Abstract
Background
After tracheostomy, the airway lacks an essential mechanism for warming and humidifying the inspired air with the consequent functional impairment and discomfort. The purpose of this study was to compare airway hydration with cold‐air nebulization versus heated high‐flow humidification on medical interventions and tracheal ciliary beat frequency (CBF).
Methods
Newly tracheostomized patients (n = 20) were treated either with cold‐air nebulization or heated humidification. The number of required tracheal suctioning procedures to clean the trachea and tracheal CBF were assessed.
Results
The number of required suctions per day was significantly lower in the heated humidification group with medians 3 versus 5 times per day. Mean CBF was significantly higher in the heated humidification group (6.36 ± 1.49 Hz) compared to the cold‐air nebulization group (3.99 ± 1.39 Hz).
Conclusion
The data suggest that heated humidification enhanced mucociliary transport leading to a reduced number of required suctioning procedures in the trachea, which may improve postoperative patient care.
Keywords: humidification, nurse care, tracheostomy, tracheostomy care ciliary beat frequency (CBF)
1. INTRODUCTION
After tracheostomy, relevant functions of the upper airway cease to exist. One major function of the upper airway is conditioning of the inspired air to body temperature and 100% relative humidity.1, 2 Consequently, tracheostomy can lead to pathological changes of the lower airways, including damage to the ciliated tracheal mucosa, thickening of airway secretions, and the loss of mucociliary transport.3 As a result, patients complain about crust and mucus plug formation in the lower airway, particularly in the early postoperative period after tracheostomy. Repeated cleaning and suctioning of the lower airway/the trachea is necessary, which results in significant patient discomfort and increases the risk of lower respiratory tract infection and airway obstruction.
Ciliated epithelial cells are the basis of mucociliary transport in the upper and lower airway. Their primary task is the elimination of dust and inhaled particles by transporting debris in a layer of mucus with a fast and synchronous ciliary beat frequency (CBF), and related mucociliary clearance are influenced by many factors, including disease, medication, long‐term exposure to nicotine or alcohol, and even temperature and humidity.4, 5, 6, 7, 8, 9, 10
The pivotal role of a functionally intact mucosa in the lower airway supports the use of heated and humidified air delivered through tracheostomy.3 To ensure this conditioning of inspired air, different techniques are used. Heated humidifiers, heated and non‐heated water nebulizers, and passive humidifiers are commonly applied.11 A common method after tracheostomy is the use of heat‐and‐moisture exchangers (HMEs), also referred to as “artificial noses.” They are passive humidifiers that retain heat and moisture from the expired air of the patient and return a part of it after inspiration. For mechanically ventilated patients, HMEs are often used due to their low cost.12 Spontaneously breathing patients with long‐term tracheostomies who use these devices have significantly fewer complaints of sputum production and coughing and report a better quality of life.13, 14 The most efficient but also most expensive heated humidifiers are those that channel the airflow through a heated water bath before inspiration.12 With this technique, 100% relative humidity and natural body temperature of inspired air can be achieved.
Despite the paramount role of airway conditioning in patients after tracheostomy, however, comparative data regarding the objective and subjective effects of different techniques of airway humidification is rarely available, especially in regard to ciliary function. The present study provides data comparing nonheated (cold air) water nebulization and heated humidification on cilia activity and nursing care in newly tracheostomized patients.
2. MATERIALS AND METHODS
The study was performed at the Department of Otorhinolaryngology, Head and Neck Surgery, at the University Hospital Mannheim, Germany. The local ethics board of the Medical Faculty Mannheim, University of Heidelberg, reviewed and approved the protocol (reference number 2013‐402M‐MA). The authors obtained written informed consent from all participants. Included in the study were 20 adult patients, predominantly with head and neck malignancies, who underwent tracheostomies for any reason. Exclusion criteria were a known ciliary dysfunction or a previous tracheostomy.
2.1. Study protocol
Patients for the study were recruited before or within the first 24 hours after tracheostomy. After informed consent, patients were divided into 2 groups; one group received cold‐air nebulization and the other heated humidification, according to availability. Randomization was not performed. The cold‐air nebulization group received a Cirrus nebulizer set (Intersurgical, Wokingham, UK), which was connected to the compressed air supply at ambient temperature, and the flow was set to 8 L/min. The heated humidification group received an AIRVO 2 humidifier (Fisher & Paykel Healthcare, Auckland, New Zealand). An air‐flow of 30 L/min was used, and the temperature was set to 37°C.
The patients were instructed to use the humidifiers at least 8 hours per day. An exchange of the tracheal cannula was performed according to the standard operating procedure on days 2, 4, 6, 8, and 10 after surgery. After 14 days, the study documentation and ciliary experiments described below ended, but patients could use their devices during the entire clinical stay.
2.2. Tracheostoma care and tracheal suction
Specially trained nurses performed tracheostoma and tracheal care (suctioning, removing of crusts, etc). The suction procedure was based on current knowledge. A suction catheter was gently inserted into the tracheostoma and the trachea for a maximum of 5.9 inches (15 cm) or until resistance was detected, for a maximum of 10 seconds.15, 16 The suctioning procedure was performed according to clinical needs (airway obstruction due to crust formation or mucus retention, as indicated by the patient or detected by the nursing staff trained in tracheostomy management). Suction procedures were documented on a daily chart for up to 14 days.
2.3. Ciliary beat frequency
The CBF in tracheal epithelial cells was measured on days 2, 4, 6, 8, and 10 after the tracheostomies. The ciliated samples were harvested by brushing the trachea with a standard cytology brush (Gynobrush Plus; Heinz Herenz, Hamburg, Germany), which was dipped in a 0.9% saline solution before brushing.8 The patient was placed in a sitting position on an examination chair and the cannula was removed. The brushing was performed 2 cm below the previous location of the cannula to ensure that the mucosa in the area of cell harvesting was not damaged directly by the cannula. After the brushing, a new cannula was inserted into the patient's tracheostoma. Ex vivo analysis of ciliary function was performed in an observer‐blind manner. Directly after brushing, CBF was analyzed, as previously described.8 Cells were dispensed by dunking and twisting the brush in 5 mL of Roswell Park Memorial Institute medium (Roswell Park Memorial Institute 1640, cell culture tested, standard, L‐glutamine: 300 mg/L; PromoCell, Heidelberg, Germany), which was heated up to 22°C. Cells were transferred into a Petri dish and placed under an inverted phase‐contrast microscope (Leica Microsystems GmbH, Wetzlar, Germany) to visualize them under 400‐fold magnification. Five to 10 sequences of 2 seconds at a rate of 100 frames per second were recorded using a high‐speed digital camera with the Sisson‐Ammons Video Analysis software. The CBF was analyzed with the Sisson‐Ammons Video Analysis software system's region of interest method. Therefore, a rectangular area containing active beating cilia was selected to analyze the recurring bright/dark changes.17 As the ciliary motor function is highly temperature‐dependent,18 experiments were performed in a stable, temperature‐controlled environment at 22°C.
2.4. Statistical analysis
Statistical analysis and plotting was done using “R” as an open source statistical environment using the Mann‐Whitney U test.19 The data for the suctioning procedure did not pass the normality test, so a Mann‐Whitney Wilcoxon test was used for statistical analysis. The data for CBF was normally distributed and an unpaired t test was used to compare means. All data were corrected for repeated measurements by the Holm's method.20
3. RESULTS
Twenty patients were initially included, and 18 patients finished the study as intended (12 men/6 women; median age was 70 years with a range of 16). One patient (in the heated humidification group) transferred to the intensive care unit due to postoperative myocardial infarction, making it impossible to follow the study protocol; the other patient (in the cold‐air nebulization group) was discharged before termination of the study. All patients but 1 were tracheostomized due to malignancies of the head and neck area, including 17 patients with squamous cell carcinoma and 1 patient with lymphoma (CD‐20 positive high‐grade B‐cell lymphoma). One patient was tracheostomized temporarily for severe obstructive sleep apnea with intolerance to the continuous positive airway pressure machine. All patients but 1 were active smokers (at least 20 pack‐years). Chronic obstructive pulmonary disease or other pulmonary diseases were not documented in the clinical history in any patient. There were no statistically significant differences between the 2 groups for age, sex, or body mass index. During the study, there were no adverse events, such as tracheostoma occlusion, hemorrhage, fever, lower or upper respiratory infection, episodes of hypoxia, or death. One myocardial infarction, however, occurred in a patient in the heated humidification group. Further details regarding the final study cohort are shown in Table 1.
Table 1.
Overview of included patients
| Sex | BMI | Age, years | Diagnosis | |
|---|---|---|---|---|
| Cold‐air nebulization group | ||||
| 1 | M | 19.6 | 52 | T2N2bM0 laryngeal SCC |
| 3 | M | 15.2 | 48 | T2N0M0 laryngeal SCC |
| 5 | F | 23.8 | 63 | T4bN2bM0 oropharyngeal SCC |
| 7 | F | 30.9 | 58 | T3N2bM0 oropharyngeal SCC |
| 9 | M | 26 | 88 | T3N0M0 laryngeal SCC |
| 11 | M | 33.6 | 56 | Obstructive sleep apnea |
| 13 | M | 19.5 | 59 | T4aN2b laryngeal SCC |
| 15 | F | 33.4 | 74 | B‐cell lymphoma |
| 17 | M | 25.7 | 55 | T3N2bM0 hypopharyngeal SCC |
| Heated humidification group | ||||
| 2 | M | 19.6 | 70 | T3N1M0 oropharyngeal SCC |
| 4 | M | 15.2 | 68 | T3N2bM0 laryngeal SCC |
| 6 | M | 23.7 | 74 | T3N1M0 Larynx SCC |
| 8 | M | 23.4 | 74 | T3N1M0 Larynx SCC |
| 10 | M | 22.8 | 70 | T3N0M0 Laryngeal SCC |
| 12 | F | 23.6 | 58 | T3N2M0 Hypopharyngeal SCC |
| 14 | F | 25 | 71 | T4bN2bM0 Hypopharyngeal SCC |
| 16 | M | 27.1 | 69 | T4aN2cM0 oropharyngeal SCC |
| 18 | F | 16.9 | 65 | T2N2cM0 laryngeal SCC |
Abbreviations: BMI, body mass index; SCC, squamous cell carcinoma.
3.1. Counting suction procedures
The number of required manual suction procedures at the trachea was higher in the cold‐air nebulization group compared with the heated humidification group during the study. The data did not pass the normality test, which is most likely due to the nature of the parameter. The number of suction procedures can be small but never <0 (realistically 1), whereas some patients would require 10 or more procedures per day causing skewness of the raw data in both groups. The overall number of required suction procedures continuously decreased from days 5 to 14 in both groups (Table 2) with statistically significant difference (P < .05) on days 9, 11, and 14. After correction by the Holm's method for repeated measurements, there were no statistical differences. The overall median number of tracheal suctions per day in the cold‐air nebulization group was 5 with a range of 12 from 1 to 13 (total number of suctions = 117). The median number of tracheal suctions per day in the heated humidification group was 3 with a range of 13 from 1 to 14 (total number of suctions = 116). The results report a significantly higher (P < .001) number of required suction procedures over 14 days in the cold‐air nebulization group compared with the heated humidification group (see Figure 1).
Table 2.
Median numbers of necessary tracheal suction procedures per day in the cold‐air nebulization and heated humidifier groups
| Cold‐air nebulization group | Heated humidification group | ||
|---|---|---|---|
| Day | Median | Median | P value |
| 1 | 5.0 | 4.0 | .1183 |
| 2 | 6.0 | 4.0 | .1145 |
| 3 | 7.0 | 3.0 | .0955 |
| 4 | 6.0 | 4.0 | .2665 |
| 5 | 6.0 | 4.0 | .1058 |
| 6 | 6.0 | 4.0 | .1266 |
| 7 | 4.5 | 3.5 | .3409 |
| 8 | 4.5 | 2.0 | .0606 |
| 9 | 5.5 | 2.0 | .0488 |
| 10 | 3.5 | 1.5 | .1562 |
| 11 | 4.5 | 1.5 | .011 |
| 12 | 3.5 | 2.0 | .0862 |
| 13 | 3.0 | 1.5 | .1585 |
| 14 | 4.0 | 1.5 | .0002 |
P value is per day. After Holm's correction before repeated measurements there was no statistical significance.
Figure 1.
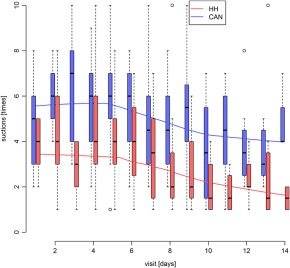
Box‐and‐whisker plot of suction procedures in the cold‐air nebulization (CAN) group and the heated humidifier (HH) group. The boxes represent the interquartile range (IQR), with the whiskers extending up to 1.5 times the IQR. The median is marked with a solid line. Outliers are marked with a circle. Visits are shown in days, and suction in necessary numbers [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Ciliary beat frequency
The CBF was lower in patients receiving cold‐air nebulization compared to the heated humidification group at all time points. On day 2, CBF was 4.2 ± 0.8 in the cold‐air nebulization group and 6.4 ± 1.2 in the heated humidification group, and the values remained stable during the 10‐day period (for details, see Table 2). After correction by the Holm's method for repeated measurements,20 the differences in CBF between the 2 groups were statistically highly significant (P < .01) on days 2 and 8, and significant (P < 0.05) on days 4 and 6. Overall, the mean CBF in the cold‐air nebulization group was 3.99 ± 1.39 Hz and in the heated humidification group it was 6.36 ± 1.49 Hz (P < .001). Figure 2 provides graphical interpretation.
Figure 2.
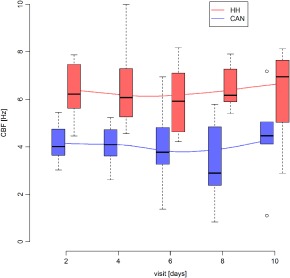
Box‐and‐whisker plot of ciliary beat frequency (CBF) changes in the cold‐air nebulization (CAN) group and the heated humidifier (HH) group. The boxes represent the interquartile range (IQR), with the whiskers extending up to 1.5 times the IQR. The median is marked with a solid line. Outliers are marked with a circle. Visits are shown in days, and CBF in Hz [Color figure can be viewed at wileyonlinelibrary.com]
Table 3.
Ciliary beat frequency (Hz) in the cold‐air nebulization and heated humidifier groups
| Heated humidification group | Cold‐air nebulization group | ||||
|---|---|---|---|---|---|
| Day | Mean | SD | Mean | SD | P value |
| 2 | 6.4 | 1.2 | 4.2 | 0.8 | .004 |
| 4 | 6.5 | 1.7 | 4.1 | 0.9 | .02 |
| 6 | 6.0 | 1.5 | 4.1 | 1.6 | .046 |
| 8 | 6.8 | 1.4 | 3.4 | 1.7 | .004 |
| 10 | 6.3 | 1.8 | 4.4 | 2.0 | .09 |
P value is after Holm's correction for repeated measurements.
4. DISCUSSION
A significant number of patients receive a tracheotomy for any reason. For example, >100 000 tracheostomies are performed annually in the United States.21 Across Europe, 7%‐16% of critical care admissions are managed with a tracheostomy, similar to data from the United States.22 Around 25% of patients with upper aerodigestive tract malignancies, such as head and neck cancers, receive a tracheostomy.23 The resulting stoma allows patients to breathe, bypassing the upper airway. Consequently, humidification of and heating the inspired air is compromised. In clinical routine, heated humidifiers, jet nebulizers, and HMEs are used for compensation. The HMEs show wide variation in water exchange performance (range 0.5‐3.6 mg/0.5 L air)24 and are the cheapest systems to use.12 Jet nebulizers can be efficient in delivering aerosolized solutions to the lungs and trachea in mechanically ventilated patients, but in spontaneously breathing patients, a heated humidifier and HME have better humidification and thermic capacities.25, 26 Nebulizers are also reported to be potentially harmful. In rabbits, long‐term exposure to ultrasonic nebulized saline (72 hours) led to pathological pulmonary changes comparable to severe bronchopneumonia.27 Additionally, in patients with chronic bronchitis, a significant decrease in 1‐second forced expiratory volume and vital capacity has been observed after saline inhalation via ultrasonic nebulizer.28 The advantage of heated humidifiers over HMEs has been shown previously. Heated humidifiers decrease adverse clinical events in children over a 10‐month period.29 Moreover, in intubated patients without a tracheostomy, heated humidification systems tested superior to HMEs. Fewer tracheostomies were needed; there was less hypothermia, and fewer thick, tenacious bronchial secretions were noted.30
In this study, the number of suction procedures and manual interventions required to clean the upper airway could be reduced in the heated humidification group by 40% from median value, 5 suction procedures per day to 3 per day. One explanation for this could be that there was higher CBF measured in this group. The mean overall CBF increased from 3.99 Hz ±1.39 in the cold‐air nebulization group to 6.36 Hz ±1.49, which corresponds to an increase of 37%. The CBF difference was also statistically significant on measurement days 2, 4, 6, and 8, but showed weak correlation with the number of suctioning procedures, but the data on correlation were not presented.
The impact of different techniques of airway humidification on tracheal epithelial CBF in tracheostomized patients has not been evaluated to date. In the present study, 20 newly tracheostomized patients, mostly due to head and neck cancer surgeries, were treated with cold‐air nebulization or heated humidification for 14 days, and CBF as well as tracheostomal suctions were assessed. The CBF measurements showed a significantly higher ciliary activity in patients supplied with the heated humidification systems in comparison to the cold‐air nebulizing systems. The normal CBF of tracheal epithelial cells collected during bronchoscopy is 11.3 beats/second when measured at a temperature of 23‐25°C. A CBF of 5.7 ± 2.5 Hz was shown in a comparable study with nasal epithelial cells using the same methodology for CBF assessment.31 The presented data, therefore, could be interpreted as an impaired ciliary function in both groups, which could be explained by epithelial irritation caused by the tracheostomy. An alternative explanation could be that harvesting the ciliated cells was performed with a cytobrush, in contrast to using a biopsy during bronchoscopy. With regard to the significantly lower CBF in the cold‐air nebulization group compared to the heated humidification group, as well as to the nasal CBF in healthy subjects, an impaired ciliary function in the cold‐air nebulization group could be demonstrated. Increased viscoelasticity in the trachea mucus layer in cold‐air nebulization could explain the impaired ciliary function, similar to increased viscoelasticity in cystic fibrosis.32 Optimal mucociliary clearance depends on the ability of the airway epithelium to hydrate secreted mucins so that mucus concentrations are optimal for cilial‐dependent mucus transport.33, 34 Additional CBF and the associated mucociliary clearance are temperature‐dependent, the higher CBF in the heated humidification group may be attributed to the more stable physiologic airway temperature with the heated system, even if the CBF measuring was at the same temperature in cold‐air nebulization group and in the heated humidification group.4, 5, 35
The key strength of the study is its prospective nature and use of a clinically relevant outcome ‐ the analyses of the number of required suction procedures. The data in this study support the theory of a protective role of heated humidification on mucociliary function in the airways. The mechanisms for improved outcome could be a reduction in mucus secretion or improved mucociliary clearance resulting in less need for suctioning in newly tracheostomized patients. Another strength of this study is a comparison of standard care procedure (cold‐air nebulization) with an alternative care provided by continuous active humidification of inspired air. The most important limitation of the study is that it is a nonrandomized study and that the CBF measurement was performed at 22°C and not with a heated microscope stage. In addition, blinding with regard to the type of humidification used was not possible considering the obvious technical difference between the humidification systems. Another limitation is that the patient comfort and preference were not assessed. There is also a lack of baseline measurements in the heated humidification group to enable analyses of the effects of heated humidification in every patient individually, particularly CBF.
5. CONCLUSION
Heated humidification demonstrated a potential advantage over cold‐air nebulization in newly tracheostomized patients with regard to physiologic and clinically relevant parameters. The number of required suction procedures was reduced, and the CBF was higher in the heated humidification group. Results indicate a potential advantage of hydration with heated and humidified air over the conventional nebulization of cold saline solution after a tracheotomy. A larger randomized trial might be required for studying the clinical outcomes of heated humidification and comparing these with the use of HME in tracheostomized patients.
AUTHOR CONTRIBUTIONS
Clinical assessment and experiments: Birk, Händel
Writing of manuscript: Aderhold, Birk, Kramer, Sommer, Stuck, Wenzel
Scientific support: Hoermann
Study initiation and study design: Birk, Sommer, Stuck
Scientific and functional support: Sommer
Birk R, Händel A, Wenzel A, et al. Heated air humidification versus cold air nebulization in newly tracheostomized patients. Head & Neck. 2017;39:2481–2487. https://doi.org/10.1002/hed.24917
Funding information The study was supported by Fisher and Paykel Health Care.
REFERENCES
- 1. Rankin N. What is optimum humidity? Respir Care Clin N Am. 1998;4(2):321‐328. [PubMed] [Google Scholar]
- 2. Sahin‐Yilmaz A, Naclerio RM. Anatomy and physiology of the upper airway. Proc Am Thorac Soc. 2011;8(1):31‐39. [DOI] [PubMed] [Google Scholar]
- 3. Heffner JE, Hess D. Tracheostomy management in the chronically ventilated patient. Clin Chest Med. 2001;22(1):55‐69. [DOI] [PubMed] [Google Scholar]
- 4. Henning A, Schneider M, Bur M, Blank F, Gehr P, Lehr CM. Embryonic chicken trachea as a new in vitro model for the investigation of mucociliary particle clearance in the airways. AAPS PharmSciTech. 2008;9(2):521‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jorissen M, Bessems A. Influence of culture duration and ciliogenesis on the relationship between ciliary beat frequency and temperature in nasal epithelial cells. Eur Arch Otorhinolaryngol. 1995;252(8):451‐454. [DOI] [PubMed] [Google Scholar]
- 6. Shah AS, Ben‐Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325(5944):1131‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stannard W, O'Callaghan C. Ciliary function and the role of cilia in clearance. J Aerosol Med. 2006;19(1):110‐115. [DOI] [PubMed] [Google Scholar]
- 8. Birk R, Aderhold C, Stern‐Sträter J, Hörmann K, Stuck BA, Sommer JU. Polyhexanide‐containing solution reduces ciliary beat frequency of human nasal epithelial cells in vitro. Eur Arch Otorhinolaryngol. 2015;272(2):377‐383. [DOI] [PubMed] [Google Scholar]
- 9. Chen M. Amended final report of the safety assessment of t‐butyl alcohol as used in cosmetics. Int J Toxicol. 2005;24 Suppl 2:1‐20. [DOI] [PubMed] [Google Scholar]
- 10. Elliott MK, Sisson JH, Wyatt TA. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am J Respir Cell Mol Biol. 2007;36(4):452‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewarski JS. Long‐term care of the patient with a tracheostomy. Respir Care. 2005;50(4):534‐537. [PubMed] [Google Scholar]
- 12. De Leyn P, Bedert L, Delcroix M, et al. Tracheotomy: clinical review and guidelines. Eur J Cardiothorac Surg. 2007;32(3):412‐421. [DOI] [PubMed] [Google Scholar]
- 13. Ackerstaff AH, Hilgers FJ, Balm AJ, Tan IB. Long‐term compliance of laryngectomized patients with a specialized pulmonary rehabilitation device: Provox Stomafilter. Laryngoscope. 1998;108(2):257‐260. [DOI] [PubMed] [Google Scholar]
- 14. Hilgers FJ, Aaronson NK, Ackerstaff AH, Schouwenburg PF, van Zandwikj N. The influence of a heat and moisture exchanger (HME) on the respiratory symptoms after total laryngectomy. Clin Otolaryngol Allied Sci. 1991;16(2):152‐156. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen CM, Rosendahl‐Nielsen M, Hjermind J, Egerod I. Endotracheal suctioning of the adult intubated patient–what is the evidence? Intensive Crit Care Nurs. 2009;25(1):21‐30. [DOI] [PubMed] [Google Scholar]
- 16. Maggiore SM, Lellouche F, Pignataro C, et al. Decreasing the adverse effects of endotracheal suctioning during mechanical ventilation by changing practice. Respir Care. 2013;58(10):1588‐1597. [DOI] [PubMed] [Google Scholar]
- 17. Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All‐digital image capture and whole‐field analysis of ciliary beat frequency. J Microsc. 2003;211(Pt 2):103‐111. [DOI] [PubMed] [Google Scholar]
- 18. Sommer JU, Schäfer K, Omran H, et al. ENT manifestations in patients with primary ciliary dyskinesia: prevalence and significance of otorhinolaryngologic co‐morbidities. Eur Arch Otorhinolaryngol. 2011;268(3):383‐388. [DOI] [PubMed] [Google Scholar]
- 19. The R Core Team . R: a language and environment for statistical computing. 2016. https://cran.r‐project.org/doc/manuals/r‐release/fullrefman.pdf. Accessed July 1, 2016.
- 20. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65‐70. [Google Scholar]
- 21. Yu M. Tracheostomy patients on the ward: multiple benefits from a multidisciplinary team? Crit Care. 2010;14(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGrath BA, Wallace S. The UK National Tracheostomy Safety Project and the role of speech and language therapists. Curr Opin Otolaryngol Head Neck Surg. 2014;22(3):181‐187. [DOI] [PubMed] [Google Scholar]
- 23. Langerman A, Patel RM, Cohen EE, Blair EA, Stenson KM. Airway management before chemoradiation for advanced head and neck cancer. Head Neck. 2012;34(2):254‐259. [DOI] [PubMed] [Google Scholar]
- 24. van den Boer C, Muller SH, Vincent AD, van den Brekel MW, Hilgers FJ. Ex vivo assessment and validation of water exchange performance of 23 heat and moisture exchangers for laryngectomized patients. Respir Care. 2014;59(8):1161‐1171. [DOI] [PubMed] [Google Scholar]
- 25. O'Riordan TG, Palmer LB, Smaldone GC. Aerosol deposition in mechanically ventilated patients. Optimizing nebulizer delivery. Am J Respir Crit Care Med. 1994;149(1):214‐219. [DOI] [PubMed] [Google Scholar]
- 26. Thomachot L, Viviand X, Arnaud S, Vialet R, Albanese J, Martin C. Preservation of humidity and heat of respiratory gases in spontaneously breathing, tracheostomized patients. Acta Anaesthesiol Scand. 1998;42(7):841‐844. [DOI] [PubMed] [Google Scholar]
- 27. Modell JH, Giammona ST, Davis JH. Effect of chronic exposure to ultrasonic aerosols on the lung. Anesthesiology. 1967;28(4):680‐688. [DOI] [PubMed] [Google Scholar]
- 28. Pflug AE, Cheney FW Jr, Butler J. The effects of an ultrasonic aerosol on pulmonary mechanics and arterial blood gases in patients with chronic bronchitis. Am Rev Respir Dis. 1970;101(5):710‐714. [DOI] [PubMed] [Google Scholar]
- 29. McNamara DG, Asher MI, Rubin BK, Stewart A, Byrnes CA. Heated humidification improves clinical outcomes, compared to a heat and moisture exchanger in children with tracheostomies. Respir Care. 2014;59(1):46‐53. [DOI] [PubMed] [Google Scholar]
- 30. Martin C, Perrin G, Gevaudan MJ, Saux P, Gouin F. Heat and moisture exchangers and vaporizing humidifiers in the intensive care unit. Chest. 1990;97(1):144‐149. [DOI] [PubMed] [Google Scholar]
- 31. Sommer JU, Gross S, Hörmann K, Stuck BA. Time‐dependent changes in nasal ciliary beat frequency. Eur Arch Otorhinolaryngol. 2010;267(9):1383‐1387. [DOI] [PubMed] [Google Scholar]
- 32. Kreda SM, Davis CW, Rose MC. CFTR, mucins, and mucus obstruction in cystic fibrosis. Cold Spring Harb Perspect Med. 2012;2(9):a009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Randell SH, Boucher RC; University of North Carolina Virtual Lung Group . Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol. 2006;35(1):20‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paisley D, Gosling M, Danahay H. Regulation of airway mucosal hydration. Expert Rev Clin Pharmacol. 2010;3(3):361‐369. [DOI] [PubMed] [Google Scholar]
- 35. Green A, Smallman LA, Logan AC, Drake‐Lee AB. The effect of temperature on nasal ciliary beat frequency. Clin Otolaryngol Allied Sci. 1995;20(2):178‐180. [DOI] [PubMed] [Google Scholar]


