Abstract
BACKGROUND
Cannabis is purported to alleviate symptoms related to cancer treatment, although the patterns of use among cancer patients are not well known. This study was designed to determine the prevalence and methods of use among cancer patients, the perceived benefits, and the sources of information in a state with legalized cannabis.
METHODS
A cross‐sectional, anonymous survey of adult cancer patients was performed at a National Cancer Institute–designated cancer center in Washington State. Random urine samples for tetrahydrocannabinol provided survey validation.
RESULTS
Nine hundred twenty‐six of 2737 eligible patients (34%) completed the survey, and the median age was 58 years (interquartile range [IQR], 46‐66 years). Most had a strong interest in learning about cannabis during treatment (6 on a 1‐10 scale; IQR, 3‐10) and wanted information from cancer providers (677 of 911 [74%]). Previous use was common (607 of 926 [66%]); 24% (222 of 926) used cannabis in the last year, and 21% (192 of 926) used cannabis in the last month. Random urine samples found similar percentages of users who reported weekly use (27 of 193 [14%] vs 164 of 926 [18%]). Active users inhaled (153 of 220 [70%]) or consumed edibles (154 of 220 [70%]); 89 (40%) used both modalities. Cannabis was used primarily for physical (165 of 219 [75%]) and neuropsychiatric symptoms (139 of 219 [63%]). Legalization significantly increased the likelihood of use in more than half of the respondents.
CONCLUSIONS
This study of cancer patients in a state with legalized cannabis found high rates of active use across broad subgroups, and legalization was reported to be important in patients' decision to use. Cancer patients desire but are not receiving information about cannabis use during their treatment from oncology providers. Cancer 2017;123:4488‐97. © 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the Creative Commons Attribution‐NonCommercial‐NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non‐commercial and no modifications or adaptations are made.
Keywords: cancer, cannabis, marijuana, pain, supportive care
Short abstract
Cannabis use is common among patients receiving treatment at a large cancer center in a state with legalized recreational and medical cannabis. Active use is reported across broad demographic and diagnostic cancer subgroups, and legalization is reported to be important in patients' decision to use.
INTRODUCTION
Cannabis is the most frequently used illicit drug in the United States.1, 2, 3 In the 2014‐2015 National Survey on Drug Use and Health, 8.3% of those who were 12 years old or older had used cannabis in the past month.2 Of adult active users, 9.8% reported use for medical reasons.4 A number of states have passed regulations that allow medicinal and/or recreational cannabis use, and this has increased local access and availability.4 In Washington State, cannabis was legalized for medicinal use in 1998 and for recreational use in November 2012; cannabis became commercially available in Washington in July 2014.
Cannabis has been purported to provide benefits for cancer patients, most frequently by alleviating anorexia, nausea, and pain.5 Positive impacts on mood and insomnia have been suggested as additional benefits.6 Research evaluating cannabis as therapy is limited,7, 8 and because of federal regulations, most studies have examined synthetic tetrahydrocannabinol (THC) analogues.9, 10, 11 THC may help to relieve pain12 and spasticity among targeted populations,13 but data evaluating other therapeutic aspects of cannabis are insufficient.5, 7, 14, 15 With insufficient data demonstrating the benefits for cancer patients, small studies and clinical observations have also raised concerns about the safety of cannabis use in immunosuppressed populations.16, 17, 18, 19 Currently, most available data on the medical uses of cannabis for cancer‐related symptom management come from nonscientific observations assembled from Web sites, lay press, and community interactions rather than rigorous scientific research.7
Increasing interest and shifting political attitudes on cannabis, coupled with a lack of knowledge of the risks and benefits in cancer care, indicate a need to understand current use patterns and to develop accurate and informative education for both cancer patients and their providers. The primary aim of this study was to better understand the extent and patterns of cannabis use among cancer patients in a state with legalized medical and recreational cannabis. We administered an anonymous survey to a representative cohort of ambulatory patients at a large National Cancer Institute–designated comprehensive cancer center in the Pacific Northwest specifically to determine the prevalence of cannabis use within a range of cancer patients. Furthermore, among active users, we assessed the methods of use, the context of their current use with medical treatment, the current reasons for use, the perceived impact of the legalization of recreational cannabis on current use, and patients' sources of information about cannabis use in cancer. Random urine samples tested for THC were used as a method of validation for survey prevalence data.
MATERIALS AND METHODS
Setting
We conducted a cross‐sectional survey of cancer patients at the Seattle Cancer Care Alliance over a 6‐week period between 2015 and 2016. The Seattle Cancer Care Alliance is the ambulatory center for a cancer consortium that includes the Fred Hutchinson Cancer Research Center, the University of Washington, and Seattle Children's Hospital; it serves patients from Alaska, Idaho, Montana, Oregon, Washington, and Wyoming as well as those referred to the center for hematopoietic cell transplantation and other research protocols. The facility includes clinical laboratories, clinics, radiology and procedure suites, and an infusion center; providers see approximately 75,000 outpatients yearly.
Participants
Patients presenting to the Seattle Cancer Care Alliance during the study period were eligible for the survey. To ensure a broad selection, surveys were offered in 3 clinical areas: radiology/special procedures, general oncology, and infusion units. Patients were eligible for the study and were given the opportunity to participate if they 1) were 18 years old or older, 2) were English‐speaking, and 3) had not previously completed the survey at a prior appointment.
Survey Development
A 44‐item questionnaire was developed to address cannabis use among cancer patients. These survey items were constructed to address key research questions on cannabis beliefs and health perceptions. An initial draft was informed by a literature review, consultations with clinical staff and patients, and study investigator experience. Independent clinical staff then assessed and modified the initial draft through one‐on‐one discussions and an e‐mail review with investigators. Health care providers, nutritionists, specialists in patient education, and the local public health department provided feedback on the survey's content and format. A draft was then presented to a caregiver and patient committee, and this allowed feedback on the survey's methods and validity; after additional modifications, this committee approved the final survey. The final survey had an introductory page describing the study goals, the anonymous nature of responses, and the estimated time for completion. The survey covered demographic and clinical information as well as issues concerning cannabis use (see online supporting information).
Study Procedures
Eligible patients were approached on arrival by trained front‐desk staff. Interested patients were given the paper survey and a prelabeled/self‐sealing privacy envelope. Completed surveys were returned directly to front‐desk staff in sealed envelopes and were picked up by the research team weekly or were sent by patients through campus/standard mail to the research team. Staff documented refusals during the first period of the survey distribution (radiology/procedures). In the other 2 areas, the denominator of eligible patients was determined from the total number of appointments/arrivals during the period of the survey distribution. An opt‐out check box was also available; opt‐outs and surveys that were returned unanswered were considered refusals. All anonymized survey responses were entered into Research Electronic Data Capture (RedCap, Nashville, Tennessee)20 and were double‐entered for accuracy.
Urine samples
In the first survey period, random leftover clinical urine samples (≥1 mL) from the center's laboratory were processed for THC. All urine samples were stored anonymously onsite in refrigerators and were then processed in bulk with the enzyme multiplied immunoassay technique; samples with detected THC concentrations ≥ 50 ng/mL were considered positive. Samples with detectable levels < 50 ng/mL were sent for confirmatory testing using gas chromatography–mass spectrometry (Mayo Clinical Reference Laboratory), which assessed them for Δ‐9‐tetrahydrocannabinol carboxylic acid. Those with samples insufficient for retesting or below the limit of detection (<3 ng/mL) were considered negative.
The survey, the methods for distribution, and all other study‐related procedures were approved by the institutional review board of the Fred Hutchinson Cancer Research Center.
Measures
Sociodemographic variables included age, sex, education level, and residential distance from the cancer center, whereas the cancer status included various indicators of the current diagnosis and treatment status. Cannabis use was assessed with multiple variables, including any lifetime use, details of the frequency and recency of use, methods of use (including inhalation, edibles, or both), reasons for stopping, and the impact of legalization on use. In addition, we assessed where patients acquired information on cannabis and where they preferred to get this information. Respondents were characterized as self‐identified active users (those patients who self‐reported cannabis use within the last year), prior users (those patients who reported cannabis at any point in their life but not within the past year), and never users (those patients who reported no history of cannabis use). Those who used cannabis 1 or more times a day were considered heavy users, those who used cannabis less than once a day but 1 or more times a week were considered moderate users, and those who used cannabis less than once per week were considered light users. Nine self‐reported reasons for using cannabis were assessed with a check‐all‐that‐apply question and, for analyses, were stratified into physical symptoms (for pain, for nausea/upset stomach, and to improve appetite), neuropsychiatric symptoms (for depression/to improve mood, to help cope with illness, to help deal with stress, and to sleep), recreational use/enjoyment, and treatment of cancer.
Statistical Methods
Survey responses and data comparisons are summarized as frequencies and percentages for categorical variables and as medians and interquartile ranges (IQRs) for continuous variables. Statistical comparisons were performed with the chi‐square test or Fisher's exact test (categorical variables), a 2‐sample t test (continuous variable vs 2‐category variable), or a 1‐way analysis of variance (continuous variable vs 3‐category variable). Because some questions allowed multiple responses, the sum was larger than the total sample of respondents; therefore, percentages represent the percentages of responses per the number of participants. To compare Likert scales, values were combined into low (1‐3), medium (4‐7), and high categories (8‐10). Analyses were performed with SAS 9.4 (SAS, Cary, North Carolina). A significance level of .05 (2‐sided) was used for all analyses.
RESULTS
Demographics of the Survey Respondents
Out of a maximum of 2737 possible participants, 926 (34%) completed the survey (Fig. 1). Of those completing the survey, the median age was 58 years (IQR, 46‐66 years), and the majority were men (Table 1). More than half reported having at least a college degree, and most lived locally (median from the center, 25 miles; IQR, 10‐60 miles); the reported distances were consistent with the center's national catchment area (Supporting Fig. 1 [see online supporting information]). The largest group of respondents had an underlying solid tumor malignancy, and the majority were receiving active cancer treatment at the time of the survey's completion (Table 1). When we compared the 2 study periods, differences in sex, the type of cancer, and the treatment status were noted (Supporting Table 1 [see online supporting information]).
Figure 1.

Schema of the survey respondents. Survey period 1 includes patients given the survey in the radiology/procedure suite waiting room (September 21 to October 9, 2015). During this period, surveyors recorded all refusals. Survey period 2 includes patients given the survey in the general oncology (January 11‐25, 2016) and infusion waiting rooms (January 4‐20, 2016). During this period, refusals were estimated on the basis of the number of unique patients seen in this area during the survey time period. *Anonymous leftover urine samples were collected during survey period 1. Declined indicates patients who declined to take the survey at the front desk, whereas opt out indicates patients who took the survey but returned the survey unanswered or after they had checked the opt‐out box on the first page of the survey.
Table 1.
Respondent Demographics (n = 926)
| Variable | Value |
|---|---|
| General | |
| Age, median (IQR), y | 58 (46‐66) |
| Age by decade, No. (%) | |
| <30 y | 55 (6) |
| 30‐39 y | 82 (10) |
| 40‐49 y | 123 (14) |
| 50‐59 y | 202 (24) |
| 60‐69 y | 254 (30) |
| ≥70 y | 141 (16) |
| Sex, No. (%) | |
| Male | 443 (52) |
| Female | 417 (48) |
| Education, No. (%) | |
| Elementary school | 5 (1) |
| High school/GED | 102 (11) |
| Some college | 252 (28) |
| College graduate | 325 (36) |
| Graduate degree | 209 (23) |
| Distance from center, median (IQR), miles | 25 (10‐60) |
| Distance from center, No. (%) | |
| ≤25 miles | 402 (52) |
| 26‐100 miles | 264 (34) |
| 101‐250 miles | 43 (6) |
| >250 miles | 61 (8) |
| Cancer diagnosis | |
| Cancer group, No. (%)a | |
| Solid tumor | 577 (66) |
| Hematologic | 349 (34) |
| Type of cancer, No. (%)a | |
| Hematologic | 298 (34) |
| Gastrointestinal | 156 (18) |
| Breast | 102 (12) |
| Lung or head/neck | 108 (12) |
| Sarcoma/bone and joint | 35 (4) |
| Skin | 32 (4) |
| Gynecologic | 24 (3) |
| Prostate | 26 (3) |
| Brain | 15 (2) |
| Genitourinary | 18 (2) |
| Other | 88 (10) |
| Cancer treatment status | |
| Treatment status, No. (%) | |
| Newly diagnosed | 40 (5) |
| Currently undergoing treatment | 580 (66) |
| Finished therapy | 185 (21) |
| Not currently receiving treatment | 79 (9) |
| First visit, No. (%) | |
| Yes | 47 (5) |
| No | 847 (95) |
| Currently receiving medication for cancer, No. (%)b | |
| Yes | 586 (66) |
| No | 250 (28) |
| Currently on radiation therapy, No. (%)b | |
| Yes | 63 (7) |
| No | 780 (88) |
| Currently receiving bone marrow transplant, No. (%)b | |
| Yes | 161 (18) |
| No | 693 (78) |
| Type of bone marrow transplant, No. (%)c | |
| Autologous | 59 (41) |
| Allogeneic | 69 (48) |
| Both | 17 (12) |
Abbreviations: GED, general educational development; IQR, interquartile range. Not all respondents completed demographic data, so percentages given as total per question. Percentages may not always equal 100% due to rounding.
Patients could choose more than one option, as some had multiple cancers.
Does not equal 100%, as <10% of respondents reported that they did not know if they were on active therapy.
Among patients reporting that they had received a bone marrow transplant.
Current and Past Cannabis Use
Sixty‐six percent of the respondents (607 of 926) had used cannabis at some point in their life, and 24% (222 of 926) considered themselves active cannabis users (Supporting Fig. 2 [see online supporting information]). Active users were younger, had less education, and were less likely to be hematopoietic cell transplant recipients in comparison with prior and never users (Table 2); the underlying type of cancer did not affect use. There were no differences in cannabis use among respondents in the 2 study periods (Supporting Table 1 [see online supporting information]).
Table 2.
Demographic Comparisons Among Cancer Patients by Cannabis Use Statusa
| Variable | Active Users, No. (%) | Prior Users, No. (%) | Never Users, No. (%) | P b | P for Active Users vs All Others |
|---|---|---|---|---|---|
| Age group (decades) | <.0001 | <.0001 | |||
| <30 y | 21 (10) | 18 (5) | 14 (5) | ||
| 30 to <40 y | 22 (10) | 36 (10) | 23 (8) | ||
| 40 to <50 y | 37 (17) | 51 (14) | 34 (12) | ||
| 50 to <60 y | 52 (25) | 96 (27) | 54 (19) | ||
| 60 to <70 y | 68 (32) | 117 (33) | 69 (24) | ||
| ≥70 y | 12 (6) | 38 (11) | 91 (32) | ||
| Sex | .02 | .04 | |||
| Male | 123 (57) | 189 (53) | 128 (45) | ||
| Female | 92 (43) | 167 (47) | 157 (55) | ||
| Legalization and use (scale, 1‐10) | <.0001 | <.0001 | |||
| 1‐3 (no change) | 65 (30) | 177 (47) | 213 (69) | ||
| 4‐7 | 37 (17) | 106 (28) | 52 (17) | ||
| 8‐10 (much more likely) | 117 (53) | 97 (26) | 45 (15) | ||
| Education | <.01 | <.01 | |||
| Elementary school | 3 (1) | 0 (0) | 2 (1) | ||
| High school/GED | 37 (17) | 36 (10) | 29 (10) | ||
| Some college | 64 (29) | 100 (27) | 85 (28) | ||
| College graduate | 80 (37) | 133 (36) | 110 (37) | ||
| Graduate degree | 35 (16) | 100 (27) | 74 (25) | ||
| Type of cancerc, d | N/A | N/A | |||
| Gastrointestinal | 45 (21) | 55 (15) | 55 (18) | ||
| Hematologic | 66 (31) | 124 (35) | 107 (36) | ||
| Gynecologic | 7 (3) | 15 (4) | 2 (1) | ||
| Lung or head/neck | 29 (13) | 40 (11) | 37 (12) | ||
| Breast | 24 (11) | 42 (12) | 36 (12) | ||
| Genitourinary | 5 (2) | 9 (3) | 4 (1) | ||
| Prostate | 9 (4) | 11 (3) | 6 (2) | ||
| Brain | 4 (2) | 9 (3) | 2 (1) | ||
| Skin | 10 (5) | 13 (4) | 9 (3) | ||
| Sarcoma/bone and joint | 10 (5) | 11 (3) | 14 (5) | ||
| Hematologic disease (nonmalignant) | 11 (5) | 21 (6) | 21 (7) | ||
| Other | 9 (4) | 14 (4) | 11 (4) | ||
| Cancer group | .43 | .20 | |||
| Solid tumor | 150 (69) | 231 (65) | 192 (64) | ||
| Hematologic | 66 (31) | 124 (35) | 107 (36) | ||
| BMT status | <.01 | <.01 | |||
| Transplant patient | 24 (11) | 84 (23) | 52 (17) | ||
| Nontransplant patient | 184 (84) | 269 (73) | 236 (79) | ||
| Unknown | 11 (5) | 14 (4) | 12 (4) | ||
| Any current information source | <.0001 | <.0001 | |||
| Yes | 178 (87) | 194 (59) | 87 (32) | ||
| No | 26 (13) | 136 (41) | 184 (68) |
Abbreviations: BMT, bone marrow transplant; GED, General Educational Development; N/A, not available.
Not all percentages equal 100% due to rounding.
P values for categorical variables were calculated with the chi‐square test of independence.
P values for the type of cancer could not be calculated because some patients had multiple cancers.
Total percentage may be greater than 100%, because respondents could select more than one option.
Most active users had used cannabis before their cancer diagnosis (147 [67%]). Of those who quit, most did so before their diagnosis (266 of 326 [82%]), and they were older (median, 59 years; IQR, 50‐66 years) than those who quit after their diagnosis (median, 48 years; IQR, 34‐58 years; P < .0001). Most who quit after their diagnosis (32 of 57 [56%]) were undergoing active treatment for a solid tumor malignancy; a small number quit on the basis of a recommendation from their cancer or primary care physician (8 of 51 [16%] and 2 of 51 [4%], respectively). The majority of the active users had told their cancer team about their use (138 of 221 [62%]).
Frequency and Methods of Cannabis Use
Of the 222 active cannabis users, 164 (74%) reported using cannabis at least once a week (moderate use), and 124 (56%) used cannabis at least daily (heavy use); 68 (31%) used cannabis multiple times a day. Among the 193 leftover urine samples selected from the ambulatory laboratory for THC testing, 27 (14%) were positive (23 by the enzyme multiplied immunoassay technique and 4 by gas chromatography–mass spectrometry), and this was consistent with the number of survey respondents who reported at least moderate (weekly) use (164 of 926 [18%]).
A similar number of active users smoked (153 of 220 [70%]) or used edibles (154 [70%]), although dual use was also common (89 [40%]; Fig. 2). Pipes were the most common method of inhalation, and they were followed by vaporizers and rolled cigarettes; the most frequent forms of edibles were store‐bought candy/other edibles, butters/oils, and homemade baked goods.
Figure 2.
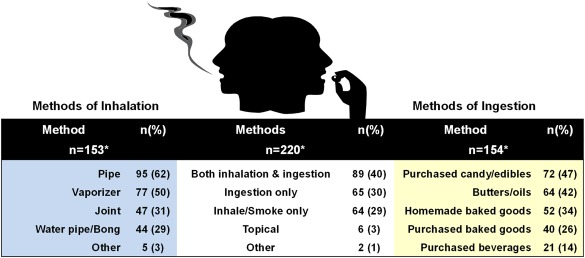
Patterns of cannabis use among active users. *Total percentages may be greater than 100%, because respondents could select more than one option.
Reasons for Marijuana Use Among Active Users
Active users reported using cannabis most frequently for pain, which was followed by nausea/upset stomach and stress (Fig. 3). Seventy‐six of 219 patients (35%) reported using cannabis for enjoyment/recreational use, but only 16 of these patients (7.3%) used cannabis for this reason exclusively. Use for neuropsychiatric reasons (139 of 219 [63%]) was nearly as common as use for physical symptoms (165 of 219 [75%]; Fig. 3). More than one‐quarter of active users (58 of 219 [26%]) believed that cannabis was helping to treat their cancer, and 10 (5%) indicated that this was their only reason for use. Regardless of symptoms, 106 of 206 (51%) felt that cannabis was a major benefit (score on a Likert scale, 8‐10), and 80 (39%) felt that it was a moderate benefit (score, 4‐7).
Figure 3.

Reasons for cannabis use among the survey respondents. The reasons for use were not mutually exclusive responses. Overall, the respondents used cannabis for physical symptoms (165 of 219 [75%]), for neuropsychiatric symptoms (139 of 219 [63%]), recreationally (76 of 219 [35%]), and to treat cancer (58 of 219 [26%]).
In comparison with prior and never users, active users reported that they were more likely to use cannabis because of its legalization (P < .001; scale, 1 [no change] to 10 [much more likely]): the median value was 8 for active users (IQR, 1‐10), 4 for prior users (IQR, 1‐8), and 1 for never users (IQR, 1‐6). Among active and prior users, women were more likely to use because of legalization in comparison with men (P = .002; Supporting Table 1 [see online supporting information]).
Knowledge and Sources of Information
The majority of the respondents wanted to learn more about cannabis and cancer (6 on a 1‐10 scale; IQR, 3‐10) but the level of interest varied with age (P < .01; Fig. 4A). Although nearly all respondents preferred to get information from their cancer team, (677/911 [74%]) less than 15% received information from their cancer physician or nurse (Fig. 4B). Most received information from friends/family, newspaper/magazine articles, Web sites/blogs, or another cancer patient; more than one‐third reported that they had not received any information. Only 73 of the 926 patients completing the survey (8%) did not want to receive more information.
Figure 4.

Cancer respondents' interest in education and sources of information about cannabis use during cancer therapy. (A) Interest in learning about cannabis during cancer therapy stratified by age (*P ≤ .05, **P < .01, and ***P < .001). In the low‐interest group, comparisons were made between ages < 30 years and other age strata. In the high‐interest group, comparisons were made between ages ≥ 70 years and other age strata. No statistical differences were found in the moderate‐interest group. (B) Desired and current sources of information about cannabis during cancer therapy. The responses were not mutually exclusive.
DISCUSSION
This survey‐based study of cancer patients at a large comprehensive cancer center within a state with medically and recreationally legalized cannabis found that nearly a quarter of patients reported active use. More than half of active users reported that legalization significantly increased their likelihood of using, and cannabis use was spread across demographic subsets, including age, sex, and cancer diagnosis subsets. Respondents reported using a diverse mix of cannabis products, which were evenly divided between inhaled and edible modalities. Cannabis was used commonly for the relief of physical symptoms, but use for neuropsychiatric symptoms was nearly as frequent. Even among never users, the respondents indicated substantial interest in learning more about the role of cannabis in cancer care. Despite nearly all respondents wanting more information/education directly from their hematology/oncology providers, most reported that they were more likely to get information from sources outside the health care system.
Self‐reported cannabis use among respondents in our study was 24% within the last year and 21% within the last month. These levels are more than double those reported in national prevalence studies, where rates vary between 1.8% and 8.3% over 1 month and between 2.8% and 12.9% over 1 year.2, 3, 4, 21, 22, 23, 24, 25 The younger demographic drives rates in most large studies, in which nearly 20% of 18‐ to 25‐year‐olds are reported to use cannabis.2 In contrast, those under the age of 30 years made up only 6% of our total respondents but had rates of active use of nearly 40%.
Studies specifically targeting older adults (≥50 years), which are more consistent with our cohort's median age, have described cannabis use rates of 1.8% per month and 2.8% to 4.8% per year.23, 26 Older adults at our cancer center were much more likely to use cannabis, with rates anywhere from 4 to 14 times those reported for the general population. The frequency of THC detection in anonymized leftover urine samples appeared to corroborate survey data for patients who reported a weekly use.
Comparing our results with the results for other cancer populations is difficult because of the limited number of studies. An Australian study evaluating cannabis use among patients with advanced cancer and/or a poor appetite at cancer and palliative care clinics found that 26 of 204 patients (13%) had previously used medical cannabis.27 A study from Israel, where medical cannabis is legal, estimated that only 1.7% of 17,000 cancer patients had acquired a permit for medical marijuana.28 Among patients receiving medical cannabis, only 7% of those in California reportedly used it for cancer‐related complications,29 and only 2.6% of those in the Netherlands reported combined medical cannabis and oncolytics.30 Although reports indicate that less than 20% of patients use cannabis for primarily medicinal purposes,31 with the rest presumably using cannabis recreationally, only 7% of the respondents in our study used cannabis only for recreational purposes.
Cannabis use in other immunosuppressed populations and in those with other chronic conditions also appears to be higher overall than usage rates reported for the general public.32, 33, 34 However, comparisons with our data may be less relevant because most studies are not conducted in locales with available recreational cannabis and/or focus on diseases that are more frequently reported in younger patient populations (eg, inflammatory bowel disease) and/or may be associated with increased substance use (eg, human immunodeficiency virus).35
Respondents in our cohort used cannabis for a wide variety of physical and neuropsychiatric symptoms that have a limited evidence base.7, 36, 37 Most frequently, patients used cannabis to treat pain. Although there is evidence for the pain‐relieving properties of cannabis, most data come from small studies that have evaluated its use for chronic neuropathic pain.37, 38, 39, 40 Other more recent studies have suggested that cannabis may help to limit opioid use in some patients with chronic pain.12, 41 However, because pain can be a persistent symptom in cancer patients, intermittent use among respondents may indicate limits to the benefits of cannabis use for pain control. Future studies evaluating cannabis in cancer‐related pain control are needed to assess its role as a potential adjunct to currently approved pain‐control strategies.
Data supporting cannabis use for nausea and appetite improvement are even less clear, with some studies suggesting possible benefits15, 42 and others suggesting none.11, 14 Despite weak evidence, nearly 50% of oncologists would still recommend cannabis for such symptoms.43 In addition, a significant number of respondents believed that cannabis helped to treat their cancer, although no trials have addressed this question.
Neuropsychiatric problems such as depression, anxiety, and insomnia are common during cancer treatment, and evidence‐based treatments are available to address these problems.44 Despite little scientific data supporting cannabis use for mental health–related symptoms during cancer treatment,45 our findings reveal that a large proportion of patients use cannabis for these issues. Cannabis use may be associated with self‐medication of serious psychiatric disorders and/or avoidance of potentially beneficial evidence‐based approaches to these problems. Research is needed to examine the potential role of cannabis as an alternative or adjunct for treating depression, anxiety,46 and insomnia47, 48 in addition to other common cancer‐related comorbidities. In future studies, it may be important to compare cannabis with alcohol and other illicit substances that may also be used by patients to mitigate some of these same symptoms
There is a need to better understand methods of cannabis use to maximize benefit and limit risk because patients are already using a wide variety of products. Prior studies using synthetic THC analogues9, 10, 11 have not incorporated the whole cannabis plant and, therefore, cannot evaluate other substances, such as terpenes and flavonoids, that may enhance or provide additional therapeutic properties.49 At the same time, the numerous potential risks of cannabis in this population, including drug‐drug interactions,17, 50 infections,16, 51, 52, 53, 54 sinopulmonary side effects,18, 54, 55, 56 neuropsychiatric sequelae,19, 57 and unintended overdoses/poisonings,58, 59, 60, 61 argue for rigorous safety studies. Currently, however, the US government continues to classify cannabis as a schedule I drug, and this restricts federal funding for safety studies and those assessing its therapeutic use in this population.62
Our study is not without limitations typically seen in survey studies. Most importantly, only 1 in 3 patients responded to the survey, so it is possible that a sampling bias may have led to either overrepresentation or underrepresentation of current use patterns among cancer patients. For example, it is possible that patients who were already interested in cannabis may have been more likely to respond to our survey, and this could have inflated the number of active users. Conversely, because cannabis remains an illicit drug, social desirability may have led respondents to underreport use. As with many survey instruments, it is also possible that because questions were asked about both recent and past use, a recall bias may have also affected responses. Furthermore, it is possible that patients taking dronabinol or other cannabinoids may have considered themselves cannabis users, and such agents may have affected urinary testing. Our data may not reflect rates of use among cancer patients in other states that have different medical/recreational cannabis laws. Finally, our survey was limited to English‐speaking patients and potentially missed segments of the population whose cannabis usage patterns may vary because of cultural differences. Despite these limitations, the moderate response rate, large sample size, and corroboration between random urinary testing and survey results suggest that this study provides a good estimate of current use practices at our center.
It is expected that many of the estimated 1.7 million patients in the United States diagnosed with cancer yearly will be exposed to increased local availability, permissiveness, and nonscientific reports suggesting benefits of cannabis. Because it is estimated that cannabis use will continue to expand nationally,24 the development of a framework for understanding the utility of cannabis among patients who are diagnosed with cancer has become important for both patients and providers. Despite the limited evidence for a medical role for cannabis in oncology, our data suggest that cannabis may be currently used frequently in this setting. Patients are interested in receiving information about how cannabis might benefit them and prefer that this information come directly from their cancer providers. There is a need for clinical trials evaluating the role of cannabis in symptom management and for the development of formalized education for patients and health care professionals about the risks and benefits of use in this population.
FUNDING SUPPORT
Study data were collected and managed using REDCap electronic data capture tools20 hosted at the Institute of Translational Health Sciences. REDCap (Research Electronic Data Capture) is a secure, web‐based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources. REDCap at ITHS is supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR002319. Maresa C. Woodfield was supported in part by a research scholarship from the Mary Gates Endowment for Students at the University of Washington. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, Fred Hutchinson Cancer Research Center, Seattle Cancer Care Alliance, or the University of Washington.
CONFLICT OF INTEREST DISCLOSURES
Steven A. Pergam has received research support from and has been a consultant for Merck Sharp & Dohme Corp. and Optimer/Cubist Pharmaceuticals outside the submitted work. Guang‐Shing Cheng has served as a consultant for Gilead Sciences. Jesse R. Fann is a consultant for Quartet Health.
AUTHOR CONTRIBUTIONS
Steven A. Pergam: Full access to all data in the study; responsibility for the integrity of the data and the accuracy of all analyses; study concept and design; acquisition, analysis, or interpretation of the data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; and study supervision. Maresa C. Woodfield: Full access to all data in the study; study concept and design; acquisition, analysis, or interpretation of the data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; and statistical analysis. Christine M. Lee: Full access to all data in the study; study concept and design; acquisition, analysis, or interpretation of the data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; and statistical analysis. Guang‐Shing Cheng: Full access to all data in the study; acquisition, analysis, or interpretation of the data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; and statistical analysis. Kelsey K. Baker: Full access to all data in the study; acquisition, analysis, or interpretation of the data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; and statistical analysis. Sara R. Marquis: Full access to all data in the study; acquisition, analysis, or interpretation of the data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; and statistical analysis. Jesse R. Fann: Full access to all data in the study; study concept and design; acquisition, analysis, or interpretation of the data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; and study supervision.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information 1
We thank Kyoko Kurosawa and Zach Stednick for their assistance with the figures and Dr. Jeff Duchin, the clinical staff at the Seattle Cancer Care Alliance, Brandelyn Bergstedt, and the Patient and Family Advisory Committee for their help in developing the survey.
REFERENCES
- 1. Caldeira KM, O'Grady KE, Vincent KB, Arria AM. Marijuana use trajectories during the post‐college transition: health outcomes in young adulthood. Drug Alcohol Depend. 2012;125:267‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Center for Behavioral Health Statistics and Quality . Key substance use and mental health indicators in the United States: results from the 2015 National Survey on Drug Use and Health. http://www.samhsa.gov/data/sites/default/files/NSDUH‐FFR1‐2015/NSDUH‐FFR1‐2015/NSDUH‐FFR1‐2015.htm Accessed February, 2016.
- 3. Azofeifa A, Mattson M, Schauer G, McAfee T, Grant A, Lyerla R. National estimates of marijuana use and related indicators—National Survey on Drug Use and Health, United States, 2002‐2014. MMWR Surveill Summ. 2016;65:1‐28. [DOI] [PubMed] [Google Scholar]
- 4. Compton WM, Han B, Hughes A, Jones CM, Blanco C. Use of marijuana for medical purposes among adults in the United States. JAMA. 2017;317:209‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abrams DI, Guzman M. Cannabis in cancer care. Clin Pharmacol Ther. 2015;97:575‐586. [DOI] [PubMed] [Google Scholar]
- 6. Robson P. Therapeutic aspects of cannabis and cannabinoids. Br J Psychiatry. 2001;178:107‐115. [DOI] [PubMed] [Google Scholar]
- 7. Wilkie G, Sakr B, Rizack T. Medical marijuana use in oncology: a review. JAMA Oncol. 2016;11:1‐6. [DOI] [PubMed] [Google Scholar]
- 8. Birdsall SM, Birdsall TC, Tims LA. The use of medical marijuana in cancer. Curr Oncol Rep. 2016;18:40. [DOI] [PubMed] [Google Scholar]
- 9. Springer YP, Gerona R, Scheunemann E, et al. Increase in adverse reactions associated with use of synthetic cannabinoids—Anchorage, Alaska, 2015‐2016. MMWR Morb Mortal Wkly Rep. 2016;65:1108‐1111. [DOI] [PubMed] [Google Scholar]
- 10. Cote M, Trudel M, Wang C, Fortin A. Improving quality of life with nabilone during radiotherapy treatments for head and neck cancers: a randomized double‐blind placebo‐controlled trial. Ann Otol Rhinol Laryngol. 2016;125:317‐324. [DOI] [PubMed] [Google Scholar]
- 11. Meiri E, Jhangiani H, Vredenburgh JJ, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy‐induced nausea and vomiting. Curr Med Res Opin. 2007;23:533‐543. [DOI] [PubMed] [Google Scholar]
- 12. Boehnke KF, Litinas E, Clauw DJ. Medical cannabis associated with decreased opiate medication use in retrospective cross‐sectional survey of chronic pain patients. J Pain. 2016;17:739‐744. [DOI] [PubMed] [Google Scholar]
- 13. Hill KP, Weiss RD. Minimal physical health risk associated with long‐term cannabis use—but buyer beware. JAMA. 2016;315:2338‐2339. [DOI] [PubMed] [Google Scholar]
- 14. Strasser F, Luftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta‐9‐tetrahydrocannabinol in treating patients with cancer‐related anorexia‐cachexia syndrome: a multicenter, phase III, randomized, double‐blind, placebo‐controlled clinical trial from the Cannabis‐In‐Cachexia‐Study‐Group. J Clin Oncol. 2006;24:3394‐3400. [DOI] [PubMed] [Google Scholar]
- 15. Machado Rocha FC, Stefano SC, De Cassia Haiek R, Rosa Oliveira LMQ, Da Silveira DX. Therapeutic use of Cannabis sativa on chemotherapy‐induced nausea and vomiting among cancer patients: systematic review and meta‐analysis. Eur J Cancer Care. 2008;17:431‐443. [DOI] [PubMed] [Google Scholar]
- 16. Verweij PE, Kerremans JJ, Voss A, Meis JF. Fungal contamination of tobacco and marijuana. JAMA. 2000;284:2875. [DOI] [PubMed] [Google Scholar]
- 17. Anderson GD, Chan LN. Pharmacokinetic drug interactions with tobacco, cannabinoids and smoking cessation products. Clin Pharmacokinet. 2016;55:1353‐1368. [DOI] [PubMed] [Google Scholar]
- 18. Martinasek M, McGrogan J, Maysonet A. A systematic review of the respiratory effects of inhalational marijuana. Respir Care. 2016;61:1543‐1551. [DOI] [PubMed] [Google Scholar]
- 19. Volkow N, Baler R, Compton W, Weiss S. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219‐2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pacula RL, Jacobson M, Maksabedian E. In the weeds: a baseline view of cannabis use among legalizing states and their neighbors. Addiction. 2016;111:973‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salas‐Wright CP, Vaughn MG, Cummings‐Vaughn LA, et al. Trends and correlates of marijuana use among late middle‐aged and older adults in the United States, 2002‐2014. Drug Alcohol Depend. 2017;171:97‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dinitto DM, Choi NG. Marijuana use among older adults in the U.S.A.: user characteristics, patterns of use, and implications for intervention. Int Psychogeriatr. 2011;23:732‐741. [DOI] [PubMed] [Google Scholar]
- 24. Grucza RA, Agrawal A, Krauss MJ, Cavazos‐Rehg PA, Bierut LJ. Recent trends in the prevalence of marijuana use and associated disorders in the United States. JAMA Psychiatry. 2016;73:10‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001‐2002 and 2012‐2013. JAMA Psychiatry. 2015;72:1235‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han BH, Sherman S, Mauro PM, Martins SS, Rotenberg J, Palamar JJ. Demographic trends among older cannabis users in the United States, 2006‐2013. Addiction. 2017;112:516‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luckett T, Phillips J, Lintzeris N, et al. Clinical trials of medicinal cannabis for appetite‐related symptoms from advanced cancer: a survey of preferences, attitudes and beliefs among patients willing to consider participation. Intern Med J. 2016;46:1269‐1275. [DOI] [PubMed] [Google Scholar]
- 28. Waissengrin B, Urban D, Leshem Y, Garty M, Wolf I. Patterns of use of medical cannabis among Israeli cancer patients: a single institution experience. J Pain Symptom Manage. 2015;49:223‐230. [DOI] [PubMed] [Google Scholar]
- 29. Ryan‐Ibarra S, Induni M, Ewing D. Prevalence of medical marijuana use in California, 2012. Drug Alcohol Rev. 2015;34:141‐146. [DOI] [PubMed] [Google Scholar]
- 30. Hazekamp A, Heerdink ER. The prevalence and incidence of medicinal cannabis on prescription in the Netherlands. Eur J Clin Pharmacol. 2013;69:1575‐1580. [DOI] [PubMed] [Google Scholar]
- 31. Lin LA, Ilgen MA, Jannausch M, Bohnert KM. Comparing adults who use cannabis medically with those who use recreationally: results from a national sample. Addict Behav. 2016;61:99‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weiss A, Friedenberg F. Patterns of cannabis use in patients with inflammatory bowel disease: a population based analysis. Drug Alcohol Depend. 2015;156:84‐89. [DOI] [PubMed] [Google Scholar]
- 33. Chong MS, Wolff K, Wise K, Tanton C, Winstock A, Silber E. Cannabis use in patients with multiple sclerosis. Mult Scler. 2006;12:646‐651. [DOI] [PubMed] [Google Scholar]
- 34. Furler MD, Einarson TR, Millson M, Walmsley S, Bendayan R. Medicinal and recreational marijuana use by patients infected with HIV. AIDS Patient Care STDS. 2004;18:215‐228. [DOI] [PubMed] [Google Scholar]
- 35. Hartzler B, Dombrowski JC, Crane HM, et al. Prevalence and predictors of substance use disorders among HIV care enrollees in the United States. AIDS Behav. 2017;21:1138‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cathcart P, de Giorgio A, Stebbing J. Cannabis and cancer: reality or pipe dream? Lancet Oncol. 2015;16:1291‐1292. [DOI] [PubMed] [Google Scholar]
- 37. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems. JAMA. 2015;313:2474‐2483. [DOI] [PubMed] [Google Scholar]
- 38. Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low‐dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo‐controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112:299‐306. [DOI] [PubMed] [Google Scholar]
- 41. Lucas P, Walsh Z. Medical cannabis access, use, and substitution for prescription opioids and other substances: a survey of authorized medical cannabis patients. Int J Drug Policy. 2017;42:30‐35. [DOI] [PubMed] [Google Scholar]
- 42. Ungerleider JT, Andrysiak T, Fairbanks L, Goodnight J, Sarna G, Jamison K. Cannabis and cancer chemotherapy. Cancer. 1982;50:636‐645. [DOI] [PubMed] [Google Scholar]
- 43. Doblin R, Kleiman M. Marijuana as antiemetic medicine: a survey of oncologists experiences and attitudes. J Clin Oncol. 1991;9:1314‐1319. [DOI] [PubMed] [Google Scholar]
- 44. Adler NE, Page AEK, eds. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 45. Kramer JL. Medical marijuana for cancer. CA Cancer J Clin. 2015;65:110‐122. [DOI] [PubMed] [Google Scholar]
- 46. Bricker J, Russo J, Stein M, et al. Does occasional cannabis use impact anxiety and depression treatment outcomes?: results from a randomized effectiveness trial. Depress Anxiety. 2007;24:392‐398. [DOI] [PubMed] [Google Scholar]
- 47. Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg. 2010;110:604‐610. [DOI] [PubMed] [Google Scholar]
- 48. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32:1605‐1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maida V, Daeninck PJ. A user's guide to cannabinoid therapies in oncology. Curr Oncol. 2016;23:398‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hauser N, Sahai T, Richards R, Roberts T. High on cannabis and calcineurin inhibitors: a word of warning in an era of legalized marijuana. Case Rep Transplant. 2016;2016:4028492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hamadeh R, Ardehali A, Locksley RM, York MK. Fatal aspergillosis associated with smoking contaminated marijuana, in a marrow transplant recipient. Chest. 1988;94:432‐433. [DOI] [PubMed] [Google Scholar]
- 52. Szyper‐Kravitz M, Lang R, Manor Y, Lahav M. Early invasive pulmonary aspergillosis in a leukemia patient linked to Aspergillus contaminated marijuana smoking. Leuk Lymphoma. 2001;42:1433‐1437. [DOI] [PubMed] [Google Scholar]
- 53. Thompson GR III, Tuscano JM, Dennis M, et al. A microbiome assessment of medical marijuana. Clin Microbiol Infect. 2017;23:269‐270. [DOI] [PubMed] [Google Scholar]
- 54. Khwaja S, Yacoub A, Cheema A, et al. Marijuana smoking in patients with leukemia. Cancer Control. 2016;23:278‐283. [DOI] [PubMed] [Google Scholar]
- 55. Tashkin DP, Simmons MS, Tseng CH. Impact of changes in regular use of marijuana and/or tobacco on chronic bronchitis. COPD. 2012;9:367‐374. [DOI] [PubMed] [Google Scholar]
- 56. Self TH, Shah S, March KL, Sands CW. Asthma associated with the use of cocaine, heroin, and marijuana: a review of the evidence. J Asthma. In press. [DOI] [PubMed] [Google Scholar]
- 57. Jouanjus E, Leymarie F, Tubery M, Lapeyre‐Mestre M. Cannabis‐related hospitalizations: unexpected serious events identified through hospital databases. Br J Clin Pharmacol. 2011;71:758‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cao D, Srisuma S, Bronstein AC, Hoyte CO. Characterization of edible marijuana product exposures reported to United States poison centers. Clin Toxicol (Phila). 2016;54:840‐846. [DOI] [PubMed] [Google Scholar]
- 59. Kim HS, Hall KE, Genco EK, Van Dyke M, Barker E, Monte A. Marijuana tourism and emergency department visits in Colorado. N Engl J Med. 2016;374:797‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang G, Le Lait M, Deakyne S, Bronstein A, Bajaj L, Roosevelt G. Unintentional pediatric exposures to marijuana in Colorado, 2009‐2015. JAMA Pediatr. 2016;170:E1‐E6. [DOI] [PubMed] [Google Scholar]
- 61. Sznitman SR, Goldberg V, Sheinman‐Yuffe H, Flechter E, Bar‐Sela G. Storage and disposal of medical cannabis among patients with cancer: assessing the risk of diversion and unintentional digestion. Cancer. 2016;122:3363‐3370. [DOI] [PubMed] [Google Scholar]
- 62. US Department of Justice . Title 21 United States Code (USC) Controlled Substances Act: Section 812. https://www.deadiversion.usdoj.gov/21cfr/21usc/812.htm. Accessed February 17, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information 1


