confluence, attached MSCs were detached using TrypleE 10× (GIBCO, USA). Cells were then replated at a seeding density of 4*103 cell per cm2 in culture media. Single and double layer cell factories (Nunc, Thermo Scientific, Waltham, MA, USA) were used to reach clinical numbers of MSCs. BM‐MCs at passages 3 or 4 were used for patient's injections, with an average number of 110*106 cells injected per patient (Table 1).
Characterization of the patients' expanded MSCs was in accordance with the International Society for Cellular Therapy (ISCT) recommendations.32 Cells were observed microscopically for the spindle shape and attachment to tissue culture flasks. Flow cytometry was performed to confirm the expression of CD73, CD90, and CD105 surface molecules at a percentage of at least 95% and the absence of CD34, CD45, CD14, and CD3 surface markers at 5% most. In addition, the differentiation assessment into adipogenic, osteogenic, and chondrogenic lineages was performed using StemPro differentiation media (GIBCO, ThermoFisher, Waltham, MA, USA).33
2.3. Transplantation of BM‐MSCs and MSC‐CM
BM‐MSCs were injected intrathecally into patients by the neurologist immediately after harvest. In addition, at least 50 mL of mycoplasma‐tested MSC‐CM was preserved at −80°C for subsequent injection and analysis. After a month interval, an average volume of 18 mL of thawed MSC‐CM corresponding to each patient's MSCs was given in a similar intrathecal manner as the MSCs.
After each injection, patients were observed in the outpatient clinic for at least one hour. They were followed up closely for the first week for any health‐related incident. They presented to the clinic at 3,6,12 months after treatment for full examinations as described below.
2.4. Patients' examinations and follow up
2.4.1. Clinical evaluation
All patients were evaluated clinically using qualitative and quantitative tests. This included Extended Disability Status Scale (EDSS) for general body functions, Mini‐Mental Status Examination (MMSE) for cognitive function, the Timed 25‐Foot Walk (T25‐FW) test for lower limb function and 9 Hole Peg (9HP) test for upper limb fine motor function.
2.4.2. Visual analysis
The eye condition was not a recruitment criteria and it was used for safety assessment as well as efficacy in an examiner blinded method. This included best corrected visual acuity, pupillary examination, ocular motility and alignment assessment, slit‐lamp examination including anterior and posterior segment examination, Contrast Sensitivity (CS) test at 2.5% and 10% charts, color vision test (Farnsworth D‐15 Hue test), Visual Evoked Potential (VEP), Optical Coherence Tomography (OCT), and automated visual fields. For each patient, the worst presenting eye at baseline was followed up and included in the analysis (Table 3). In the electrophysiology test, the VEP latency‐100 was used which measures the speed of signal transmission in milliseconds (ms), with a normal range of 100 ± 7 ms. For the OCT, retinal nerve fiber layer thickness (μm) was measured with 100 um being normal.
Table 3.
Efficacy evaluation of MS patients at 12 mo posttreatment
| M0 | M12 | Change from M0 to M12 | P‐valueb | |
|---|---|---|---|---|
| EDSS | 5.1 (±1.73) | 5 (±1.86) | ‐0.1 (±2.16) | .94 |
| Volume of MRI‐WM lesions (mm3) | 34.5 (±30.3) | 44.7 (±32.9) | 10.2 (±17.5) | .04 |
| Number of B MRI‐WM lesions | 130 (±91) | 134 (±96) | 4.3 (±7.01) | .10 |
| Number of SC MRI‐WM lesions | 14.3 (±5.2) | 14.4 (±5.3) | 0.1 (±0.32) | .99 |
| Number of enhanced MRI‐WM lesions | 5.5 (±11.2) | 1.5 (±1.96) | ‐4 (±9.38) | .78 |
| VEPa (ms) | 127.3 (±23.4) | 127.5 (±26.3) | 0.22 (±7.25) | .84 |
| OCTa μm | 85.2 (±15.3) | 84.4 (±16.29) | ‐0.8 (±4.15) | .69 |
| 9‐hole peg test (s) | 32.2 (±5.19) | 29.9 (±4.66) | ‐2.31 (±3.08) | .06 |
| T25FW test (min) | 12.6 (±10.2) | 11.5 (±9.1) | ‐1.1 (±11.2) | .62 |
| MMSE | 29.1 (±.73) | 29 (±3.16) | ‐0.1 (±1.97) | .98 |
Worst eye measurement (right or left).
Wilcoxon paired test.
T25FW, Timed 25‐Foot Walk; MMSE, Mini‐Mental Status Examination; EDSS, Extended Disability Status Scale; WM, white matter; VEP, visual evoked potential; OCT, optical coherence tomography; R, right; L, left; M, month, s, seconds, ms, milliseconds.
2.4.3. Radiological evaluation
Magnetic resonance imaging (MRI) was performed on a 3‐Tesla Verio system (Siemens Medical Systems, Erlangen, Germany). All patients underwent an MRI scanning at baseline (less than 72 hours prior to stem cell injection) then at 3, 6, and 12 months post‐MSCs injection. The detailed imaging sequences protocol of the brain and those of the cervical and thoracic spine can be found in Appendix S2. T2‐weighted FLAIR and T1‐weighted MPRAGE images were generated for all patients.
The T2‐hyperintense lesion volume measurements were obtained using a semi‐automated segmentation technique based on Fuzzy connections algorithm in JIM software (Jim version 3; Xinapse Systems, Colchester, England).34
2.5. Measuring inflammatory biomarkers CM content
Cryopreserved BM‐MSCs from 7 enrolled patients were thawed, cultured until they reached 80% confluence after which MSC‐CM was removed to analyze its content. The Luminex multiplex ELISA technology was used, samples were tested in triplicates, and culture media were used as a reference. The concentration in pg/mL of 27 inflammatory biomarkers was assayed according to the manufacturer's directions (Bio‐Rad, Pro‐Human Cytokine 27‐Plex Immunoassay).35 The analytes included FGF basic, eotaxin, G‐CSF, GM‐CSF, IFN‐γ, IL‐1β, IL‐1ra, IL‐2, IL‐4, IL‐5, IL‐6, IL‐7, IL‐8, IL‐9, L‐10, IL‐12 (p70), IL‐13, IL‐15, IL‐17, IP‐10, MCP‐1 (MCAF), MIP‐1α, MIP‐1β, PDGF‐BB, RANTES, TNF‐α, and VEGF.
2.6. Statistics
Wilcoxon matched‐pairs signed rank test was used to assess differences between baseline and 12 months posttreatment presented in Table 3. Results were also analyzed for 3 and 6 months (Table S2). For analytes concentrations, Mann‐Whitney test was used in the DataPro analysis software.
3. RESULTS
3.1. Safety assessments
No patient had any treatment‐related life‐threatening adverse event (AE) from the time of injection through the follow‐up year. Table 2 lists the recorded AEs for the 15 treated patients. The transient minor AEs experienced by at least one patient following the administration of MSCs are summarized in Table 2. No cases of meningitis, encephalopathy, seizures, or allergic reactions were experienced by any patient. All patients' records were filed in a paper format.
Table 2.
Reported adverse events following autologous MSC administration (n = 15)
| Adverse event | n | % |
|---|---|---|
| Injection site bruising | 1 | 6.7 |
| Injection site pain | 9 | 60 |
| Injection site swelling | 4 | 26.7 |
| Fever | 6 | 40 |
| Headache | 8 | 53.3 |
| Constipation | 1 | 6.7 |
| Tremor | 1 | 6.7 |
3.2. Efficacy assessments
Table 3 summarizes the clinical, ophthalmology, and radiological assessment results at 12 months compared to baseline, while the results of 3 and 6 months are summarized in Table S2.
3.3. Clinical assessment
The EDSS was the primary efficacy parameter in this study; it is the most widely used test by neurologists for MS patients. The average overall change in the EDSS was ‐0. 1 (±2.16) points with a change range of (−4 to 3) points (Table 2). Of the 10 patients, 4 corresponding to 40% showed no change in their EDSS, another 40% of patients deteriorated while 20% improved (MS1, MS9) (Figure 2 and Table S1).
Figure 2.
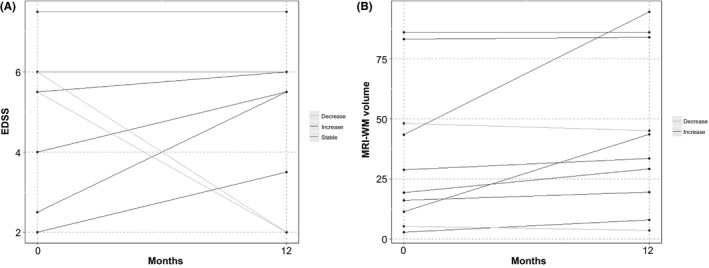
Expanded Disability Status Scale (EDSS) and white matter (WM) lesions volume outcomes at 12 mo posttreatment
The secondary efficacy parameters assessment of upper limbs function using the 9‐PH test for fine hand motor function showed a trend of improved function of −2.31 (±3.08) seconds. Furthermore, the lower limb function assessment by the T‐25‐FWT showed an overall improvement trend of −1.1 (±11.22) seconds. Finally, the group of patients enrolled had a normal cognitive score measured by MMSE (29.1 ± 0, range 25‐30) which has remained the same with a change of −0.1 (±1.97) at 12 month follow‐up.
3.4. MRI assessments
The number of T2 brain white matter lesions remained unchanged between baseline and 12‐month scans in 60% of patients, and it increased in 40% with an overall rise of 4.3 ±7.0 lesions (Table 3). On the other hand, 90% of patients did not show any change in the number of spinal cord white matter (SC‐WM) lesions, while the number increased in 10%. However, the number of enhancing T1‐lesions in both the brain and spinal cord has decreased in 20%, increased in 30%, and has not changed in 50% of patients. Volumetric studies of the white matter lesions showed that the T2‐lesion volume did not change in 20% of patients, and it increased in 70% and decreased in 10% of patients (Figure 2). There was a significant overall increase of 10.22 (±17.51) mm3 with a P‐value of .037 (Table 3).
3.5. Visual assessments
Although many patients reported subjective improvement in the quality of vision, there were no positive statistically significant changes in all of the studied parameters including vision, color vision, and the objective measures such as VEP and OCT (Figure 3). On the contrary, Table 3 shows a trend of a decreased mean retinal nerve fiber layer (RNFL) on OCT with a decrease of −0.8 (±4.15) μm. In addition, the VEP results showed a trend of increased latency of the electrical conduction of the optic nerves with 0.22 (±7.25) milliseconds (ms) which was noticeable even at 3 and 6 months posttreatment (Table S2). Despite minimal changes in VEP and OCT, the vision remained stable in all patients throughout the study.
Figure 3.
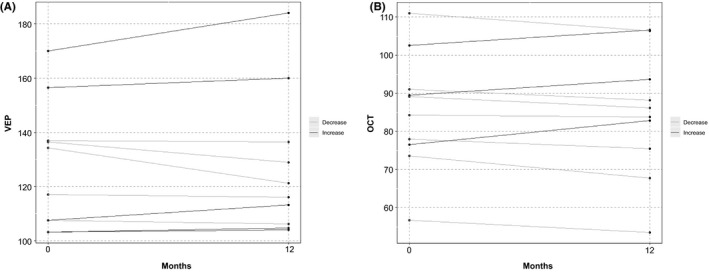
Visual outcomes at 12 mo posttreatment
3.6. MSC‐CM inflammatory profile
The human inflammatory panel was used to examine any difference in concentrations of biomarkers released by BM‐MSCs in the 7 tested patients. Of the 27 biomarkers, two were excluded from analysis due to high background and the remaining 25 were plotted against each other across samples. Interleukins (IL‐6, IL‐8), vascular endothelial growth factor (VEGF), and monocyte chemoattractant protein‐1 (MCP‐1) were the biomarkers produced with the highest concentrations in all the 7 samples (Figure 4). The concentration of IL6, IL‐8, and VEGF was higher in two of the samples (MS3, MS8). Ten more cytokines were detected in varying and lower concentrations of less than 300 pg/mL including IL‐10, IL‐12, IL‐15 IL‐2, FGF, and tumor necrosis factor‐alpha (TNF‐alpha) (data not shown).
Figure 4.
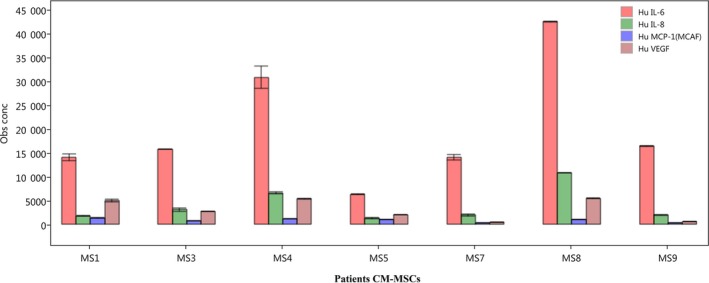
Top analytes secreted by MS patients' BM‐MSCs
4. DISCUSSION
MS is still difficult to treat, and many patients will become disabled at some point, and thus, the need for a treatment that is both safe and effective is beyond any doubt.
MSCs continue to be investigated as a treatment option for several neurological diseases. The rationale behind adding the MSC‐CM in this study was to boost the dual; regenerative, and immune‐modulatory benefits which have been attributed to the secretome of MSCs as well as the cell‐cell contact.36 Immune‐modulating soluble cytokines, growth factors, and brain repair molecules in the MSC‐CM have been investigated in vitro.37 In addition, the importance of MSC‐CM in the repair process was demonstrated by the intravenous injection of MSC‐CM without MSCs in the EAE MS model, which promoted the development of oligodendrocytes and neurons.20
In previous clinical studies, the optimal route of stem cell injection, frequency, and number of cells injected has varied and is yet to be determined.22, 23, 24, 25, 26, 27, 28 In a preclinical study, direct intraventricular MSCs injection has been reported to be more effective in the EAE model compared to the intravenous route.38 The intrathecal route has been previously used for MS patients but with lower numbers of injected MSCs.23, 24
In this trial, we report injecting safely the highest reported dose of autologous MSCs into MS patients (93‐168*106) via the intrathecal route. It was followed a month later with the MS‐CM (15‐20 mL). In the present study—to the best of our knowledge—a cellular treatment protocol was used for the first time in any human neurological disease. No serious adverse events were reported after the injection of BM‐MSCs with transient localized pain and headache being the most common complaints. A similar trial had reported transient encephalopathy and seizures postcryopreserved BM‐MSCs' intrathecal injection in the only patient who had received a high dose (100*106) while no adverse effects were reported when using lower doses of BM‐MSCs (30‐50*106 MSCs).23 Others have used cryopreserved BM‐MSCs intravenously without reporting any serious adverse events.26 In this study, MSCs were given immediately after expansion to limit cell death due to cryopreservation. The MSCs were also cultured using a matching blood group human platelet lysate media supplement instead of the usually used fetal calf serum. These are technical aspects of the MSCs culture that might have contributed to the safety outcome and reduced sensitization or inflammatory reactions.
Due to the small number and heterogeneity of enrolled patients in terms of the type of MS, duration of the disease, and baseline EDSS score, it was not possible to reach statistical significance and draw definite conclusions. The withdrawal of 3 of the 15 enrolled patients may indicate some hurdles to the intrathecal route in some patients which needs to be considered in planning future trials. For the remaining 10 patients, a trend of decreased EDSS in patients who had failed initial first‐ or second‐line therapy with a percentage of 60% stabilization or improvement is encouraging. Two patients had a remarkable decrease in their EDSS. Both were SPMS, the first patient presented with an EDSS of 5.5 which became 2 at twelve months, while the other patient went from a score of 6 to 1.5 (Table S1). This had a great impact on all aspects of their lives which was also detected by improved 9‐HPT and the T25FW of more than 20% in each test for each patient (Table S1).
The original Multiple Sclerosis Functional Composite (MSFC) test consists of three components, and the composite score is created by converting the score of each of the three components into a z‐score. However, the original MSFC test does not have a clear meaning in MS progression.39 In the present study, we used two components of its components, the 9‐HPT and the T25FW. With these two tests, a 20% change had been shown to be sensitive in assessing disease progression.40
The cognitive aspect of MS has been well documented as part of the MS manifestations.41 It is nevertheless disregarded in the efficacy assessments of many therapeutic options. The (MMSE) psychocognitive test indicated patients were normal at baseline, and 30% of patients showed a decrease in the score which could reflect a progression in the disease, although a report of the MMSE's low sensitivity in assessing cognitive decline in patients would make it less conclusive.42
One of the two patients who had improved on the EDSS scale had a decrease of 18 enhanced lesions while the total number and volume of brain lesions increased. While the other patient had one more enhanced lesion at 12 months with an increase in total number and volume of brain lesions (Table S1), no correlation was found between the clinical and radiological findings. There was a progression in the lesion volume in most patients who showed improved clinical scores. In addition, MS patients who had a decreased lesion volume did not show any measurable clinical improvement with the tests used. Also, MS2 who had 25 less enhancing lesions (30 to 5) but her total lesions increased by 21 (350 to 371) showed significant clinical worsening. This phenomenon is not unusual in MS patients where the MRI results do not always reflect the prognosis or the course of the disease. This could be attributed to the low specificity and the inability of such MRI measures to detect and quantify the extent of the diffuse, and occult damage beyond the visible white matter and gray matter lesions in normal appearing brain tissues.43, 44 The cellular treatment might have a positive effect on the normal appearing brain tissue rather than the MS lesions themselves. This implies that the hidden disease in normal appearing brain tissue could play an important role in the clinical status of MS patients which can only be elucidated using quantitative MRI techniques.
Moreover, the ophthalmological results did not correlate with the EDSS score, while some patients improved in these outcome measures, their overall assessment was either stable or worse. Our results were similar to a previous report that used autologous MSCs and could not identify any significant effects on color vision, visual fields, macular volume, and retinal nerve fiber layer thickness.25 However, that study documented minimal improvement in visual acuity and visual evoked response latency, with an increase in optic nerve area. On the other hand, results of another study showed vision and visual contrast testing improvement in 2 of 4 patients with unchanged RNFL thickness in 3 patients and a decrease in one.23
The lack of a control group is a drawback in this cellular therapy on MS which is due to the clinical and pathological heterogeneity of the MS patients that makes it difficult to identify a matching group. In one study, this was bypassed by a latency period of 6 months between groups so that every patient is his own control, a strategy worth considering in future MS trials but may be with a longer latency period.26 Nevertheless, it can be clearly observed that most patients improved in one of the efficacy endpoints reflecting diversity of responses (Table S1). This could reflect (i) the heterogeneity of patients included in terms of type, areas affected, and severity of MS, (ii) the different homing and therapeutic potential of the MSCs injected, and (iii) the different composition of MSC‐CM and its potential to reverse differently the disease aspects examined in this study.
In a preliminary attempt to determine the therapeutic effect the stem cell secretome present in the MSC‐CM, the analysis of key inflammatory biomarkers was performed. There was a consistent expression of the highly secreted proteins: IL‐6, IL‐8, VEGF, and MCP‐1 in MSC‐CM of MS patients. This expression pattern is in accordance with a previous work comparing normal MSC‐CMs content from different sources.45 Although all four analytes fall into the category of mainly pro‐inflammatory effectors, some studies have provided evidence of their pleotropic, antiinflammatory, and neuroprotective roles. IL‐6 is suggested to have antiinflammatory properties with enhancing neurogenesis abilities in the CNS 46, 47, 48 While IL‐8 and VEGF are potent angiogenic factors with a recently investigated neuroprotective effect,49, 50 some evidence has also supported the neuromodulatory effects of MCP‐1 in the CNS.13, 51 Despite this similar trend, the concentration of the top analytes produced varied greatly between patients. In search of a relationship between the protein expression profile and the clinical outcome, it was surprising not to find a similar expression profile between the patients with the EDSS improvement (MS1, MS9). Although MS1 MSCs produced the highest amount of MCP1, MS9 MSCs did not show the same secretion pattern. The cytokines and growth factors' analysis did not detect any correlation with any of the secondary outcome measures. Nevertheless, an interesting finding was that the highest secretion of IL‐6, IL‐8, and VEGF was detected in MSC‐CM of patients with the lowest number of WM lesions at baseline. As MS8 had the lowest number of lesions28 and his MSCs expressed the highest amount of the top analytes, he was followed by MS4 who had 60 lesions and expressed the 2nd highest amount. While this correlation was not reflected in the efficacy of treatment in this study, it would be valuable to investigate the biological and therapeutic properties of the MS‐MSCs compared to control individuals and the effect of disease severity in terms of loss of white matter on MSCs and CM‐MSCs content.
In conclusion, both BM‐MSC and MSC‐CM are safe with relative efficacy in stabilizing the disease and reversing symptoms. The results encourage us and others to launch larger clinical trials to better determine the effectiveness of MSCs and their MSC‐CM both combined and separate. Studies like this one examining the cellular components used for treating MS are needed to help understand the different responses in patients and improve the outcomes.
Supporting information
Dahbour S, Jamali F, Alhattab D, et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci Ther. 2017;23:866–874. 10.1111/cns.12759
NIH clinical trials registry identifier: NCT01895439.
REFERENCES
- 1. Charcot J‐M. Histologie de la sclérose en plaques (leçon recueillie par Bourneville). Gaz Hop Civ. 1868; 1051: 1868. [Google Scholar]
- 2. Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502‐1517. [DOI] [PubMed] [Google Scholar]
- 3. Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938‐952. [DOI] [PubMed] [Google Scholar]
- 4. Giovannoni G, Southam E, Waubant E. Systematic review of disease‐modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Multi Sclerosis J. 2012;18:932‐946. [DOI] [PubMed] [Google Scholar]
- 5. Rudick RA, Polman CH. Current approaches to the identification and management of breakthrough disease in patients with multiple sclerosis. Lancet Neurol. 2009;8:545‐559. [DOI] [PubMed] [Google Scholar]
- 6. Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab‐associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870‐1880. [DOI] [PubMed] [Google Scholar]
- 7. Lindsey JW, Haden‐Pinneri K, Memon NB, Buja LM. Sudden unexpected death on fingolimod. Mult Scler. 2012;18:1507‐1508. [DOI] [PubMed] [Google Scholar]
- 8. Callera F, de Melo CM. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells' migration into the injured site. Stem Cells Dev. 2007;16:461‐466. [DOI] [PubMed] [Google Scholar]
- 9. Chotivichit A, Ruangchainikom M, Chiewvit P, Wongkajornsilp A, Sujirattanawimol K. Chronic spinal cord injury treated with transplanted autologous bone marrow‐derived mesenchymal stem cells tracked by magnetic resonance imaging: a case report. J Med Case Rep. 2015;9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trubiani O, Giacoppo S, Ballerini P, et al. Alternative source of stem cells derived from human periodontal ligament: a new treatment for experimental autoimmune encephalomyelitis. Stem Cell Res Ther. 2016;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364‐370. [DOI] [PubMed] [Google Scholar]
- 12. Alexanian AR. An efficient method for generation of neural‐like cells from adult human bone marrow‐derived mesenchymal stem cells. Regen Med. 2010;5:891‐900. [DOI] [PubMed] [Google Scholar]
- 13. Kyurkchiev D, Bochev I, Ivanova‐Todorova E, et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cell. 2014;6:552‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ivanova‐Todorova E, Bochev I, Dimitrov R, et al. Conditioned medium from adipose tissue‐derived mesenchymal stem cells induces CD4+FOXP3+ cells and increases IL‐10 secretion. J Biomed Biotechnol. 2012;2012:295167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T‐cell anergy. Blood. 2005;106:1755‐1761. [DOI] [PubMed] [Google Scholar]
- 16. Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10:649‐656. [DOI] [PubMed] [Google Scholar]
- 17. Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro‐regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54‐64. [DOI] [PubMed] [Google Scholar]
- 18. Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow‐derived mesenchymal stem cells secrete brain‐derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63‐70. [DOI] [PubMed] [Google Scholar]
- 19. Ballerini P, Diomede F, Petragnani N, et al. Conditioned medium from relapsing‐remitting multiple sclerosis patients reduces the expression and release of inflammatory cytokines induced by LPS‐gingivalis in THP‐1 and MO3.13 cell lines. Cytokine. 2017;96:261‐272. [DOI] [PubMed] [Google Scholar]
- 20. Bai L, Lennon DP, Caplan AI, et al. Hepatocyte growth factor mediates mesenchymal stem cell‐induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15:862‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suto N, Mieda T, Iizuka A, Nakamura K, Hirai H. Morphological and functional attenuation of degeneration of peripheral neurons by mesenchymal stem cell‐conditioned medium in spinocerebellar ataxia type 1‐knock‐in mice. CNS Neurosci Ther. 2016;22:670‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. 2007;4:50‐57. [DOI] [PubMed] [Google Scholar]
- 23. Yamout B, Hourani R, Salti H, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. 2010;227:185‐189. [DOI] [PubMed] [Google Scholar]
- 24. Karussis D, Karageorgiou C, Vaknin‐Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open‐label phase 2a proof‐of‐concept study. Lancet Neurol. 2012;11:150‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Llufriu S, Sepulveda M, Blanco Y, et al. Randomized placebo‐controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS ONE. 2014;9:e113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Odinak MM, Bisaga GN, Novitskii AV, et al. Transplantation of mesenchymal stem cells in multiple sclerosis. Zh Nevrol Psikhiatr Im S S Korsakova. 2011;111:72‐76. [PubMed] [Google Scholar]
- 28. Cohen JA, Imrey PB, Planchon SM, et al. Pilot trial of intravenous autologous culture‐expanded mesenchymal stem cell transplantation in multiple sclerosis. Mult Scler. 2017. [Epub ahead of print] 10.1177/1352458517703802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840‐846. [DOI] [PubMed] [Google Scholar]
- 30. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fekete N, Rojewski MT, Furst D, et al. GMP‐compliant isolation and large‐scale expansion of bone marrow‐derived MSC. PLoS ONE. 2012;7:e43255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315‐317. [DOI] [PubMed] [Google Scholar]
- 33. Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311‐324. [DOI] [PubMed] [Google Scholar]
- 34. Udupa JK, Samarasekera S. Fuzzy connectedness and object definition: theory, algorithms, and applications in image segmentation. Graph Model Image Process. 1996;58:246‐261. [Google Scholar]
- 35. Christiansson L, Mustjoki S, Simonsson B, Olsson‐Strömberg U, Loskog ASI, Mangsbo SM. The use of multiplex platforms for absolute and relative protein quantification of clinical material. EuPA Open Proteom. 2014;3:37‐47. [Google Scholar]
- 36. Luk F, de Witte SF, Korevaar SS, et al. Inactivated mesenchymal stem cells maintain immunomodulatory capacity. Stem Cells Dev. 2016;25:1342‐1354. [DOI] [PubMed] [Google Scholar]
- 37. Drago D, Cossetti C, Iraci N, et al. The stem cell secretome and its role in brain repair. Biochimie. 2013;95:2271‐2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kassis I, Grigoriadis N, Gowda‐Kurkalli B, et al. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Arch Neurol. 2008;65:753‐761. [DOI] [PubMed] [Google Scholar]
- 39. Rudick R, Polman C, Cohen J, et al. Assessing disability progression with the multiple sclerosis functional composite. Mult Scler. 2009;15:984‐997. [DOI] [PubMed] [Google Scholar]
- 40. Kragt J, van der Linden FA, Nielsen J, Uitdehaag BM, Polman C. Clinical impact of 20% worsening on Timed 25‐foot Walk and 9‐hole Peg Test in multiple sclerosis. Mult Scler. 2006;12:594‐598. [DOI] [PubMed] [Google Scholar]
- 41. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139‐1151. [DOI] [PubMed] [Google Scholar]
- 42. Swirsky‐Sacchetti T, Field HL, Mitchell DR, et al. The sensitivity of the Mini‐Mental State Exam in the white matter dementia of multiple sclerosis. J Clin Psychol. 1992;48:779‐786. [DOI] [PubMed] [Google Scholar]
- 43. Kappos L, Moeri D, Radue EW, et al. Predictive value of gadolinium‐enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta‐analysis. Gadolinium MRI Meta‐analysis Group. Lancet. 1999;353:964‐969. [DOI] [PubMed] [Google Scholar]
- 44. Molyneux P, Barker G, Barkhof F, et al. Clinical–MRI correlations in a European trial of interferon beta‐1b in secondary progressive MS. Neurology. 2001;57:2191‐2197. [DOI] [PubMed] [Google Scholar]
- 45. Hsiao ST‐F, Asgari A, Lokmic Z, et al. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012;21:2189‐2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tilg H, Dinarello CA, Mier JW. IL‐6 and APPs: anti‐inflammatory and immunosuppressive mediators. Immunol Today. 1997;18:428‐432. [DOI] [PubMed] [Google Scholar]
- 47. Casella G, Garzetti L, Gatta AT, et al. IL4 induces IL6‐producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J Neuroinflamm. 2016;13:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Erta M, Quintana A, Hidalgo J. Interleukin‐6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou L, Lin Q, Wang P, et al. Enhanced neuroprotective efficacy of bone marrow mesenchymal stem cells co‐overexpressing BDNF and VEGF in a rat model of cardiac arrest‐induced global cerebral ischemia. Cell Death Dis. 2017;8:e2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Horie N, Pereira MP, Niizuma K, et al. Transplanted stem cell‐secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou Y, Tang H, Liu J, Dong J, Xiong H. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. J Neurochem. 2011;116:406‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


