Abstract
Background: Little is known about bone mineral density (BMD) during pregnancy. Advances in technology with lower radiation emissions by dual-energy X-ray absorptiometry instruments now permit the safe measurement of BMD during pregnancy.
Objective: We evaluated maternal BMD during pregnancy as a function of vitamin D status in women of diverse racial/ethnic backgrounds.
Design: A total of 301 women who underwent BMD measurements at 12–20 wk of gestation and again at 0–14 wk postpartum were included in this analysis. Women were a subset of subjects who were recruited for a randomized, controlled, double-blind trial of vitamin D supplementation in pregnancy (400, 2000, or 4000 IU/d).
Results: Treatment had no significant effect on changes in BMD that occurred between 12–20 wk of gestation and 0–14 wk postpartum. Similarly, changes in spine and femoral neck bone mineral contents (BMCs) were not significantly different in the treatment groups. In addition, vitamin D inadequacy (serum 25-hydroxyvitamin D concentration, averaged across pregnancy, <50 nmol/L) was not associated with changes in BMD or BMC. There were significant racial/ethnic differences in spine BMD. African Americans lost more spine BMD than did Caucasians (−0.04 ± 0.04 compared with −0.02 ± 0.04 g/cm2; P = 0.033). In addition, baseline obesity was associated with a greater loss of femoral neck BMD. The means ± SDs of femoral neck BMD loss were −0.02 ± 0.05 and 0.0 ± 0.03 g/cm2 for groups with baseline body mass index (BMI; in kg/m2) ≥30 and <30, respectively.
Conclusion: These findings do not support a dose effect of vitamin D supplementation on bone health and suggest that race/ethnicity and BMI play an important role in pregnancy bone health. This trial was registered at clinicaltrials.gov as NCT00292591.
Keywords: bone mineral content, bone mineral density, cholecalciferol, pregnancy, vitamin D
INTRODUCTION
Little is known about changes in bone mineral density (BMD) during pregnancy. Early model densitometers emitted substantial radiation that was not considered safe for evaluation during pregnancy. With the advent of fan beam densitometers that emit low ionizing radiation, their use at the beginning of the second trimester of pregnancy became possible. Although earlier studies showed little to no effect of vitamin D on maternal bone loss during pregnancy (1–4), other authors have suggested that vitamin D status is a modifying factor in bone loss; however, this possibility has not been studied systematically in pregnant women to our knowledge.
As part of a large randomized controlled trial of vitamin D supplementation during pregnancy [safety and efficacy were previously reported (5)], BMD changes during pregnancy were sought. It was hypothesized that high-dose prenatal vitamin D supplementation would result in the optimization of maternal calcium homeostasis and skeletal mineralization that have not been shown with the present dietary recommended intake of 400 IU/d. In addition, it was predicted that when a higher daily vitamin D supplementation dose (2000 and 4000 IU) compared with the lower daily dose (400 IU) was given to pregnant mothers, it would result in optimal nutritional vitamin D status during pregnancy that, in turn, would result in more optimal skeletal mineralization in both the mother and her developing fetus. Thus, less loss of skeletal density in the mothers who would be randomly assigned to receive the higher doses of vitamin D at the end of pregnancy was predicted. In addition, because of the stratification of treatment by racial/ethnic groups in this clinical trial and known differences in BMD between nonpregnant African American and Caucasian women, it was predicted that there would be significant differences in bone mineralization of the women on the basis of their race/ethnicity. Because little information has been reported about bone mineralization in Hispanic women, this trial also served to define bone mineralization in that subcohort. To expand the data that are available regarding the effects of vitamin D and race/ethnicity on BMD during pregnancy, the results of BMD aspects of the trial are presented here.
METHODS
Subjects and study design
This study was approved by the Medical University of South Carolina Institutional Review Board for Human Research (HR #10727), registered at clinicaltrials.gov as NCT00292591, and conducted from 4 January 2004 to 31 July 2009 at the Medical University of South Carolina (Charleston, South Carolina). All subjects gave written, informed consent and were <16 wk of gestation at the time of consent. Subjects were participants in a double-blind randomized controlled trial of vitamin D supplementation during pregnancy and were randomly assigned to 1 of 3 vitamin D–supplementation groups [400, 2000, or 4000 IU vitamin D (as cholecalciferol)/d] at 12–16 wk of gestation; the biochemical and clinical results of the trial have been previously published (5). Inclusion criteria were that the women were in good general health, with a singleton pregnancy between 12 and 16 wk of gestation, and were between the ages of 16–45 y. The exclusion criteria were chronic hypertension, diabetes, nonsingleton pregnancy (twins and multiples), thyroid disease (new onset or not well controlled), and any calcium metabolism abnormality. The Investigational New Drug number 66,346 was obtained from the Food and Drug Administration for the use of the 2000- and 4000-IU doses of vitamin D during pregnancy. All participants were provided a standard prenatal vitamin containing 400 IU vitamin D and either 100 or 200 mg Ca. Women from the following 3 racial/ethnic groups were equally recruited: African American, Hispanic, and Caucasian.
Compliance or adherence to the protocol was tracked via monthly vitamin D pill counts. Although differences in prenatal vitamin compliance were not recorded in this study, in previous studies, we showed the compliance rates between a vitamin D tablet and prenatal vitamin pill to be comparable, and thus for this study, prenatal vitamin D intake was assumed to mirror the vitamin D tablet intake.
Maternal dual-energy X-ray absorptiometry measurements
The cohort of women who are presented in this publication (n = 301) represents a subset of the total randomized controlled trial (Figure 1). The participants had BMD determinations [dual-energy X-ray absorptiometry (DXA) scans] of the hip and lumbar spine (L1–L4) during gestation (12–20 wk of gestation) and again postpartum (0–14 wk postpartum). BMD was expressed as g/cm2; bone mineral content (BMC) was expressed as g. All DXA scans were done by the same technologist with a Hologic Discovery A densitometer (Hologic). Machine calibration and subject positioning during DXA were standardized. At each visit, scale body weight was measured with the use of the same scale (Healthometer ProPlus; Welch Allyn Inc.); height was measured with the use of a stadiometer (Harpenden stadiometer; Holtain Ltd.). Quality assurance of the densitometric technique followed the Hologic protocol with the use of the Hologic spine phantom (Hologic). The long-term interassay and intra-assay CVs for the spine phantom were ≤1%.
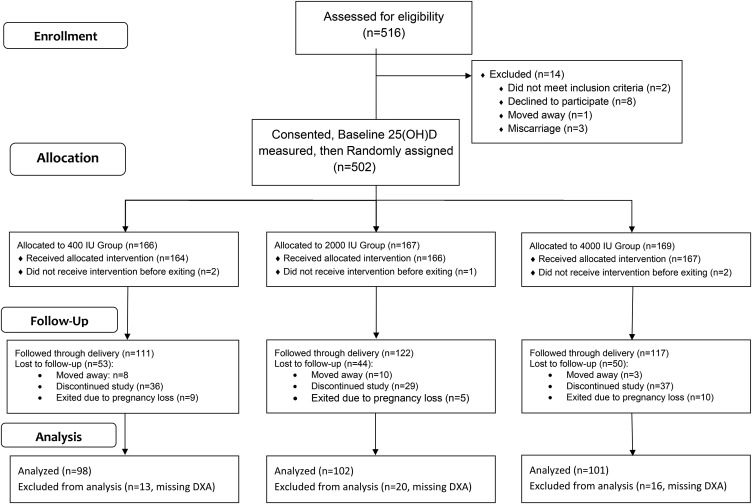
FIGURE 1.
Consolidated Standards of Reporting Trials diagram of pregnancy study bone mineral density subset. DXA, dual-energy X-ray absorptiometry; 25(OH)D, 25-hydroxyvitamin D.
Maternal sociodemographic and clinical characteristics
Questionnaires were completed during the study to ascertain maternal sociodemographic and clinical characteristics and findings throughout pregnancy as previously described (5). Reported physical activity was assessed at baseline (8–12 wk of gestatation), 22–26 wk of gestation, and 34–37 wk of gestation with the use of the Minnesota Leisure Time Physical Activity Questionnaire (6). Note that, in women who delivered <34 wk of gestation, this last physical activity assessment was not available. Maternal weight was measured monthly. Maternal height was measured at baseline and at the first DXA-scan visit. BMI (in kg/m2) was calculated with the use of prepregnancy height and weight as weight divided by the square of height.
Maternal vitamin D status
For the purposes of this study, vitamin D inadequacy was defined as a 25-hydroxyvitamin D [25(OH)D] concentration <50 nmol/L (<20 ng/mL), and vitamin D adequacy was defined as a 25(OH)D concentration ≥50 nmol/L (≥20 ng/mL). Maternal blood samples were collected monthly during gestation. A rapid, direct radioimmunoassay that was developed in the Hollis laboratory and manufactured by Diasorin Corp. was used to measure the total circulating 25(OH)D concentration in serum samples. This radioimmunoassay is a Food and Drug Administration–cleared device. Throughout the study, the laboratory participated in the Vitamin D External Quality Assessment Scheme (7, 8) to ensure reliability of the 25(OH)D assays.
Statistics
Maternal baseline characteristics for this study cohort were compared between treatment groups and racial/ethnic groups with the use of a Kruskal-Wallis rank-sum test for continuous variables and a Fisher’s exact test for categorical variables. The main outcome of interest was the change in bone measures between 12–20 wk of gestation and 0–14 wk postpartum that were measured at the spine and femoral neck of the hip. Spearman rank correlations (ρ) were used to assess the associations between bone measures and other continuous variables of interest. The effects of categorical variables on bone measures were assessed via a Kruskal-Wallis rank-sum test. Tukey’s method was used to adjust for multiple comparisons of means. The effect of the total circulating 25(OH)D concentration on the change in bone measures during pregnancy was examined in multiple regression models that were adjusted for 12–20-wk gestational bone measures, race/ethnicity, obesity, the timing of gestational and postpartum visits, and other variables that were shown to be significant in bivariate analysis. Normality and equal-variance assumptions were examined with the use of graphical methods. Results are presented as means ± SDs unless otherwise specified.
A sample size of 100 participants/treatment group would have enabled the ANOVA to have 90% power to detect a standardized effect size of 0.20 in bone measure changes at a 2-sided significance level of 0.05. Data were analyzed with the use of R version 3.1.2 software (R Foundation for Statistical Computing).
RESULTS
The maternal characteristics of the 301 participants are shown in Table 1. Baseline characteristics were compared between treatment groups on the basis of race/ethnicity, BMI, obesity (BMI ≥30), age at study entry, the concentration of serum 25(OH)D, vitamin D inadequacy [25(OH)D concentration <50 nmol/L], calcium intake, the level of physical activity, the season of study entry, and compliance. No significant differences were shown between treatment groups at baseline.
TABLE 1.
Baseline maternal characteristics between treatment groups1
| Vitamin D supplementation, IU |
||||
| Maternal baseline characteristic (n = 301) | 400 (n = 98) | 2000 (n = 102) | 4000 (n = 101) | P |
| Race, n (%) | 0.753 | |||
| African Americans | 25 (25.5) | 29 (28.4) | 26 (25.7) | |
| Hispanics | 41 (41.8) | 47 (46.1) | 41 (40.6) | |
| Caucasians | 32 (32.7) | 26 (25.5) | 34 (33.7) | |
| BMI, kg/m2 | 28.3 ± 7.12 | 28.4 ± 7.4 | 26.8 ± 5.6 | 0.467 |
| Obesity (BMI ≥30), n (%) | 31 (34.8) | 33 (35.1) | 25 (27.4) | 0.458 |
| Age, y | 27.6 ± 5.8 | 28.2 ± 5.4 | 27.2 ± 5.5 | 0.426 |
| Serum 25(OH)D, nmol/L | 62.8 ± 27.4 | 56.4 ± 21.5 | 58.2 ± 21.3 | 0.214 |
| Vitamin D inadequacy,3 n (%) | 31 (31.6) | 43 (42.2) | 35 (35.4) | 0.291 |
| Calcium intake, mg/d | 1089 ± 631 | 1004 ± 546 | 1103 ± 573 | 0.520 |
| Physical activity, h/d | 2.1 ± 3.4 | 1.5 ± 1.5 | 1.7 ± 2.7 | 0.880 |
| Season at study entry, n (%) | 0.813 | |||
| April through September | 50 (51.0) | 53 (52.0) | 48 (47.5) | |
| October through March | 48 (49.0) | 49 (48.0) | 53 (52.5) | |
| Compliance,4 n (%) | 53 (54.1) | 57 (55.9) | 55 (54.5) | 0.964 |
| Gestational visit, wk | 15.9 ± 1.3 | 15.9 ± 1.2 | 16.0 ± 1.2 | 0.664 |
| Postpartum visit, wk | 2.9 ± 2.1 | 2.8 ± 2.0 | 2.7 ± 2.0 | 0.332 |
There were no significant differences in baseline characteristics between treatment groups. Overall comparisons between treatment groups were based on a Kruskal-Wallis rank-sum test for continuous variables (mean ± SD) and a Fisher’s exact test for categorical variables [n (%)]. 25(OH)D, 25-hydroxyvitamin D.
Mean ± SD (all such values).
25(OH)D concentration <50 nmol/L (<20 ng/mL).
Taking ≥75% of vitamin D pills during the course of the study.
The maternal characteristics of the 301 participants also were compared between racial/ethnic groups (Table 2). Notably, African Americans had higher baseline BMI and lower baseline serum 25(OH)D than did Caucasians and Hispanics. African Americans were also less likely to be compliant than were other race/ethnicities. Hispanics had significantly higher calcium intake than that of Caucasians but were less active than were African Americans.
TABLE 2.
Baseline maternal characteristics between racial/ethnic groups1
| Maternal baseline characteristic (n = 301) | African Americans (n = 80) | Hispanics (n = 129) | Caucasians (n = 92) | P |
| BMI, kg/m2 | 31.7 ± 7.42,H,C | 26.3 ± 6.0 | 26.3 ± 5.7 | <0.001 |
| Obesity (BMI ≥30), n (%) | 46 (57.5)H,C | 25 (19.4) | 18 (19.6) | <0.001 |
| Age, y | 26.3 ± 4.8C | 25.7 ± 4.6C | 31.6 ± 5.4 | <0.001 |
| Serum 25(OH)D, nmol/L | 40.5 ± 19.0H,C | 59.4 ± 19.7C | 74.9 ± 20.6 | <0.001 |
| Vitamin D inadequacy,3 n (%) | 62 (77.5)H,C | 40 (31.0)C | 7 (7.6) | <0.001 |
| Calcium intake, mg/d | 1035 ± 687 | 1178 ± 591C | 925 ± 409 | 0.005 |
| Physical activity, h/d | 2.7 ± 3.2H | 1.2 ± 1.5 | 2.0 ± 3.2 | 0.001 |
| Season at study entry, n (%) | 0.223 | |||
| April through September | 38 (47.5) | 72 (55.8) | 41 (44.6) | |
| October through March | 42 (52.5) | 57 (44.2) | 51 (55.4) | |
| Compliance,4 n (%) | 29 (36.3)H,C | 73 (56.6) | 63 (68.5) | <0.001 |
| Gestational visit, wk | 16.1 ± 1.4H | 15.6 ± 1.1C | 16.2 ± 1.2 | <0.001 |
| Postpartum visit, wk | 3.8 ± 2.6H,C | 2.1 ± 1.1C | 2.9 ± 2.1 | <0.001 |
There were significant differences between racial/ethnic groups in baseline BMI, obesity, age, serum 25(OH)D, vitamin D inadequacy, calcium intake, physical activity, compliance rate, and the timing of gestational and postpartum visits. Overall comparisons between racial/ethnic groups were based on a Kruskal-Wallis rank-sum test for continuous variables (means ± SDs) and Fisher’s exact test for categorical variables [n (%)]. Superscript H or superscript C indicates a significant difference from Hispanics or Caucasians, respectively. Tukey’s test was used to adjust for multiple comparisons between racial/ethnic groups. 25(OH)D, 25-hydroxyvitamin D.
Mean ± SD (all such values).
Baseline 25(OH)D concentration <50 nmol/L (<20 ng/mL).
Taking ≥75% of vitamin D pills during the course of study.
The timing of gestational and postpartum visits was not significantly different by treatment; however, there were significant racial/ethnic differences in the timing of gestational and postpartum visits when DXAs were performed. This finding was taken into account in subsequent analyses in the multiple regression models as later discussed.
Data on breastfeeding status for participating women were obtained at the time of hospital discharge. In this cohort, breastfeeding status was not associated with changes in BMD or BMC from the spine or femoral neck between the 2 visits. Caucasian women were significantly more likely to breastfeed than were African Americans; however, breastfeeding status was not related to changes in any of the bone measures in Caucasians or African American women in this cohort.
The concentration of 25(OH)D was measured at monthly visits until 1 mo before delivery. The pregnancy 25(OH)D-concentration profile of each woman was summarized by averaging her 25(OH)D concentrations across visits and is referred to as mean 25(OH)D. The 4000-IU group had a significantly higher mean 25(OH)D concentration than that of the 2000-IU group (101.2 ± 23.8 compared with 86.1 ± 20.7 nmol/L, respectively; P < 0.001) and that of the 400-IU group (101.2 ± 23.8 compared with 76.4 ± 27.8 nmol/L, respectively; P < 0.001). The 2000-IU group also had a significantly higher mean 25(OH)D concentration than that of the 400-IU group (P = 0.007). The effect of vitamin D supplementation on serum 25(OH)D has been described in detail in our previous article (5).
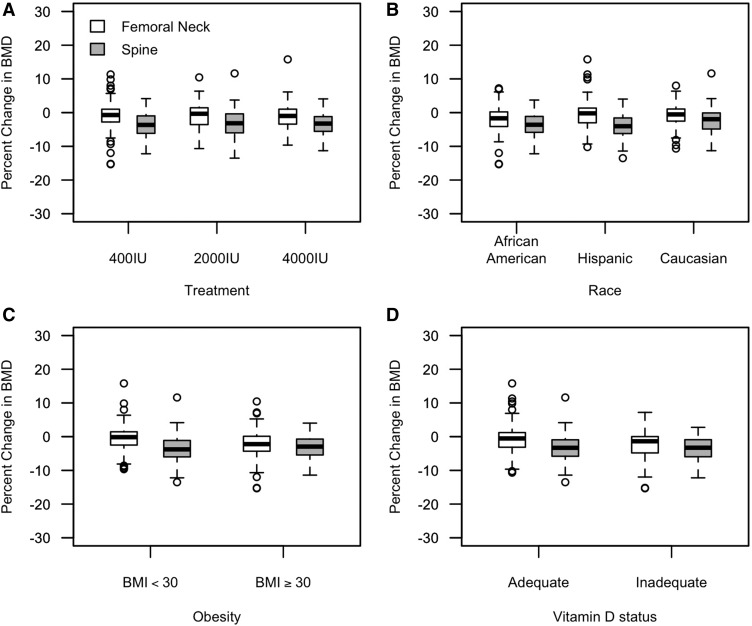
As summarized in Table 3, no significant differences in spine or femoral neck BMDs were shown in the 3 treatment groups at 12–20 wk of gestation or at 0–14 wk postpartum. The change in BMD (expressed as g/cm2) between these 2 visits was similar by treatment group for the spine (P = 0.779) and femoral neck (P = 0.886). The percentages of changes of BMD at 0–14 wk postpartum relative to BMD at 12–20 wk of gestation are also shown in Figure 2A. As shown in Table 3, treatment also appeared to have no significant effect on spine and femoral neck BMCs at either visit or the changes between the 2 visits.
TABLE 3.
BMD and BMC between treatment groups1
| Vitamin D supplementation, IU |
||||
| 400 (n = 98) | 2000 (n = 102) | 4000 (n = 101) | P | |
| BMD, g/cm2 | ||||
| Spine | ||||
| At 12–20 wk of gestation | 1.05 ± 0.12 | 1.06 ± 0.12 | 1.05 ± 0.11 | 0.910 |
| At 0–14 wk postpartum | 1.02 ± 0.13 | 1.03 ± 0.14 | 1.02 ± 0.12 | 0.878 |
| Change | −0.04 ± 0.04 | −0.03 ± 0.04 | −0.03 ± 0.03 | 0.779 |
| Femoral neck | ||||
| At 12–20 wk of gestation | 0.89 ± 0.12 | 0.9 ± 0.13 | 0.89 ± 0.12 | 0.903 |
| At 0–14 wk postpartum | 0.88 ± 0.12 | 0.89 ± 0.13 | 0.88 ± 0.13 | 0.916 |
| Change | −0.01 ± 0.04 | −0.01 ± 0.04 | −0.01 ± 0.03 | 0.886 |
| BMC, g | ||||
| Spine | ||||
| At 12–20 wk of gestation | 59.44 ± 10.58 | 58.92 ± 11.27 | 59 ± 9.83 | 0.829 |
| At 0–14 wk postpartum | 56.33 ± 11.21 | 56.26 ± 11.6 | 55.99 ± 9.98 | 0.974 |
| Change | −3.10 ± 2.73 | −2.67 ± 2.5 | −3.01 ± 2.45 | 0.357 |
| Femoral neck | ||||
| At 12–20 wk of gestation | 4.26 ± 0.74 | 4.28 ± 0.8 | 4.19 ± 0.62 | 0.726 |
| At 0–14 wk postpartum | 4.18 ± 0.67 | 4.25 ± 0.76 | 4.16 ± 0.61 | 0.818 |
| Change | −0.07 ± 0.42 | −0.03 ± 0.28 | −0.02 ± 0.23 | 0.850 |
All values are means ± SDs. No significant differences in BMD or BMC measures were detected between the treatment groups. Overall comparisons between treatment groups were based on a Kruskal-Wallis rank-sum test. BMC, bone mineral content; BMD, bone mineral density.
FIGURE 2.
Percentages of changes of BMD at 0–14 wk postpartum relative to BMD at 12–20 wk of gestation are shown by treatment, race, obesity status, and vitamin D status, respectively, for the cohort of 301 women. Circles represent outliers, and upper and lower bars represent ranges of data without including outliers. The upper, middle, and bottom of each box represent the 75%, 50%, and 25% quantiles of data, respectively. (A) Percentages of changes were not significantly different between treatment groups for the spine (P = 0.769) and femoral neck (P = 0.859). (B) Percentages of changes in spine BMD (P = 0.002) were significantly different in race/ethnicities, and percentages of changes in femoral neck BMD (P = 0.058) were trending. (C) Obesity tended to be associated with greater loss of spine BMD (P = 0.054) and was associated with femoral neck BMD (P < 0.001). (D) Vitamin D status was not associated with percentages of changes in spine BMD (P = 0.867) or femoral neck BMD (P = 0.104). BMI is expressed as kg/m2. BMD, bone mineral density.
There were significant racial/ethnic differences with regard to the change in BMD (Figure 2B). In view of the fact that African Americans had higher BMD at both 12–20 wk of gestation and 0–14 wk postpartum, they lost more spine BMD than did Caucasians (−0.04 ± 0.04 compared with −0.02 ± 0.04 g/cm2, respectively; P = 0.033) and lost more femoral neck BMD than did Hispanics (−0.02 ± 0.04 compared with 0.00 ± 0.04 g/cm2, respectively; P = 0.023) between the 2 visits (Table 4). This trend remained in the compliant women who took >75% of vitamin D pills, whereby compliant African Americans lost more spine BMD than compliant Caucasians did (−0.04 ± 0.04 compared with −0.02 ± 0.04 g/cm2, respectively; P = 0.023). Spine and femoral neck BMC measures by race/ethnicity also are summarized in Table 4.
TABLE 4.
BMD and BMC between racial/ethnic groups1
| African American (n = 80) | Hispanic (n = 129) | Caucasian (n = 92) | P | |
| BMD, g/cm2 | ||||
| Spine | ||||
| At 12–20 wk of gestation | 1.13 ± 0.12H,C | 0.99 ± 0.10C | 1.08 ± 0.10 | <0.001 |
| At 0–14 wk postpartum | 1.10 ± 0.14H | 0.95 ± 0.10C | 1.06 ± 0.10 | <0.001 |
| Change | −0.04 ± 0.04C | −0.04 ± 0.03C | −0.02 ± 0.04 | 0.006 |
| Femoral neck | ||||
| At 12–20 wk of gestation | 0.96 ± 0.15H,C | 0.87 ± 0.11 | 0.87 ± 0.11 | <0.001 |
| At 0–14 wk postpartum | 0.94 ± 0.15H,C | 0.86 ± 0.11 | 0.86 ± 0.11 | <0.001 |
| Change | −0.02 ± 0.04H | 0.00 ± 0.04 | −0.01 ± 0.03 | 0.056 |
| BMC, g | ||||
| Spine | ||||
| At 12–20 wk of gestation | 63.46 ± 10.17H | 52.29 ± 7.67C | 64.90 ± 8.80 | <0.001 |
| At 0–14 wk postpartum | 60.57 ± 10.66H | 49.2 ± 7.97C | 62.2 ± 9.08 | <0.001 |
| Change | −2.89 ± 2.84 | −3.1 ± 2.44 | −2.7 ± 2.47 | 0.478 |
| Femoral neck | ||||
| At 12–20 wk of gestation | 4.57 ± 0.80H,C | 4.03 ± 0.69C | 4.26 ± 0.59 | <0.001 |
| At 0–14 wk postpartum | 4.5 ± 0.83H,C | 4.02 ± 0.60 | 4.2 ± 0.55 | <0.001 |
| Change | −0.07 ± 0.25 | −0.02 ± 0.37 | −0.05 ± 0.29 | 0.453 |
All values are means ± SDs. Significant racial differences in BMD and BMC were shown at gestation and postpartum. The loss of spine BMD was also significantly different between groups. Overall comparisons between racial/ethnic groups were based on a Kruskal-Wallis rank-sum test. Superscript H or superscript C indicate a significant difference from Hispanics or Caucasians, respectively. Tukey’s test was used to adjust for multiple comparisons between racial/ethnic groups. BMC, bone mineral content; BMD, bone mineral density.
Obesity (baseline BMI ≥30) was associated with a greater loss of femoral neck BMD (Figure 2C). The means ± SDs of femoral neck BMD loss was −0.02 ± 0.05 and 0.0 ± 0.03 g/cm2 for the obese and nonobese groups, respectively (P < 0.001). Being obese compared with not being obese was associated with higher femoral neck BMD at both gestation (0.98 ± 0.12 compared with 0.86 ± 0.11, respectively, P < 0.001) and postpartum visits (0.95 ± 0.12 compared with 0.85 ± 0.11, respectively, P < 0.001). Similarly, obesity was associated with higher spine BMD at both gestation (1.12 ± 0.11 compared with 1.03 ± 0.11, respectively; P < 0.001) and postpartum visits (1.09 ± 0.12 compared with 1.00 ± 0.12, respectively; P < 0.001). Note that African Americans were more likely to be obese than were Hispanics (59.7% compared with 21.9%, respectively; P < 0.001) and Caucasians (59.7% compared with 21.7%, respectively; P < 0.001). The prevalence of obesity in African Americans might have contributed to the observation that African Americans have higher BMD at both gestation and postpartum visits.
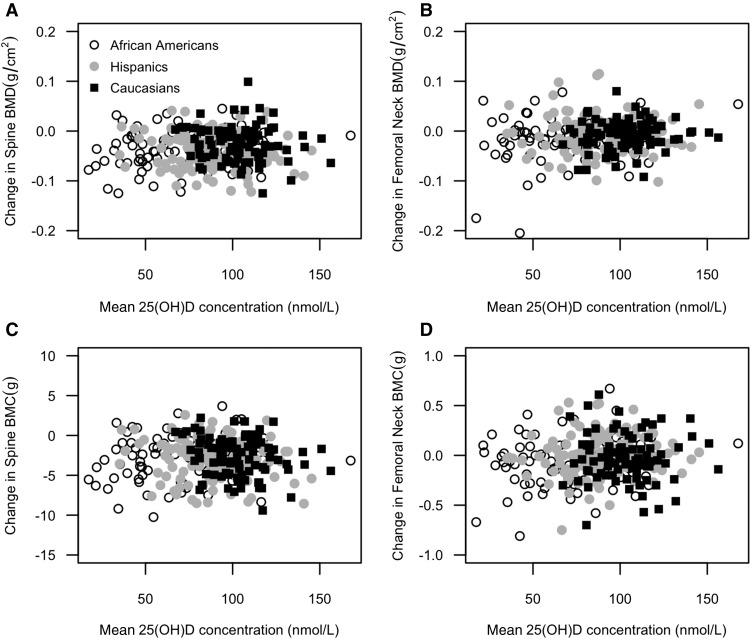
Changes in BMD and BMC were plotted as a function of the mean 25(OH)D concentration in Figure 3. The mean 25(OH)D concentration during pregnancy was not associated with changes in spine BMD (r = 0.03, P = 0.591), femoral neck BMD (r = 0.07, P = 0.211), spine BMC (r = −0.05, P = 0.352), or femoral neck BMC (r = 0.07, P = 0.200). Vitamin D inadequacy [mean 25(OH)D concentration <50 nmol/L] was not associated with the percentages of changes in BMD (Figure 2D).
FIGURE 3.
Changes in BMD and BMC as a function of mean 25(OH)D (n = 301). On the basis of Spearman’s rank correlation test, the mean 25(OH)D concentration during pregnancy was not associated with changes in spine BMD (r = 0.03, P = 0.591), femoral neck BMD (r = 0.07, P = 0.211), spine BMC (r = −0.05, P = 0.352), or femoral neck BMC (r = 0.07, P = 0.200), respectively. BMC, bone mineral content; BMD, bone mineral density; 25(OH)D, 25-hydroxyvitamin D.
The majority of vitamin D–inadequate women were African Americans. Of the 33 women who met the definition of vitamin D inadequacy [mean 25(OH)D concentration <50 nmol/L], 22 women were in the 400-IU group, and 26 women were African Americans. Vitamin D–inadequate African Americans compared with vitamin D–adequate African Americans had similar BMD losses from the spine (−0.04 ± 0.04 compared with −0.04 ± 0.04 g/cm2, respectively; P = 0.508) and femoral neck (−0.03 ± 0.06 compared with −0.02 ± 0.04 g/cm2, respectively; P = 0.570). At 12–20 wk of gestation, vitamin D–inadequate African Americans had higher femoral neck BMD than vitamin D–adequate African Americans did (1.04 ± 0.11 compared with 0.94 ± 0.15 g/cm2, respectively; P = 0.047). Because vitamin D–inadequate African Americans also were more likely to be obese than were vitamin D–adequate African Americans (76.9% compared with 50.9%, respectively; P = 0.048), the observed bivariate association between BMD and vitamin D inadequacy might have been due to the confounding factor of obesity. After adjusting for obesity, vitamin D inadequacy was no longer associated with femoral neck BMD in African Americans. Vitamin D inadequacy was not associated with a loss in spine BMC or femoral neck BMC in African Americans either (Supplemental Table 1).
A multiple regression analysis on change in BMD revealed that race/ethnicity and baseline BMI appeared to play a role in pregnancy bone health after adjusting for vitamin D status, baseline obesity status, spine BMD at 12–20 wk of gestation, and the timing of gestational and postpartum visits, whereas African Americans, on average, lost 0.016 ± 0.007 g/cm2 more spine BMD between gestation and postpartum visits than did Caucasians (Table 5). The protective effect of higher obesity in African American women in the sample did not result in a lower spine BMD in African American women postpartum than in Caucasian and Hispanic women.
TABLE 5.
Multiple linear regression analysis assessing the effect of predictor variables on changes in spine BMD and femoral neck BMD1
| Change in BMD, g/cm2 |
||||
| Spine (n = 274, r2 = 0.07) |
Femoral neck (n = 274, r2 = 0.08) |
|||
| Coefficient ± SE | P | Coefficient ± SE | P | |
| Racial/ethnic group | ||||
| African American (reference) | — | — | — | — |
| Hispanic | 0.004 ± 0.007 | 0.534 | 0.000 ± 0.007 | 0.965 |
| Caucasian | 0.016 ± 0.007 | 0.016 | −0.002 ± 0.007 | 0.801 |
| Mean 25(OH)D, nmol/L | ||||
| ≥50 (reference) | — | — | — | — |
| <50 | −0.001 ± 0.008 | 0.855 | −0.009 ± 0.008 | 0.263 |
| BMI, kg/m2 | ||||
| <30 (reference) | — | — | — | — |
| ≥30 | 0.007 ± 0.005 | 0.196 | −0.014 ± 0.006 | 0.015 |
| Gestational BMD, g/cm2 | 0.041 ± 0.022 | 0.063 | −0.031 ± 0.02 | 0.124 |
| Time of gestational visit, wk | −0.001 ± 0.001 | 0.355 | 0.000 ± 0.001 | 0.886 |
| Time of postpartum visit, wk | 0.000 ± 0.002 | 0.927 | −0.003 ± 0.002 | 0.174 |
Age, calcium intake, physical activity, season of study entry, and compliance status were not significant in bivariate analysis and were not included in multiple regression models. Gestational weight gain was not significantly associated with the change in spine or femoral neck BMD after adjusting for gestational BMD, and the timing of gestational and postpartum visits. BMD, bone mineral density; 25(OH)D, 25-hydroxyvitamin D.
In addition, baseline obesity was associated with a greater loss of femoral neck BMD after adjusting for other variables in the model (Table 5). The timing of gestational and postpartum visits had no significant effect on spine or femoral neck BMD (Table 5), possibly because the timing varied significantly between racial/ethnic groups, and we were not powered to test this question. Vitamin D inadequacy was not associated with changes in spine or femoral neck BMD (Table 5). We also modeled changes in bone measures in the African American cohort because the majority of vitamin D–inadequate women were African Americans. The effect of vitamin D inadequacy on spine BMC trended toward significance, whereby vitamin D–inadequate African American women, on average, lost 1.414 ± 0.754 g (P = 0.066) (Supplemental Table 2) more spine BMC compared with that of vitamin D–adequate African American women while controlling for baseline obesity status, gestational weight gain, spine BMC at gestational DXA, and the timing of gestational and postpartum visits. Age, calcium intake, physical activity, season of study entry, and compliance status were not associated with any of the bone measures in the bivariate analysis (P > 0.2) and were not included in the aforementioned multiple regression models.
DISCUSSION
To our knowledge, this is the first report of a randomized controlled trial that examined BMD and BMC during pregnancy as a function of maternal vitamin D status. The change in BMD and BMC was similar between treatment groups for both the femoral neck and spine. Vitamin D inadequacy [serum 25(OH)D concentration, averaged across pregnancy, <50 nmol/L] was not associated with changes in BMD or BMC. In addition, the role of BMI was significant, whereby baseline obesity was associated with a greater loss of femoral neck BMD but not a lower postpartum BMD. Another important finding of this study was the strong racial disparity in bone health changes during pregnancy. African Americans, on average, had a greater loss of spine BMD than did Caucasians. Because of the considerable loss to follow-up of the 502 subjects who were originally randomly assigned to treatment and the inverse associations between vitamin D status and BMD at baseline in one group compared with others, the outcomes may have been influenced by the initial lower vitamin D status and higher BMD in the African American women.
Often plagued with small sample sizes, earlier pregnancy studies also were limited by the DXA technology and the potential adverse effects of ionizing radiation exposure to the developing fetus. Three previous studies measured BMD in women prepregnancy and after pregnancy but not during pregnancy (1–4). More et al. (9) studied the forearm during pregnancy as well as the lumbar spine prepregnancy and after pregnancy. In the largest cohort to date, Møller et al. (10) measured BMD in 153 women preconception, at each trimester during pregnancy, and postpartum and showed that pregnancy caused a reversible bone loss with a return to prepregnancy BMD by 19 mo postpartum, independent of the breastfeeding length. Dahlman et al. (4) reported on the association between vitamin D status and maternal bone health and BMD in a group of immigrant and Swedish women with the measurement of BMD at pregnancy week 12 and 6–12 mo postpartum and showed no significant association between 25(OH)D concentrations and BMD measurements. Similarly, Shao et al. (11), in their observational study of 130 Chinese women, showed that BMD loss, as measured via calcaneus quantitative ultrasound between early and late pregnancy, was associated with serum calcium, phosphorus, and alkaline phosphatase but not with vitamin D status. When viewed together, these previous studies showed minimal bone loss during pregnancy (4, 9–11). The findings of this study are consistent with previous studies that evaluated BMD using DXA and bone ultrasound with comparisons either preconception or early in pregnancy and typically near delivery or 1–6 wk postdelivery (1–4, 9–11).
Although large epidemiologic studies have shown that 25(OH)D concentrations below a certain threshold (e.g., <30 nmol/L or <12 ng/mL) are associated with diminished BMD, there has been mixed evidence about the benefit of raising circulating 25(OH)D concentrations to prevent bone loss. To our knowledge, this is the first report of that question that has been applied to pregnant women, and the findings suggest that there is little effect of vitamin D supplementation on bone loss during pregnancy. We did not find a significant association between the mean 25(OH)D concentration and bone health; however, only 11.0% of participants had a mean 25(OH)D concentration <50 nmol/L, which led to reduced power to detect the effect of vitamin D inadequacy on bone health.
The role of BMI in BMD has been clearly documented and supports the premise that body weight affects bone mineralization during pregnancy. In this study, higher BMI was associated with a greater loss of femoral neck BMD during pregnancy. The significance of this finding remains unknown.
There were certain limitations of the study that should be noted. First, maternal baseline BMD before pregnancy was not known. The first DXA was performed at 12–20 wk of gestation. There may have been some bone loss earlier in pregnancy; however, the flux of calcium from the mother to the fetus is increased in the third trimester and not at this early gestational age (12). Although we did not measure vitamin D receptor, vitamin D–binding protein, and calcium receptor gene polymorphisms, which could affect serum 25(OH)D concentrations and risk of vitamin D inadequacy, the trial was designed with stratification by race/ethnicity to account for the differences in race/ethnicity. Although we examined the effect of race/ethnicity, vitamin D status, and other categorical variables on bone health, this study was not powered to test these categorical variables. Another limitation of this study is the lack of precision in physical activity measurements that were obtained by patient recall over the past month. Although the Minnesota Leisure Time Physical Activity Questionnaire has been previously validated, it does not take into account physical activities at work and other activities such as aerobics and yoga and, thus, might not be a good instrument for measuring physical activities of pregnant women. Although the percentage of mothers who were breastfeeding at the postpartum visit was not obtained, we had information about breastfeeding status at the time of discharge after delivery, and these data were analyzed. Although breastfeeding status was not related to changes in any bone measures in Caucasians or African Americans, the measures were taken shortly after delivery when changes in bone that are associated with breastfeeding would not yet have occurred.
Because of the longer turnover time of bone and bone remodeling, another limitation of the study is the short duration between evaluations. Although one-third of the women were lost to follow-up, participants who were lost to follow-up did not differ significantly from those who continued in terms of racial/ethnic makeup, obesity, and vitamin D status. While there was some variation in the timing of postpartum visits, when the number of days postpartum was used in the regression model, it did not change the findings. Last, there was no follow-up beyond 14 wk postpartum, and therefore, the long-term effect of bone loss during pregnancy for this cohort remains unknown.
Although there might be greater bone loss in African American women with low 25(OH)D concentrations during pregnancy, vitamin D alone may not account for this observation. Also, because the African American women had higher baseline BMD, it is surprising that they would lose more BMD over time if, in fact, all women are losing ∼2–3% of their BMDs during pregnancy. Other factors such as genetic differences in vitamin D metabolism and calcium receptor polymorphisms may play a role. We acknowledge that our study provides no conclusive evidence for the benefit of vitamin D supplementation on bone health, but the importance of vitamin D on pregnancy bone health in a certain subset of women who are presented here should be considered. Further investigation is necessary to delineate additional risk factors.
In conclusion, this study shows significant racial/ethnic differences in bone health, whereby African American women have greater BMD loss than do Caucasian women. In addition, we show that obesity is associated with a greater loss of femoral neck BMD. Last, this study suggests that vitamin D supplementation is not associated with improved bone health, although vitamin D inadequacy might lead to a greater loss of spine BMC in African Americans. Additional studies are warranted to determine the interplay between genetic and metabolic interactions and their effects on bone metabolism and health.
Acknowledgments
We thank study coordinators Pamela Smith and Martha Murphy for their assistance during the study and Myla Ebeling for her assistance with data access and management.
The authors’ responsibilities were as follows—WW, EG-M, and NEF: contributed to the data analysis and writing of the manuscript; JRS, BWH, and CLW: contributed to the study design, conduct of the study, data management and analysis, and writing of the manuscript; BA: contributed to the study design and conduct of the study; and all authors: read and approved the final manuscript. BWH was a consultant for Diasorin Corp. but that agreement ended in April 2012. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: BMC, bone mineral content; BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Drinkwater BL, Chesnut CH III. Bone density changes during pregnancy and lactation in active women: a longitudinal study. Bone Miner 1991;14:153–60. [DOI] [PubMed] [Google Scholar]
- 2.Naylor KE, Iqbal P, Fledelius C, Fraser RB, Eastell R. The effect of pregnancy on bone density and bone turnover. J Bone Miner Res 2000;15:129–37. [DOI] [PubMed] [Google Scholar]
- 3.Kaur M, Pearson D, Godber I, Lawson N, Baker P, Hosking D. Longitudinal changes in bone mineral density during normal pregnancy. Bone 2003;32:449–54. [DOI] [PubMed] [Google Scholar]
- 4.Dahlman I, Gerdhem P, Bergstrom I. Vitamin D status and bone health in immigrant versus Swedish women during pregnancy and the post-partum period. J Musculoskelet Neuronal Interact 2013;13:464–9. [PubMed] [Google Scholar]
- 5.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011;26:2341–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson MT, Leon AS, Jacobs DRJ, Ainsworth BE, Serfass R. Comprehensive evaluation of the Minnesota leisure time physical activity questionnaire. J Clin Epidemiol 1994;47:271–81. [DOI] [PubMed] [Google Scholar]
- 7.Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets 2011;12:19–28. [DOI] [PubMed] [Google Scholar]
- 8.Food and Nutrition Board. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for vitamin D and calcium. Washington (DC): National Academy Press; 2010. [Google Scholar]
- 9.More C, Bettembuk P, Bhattoa HP, Balogh A. The effects of pregnancy and lactation on bone mineral density. Osteoporos Int 2001;12:732–7. [DOI] [PubMed] [Google Scholar]
- 10.Møller UK, Við Streym S, Mosekilde L, Rejnmark L. Changes in bone mineral density and body composition during pregnancy and postpartum. A controlled cohort study. Osteoporos Int 2012;23:1213–23. [DOI] [PubMed] [Google Scholar]
- 11.Shao H, Tao M, Fan Y, Jing J, Lu J. Vitamin D levels and other factors related to bone mineral density during pregnancy. Aust N Z J Obstet Gynaecol 2012;52:571–5. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs CS. The role of vitamin D in pregnancy and lactation: insights from animal models and clinical studies. Annu Rev Nutr 2012;32:97–123. [DOI] [PubMed] [Google Scholar]