Abstract
BACKGROUND
Limited information is available about testosterone concentrations representative of the general US population, especially children, women, and non-Hispanic Asians.
METHODS
We obtained nationally representative data for total testosterone (totalT), measured with standardized LC-MS/MS, for the US population age 6 years and older from the 2011–2012 National Health and Nutrition Examination Survey (NHANES). We analyzed 6746 serum samples and calculated the geometric means, distribution percentiles, and covariate-adjusted geometric means by age, sex, and race/ethnicity.
RESULTS
The 10th–90th percentiles of totalT values in adults (≥20 years) was 150–698 ng/dL (5.20–24.2 nmol/L) in men, 7.1–49.8 ng/dL (0.25–1.73 nmol/L) in women, and 1.0–9.5 ng/dL (0.04–0.33 nmol/L) in children (6–10 years old). Differences among race/ethnic groups existed in children and men: covariate-adjusted totalT values in non-Hispanic Asians were highest among children (58% compared to non-Hispanic black children) and lowest among men (12% compared to Mexican-American men). Covariate-adjusted totalT values in men were higher at age 55–60 years compared to ages 35 and 80 years, a pattern different from that observed in previous NHANES cycles.
CONCLUSIONS
TotalT patterns were different among age groups in men compared with previous NHANES cycles. Covariate-adjusted totalT values peaked at age 55–60 years in men, which appeared to be consistent with the increased use of exogenous testosterone. Differences among race/ethnic groups existed and appeared more pronounced in children than adults.
Testosterone is a key hormone for the regulation of sexual differentiation and development. It can affect the metabolism of blood lipids and glucose (1, 2). Serum concentrations of testosterone are associated with chronic diseases such as cardiovascular disease (3), diabetes mellitus (4), metabolic syndrome (5), and osteoporosis (6) and with mortality (7). Testosterone measurements are necessary for the diagnosis of classic hypogonadism in men and polycystic ovary syndrome in women (8, 9).
Despite the increasing use of testosterone measurements in patient care and research, only limited information is available about testosterone concentrations representative of the general US population, especially women, children, and non-Hispanic Asians (NHAs).2 The National Health and Nutrition Examination Survey (NHANES) is a population-based survey designed to collect nationally representative data in the US household population. The sample for this survey represents the US population of all ages and major race/ethnic groups for the years 2011 and 2012. Data on testosterone concentrations in serum from male participants were reported in earlier NHANES cycles (10). The measurements were limited to men, did not provide information on Asian Americans, and were performed with an assay reported to be inaccurate, especially at low testosterone concentrations (11, 12). In addition, recent studies report an increase in testosterone prescriptions, suggesting changes in testosterone concentrations in the US population (13, 14). This situation has created the need for new data on testosterone concentrations in the US population with an accurate and specific analytical method.
The aim of this study was to obtain nationally representative information about current total testosterone (totalT) concentrations in serum from participants age ≥6 years during the NHANES 2011–2012 survey period with a LC-MS/MS method standardized by the CDC Hormone Standardization Program.
Methods
PARTICIPANTS
Samples were obtained from participants of the 2011–2012 NHANES (15). Demographic data were collected according to standardized protocols (13). Each participant provided 1 sample. Serum was collected in non–anticoagulant-containing (red top) vacuum tubes, processed by a standard protocol (16), shipped frozen on dry ice, and stored at −70 °C until analysis. Samples were analyzed within 4 weeks after collection. NHANES obtained a stratified, multistage probability cluster sample designed to represent the US population on the basis of age, sex, and race/ethnicity (17). All participants in the survey gave written informed consent. The NHANES protocol was reviewed and approved by the National Center for Health Statistics Research Ethics Review Board.
TotalT MEASUREMENT
We used 6746 serum samples from individuals ≥6 years old. TotalT was measured with LC-MS/MS as previously described (18). In brief, testosterone was isolated from 100 μL serum by 2 serial liquid–liquid extraction steps and quantified with [13C] stable isotope–labeled testosterone as the internal standard. The method had a non-significant bias to established reference methods at National Institute for Standards and Technology and the University of Ghent. Imprecision over 2 years was <4.8%, and the limit of detection was 0.3 ng/dL (0.01 nmol/L).
BIOMARKER MEASUREMENTS
Diabetes status was determined according to glycohemoglobin A1c (Tosoh Automated Glycohemoglobin Analyzer HLC-723G8, standardized by the National Glycohemglobin Standardization Program) with a maximum imprecision of 1.2% (19). We categorized diabetes status (nondiabetes, A1c <5.6%; prediabetes and diabetes, A1c ≥5.6%) according to recommendations from the American Diabetes Association (20). Total cholesterol (TC) and HDL cholesterol (HDLC) were measured with a Roche Modular P chemistry analyzer (Roche Diagnostics) in a laboratory standardized by the CDC Lipids Standardization Program, with a maximum imprecision of 3.2% and 1.5% for HDLC and TC, respectively. A previously described LC-MS/MS method (21) was used to quantify cotinine, a biomarker for tobacco smoke exposure. We categorized individuals into presumed smokers [cotinine ≥10 ng/mL (56.8 nmol/L)] and nonsmokers [cotinine <10 ng/mL (56.8 nmol/L)] on the basis of the classification described by Benowitz (21). We categorized body mass index (BMI) according to WHO criteria (BMI <18.5, underweight; 18.5–24.9, healthy weight; 25.0–29.9, overweight; ≥30, obese) (22).
STATISTICAL EVALUATION
Geometric means (GMs) and distribution percentiles were calculated for totalT by age groups, sex, and self-reported race/ethnicity [Mexican American (MA), non-Hispanic black (NHB), non-Hispanic white (NHW), and NHA]. Individuals not included in one of these race/ethnicity groups (“other” in Table 1) were included in the total population estimates. GM values were calculated for different collection times (morning, afternoon, evening) and fasting status (≥8 h, <8 h). GMs and percentiles were calculated with SUDAAN version 11.0.1 (Research Triangle Institute). We estimated 95% CIs for GMs on the basis of the Taylor series linearization method (23) and adapted CIs for percentiles from the methods of Korn and Graubard (24) and Woodruff (25). We calculated covariate-adjusted GMs (CGMs) for selected demographic groups separately for men (age ≥20 years), women (age ≥20 years), and children (6 – 10 years) with least-squares multiple regression adjusted for age (6 – 10, 20 – 29, 30 – 39, 40 – 49, 50 – 59, 60 – 69, ≥70 years), BMI categories, race/ethnicity, physical activity (ideal, intermediate, sedentary), and alcohol consumption (nondrinker, 0 drinks per day; moderate drinker, >0 but <1 drink per day; heavy drinker, ≥1 drink per day). Because of differences in the questionnaires, physical activity in adults and children was defined as follows: for adults: ideal, ≥20 min per day (mpd) of vigorous activity, ≥30 mpd moderate activity, or ≥30 mpd of combined vigorous and moderate activity; intermediate, <20 mpd vigorous and <30 mpd moderate but ≥5 mpd combined; sedentary, ≤5 mpd of vigorous, moderate, or combined activity; and for children: ideal, TV/computer use <60 mpd and physically active ≥60 mpd; intermediate, TV/computer use <60 mpd and physically active <60 mpd or TV/computer use ≥60 mpd and physically active ≥60 mpd; sedentary, TV/computer use ≥60 mpd and physically active <60 mpd. Alcohol consumption was not considered in the model for children. CGM was not calculated for age group 11–19 years because of the high heterogeneity in this group and the lack of data on pubertal status. To arrive at the final model, we used backward elimination to remove nonsignificant interactions (P > 0.05). Nonsignificant main effects were kept in the model as confounders when they changed the β-coefficients for significant main effects or interactions by >10%. Once the backward procedure was completed, main effects and interactions were added back into the model 1 at a time and kept if they were significant (P < 0.05). Information about prescription testosterone use was not collected in this survey and therefore could not be included in the models.
Table 1.
Characteristics of NHANES 2011–2012 participants.a
| Variable | Female | Male | Total |
|---|---|---|---|
| n | 3327 | 3419 | 6746 |
| Median age, years | 37 | 36 | 36 |
| Ethnicityb | |||
| MA | 406 | 454 | 860 |
| NHA | 437 | 450 | 887 |
| NHB | 917 | 874 | 1791 |
| NHW | 1092 | 1164 | 2256 |
| Other | 475 | 477 | 952 |
| Time of collection | |||
| Morning | 1657 | 1649 | 3306 |
| Afternoon | 1174 | 1236 | 2410 |
| Evening | 496 | 534 | 1030 |
| Fasting statusc | |||
| Fasting | 1589 | 1636 | 3225 |
| Nonfasting | 1738 | 1783 | 3521 |
| BMId | |||
| Underweight | 76 | 70 | 146 |
| Normal weight | 1270 | 1280 | 2550 |
| Overweight | 812 | 1028 | 1840 |
| Obese | 1124 | 1002 | 2126 |
| Unknown | 45 | 39 | 84 |
| Diabetes statuse | |||
| Nondiabetic | 1959 | 1872 | 3831 |
| Prediabetic or diabetic | 987 | 1086 | 2073 |
| Unknown | 381 | 461 | 842 |
| Median BMI, kg/m2f | 28 | 27.5 | 27.7 |
| Median direct HDLC, mmol/L | 1.40 | 1.24 | 1.32 |
| Median TC, mmol/L | 4.71 | 4.53 | 4.63 |
| Physical activityf,g | |||
| Ideal | 617 | 767 | 1384 |
| Intermediate | 496 | 436 | 932 |
| Sedentary | 1320 | 1206 | 2526 |
| Alcohol usef,h | |||
| Nondrinker | 812 | 569 | 1381 |
| Moderate drinker | 1177 | 1201 | 2378 |
| Heavy drinker | 134 | 427 | 561 |
Data are n unless noted otherwise.
Self-reported.
Fasting, no meal within the last ≥8 h; nonfasting, meal within the past 8 h.
Adults ≥20 years of age: underweight, BMI <18.5; normal, 18.5–24.9; overweight, 25–29.9; obese, ≥30.0.
Nondiabetic, glycohemoglobin A1c ≤5.6%; prediabetic and diabetic, glycohemoglobin A1c ≥5.6–6.5%.
Adults aged ≥20 years.
Ideal, ≥20 min per day (mpd) vigorous activity, ≥30 mpd moderate activity, or ≥30 mpd combined vigorous and moderate activity; intermediate, <20 mpd vigorous and <30 mpd moderate but ≥5 mpd combined; sedentary, 0 to <5 mpd vigorous, moderate, or combined activity.
Nondrinker, 0 drinks per day; moderate drinker, >0 but < 1 drinks per day; heavy drinker, ≥1 drinks per day.
Results
We analyzed serum samples from 6746 participants for totalT. Further details about this population are listed in Table 1.
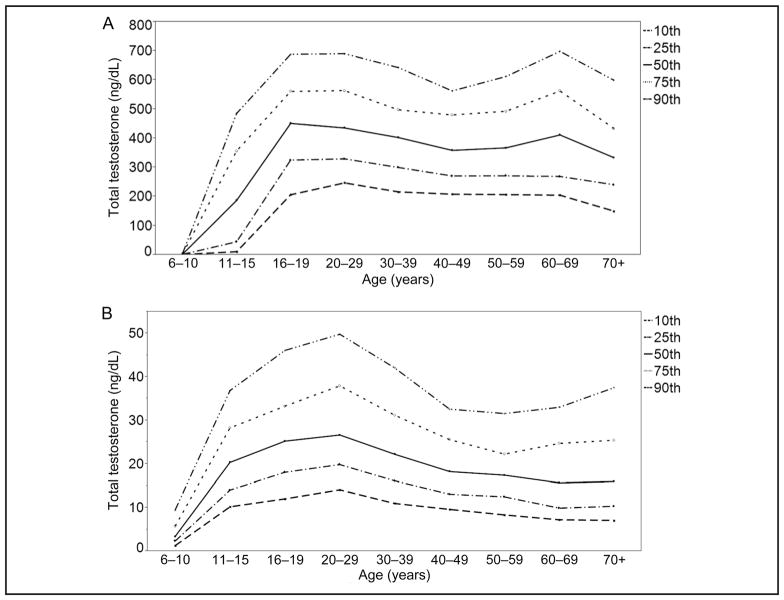
Percentile patterns across age groups were different in men (Fig. 1A) and similar in women (Fig. 1B). The percentiles in men showed a first peak at age 16–19 years and then appeared to decline with increasing age group for the 10th and 25th percentiles, whereas after an initial decline, the higher percentiles showed a second peak at age 60–69 years.
Fig. 1. Percentile values for total testosterone in male (A) and female (B) participants by age group.
Testosterone, 1 ng/dL = 0.0347 nmol/L.
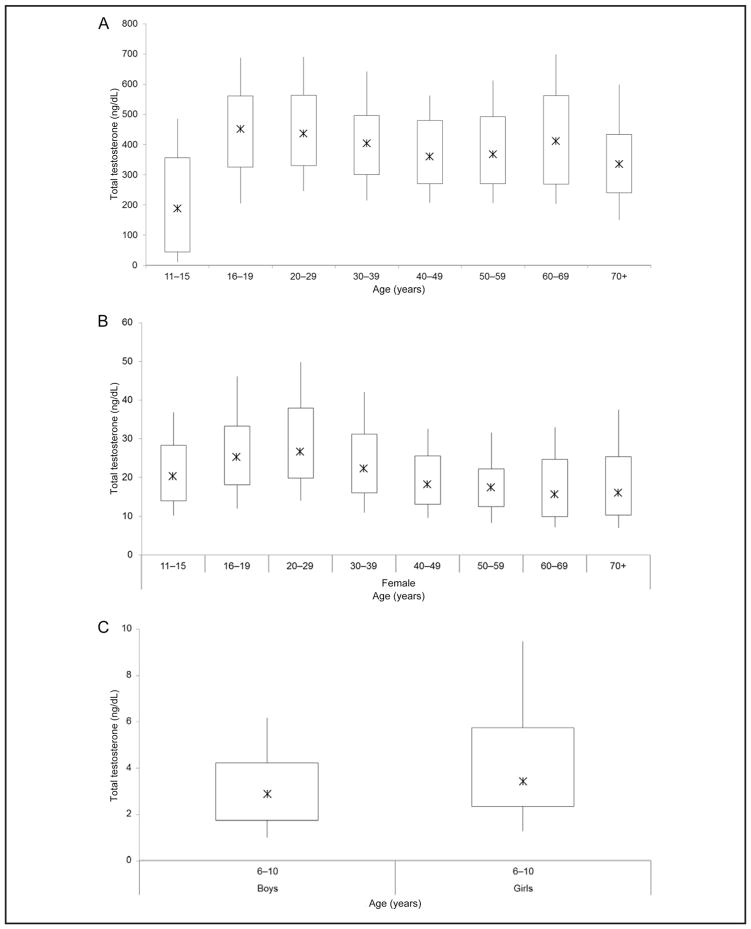
The 10th–90th percentiles of totalT values were 150 – 699 ng/dL (5.20 – 24.2 nmol/L) in men, 7.1–49.8 ng/dL (0.25–1.73 nmol/L) in women, and 1.0 – 9.5 ng/dL (0.04 – 0.33 nmol/L) in children (Fig. 2, A and B; Supplemental Table 1, which accompanies the online version of this article at http://www.clinchem.org/content/vol61/issue12). The interindividual variability of totalT values, as expressed in the ratio of 90th to 10th percentiles, was highest in boys age 11–15 years (ratio of 44), followed by boys and girls age 6 – 10 years (6 for boys and 7 for girls), and was similar among participants ≥16 years old (3 among men and 4 among women). Ratios were similar among race/ethnic groups.
Fig. 2. Testosterone distribution (10th, 25th, 50th, 75th, and 90th percentile) in men (A), women (B), and children ages 6 – 10 years (C).
Testosterone, 1 ng/dL = 0.0347 nmol/L.
In this population, the GM totalT concentrations collected from fasting adults (age ≥20 years) were not significantly different for samples collected in the morning, afternoon, or evening. Samples collected from fasting adults were consistently higher than those from non-fasting adults. These differences were significant only in men and only for samples collected in the afternoon (P = 0.0183) and evening (P = 0.0001) (see online Supplemental Table 2).
MODEL TO ESTIMATE CGM VALUES
To estimate CGM totalT values, the initial models for men and nonpregnant women included race/ethnicity, smoking, fasting status, physical activity, alcohol consumption, and diabetes status as categorical variables and age, age2, age3, log10(BMI), log10(TC), and log10(HDLC) as continuous variables and 2-way interactions. The final model included all of the main effects except log10(TC) and alcohol consumption, and in women, physical activity and age3. In women, there were interactions between race/ethnicity and diabetes (P = 0.0008) and between fasting status and log10(BMI) (P = 0.0222). In men, there was an interaction between smoking status and fasting status (P = 0.0192). In children, the initial model included sex, race/ethnicity, physical activity, and fasting status as categorical variables; age, age2, log10(BMI), log10(TC), and log10(HDLC) were included as continuous variables. The final model included all of the main effects except log10(TC), physical activity, and age2 and no interactions (Table 2). The data set for children was too small to calculate CGM values for boys and girls separately.
Table 2.
P values for the statistically significant terms in the models used to estimate covariate-adjusted totalT values.
| Variable | Men | Nonpregnant women | Children |
|---|---|---|---|
| Race/ethnicity | 0.0294 | NRa | 0.0026 |
| Smoking status | NR | 0.0390 | NA |
| Fasting status | NR | NR | 0.0041 |
| Diabetes | 0.0034 | NR | NA |
| Alcohol use | NS | NS | NA |
| Physical activity | 0.0005 | NS | NS |
| Age | 0.0122 | <0.0001 | <0.0001 |
| Age2 | 0.0121 | 0.0002 | NS |
| Age3 | 0.0092 | NS | NA |
| log10(BMI) | <0.0001 | NR | <0.0001 |
| log10(HDLC) | 0.0364 | 0.0003 | 0.0235 |
| Sex | NA | NA | 0.0001 |
| Smoking status × fasting status | 0.0192 | NS | NS |
| Race/ethnicity × diabetes | NS | 0.0008 | NS |
| Fasting status × log10(BMI) | NS | 0.0222 | NS |
NR, not reported (main effect significance not reported when the effect is involved in an interaction); NA, not applicable (main effect not included in model); NS, not significant (effect was not statistically significant at P >0.05).
In this survey, the majority (88.8%) of morning serum collections were obtained from fasting participants, and the majority (91.7%) of afternoon and evening collections were nonfasting. As a result, the effect of collection time was confounded by fasting status, preventing inclusion of both fasting status and collection time in our models. We initially restricted our analyses to fasting participants and could not detect a significant difference in totalT values among collection times. We then evaluated the effect of fasting status separately for morning, afternoon, and evening collection and found a statistically significant effect of fasting status only for adult men (see online Supplemental Table 2). Therefore, we included fasting status in our final model with the understanding that the observed effects may not be due entirely to fasting status alone, but could also be related to collection time.
MEN
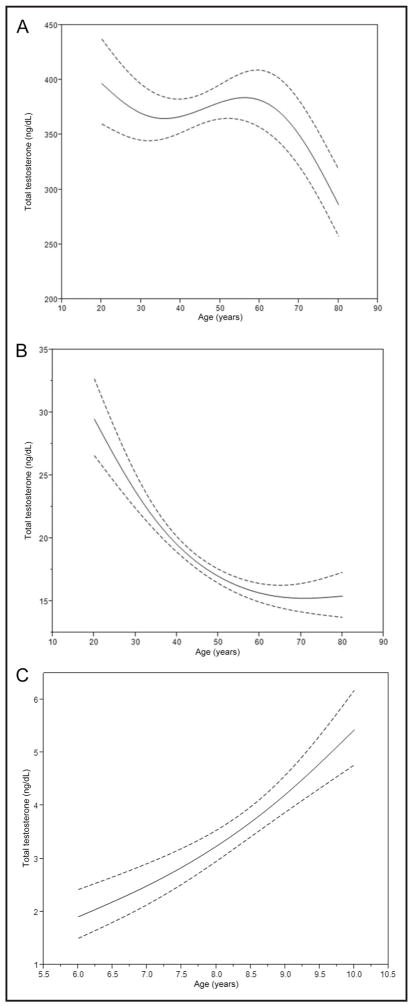
CGM totalT concentrations in men peaked at ages 20 and 55–60 years. At age 80 years, concentrations were 30% lower than at age 20 (Fig. 3A). TotalT concentrations in MA men were 13% higher than in NHA men (P = 0.0038) and 10% higher than in NHW men (P = 0.0051) (see online Supplemental Table 3). The concentrations for men without diabetes were higher than for participants with prediabetes or diabetes (15%, P = 0.0034) (see online Supplemental Table 3). Smokers also had higher concentrations than nonsmokers, but the results were only statistically significant for nonfasting participants (15% when fasting <8 h, P < 0.0001; 3.6% when fasting ≥8 h, P = 0.1769). The CGM totalT concentration in men with ideal physical activity was 392 ng/dL, significantly greater than that for men with intermediate (343 ng/dL, P = 0.0017) or sedentary (355 ng/dL, P =0.0031) physical activity. The concentrations for intermediate and sedentary men were not significantly different (P = 0.5076). BMI was negatively associated with totalT concentrations. Every 10% increase in BMI was associated with a 7.5% (95% CI, 5.9%–9.0%) decrease in totalT. HDLC was positively associated with totalT. Every 10% increase in HDLC was associated with a 1.7% (95% CI, 0.12%–3.3%) increase in totalT.
Fig. 3. Model predicted testosterone values as a function of age with 95% confidence limits (dashed lines).
(A), Men. (B), Women. (C), Children. Testosterone, 1 ng/dL = 0.0347 nmol/L.
WOMEN
The CGM totalT concentrations in women were lower with increasing age (48% lower at age 80 years compared with age 20) (Fig. 3B). We observed no differences in totalT concentrations among race/ethnic groups for women without diabetes (Table 2). TotalT values were higher in smokers compared to nonsmokers (P = 0.039) (see online Supplemental Table 4) and were positively associated with HDLC. Every 10% increase in HDLC was associated with a 2.7% (95% CI, 1.4%–4.0%) increase in totalT. There was a positive association with BMI. Among fasting participants, we observed a 4.85% (95% CI, 4.77%–4.93%) increase in totalT with every 10% increase in BMI, and among nonfasting participants, the increase was 2.34% (95% CI, 2.29%–2.40%) with every 10% increase in BMI.
CHILDREN
CGM totalT concentrations in children were higher with increasing age (Fig. 3C) and were 39% higher in girls than boys (P = 0.0001) (see online Supplemental Table 5). The highest concentrations were observed in NHA children and the lowest in NHB children (difference between groups 58%, P = 0.0071). BMI and HDLC were positively associated with totalT concentrations. Every 10% increase in BMI was associated with a 12.1% (95% CI, 8.2%–16.2%) increase in totalT, and every 10% increase in HDLC was associated with a 7.0% (95% CI, 1.0%–12.9%) increase in totalT.
Discussion
Limited information is available about totalT concentrations representative of the general US population. Here we report on totalT values in women, children, and NHAs in the NHANES population, which is designed to represent the US population on the basis of age, sex, and race/ethnicity. We observed differences in totalT values among race/ethnic groups in men and children. NHA children had the highest totalT values among children, and NHA men had the lowest totalT values among men. No significant differences were detected among race/ethnic groups in women without diabetes. Our findings on totalT values in NHB, NHW, and MA men are consistent with those reported in an earlier NHANES population (26). The reasons for differences among race/ethnic groups are not fully understood and might be related to differences in metabolism of testosterone (27, 28). Further studies are needed to assess whether these differences are associated with different health outcomes.
We found that CGM totalT values in men were higher at age 55–60 years than ages 35 and 80 years. This pattern has not been observed in previous NHANES cycles and other study populations, which reported a consistent decline in totalT values with increasing age in men (10, 29). Over the past few years, an increase in the use of androgen therapy in men age ≥40 years has been reported (13, 14). Information about androgen use was not available for this NHANES cycle, and our data likely included patients on androgen therapy. Higher totalT values were observed only in the higher percentiles of unadjusted totalT distributions, which most likely included patients on androgen therapy. Thus, the observed pattern could be explained in part by the increased use of androgen therapy. Consistent with observations in other study groups (30), totalT values declined with increasing age in women. Our initial assessments found a positive association with HDLC values in men, women, and children, consistent with findings from other studies (1). The increase in HDLC with increase in totalT was highest in children and lowest in men. Further investigations are needed to assess how these associations correspond with disease risk and health outcomes.
Serum totalT concentrations exhibit a circadian rhythm, with a peak in the morning and a nadir in the evening (31, 32). We observed similar patterns in fasting adults, which were nonsignificant. The heterogeneity among individuals in this cross-sectional population, as indicated in the ratios of 90th to 10th percentile values, might prevent the detection of significant differences between collection times. Testosterone values in nonfasting adults were consistently lower than in fasting participants independent of the time of sample collection. However, the large difference in the number of participants in each subgroup (see online Supplemental Table 1) prevents further, more detailed analysis of the effect of fasting and collection time on totalT values. Our findings on fasting status show different associations between totalT and BMI in women and totalT and smoking status in men, suggesting that fasting status in addition to collection time should be assessed in patients to facilitate the interpretation of totalT values.
The unadjusted percentile values for totalT in men were lower than those reported in other population studies (27, 29). This can be explained in part by differences in analytical methodologies, such as assay calibration, and differences in population characteristics. Other studies aimed toward establishing reference ranges and investigating clinical outcomes, whereas our study aimed at assessing totalT values in a representative sample of the US population. Therefore, we did not apply exclusion or selection criteria as was done in other studies.
The association of BMI and diabetes with totalT concentrations was different for men and women. The positive association between totalT values and BMI was similar in children ages 6–10 years and women. However, although boys showed a positive association, it was negative for men. These findings are consistent with previous reports (29, 33–35). CGM totalT values were higher in men without diabetes than in those with diabetes, whereas no difference was observed in women. Smokers showed higher totalT concentrations than non-smokers in both men and women. Men with ideal physical activity had higher totalT values then those with less activity. All these observations about diabetes, smoking, and physical activity are consistent with previous reports (30, 36 –39).
The aim of this study was to describe totalT values in a representative sample of the US population with an accurate, standardized analytical method. This information was used to detect changes in totalT values in the population and evaluate factors affecting totalT values. The study design and the information collected in the 2011–2012 NHANES is insufficient to appropriately define clinical decision levels or explain in detail the underlying causes of our observations. Because of the cross-sectional design of this study, further longitudinal studies are needed to assess the impact of lifestyle, age, and other factors on testosterone concentrations in individuals.
In summary, we quantified totalT concentrations in children, men, and women in a representative, cross-sectional sample of the noninstitutionalized US population for the years 2011 and 2012, including NHAs. We observed a peak in CGM totalT values in men at approximately age 55 years and identified differences among race/ethnic groups in children and men that warrant further detailed investigations to assess whether these differences are associated with different health outcomes. These data were generated with an analytical method standardized by the CDC Hormone Standardization Program, which facilitates comparability of data generated by other assays standardized by this program.
Supplementary Material
Table 3.
Estimated CGMs for totalT in adults in NHANES 2011–2012 age ≥20 years and children 6–10 years by race/ethnicity; estimated GM by age.a
| Variable | Total testosterone, ng/dL | ||
|---|---|---|---|
| Men | Womenb | Children | |
| Race/ethnicity | |||
| MA | 398 (377–419) | 18.3 (16.8–20.0) | 3.0 (2.7–3.3) |
| NHW | 361 (349–373) | 18.3 (17.4–19.3) | 3.6 (3.3–4.0) |
| NHB | 376 (355–399) | 18.2 (17.0–19.5) | 2.8 (2.4–3.2) |
| NHA | 351 (337–366) | 18.3 (16.7–19.9) | 4.4 (3.2–5.8) |
| Age, years | |||
| 6.0 | 1.9 (1.5–2.4) | ||
| 6.5 | 2.2 (1.8–2.7) | ||
| 7.0 | 2.5 (2.1–2.9) | ||
| 7.5 | 2.8 (2.5–3.2) | ||
| 8.0 | 3.2 (3.0–3.5) | ||
| 8.5 | 3.7 (3.4–4.0) | ||
| 9.0 | 4.2 (3.9–4.5) | ||
| 9.5 | 4.8 (4.3–5.3) | ||
| 10.0 | 5.5 (4.8–6.2) | ||
| 20 | 393 (356–433) | 29.9 (26.8–33.4) | |
| 25 | 368 (339–399) | 26.3 (24.4–28.5) | |
| 30 | 356 (330–384) | 23.5 (22.3–24.8) | |
| 35 | 354 (332–377) | 21.3 (20.5–22.0) | |
| 40 | 358 (343–373) | 19.5 (18.9–20.1) | |
| 45 | 365 (356–375) | 18.1 (17.5–18.7) | |
| 50 | 373 (359–387) | 17.0 (16.4–17.7) | |
| 55 | 378 (355–403) | 16.2 (15.6–16.9) | |
| 60 | 378 (348–411) | 15.7 (14.9–16.4) | |
| 65 | 369 (336–405) | 15.3 (14.5–16.2) | |
| 70 | 349 (320–382) | 15.2 (14.2–16.3) | |
| 75 | 318 (292–345) | 15.3 (13.9–16.7) | |
| 80 | 275 (246–308) | 15.5 (13.8–17.5) | |
Data are mean (95% CI). Testosterone 1 ng/dL = 0.0347 nmol/L.
Women without diabetes.
Acknowledgments
The authors acknowledge Jacob Farris and Gabrielle Gay for their support with processing the samples.
Footnotes
Nonstandard abbreviations: NHA, non-Hispanic Asian; NHANES, National Health and Nutrition Examination Survey; totalT, total testosterone; TC, total cholesterol; HDLC, HDL cholesterol; BMI, body mass index; GM, geometric mean; MA, Mexican American; NHB, non-Hispanic black; NHW, non-Hispanic white; CGM, covariate-adjusted geometric mean.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: No authors declared any potential conflicts of interest.
Role of Sponsor: No sponsor was declared.
References
- 1.Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217:R47–71. doi: 10.1530/JOE-12-0582. [DOI] [PubMed] [Google Scholar]
- 2.Grossmann M. Testosterone and glucose metabolism in men: current concepts and controversies. J Endocrinol. 2014;220:R37–55. doi: 10.1530/JOE-13-0393. [DOI] [PubMed] [Google Scholar]
- 3.Haring R, Travison TG, Bhasin S, Vasan RS, Wallaschofski H, Davda MN, et al. Relation between sex hormone concentrations, peripheral arterial disease, and change in ankle-brachial index: findings from the Framingham Heart Study. J Clin Endocrinol Metab. 2011;96:3724–32. doi: 10.1210/jc.2011-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corona G, Monami M, Rastrelli G, Aversa A, Sforza A, Lenzi A, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl. 2011;34:528–40. doi: 10.1111/j.1365-2605.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- 5.Brand JS, Rovers MM, Yeap BB, Schneider HJ, Tuomainen TP, Haring R, et al. Testosterone, sex hormone-binding globulin and the metabolic syndrome in men: an individual participant data meta-analysis of observational studies. PLoS One. 2014;9:e100409. doi: 10.1371/journal.pone.0100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, Finkelstein JS. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802–22. doi: 10.1210/jc.2011-3045. [DOI] [PubMed] [Google Scholar]
- 7.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–19. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–92. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 10.Nyante SJ, Graubard BI, Li Y, McQuillan GM, Platz EA, Rohrmann S, et al. Trends in sex hormone concentrations in US males: 1988–1991 to 1999–2004. Int J Androl. 2012;35:456–66. doi: 10.1111/j.1365-2605.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taieb J, Mathian B, Millot F, Patricot MC, Mathieu E, Queyrel N, et al. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem. 2003;49:1381–95. doi: 10.1373/49.8.1381. [DOI] [PubMed] [Google Scholar]
- 12.Vesper HW, Bhasin S, Wang C, Tai SS, Dodge LA, Singh RJ, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids. 2009;74:498–503. doi: 10.1016/j.steroids.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Layton JB, Li D, Meier CR, Sharpless JL, Stürmer T, Jick SS, Brookhart MA. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835–42. doi: 10.1210/jc.2013-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465–6. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC, National Center for Health Statistics. [Accessed November 2015];National Health and Nutrition Examination Survey. 2013 http://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
- 16.CDC. [Accessed November 2015];National Health and Nutrition Examination Survey (NHANES): examination and laboratory procedure manuals. 2011–2012 http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/manuals11_12.htm.
- 17.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National Health and Nutrition Examination Survey: sample design, 2011–2014. National Center for Health Statistics. Vital Health Stat. 2014;162:1–25. [PubMed] [Google Scholar]
- 18.Wang Y, Gay GG, Botelho JC, Caudill SP, Vesper HW. Total testosterone quantitative measurement in serum by LC-MS/MS. Clin Chim Acta. 2014;436:263–7. doi: 10.1016/j.cca.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC. [Accessed November 2015];National Health and Nutrition Examination Survey: 2011–2012 lab methods. http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/lab_methods_11_12.htm.
- 20.American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36:S11– 66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999;107:349–55. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Consultation on Obesity. Obesity: preventing and managing the global epidemic: report of a WHO consultation. WHO; Geneva: 1999. [Accessed November 2015]. http://whqlibdoc.who.int/trs/WHO_TRS_894.pdf. (WHO technical report series; 894) [Google Scholar]
- 23.Shah BV, Barnwell BG, Bieler GS. SUDAAN user’s manual, release 7. Research Triangle Park (NC): Research Triangle Institute; 1996. [Google Scholar]
- 24.Korn EL, Graubard BI. Confidence intervals for proportions with small expected number of positive counts estimated from survey data. Surv Methodol. 1998;24:193–201. [Google Scholar]
- 25.Woodruff RS. Confidence intervals for medians and other position measures. J Am Stat Assoc. 1952;47:635–47. [Google Scholar]
- 26.Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92:2519–25. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 27.Litman HJ, Bhasin S, Link CL, Araujo AB, McKinlay JB. Serum androgen levels in black, Hispanic, and white men. J Clin Endocrinol Metab. 2006;91:4326–34. doi: 10.1210/jc.2006-0037. [DOI] [PubMed] [Google Scholar]
- 28.Orwoll ES, Nielson CM, Labrie F, Barrett-Connor E, Cauley JA, Cummings SR, et al. Osteoporotic Fractures in Men (MrOS) Research Group. Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrinol Metab. 2010;95:E151–60. doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol. 2006;65:125–31. doi: 10.1111/j.1365-2265.2006.02560.x. [DOI] [PubMed] [Google Scholar]
- 30.Dandona P, Dhindsa S. Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96:2643–51. doi: 10.1210/jc.2010-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raff H, Sluss PM. Pre-analytical issues for testosterone and estradiol assays. Steroids. 2008;73:1297–304. doi: 10.1016/j.steroids.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Diver MJ. Analytical and physiological factors affecting the interpretation of serum testosterone concentration in men. Ann Clin Biochem. 2006;43:3–12. doi: 10.1258/000456306775141803. [DOI] [PubMed] [Google Scholar]
- 33.Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96:2430–9. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakrabarti J. Serum leptin level in women with polycystic ovary syndrome: correlation with adiposity, insulin, and circulating testosterone. Ann Med Health Sci Res. 2013;3:191–6. doi: 10.4103/2141-9248.113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braunstein GD, Reitz RE, Buch A, Schnell D, Caulfield MP. Testosterone reference ranges in normally cycling healthy premenopausal women. J Sex Med. 2011;8:2924–34. doi: 10.1111/j.1743-6109.2011.02380.x. [DOI] [PubMed] [Google Scholar]
- 36.Andersson B, Mårin P, Lissner L, Vermeulen A, Björntorp P. Testosterone concentrations in women and men with NIDDM. Diabetes Care. 1994;17:405–11. doi: 10.2337/diacare.17.5.405. [DOI] [PubMed] [Google Scholar]
- 37.Soldin OP, Makambi KH, Soldin SJ, O’Mara DM. Steroid hormone levels associated with passive and active smoking. Steroids. 2011;76:653–9. doi: 10.1016/j.steroids.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiels MS, Rohrmann S, Menke A, Selvin E, Crespo CJ, Rifai N, et al. Association of cigarette smoking, alcohol consumption and physical activity with sex steroid hormone levels in U.S. men. Cancer Causes Control. 2009;20:877–86. doi: 10.1007/s10552-009-9318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svartberg J, Jorde R. Endogenous testosterone levels and smoking in men. The fifth TROMSO study. Int J Androl. 2007;30:137–43. doi: 10.1111/j.1365-2605.2006.00720.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.