Abstract
Background: Little is known about placental vitamin D metabolism and its impact on maternal circulating vitamin D concentrations in humans.
Objective: This study sought to advance the current understanding of placental vitamin D metabolism and its role in modulating maternal circulating vitamin D metabolites during pregnancy.
Design: Nested within a feeding study, 24 healthy pregnant women (26–29 wk of gestation) consumed a single amount of vitamin D (511 IU/d from diet and a cholecalciferol supplement) for 10 wk. Concentrations of placental and blood vitamin D metabolites and placental messenger RNA (mRNA) abundance of vitamin D metabolic pathway components were quantified. In addition, cultured human trophoblasts were incubated with 13C-cholecalciferol to examine the intracellular generation and secretion of vitamin D metabolites along with the regulation of target genes.
Results: In placental tissue, 25-hydroxyvitamin D3 [25(OH)D3] was strongly correlated (r = 0.83, P < 0.001) with 24,25-dihydroxyvitamin D3. Moreover, these placental metabolites were strongly correlated (r ≤ 0.85, P ≤ 0.04) with their respective metabolites in maternal circulation. Positive associations (P ≤ 0.045) were also observed between placental mRNA abundance of vitamin D metabolic components and circulating vitamin D metabolites [i.e., LDL-related protein 2 (LRP2, also known as megalin) with 25(OH)D3 and the C3 epimer of 25(OH)D3 [3-epi-25(OH)D3]; cubilin (CUBN) with 25(OH)D3; 25-hydroxylase (CYP2R1) with 3-epi-25(OH)D3; 24-hydroxylase (CYP24A1) with 25(OH)D3, 3-epi-25(OH)D3, and 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]; and 1α-hydroxylase [(CYP27B1) with 3-epi-25(OH)D3 and 1,25(OH)2D3]. Notably, in vitro experiments with trophoblasts showed increased production and secretion of 25(OH)D3 and higher CYP24A1 gene transcript abundance in response to cholecalciferol treatment.
Conclusions: The numerous associations of many of the placental biomarkers of vitamin D metabolism with circulating vitamin D metabolites among pregnant women [including a CYP27B1–associated increase in 1,25(OH)2D3] and the evidence of trophoblast production and secretion of vitamin D metabolites, especially 25(OH)D3, suggest that the placenta may play an active role in modulating the vitamin D metabolite profile in maternal circulation in human pregnancy. This trial was registered at clinicaltrials.gov as NCT03051867.
Keywords: vitamin D, pregnancy, placenta, trophoblasts, vitamin D metabolism, 25-hydroxyvitamin D, megalin/cubilin, CYP2R1, CYP27B1, CYP24A1
INTRODUCTION
Substantial alterations in the circulating concentrations of vitamin D metabolites and related proteins have been observed during human pregnancy. For example, the circulating concentrations of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], a bioactive vitamin D metabolite, and vitamin D binding protein (DBP), a carrier for circulating vitamin D metabolites, have been shown to increase by 1.5–2.0 times across gestation (1–4). Similarly, increases in 25-hydroxyvitamin D3 [25(OH)D3] (2, 5–7), the major form of vitamin D in the blood, and in the C3 epimer of 25(OH)D3 [3-epi-25(OH)D3] (7–9) have also been observed in some studies. Nonetheless, the underlying mechanisms of these pregnancy-induced changes in circulating vitamin D metabolites are largely unknown.
The placenta is an organ of pregnancy that expresses all components of the vitamin D metabolic pathway [i.e., LDL-related protein 2 (LRP2, also known as megalin), cubilin (CUBN) receptors (10, 11), 25-hydroxylase (CYP2R1) (12), 1α-hydroxylase (CYP27B1) (13, 14), and 24-hydroxylase (CYP24A1) (12, 15)] and thus could mediate the observed pregnancy-induced alterations in maternal circulating vitamin D metabolites. Indeed, placental production of 1,25(OH)2D3 and 24,25-dihydroxyvitamin D3 [24,25(OH)2D3] by the actions of CYP27B1 and CYP24A1 on 25(OH)D3 in vitro have been established (13, 16, 17). Moreover, although the extent to which the placenta contributes to a doubling of circulating 1,25(OH)2D3 as compared with the kidneys is still debated, it is unequivocally confirmed in nephrectomized animals that placentally produced 1,25(OH)2D3 can enter the maternal circulation (18, 19). Nonetheless, the contribution of the placenta to 1,25(OH)2D3 and 24,25(OH)2D3 concentrations in humans remains uncertain. In addition, it is unknown whether placental CYP2R1 produces 25(OH)D3 from vitamin D3 (cholecalciferol) and whether this metabolite can enter the maternal blood. Furthermore, because the kidneys internalize DBP-bound 25(OH)D through the LRP2-CUBN receptors from the glomerular filtrate (20), the presence of this receptor complex in the placental membrane along with a dramatic increase in DBP during pregnancy support the possibility that the placenta takes up the DBP-bound 25(OH)D in a tissue-specific manner and regulates the production of several forms of vitamin D metabolites. Lastly, because 3-epi-25(OH)D3 is present in nearly all blood samples of pregnant women (21) and in human placental tissue (22), the role of the placenta in mediating maternal circulating 3-epi-25(OH)D3 is of interest.
To advance the current understanding of placental vitamin D metabolism and its impact on maternal circulating vitamin D concentrations in humans, this study examined the associations of the biomarkers of vitamin D metabolism in placental tissue with maternal blood obtained from pregnant women participating in a long-term controlled feeding study. The associations of placental vitamin D metabolites with the placental expression of vitamin D metabolizing genes were also examined. In addition, a human placental cell culture model was used to explore the placental uptake of 13C-labeled cholecalciferol and its metabolic fate and the impact of cholecalciferol on the target gene transcript abundance.
METHODS
Human feeding study
Study design, participants, and sample collection
Twenty-six third-trimester singleton pregnant women (26–29 wk of gestation, ≥21 y of age) with good health status (based on a health-related questionnaire, blood chemistry profile, and a complete blood count) were recruited from Ithaca, New York, from 2009 to 2010 to participate in a 12-wk controlled feeding study that randomly assigned 2 choline intake amounts and provided a mean total intake of 511 IU vitamin D/d [311 IU from diet and 200 IU from a prenatal multivitamin supplement containing cholecalciferol (Pregnancy Plus; Fairhaven Health, LLC)], 1.6 g Ca/d, and 1.9 g P/d (7, 23, 24). Pregnant women consumed 1 study meal along with supplements each weekday at the Cornell Human Metabolic Research Unit, where all meals were prepared, and food items were weighed to the gram. Carry-out meals and supplements were provided at all other times, and the daily return of empty containers was used to monitor compliance throughout the study period. Venous blood samples obtained at week 0 (study baseline) and week 10 (representing the study end point) and placental tissue samples collected at delivery were used for measurements of vitamin D metabolic and genomic parameters. Because 2 of the participants delivered at home and their placental tissue was unavailable, this study included 24 pregnant women in the final analysis. Additional information regarding the flow of study participants through the screening, controlled feeding, and analysis phases is found in Supplemental Figure 1. The study protocol was approved by the Institutional Review Board for Human Study Participant Use at Cornell University and at Cayuga Medical Center, where babies were delivered. All participants provided their informed consent before study entry.
Blood and placental vitamin D metabolite measurements
We quantified serum 25(OH)D3, serum 3-epi-25(OH)D3, and plasma 24,25(OH)2D3 with the use of a stable isotope dilution liquid chromatography–tandem mass spectrometry (LC-MS/MS) methodology that was validated in part through participation in the Vitamin D External Quality Assessment Scheme, as previously described (7). Plasma 1,25(OH)2D3 (Immunodiagnostic Systems) and DBP (R&D Systems) concentrations were quantified through the use of ELISA kits. The estimation of free 25(OH)D3 concentrations was performed with the use of a previously published equation (25).
Placental 25(OH)D3 and 24,25(OH)2D3 were extracted from homogenized placental tissues (0.4 g) in 1 mL of methanol following liquid-liquid extraction (addition of 600 μL acetonitrile, 1 mL methyl tert-butyl ether, and 25 μL internal standard), solid-phase extraction with the use of Oasis HLB cartridges (3 cc/60 mg), and derivatization with 50 μL of 0.1 mg/mL 4-(2-(6,7-dimethoxy-4-methyl-3-oxo-3,4-dihydroquinoxalinyl)ethyl)-1,2,4-triazoline-3,5-dione (26, 27). An internal standard solution containing 215 μmol d3-25(OH)D3/L (IsoSciences) and 1.7 μmol d6-24,25(OH)2D3/L (Toronto Research Chemicals Inc.) was added to the homogenized tissues at the beginning of the extraction (27). Extracts were resuspended in 110 μL of a 60:40 methanol-water solution and injected onto an LC-MS/MS system to quantify the vitamin D metabolites. LC-MS/MS conditions followed the protocol for circulating 24,25(OH)2D3 (7).
Genotyping
Genotypes of single nucleotide polymorphisms in vitamin D metabolizing genes [i.e., CYP2R1 and DBP genes] were determined by the EndPoint Genotyping software on a Roche LightCycler 480 with the use of the Applied Biosystems TaqMan Genotyping Assays (Life Technologies), as previously described (7).
Messenger RNA quantification of the vitamin D metabolic pathway components in placental tissues
RNA was extracted from placental tissue samples with the use of the RNeasy Mini Kit (Qiagen), and the concentrations were determined with the use of a NanoDrop ND-1000 instrument. Reverse transcription was performed with the use of the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific), and quantitative real-time polymerase chain reaction (qRT-PCR) was conducted (Applied Biosystems ABI 7300 system). The TaqMan Gene Expression Assays (Thermo Fisher Scientific) were Hs00167999_m1 (CYP24A1), Hs00168017_m1 (CYP27B1), Hs01379776_m1 (CYP2R1), Hs01119018_g1 (LRP2), and Hs00153607_m1 (CUBN). The reaction conditions were 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. Based on the 2−ΔΔ cycle threshold method (28), data for the target genes were expressed as fold changes in messenger RNA (mRNA) abundance, which were normalized to the housekeeping gene [β-glucuronidase (Hs99999908_m1)] and relative to the calibrator.
In vitro placental trophoblast cell culture model
HTR-8/SVneo cell culture
HTR-8/SVneo cells (immortalized human first-trimester placental extravillous trophoblasts) were a gift from Charles H. Graham (Queen’s University) (29). The cells (passage numbers 17–21) were plated on a culture dish (BD Biosciences) at a seeding density of 1.39 × 106 cells/dish, and cultured in standard RPMI 1640 medium, which contained 1.25–5% fetal bovine serum, 2 mM l-glutamine (all from Corning Life Sciences), and each vitamin D treatment [i.e., 13C2-cholecalciferol or 13C5-25(OH)D3; unlabeled cholecalciferol, 25(OH)D3, or 1,25(OH)2D3] at 37°C in a humidified atmosphere of 5% carbon dioxide and 95% air. One hundred percent ethanol was added to the standard medium to serve as a control treatment (0.1% ethanol). Cell counts and viability were determined in duplicate with the use of a TC10 cell counter (Bio-Rad) and a trypan blue exclusion test, respectively. The experiment was repeated 3 times. Within each replication, each treatment was performed in triplicate.
Unlabeled vitamin D treatment and qRT-PCR of vitamin D metabolic enzymes
To examine the effects of vitamin D exposure on the gene expression response of vitamin D metabolic enzymes in the placenta, HTR-8/SVneo cells were cultured for 72 h with unlabeled forms of cholecalciferol, 25(OH)D3, or 1,25(OH)2D3. The final vitamin D concentrations of each treatment were 2500, 5000, and 10,000 nM cholecalciferol (Cayman Chemical), 500 nM 25(OH)D3 (Cayman Chemical), and 100 nM 1,25(OH)2D3 (Sigma-Aldrich).
The cell pellets from each sample were harvested, and total RNA was extracted (PerfectPure RNA Cell and Tissue Kit), quantified, and subjected to reverse transcription and qRT-PCR. The TaqMan Gene Expression Assays for CYP24A1, CYP27B1, CYP2R1, and β-glucuronidase along with the reaction conditions and data expression method were the same as those described above in the qRT-PCR protocol used for the placental tissues.
13C2-cholecalciferol and 13C5-25(OH)D3 treatments and 13C-labeled vitamin D metabolite measurements from cells and media
To explore the metabolic fate of cholecalciferol, HTR-8/SVneo cells were cultured for 24-, 72-, and 96 h with either 13C2-cholecalciferol (Cambridge Isotope Laboratories) or 13C5-25(OH)D3 (IsoSciences LLC), resulting in final concentrations of 5000 or 500 nM, respectively. These dosing concentrations of 13C2-cholecalciferol and 13C5-25(OH)D3 were selected with the use of data from the unlabeled vitamin D treatment. A sample of each treatment at each culture time point consisted of the cell pellets and media pooled from 3 culture dishes. The cell pellets and media from each sample were harvested separately and stored at −80°C until measurement.
13C-labeled vitamin D metabolites [i.e., 13C-cholecalciferol, 13C-25(OH)D3, 13C-1,25(OH)2D3, and 13C-24,25(OH)2D3] were extracted from the cells, which were sonicated in 200 μL deionized water, using liquid-liquid (400 μL acetonitrile, 500 μL methyl tert-butyl ether, and 25 μL internal standard) and solid-phase extractions as described for placental tissue vitamin D metabolites, but followed by derivatization with 50 μL of 1 mg/mL 4-phenyl-1,2,4-triazoline-3,5-dione (26, 30). Media (15 mL) was mixed with 15 mL methanol and 25 μL internal standard and subsequently underwent the solid-phase extraction and the derivatization. An internal standard solution mix for the 13C2-cholecalciferol treatment and ethanol control contained d6-cholecalciferol, d3-25(OH)D3, d3-1,25(OH)2D3, and d6-24,25(OH)2D3, whereas an internal standard solution for 13C5-25(OH)D3 treatment contained d3-25(OH)D3, d3-1,25(OH)2D3, and d6-24,25(OH)2D3. A total of 10 μL of extracts diluted with 50 μL of 0.1% formic acid in water (+5 mM methylamine) were injected and separated by an LC-MS/MS system (Ultimate 3000 UHPLC coupled to Thermo Scientific Q Exactive Mass Spectrometer) with Waters BEH C18 columns (2.5 μm; 2.1 × 50 mm) (30). Extracts from the ethanol control were injected twice with extracts from either 13C2-cholecalciferol or 13C5-25(OH)D3 treatment of separate comparisons. Elution of the analytes of interest was performed at a flow rate of 250 μL/min under the following conditions: 40% of 0.1% formic acid in methanol and 60% of 0.1% formic acid in water (+5 mM methylamine) at 0 min; linear gradient from 40% to 70% of 0.1% formic acid in methanol (minutes 0–5); 70% of 0.1% formic acid in methanol (minutes 5–9); linear gradient from 70% to 40% of 0.1% formic acid in methanol (minutes 9–12); and 40% of 0.1% formic acid in methanol (minutes 12–17). The analytes of interest were detected in electrospray ionization-positive ion mode, and their transition pairs are provided in Supplemental Table 1.
Statistical analysis
All analyses were conducted with the use of JMP Pro 12 software (SAS Institute), STATA version 14 (StataCorp), and SigmaPlot version 11 software (Systat Software). Data not normally distributed were ln-transformed and are presented as arithmetic means ± SDs or geometric means (95% CIs) unless otherwise specified. P values were 2-sided tests and considered significant at <0.05.
The differences between baseline and study-end circulating vitamin D metabolites that were normally distributed [i.e., 25(OH)D3, 1,25(OH)2D3, 24,25(OH)2D3, free 25(OH)D3, the ratio of 24,25(OH)D3 to 25(OH)D3, and DBP) were tested with the use of a paired Student’s t test. The concentrations of 3-epi-25(OH)D3 were analyzed by Wilcoxon’s signed-rank test due to nonnormal distribution after replacing values less than the limit of detection (1.0 nmol/L) with the value of 1.0. To examine the associations of placental mRNA abundance of the vitamin D metabolic components with vitamin D metabolites in blood and placental tissues, a linear mixed model (LMM) was constructed. Each placental and circulating vitamin D metabolite were entered into the model as a fixed effect, whereas the participant identifier was entered as a random effect. The covariates considered in each initial LMM are shown in Table 1 and Supplemental Table 2, and those with a significance level of P < 0.2 were retained in the final models. In addition, the associations between placental and circulating vitamin D metabolites were assessed with the use of a Pearson’s correlation analysis, whereas the associations of serum 3-epi-25(OH)D3 (nonnormal distribution) with placental vitamin D metabolites were examined with the use of a Spearman rank correlation test.
TABLE 1.
Baseline characteristics and gestational outcomes of pregnant women in the third trimester1
| Characteristics | Pregnant women (n = 24) |
| Age, y | 29 ± 3 |
| Prepregnancy BMI, kg/m2 | 24 ± 3 |
| Ethnicity, n | |
| Caucasian | 14 |
| African American | 1 |
| Latino | 4 |
| Asian | 4 |
| Other | 1 |
| Parity, n | |
| Primiparas | 11 |
| Multiparas | 13 |
| Multivitamin supplement use before study entry, n | |
| Yes | 20 |
| No | 4 |
| Season at study entry, n | |
| April to September | 13 |
| October to March | 11 |
| GC rs7041 G>T polymorphism, n | |
| GG | 8 |
| GT | 10 |
| TT | 6 |
| CYP2R1 rs10741657 A>G polymorphism, n | |
| AA | 4 |
| AG | 18 |
| GG | 2 |
| CYP2R1 rs12794714 A>G polymorphism, n | |
| AA | 5 |
| AG | 18 |
| GG | 1 |
| Length of gestation, wk | 39.9 ± 0.7 |
| Gestational weight gain, kg | 15.9 ± 4.6 |
| Mode of delivery, n | |
| Vaginal | 17 |
| Cesarean | 5 |
Values are means ± SDs unless otherwise indicated. CYP2R1, 25-hydroxylase gene; GC, vitamin D binding protein gene.
To assess the changes in the concentrations of intracellular and extracellular 13C-labeled vitamin D metabolites from HTR-8/SVneo cells treated with 13C-labeled vitamin D, the LMM and the Bonferroni correction were used. Culture time (24, 72, or 96 h), the location of the metabolite extraction (cell or medium), and their interaction were fixed effects, and the experiment number (first, second, or third experiments) and sample identifier nested within the experiment number were random effects. The LMM was also used to assess changes in product: precursor ratios in the vitamin D metabolic pathway. The differences in enzyme gene expression among the vitamin D treatment groups (unlabeled) in the cells were tested with the use of 1-factor ANOVA.
RESULTS
Characteristics and measures of vitamin D metabolites in blood and placentas of pregnant women
Table 1 depicts the baseline characteristics and gestational outcomes of the third-trimester pregnant women, whereas Table 2 shows their vitamin D metabolite concentrations in blood at baseline and study end. Concentration differences were detected between baseline and study end for 25(OH)D3, free 25(OH)D3, 3-epi-25(OH)D3, and DBP, whereas no differences were detected between these study time points for 1,25(OH)2D3 and 24,25(OH)2D3 concentrations and the 24,25(OH)2D3:25(OH)D3 ratio.
TABLE 2.
Baseline and study-end concentrations of circulating vitamin D metabolites among pregnant women in the third trimester1
| Circulating vitamin D metabolites | Baseline (n = 24) | End of study (n = 24) |
| 25(OH)D3, nmol/L | 85.3 ± 28.6a | 96.8 ± 33.0b |
| 1,25(OH)2D3, pmol/L | 290 (235, 357)a | 312 (258, 379)a |
| 24,25(OH)2D3, nmol/L | 11.4 ± 6.1a | 12.8 ± 5.2a |
| Free 25(OH)D3, pmol/L | 15.7 (11.8, 20.9)a | 19.4 (15.0, 25.1)b |
| 3-epi-25(OH)D3, nmol/L | 3.2 ± 2.2a | 4.5 ± 3.5b |
| 24,25(OH)2D3:25(OH)D3 | 0.13 ± 0.04a | 0.13 ± 0.03a |
| DBP, μg/mL | 461 ± 247b | 422 ± 233a |
Values are means ± SDs or geometric means (95% CIs). For circulating vitamin D metabolites, data were analyzed by a paired Student’s t test [except for 3-epi-25(OH)D3, which was analyzed by Wilcoxon’s signed-rank test], and values in a row with a superscript letter indicate significant differences between baseline and study end (i.e., a < b). P < 0.05. DBP, vitamin D binding protein; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 3-epi-25(OH)D3, C3 epimer of 25-hydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3.
Placental concentrations (means ± SDs) of 25(OH)D3 and 24,25(OH)2D3 at delivery were 20.9 ± 8.2 and 2.2 ± 1.5 pmol/g, respectively, and the 24,25(OH)2D3:25(OH)D3 ratio was 0.10 ± 0.04.
Associations between placental and circulating vitamin D metabolites
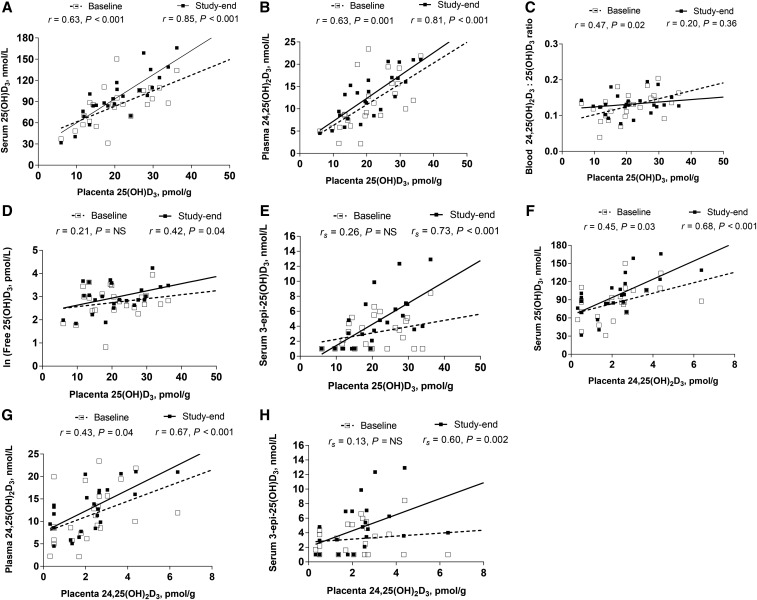
Placental vitamin D metabolites [i.e., 25(OH)D3 and 24,25(OH)2D3] were significantly correlated with the majority of maternal circulating vitamin D metabolites through time (Figure 1). Placental 25(OH)D3 showed strong positive correlations with circulating 25(OH)D3 (Figure 1A) and 24,25(OH)2D3 (Figure 1B) at both baseline and study end. Placental 25(OH)D3 was also positively correlated with baseline circulating 24,25(OH)2D3:25(OH)D3 ratio (Figure 1C), study-end free 25(OH)D3 (Figure 1D), and study-end 3-epi-25(OH)D3 (Figure 1E). In addition, placental 24,25(OH)2D3 showed positive correlations with circulating 25(OH)D3 (Figure 1F) and 24,25(OH)2D3 (Figure 1G) throughout the study, and placental 24,25(OH)2D3 was positively correlated with study-end 3-epi-25(OH)D3 (Figure 1H). Moreover, a strong positive correlation (r = 0.83, P < 0.001) was detected between placental 25(OH)D3 and placental 24,25(OH)2D3 at delivery.
FIGURE 1.
The relations of placental and circulating vitamin D metabolites in pregnant women (n = 24) by study time point: (A) placental 25(OH)D3 and serum 25(OH)D3 concentrations; (B) placental 25(OH)D3 and plasma 24,25(OH)2D3 concentrations; (C) placental 25(OH)D3 and circulating 24,25(OH)2D3:25(OH)D3 ratio; (D) placental 25(OH)D3 and circulating free 25(OH)D3 concentrations; (E) placental 25(OH)D3 and serum 3-epi-25(OH)D3 concentrations; (F) placental 24,25(OH)2D3 and serum 25(OH)D3 concentrations; (G) placental 24,25(OH)2D3 and plasma 24,25(OH)2D3 concentrations; and (H) placental 24,25(OH)2D3 and serum 3-epi-25(OH)D3 concentrations. Data were derived from Pearson and Spearman correlation tests. Study baseline values are presented in open squares with dashed line slopes, and study-end values are presented in closed squares with solid line slopes. 3-epi-25(OH)D3, C3 epimer of 25-hydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3.
Associations of placental gene expression of the vitamin D metabolic pathway components with placental vitamin D metabolites
Placental LRP2 mRNA abundance was positively associated with the placental 24,25(OH)2D3:25(OH)D3 ratio [R2 = 0.62; β = 1873.54 (235.39, 3511.68); P = 0.028] and placental 25(OH)D3 [R2 = 0.44; β = 6.51 (1.67, 11.34); P = 0.011]. Further, placental CYP2R1 and CYP24A1 mRNA abundance tended to be inversely associated with the placental 24,25(OH)2D3:25(OH) D3 ratio [for CYP2R1, R2 = 0.52, β = −9.06 (−18.47, 0.34), P = 0.058; for CYP24A1, R2 = 0.20, β = −8.72 (−17.43, −0.0042), P = 0.050].
Associations of placental gene expression of the vitamin D metabolic pathway components with circulating vitamin D metabolites
Placental LRP2 mRNA abundance was positively associated with circulating 25(OH)D3 at both baseline and study end and with study-end free 25(OH)D3 and 3-epi-25(OH)D3 concentrations (Table 3). Placental LRP2 mRNA abundance also tended to be associated with baseline circulating 3-epi-25(OH)D3 concentration [R2 = 0.58; β = 22.0 (−0.61, 44.0); P = 0.06]. Placental CUBN mRNA abundance was positively associated with circulating 25(OH)D3 and 1,25(OH)2D3 at both study time points (Table 3), whereas placental CYP2R1 mRNA abundance was positively associated with circulating 1,25(OH)2D3 concentration throughout the study and with baseline 3-epi-25(OH)D3 concentration (Table 3). Placental CYP27B1 mRNA abundance was positively associated with baseline 3-epi-25(OH)D3 and study-end 1,25(OH)2D3 concentrations (Table 3) and tended to be associated with baseline 25(OH)D3 concentrations [R2 = 0.54; β = 0.0095 (−0.0007, 0.020); P = 0.06]. Finally, placental CYP24A1 mRNA abundance was positively associated with circulating 1,25(OH)2D3 at both baseline and the end of the study (Table 3), and with baseline circulating 25(OH)D3, free 25(OH)D3, and 3-epi-25(OH)D3 concentrations. Placental CYP24A1 mRNA abundance also tended to be associated with baseline circulating 24,25(OH)2D3 concentrations [R2 = 0.16; β = 0.059 (−0.0014, 0.12); P = 0.06; data not presented in Table 3].
TABLE 3.
Associations of placental gene transcript abundance of the vitamin D metabolic pathway components with circulating vitamin D metabolites among pregnant women (n = 24)1
| Placental genes | Circulating vitamin D metabolites | R2 | β (95% CI) | P |
| LRP2 | Baseline | |||
| 25(OH)D3 | 0.51 | 3.3 (1.7, 5.0) | <0.001 | |
| End of study | ||||
| 25(OH)D3 | 0.57 | 1.9 (0.72, 3.0) | 0.003 | |
| Free 25(OH)D3 | 0.32 | 8.7 (2.2, 15.0) | 0.012 | |
| 3-epi-25(OH)D3 | 0.63 | 23 (10, 36) | 0.002 | |
| CUBN | Baseline | |||
| 25(OH)D3 | 0.25 | 0.014 (0.0021, 0.026) | 0.023 | |
| 1,25(OH)2D3 | 0.47 | 0.0035 (0.0015, 0.0052) | 0.001 | |
| End of study | ||||
| 25(OH)D3 | 0.37 | 0.016 (0.0043, 0.028) | 0.010 | |
| 1,25(OH)2D3 | 0.47 | 0.0034 (0.0015, 0.0052) | 0.001 | |
| CYP2R1 | Baseline | |||
| 1,25(OH)2D3 | 0.34 | 0.0025 (0.0009, 0.0042) | 0.004 | |
| 3-epi-25(OH)D3 | 0.56 | 0.23 (0.11, 0.34) | <0.001 | |
| End of study | ||||
| 1,25(OH)2D3 | 0.32 | 0.0021 (0.0007, 0.0034) | 0.006 | |
| CYP27B1 | Baseline | |||
| 3-epi-25(OH)D3 | 0.40 | 0.19 (0.079, 0.31) | 0.002 | |
| End of study | ||||
| 1,25(OH)2D3 | 0.28 | 0.0014 (3.8 × 10−5, 0.0028) | 0.045 | |
| CYP24A1 | Baseline | |||
| 25(OH)D3 | 0.43 | 0.024 (0.010, 0.037) | 0.002 | |
| 1,25(OH)2D3 | 0.40 | 0.0043 (0.0019, 0.0067) | 0.001 | |
| Free 25(OH)D3 | 0.33 | 0.075 (0.024, 0.13) | 0.007 | |
| 3-epi-25(OH)D3 | 0.56 | 0.38 (0.22, 0.54) | <0.001 | |
| End of study | ||||
| 1,25(OH)2D3 | 0.45 | 0.0036 (0.0016, 0.0056) | 0.001 |
Data were derived from the covariate-adjusted linear mixed models. All placental vitamin D–related gene transcript abundance (dependent variables) were ln-transformed except for LRP2. No association achieved statistical significance between placental gene expression and circulating vitamin D metabolites unless listed in this table. CUBN, cubilin; CYP2R1, 25-hydroxylase; CYP24A1, 24-hydroxylase; CYP27B1, 1α-hydroxylase; LRP2, LDL-related protein 2; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 3-epi-25(OH)D3, C3 epimer of 25-hydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3.
Effects of vitamin D exposure on placental gene expression of the vitamin D metabolic enzymes in HTR-8/SVneo cell cultures
We examined the effects of vitamin D treatment [unlabeled cholecalciferol, 25(OH)D3, and 1,25(OH)2D3] on the gene expression response of vitamin D metabolic enzymes in the placental cells. The 1,25(OH)2D3 (100 nM) and 25(OH)D3 (500 nM) treatments upregulated (P < 0.05) CYP24A1 gene expression by 734- and 566-fold, respectively, compared with the ethanol control at 72 h (Figure 2). Similarly, the higher concentrations of cholecalciferol treatments yielded a dose-response increase (P < 0.05) in CYP24A1 mRNA abundance (43-fold at 2500 nM, 114-fold at 5000 nM, and 142-fold at 10,000 nM compared with the control; Figure 2). In contrast, cholecalciferol, 25(OH)D3, or 1,25(OH)2D3 treatment did not influence CYP27B1 and CYP2R1 mRNA expression (Supplemental Table 3).
FIGURE 2.
The responses of CYP24A1 mRNA abundance in HTR-8/SVneo cells in response to vitamin D (unlabeled) treatment at 72 h. Values are means ± SEs. Data were obtained from 3 separate experiments, with each experiment containing 3 replicates/vitamin D treatment, and were analyzed after log10 transformation with the use of ANOVA. Means without a common lowercase letter differ, P < 0.05. CYP24A1, 24-hydroxylase gene; HTR-8/SVneo, immortalized human placental extravillous trophoblast cell line; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3.
Intracellular and extracellular concentrations of 13C-labeled vitamin D metabolites in HTR-8/SVneo cell cultures
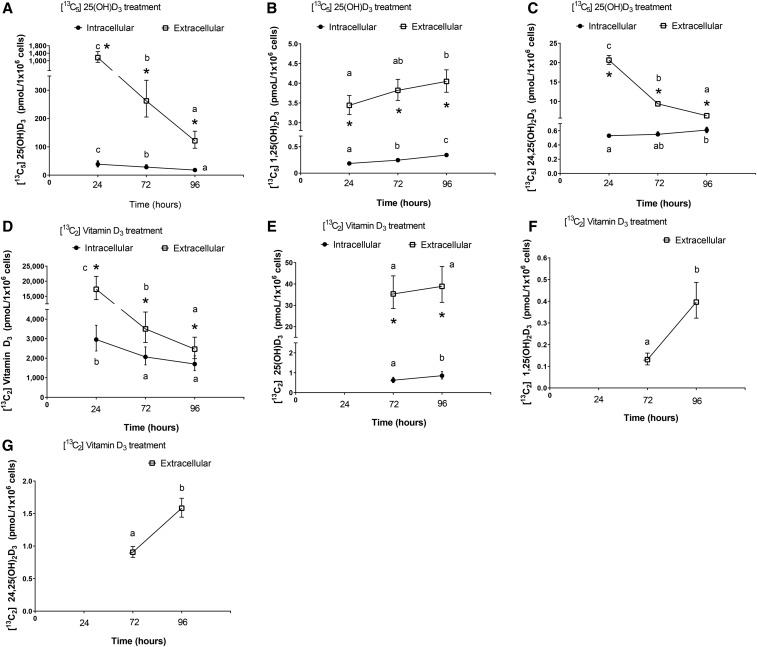
In cells treated with 13C5-25(OH)D3 (Figure 3A–C), intracellular 13C5-25(OH)D3 decreased by 53% (P < 0.001) (Figure 3A), and extracellular 13C5-25(OH)D3 (i.e., treatment concentrations in the media) decreased by 90% (P < 0.001) from 24 to 96 h. In contrast, intracellular and extracellular 13C5-1,25(OH)2D3 increased by 86% (P < 0.001) and 18% (P = 0.017), respectively, from 24 to 96 h (Figure 3B). Although intracellular 13C5-24,25(OH)2D3 increased slightly (15%; P = 0.008) from 24 to 96 h (Figure 3C), extracellular 13C5-24,25(OH)2D3 decreased (−70%; P < 0.001).
FIGURE 3.
Intracellular and extracellular concentrations of 13C-labeled vitamin D metabolites from HTR-8/SVneo cells incubated with 13C5-25(OH)D3 (A–C) or 13C2-vitamin D3 (cholecalciferol) (D–G) for 24-, 72-, and 96-h: (A) 13C5-25(OH)D3; (B) 13C5-1,25(OH)2D3; (C) 13C5-24,25(OH)2D3; (D) 13C2-cholecalciferol; (E) 13C2-25(OH)D3; (F) 13C2-1,25(OH)2D3; (G) 13C2-24,25(OH)2D3. All concentrations are predicted geometric means (95% CIs) derived from the covariate-adjusted linear mixed models. Values were obtained from 3 separate experiments with each experiment containing 3 replicates/vitamin D treatment at a time point. Different letters denote changes in concentrations through time within cells or media, P < 0.05. Asterisks denote differences in concentrations between cells and media at a culture time point, P < 0.05. None of the 13C-labeled vitamin D metabolites were detected in the ethanol control treatment. HTR-8/SVneo, immortalized human placental extravillous trophoblast cell line; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3.
In cells treated with 13C2-cholecalciferol (Figure 3D–G), intracellular 13C2-cholecalciferol decreased (−42%; P < 0.001) from 24 to 96 h (Figure 3D) as did extracellular 13C2- cholecalciferol (−86%; P < 0.001). Intracellular and extracellular 13C2-25(OH)D3 were not detected at 24 h. However, intracellular 13C2-25(OH)D3 increased by 37% (P < 0.001) from 72 to 96 h (Figure 3E), whereas extracellular 13C2-25(OH)D3 remained stable across these time points. Finally, although intracellular (at all time points) and extracellular (at 24 h) 13C2-1,25(OH)2D3 and 13C2-24,25(OH)2D3 were not detected, extracellular 13C2-1,25(OH)2D3 and 13C2-24,25(OH)2D3 increased by 203% (P < 0.001) and 75% (P < 0.001), respectively, from 72 to 96 h (Figure 3F, G). None of the 13C-labeled vitamin D metabolites were detected in the ethanol control.
Ratios of product to precursor in the 13C-labeled vitamin D metabolic pathway in HTR-8/SVneo cell cultures
In cells treated with 13C5-25(OH)D3, the ratios of intracellular 13C5-1,25(OH)2D3:13C5-25(OH)D3 and 13C5-24,25(OH)2D3:13C5-25(OH)D3 increased (P < 0.001) from 24 to 96 h (Figure 4A). In cells treated with 13C2-cholecalciferol, the extracellular:intracellular ratios for 13C2-1,25(OH)2D3:13C2-25(OH)D3 and 13C2-24,25(OH)2D3:13C2-25(OH)D3 increased (P < 0.001) from 72 to 96 h (Figure 4B). At each time point, the 24,25(OH)2D3:25(OH)D3 ratio was significantly higher (P < 0.001) than the 1,25(OH)2D3:25(OH)D3 ratio in both the 13C5-25(OH)D3 and 13C2-cholecalciferol treatments.
FIGURE 4.
The product to precursor ratios of 13C-1,25(OH)2D3 and 13C-24,25(OH)2D3 in HTR-8/SVneo cells incubated with 13C5-25(OH)D3 (A) or 13C2-vitamin D3 (cholecalciferol) (B) for 24, 72, and 96 h. All ratios are predicted geometric means (95% CIs) derived from the covariate-adjusted linear mixed models. Values were obtained from 3 separate experiments with each experiment containing 3 replicates/vitamin D treatment at a time point. Different letters denote changes within 1,25(OH)2D3:25(OH)D3 ratio or 24,25(OH)2D3:25(OH)D3 ratio through time, P < 0.05. Asterisks denote the differences between the 1,25(OH)2D3:25(OH)D3 ratio and the 24,25(OH)2D3:25(OH)D3 ratio at a culture time point, P < 0.05. None of the 13C-labeled vitamin D metabolites were detected in the ethanol control treatment. HTR-8/SVneo, immortalized human placental extravillous trophoblast cell line; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3.
DISCUSSION
The modulatory role of the placenta on maternal circulating vitamin D metabolites is poorly understood due to a scarcity of human studies that include placental measurements of vitamin D biomarkers. Thus, we assessed the associations between placental vitamin D metabolites and placental gene expression of vitamin D metabolic components and their relations with circulating vitamin D metabolites in samples obtained from pregnant women throughout their third trimester. An in vitro investigation of the uptake, metabolism, and secretion of a 13C- cholecalciferol tracer by human placental trophoblasts was also conducted.
Characterization of vitamin D metabolism in placental tissue and its putative role in modulating maternal circulating concentrations of vitamin D metabolites
In this human study, which quantified placental tissue and blood vitamin D metabolites, numerous positive associations between placental and circulating vitamin D metabolites were detected (Figure 1). Further, and more notably, positive associations (P ≤ 0.045) were observed between placental tissue mRNA abundance of vitamin D metabolic components and circulating vitamin D metabolites, which were independent of season, BMI, vitamin D–related genetic variants, and gestational and neonatal outcomes (Table 3). Overall, these findings support the overarching notion that the placenta has a possible modulatory role on maternal circulating vitamin D metabolites in pregnant women.
The placenta appears to preferentially uptake DBP-bound 25(OH)D3 through the LRP2-CUBN receptor system
Interestingly, DBP-bound 25(OH)D3, rather than free 25(OH)D3, appears to be the form of vitamin D that is preferentially taken up by the placenta. For example, the serum total 25(OH)D3 concentration (comprised of DBP-bound and free forms) showed a stronger positive correlation with placental 25(OH)D3 than that between free serum 25(OH)D3 and placental 25(OH)D3 at both baseline and the end of the study. This finding is consistent with a recent study (22) that reported a stronger correlation between DBP-bound serum 25(OH)D3 and placental 25(OH)D3 than that of free serum 25(OH)D3 with placental 25(OH)D3. Furthermore, the serum total 25(OH)D3 concentration was positively associated with placental mRNA abundance of LRP2 (megalin) and CUBN throughout the third trimester. Conversely, no associations were detected between free serum 25(OH)D3 and placental CUBN mRNA abundance. Taken together, these data are suggestive of a preferential uptake of DBP-bound 25(OH)D3 by the placenta via a regulated process involving the LRP2-CUBN receptors.
Placenta CYP27B1 appears to produce and contribute to maternal circulating 1,25(OH)2D3 concentration in a substrate-dependent manner
We previously reported a positive linear correlation of circulating 25(OH)D and 1,25(OH)2D3 concentrations among pregnant women (7), which is consistent with a previous large randomized controlled trial study (31). As such, a substrate [i.e., 25(OH)D3]-dependent production of 1,25(OH)2D3 by kidney or placenta CYP27B1 during this reproductive state has been suggested. In the present study with the same cohort of pregnant women (7), we found that the circulating baseline 25(OH)D3 concentration was positively associated with placental CYP27B1 mRNA abundance as were circulating study-end 1,25(OH)2D3 concentrations. These findings support the notion that placental CYP27B1 contributes to maternal circulating 1,25(OH)2D3 in a manner dependent on circulating 25(OH)D3. These human data from pregnant women are well aligned with previous findings from in vitro and animal studies that showed placental production and secretion of 1,25(OH)2D3 from 25(OH)D3 (13–16, 32).
Placental trophoblasts increase production and secretion of 25(OH)D3 in response to cholecalciferol treatment
The cell culture component of this study shows that human placental trophoblasts in vitro are capable of synthesizing and releasing 25(OH)D3 with the use of its precursor cholecalciferol, as evidenced by the appearance of intracellular and extracellular 13C2-25(OH)D3 from the HTR-8/SVneo cells incubated with 13C2-cholecalciferol. Although previous work reported 25(OH)D3 production by placental mitochondria (33), our finding of 25(OH)D3 production and export by trophoblasts shows that the placenta could be an extrahepatic source of circulating 25(OH)D3 in maternal blood.
Furthermore, beyond the appearance of 13C2-25(OH)D3, its concentrations were increased through time in both HTR-8/SVneo cell pellets and media after 13C2-cholecalciferol treatment, suggesting an increase of trophoblast production and secretion of 25(OH)D3 with cholecalciferol availability. A subsequent increase in 13C-1,25(OH)2D3 was also observed. These in vitro data, along with our human data that show positive associations of placental CYP27B1 transcript abundance with circulating 25(OH)D3 and 1,25(OH)2D3 concentrations, collectively support a role of the placenta in modulating maternal circulating concentrations of both 25(OH)D3 and 1,25(OH)2D3.
Lastly, we show a dose-dependent upregulation of CYP24A1 in HTR-8/SVneo cells exposed to various concentrations of cholecalciferol. This in vitro finding suggests that, like 1,25(OH)2D3, cholecalciferol can regulate target genes in human placental trophoblasts, thus highlighting a possible functional importance of the initial form of vitamin D in the placenta.
Study limitations
This human study had a relatively small sample size which limited the statistical power of the study. In addition, most of the pregnant women (88% at baseline and 92% at the end of the study) showed sufficient vitamin D status [serum 25(OH)D ≥50 nmol/L] throughout the study period. Thus, we cannot exclude the possibility that the associations of maternal and placental vitamin D biomarkers might differ in vitamin D insufficiency. Moreover, because the study was done in the third trimester when fetal calcium demands are high, it is unclear whether the observed relations of placental and maternal vitamin D metabolites would transpire in other stages of human pregnancy. Finally, our cell culture experiments, which demonstrated the production and secretion of 25(OH)D3 by human placental trophoblasts, used nonphysiologic doses of the precursor, cholecalciferol. As such, this finding may not necessarily be extrapolated to placental cells exposed to physiologic concentrations of vitamin D. However, the static nature of the media at a high concentration relative to the cells may simulate the in vivo dynamic exposure of placental cells to a continuous flow of blood providing vitamin D throughout the gestational period.
Conclusions
Numerous associations between placental and maternal vitamin D biomarkers from this human study support an active role of the placenta in the modulation of circulating vitamin D metabolites. Nonetheless, additional human studies that use stable isotope methodology are needed to fully dissect the processes behind the associations. Based on the cell culture component of this study, human trophoblasts can produce and secrete 25(OH)D3 and 1,25(OH)2D3 with the use of cholecalciferol, raising the possibility that the placenta may be a source of these vitamin D metabolites during human pregnancy.
Acknowledgments
The authors’ responsibilities were as follows—HP, PMB, and MAC: designed the research; HP, MRW, OVM, and SJ: conducted the research; HP: analyzed the data; HP and MAC: wrote the manuscript; SM: contributed to the methodology development and data interpretation; PMB and MAC: had primary responsibility for the final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: CUBN, cubilin; CYP2R1, 25-hydroxylase; CYP24A1, 24-hydroxylase; CYP27B1, 1α-hydroxylase; DBP, vitamin D binding protein; LC-MS/MS, liquid chromatography–tandem mass spectrometry; LMM, linear mixed model; LRP2, LDL-related protein 2; mRNA, messenger RNA; qRT-PCR, quantitative real-time polymerase chain reaction; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 3-epi-25(OH)D3, C3 epimer of 25(OH)D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3.
REFERENCES
- 1.Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest 1984;74:1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis pregnancy, lactation, and bone metabolism during and postweaning: a longitudinal study. Am J Clin Nutr 1995;61:514–23. [DOI] [PubMed] [Google Scholar]
- 3.Wilson SG, Retallack RW, Kent JC, Worth GK, Gutteridge DH. Serum free 1,25-dihydroxyvitamin D and the free 1,25-dihydroxyvitamin D index during a longitudinal study of human pregnancy and lactation. Clin Endocrinol (Oxf) 1990;32:613–22. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr 1998;67:693–701. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez PA, Idrisa A, Bobzom DN, Airede A, Hollis BW, Liston DE, Jones DD, Dasgupta A, Glew RH. Calcium and vitamin D status of pregnant teenagers in Maiduguri, Nigeria. J Natl Med Assoc 1997;89:805–11. [PMC free article] [PubMed] [Google Scholar]
- 6.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab 2006;91:906–12. [DOI] [PubMed] [Google Scholar]
- 7.Park H, Brannon PM, West AA, Yan J, Jiang X, Perry CA, Malysheva OV, Mehta S, Caudill MA. Vitamin D metabolism varies among women in different reproductive states consuming the same intakes of vitamin D and related nutrients. J Nutr 2016;146:1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schleicher RL, Encisco SE, Chaudhary-Webb M, Paliakov E, McCoy LF, Pfeiffer CM. Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin Chim Acta 2011;412:1594–9. [DOI] [PubMed] [Google Scholar]
- 9.Granado-Lorencio F, Garcia-Heras LM, Blanco-Navarro I, Pérez-Sacristán B. Assessment of 3-epi-25-OH-D3 in preterm and full term infant samples and its relationship to demographic, anthropometric and biochemical determinants. Clin Biochem 2014;47:853–6. [DOI] [PubMed] [Google Scholar]
- 10.Lundgren S, Carling T, Hjälm G, Juhlin C, Rastad J, Pihlgren U, Rask L, Akerström G, Hellman P. Tissue distribution of human gp330/megalin, a putative Ca(2+)-sensing protein. J Histochem Cytochem 1997;45:383–92. [DOI] [PubMed] [Google Scholar]
- 11.Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol 2002;3:256–66. [DOI] [PubMed] [Google Scholar]
- 12.Ma R, Gu Y, Zhao S, Sun J, Groome LJ, Wang Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol Endocrinol Metab 2012;303:E928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Díaz L, Sánchez I, Avila E, Halhali A, Vilchis F, Larrea F. Identification of a 25-hydroxyvitamin D3 1alpha-hydroxylase gene transcription product in cultures of human syncytiotrophoblast cells. J Clin Endocrinol Metab 2000;85:2543–9. [DOI] [PubMed] [Google Scholar]
- 14.Zehnder D, Evans KN, Kilby MD, Bulmer JN, Innes BA, Stewart PM, Hewison M. The ontogeny of 25-hydroxyvitamin D(3) 1alpha-hydroxylase expression in human placenta and decidua. Am J Pathol 2002;161:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental-decidual function. J Soc Gynecol Investig 2004;11:263–71. [DOI] [PubMed] [Google Scholar]
- 16.Weisman Y, Harell A, Edelstein S, David M, Spirer Z, Golander A. 1 alpha, 25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature 1979;281:317–9. [DOI] [PubMed] [Google Scholar]
- 17.Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, Hewison M. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod 2006;75:816–22. [DOI] [PubMed] [Google Scholar]
- 18.Gray TK, Lester GE, Lorenc RS. Evidence for extra-renal 1 alpha-hydroxylation of 25-hydroxyvitamin D3 in pregnancy. Science 1979;204:1311–3. [DOI] [PubMed] [Google Scholar]
- 19.Blum M, Weisman Y, Turgeman S, Cabili S, Wollman Y, Peer G, Stern N, Silverberg D, Schwartz D, Iaina A. Pregnancy decreases immunoreactive parathyroid hormone level in rats with chronic renal failure. Clin Sci 1999;96:427–30. [PubMed] [Google Scholar]
- 20.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999;96:507–15. [DOI] [PubMed] [Google Scholar]
- 21.Kiely ME, Zhang JY, Kinsella M, Khashan AS, Kenny LC. Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in Ireland with low vitamin D status. Am J Clin Nutr 2016;104:354–61. [DOI] [PubMed] [Google Scholar]
- 22.Tamblyn JA, Susarla R, Jenkinson C, Jeffery LE, Ohizua O, Chun RF, Chan SY, Kilby MD, Hewison M. Dysregulation of maternal and placental vitamin D metabolism in preeclampsia. Placenta 2017;50:70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Devapatla S, Pressman E, Vermeylen F, Stabler SP, Allen RH, et al. . Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr 2012;95:1060–71. [DOI] [PubMed] [Google Scholar]
- 24.West AA, Yan J, Perry CA, Jiang X, Malysheva OV, Caudill MA. Folate-status response to a controlled folate intake in nonpregnant, pregnant, and lactating women. Am J Clin Nutr 2012;96:789–800. [DOI] [PubMed] [Google Scholar]
- 25.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab 1986;63:954–9. [DOI] [PubMed] [Google Scholar]
- 26.Lipkie TE, Janasch A, Cooper BR, Hohman EE, Weaver CM, Ferruzzi MG. Quantification of vitamin D and 25-hydroxyvitamin D in soft tissues by liquid chromatography–tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2013;932:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufmann M, Gallagher C, Peacock M, Schlingmann K-P, Konrad M, Deluca HF, Sigueiro R, Lopez B, Mourino A, Maestro M, et al. . Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D & 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J Clin Endocrinol Metab 2014;99:2567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 29.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 1993;206:204–11. [DOI] [PubMed] [Google Scholar]
- 30.Ding S, Schoenmakers I, Jones K, Koulman A, Prentice A, Volmer DA. Quantitative determination of vitamin D metabolites in plasma using UHPLC-MS/MS. Anal Bioanal Chem 2010;398:779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011;26:2341–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin LP, Yeung B, Vouros P, Vilner LM, Reddy GS. Evidence for human placental synthesis of 24,25-dihydroxyvitamin D3 and 23,25-dihydroxyvitamin D3. Pediatr Res 1993;34:98–104. [DOI] [PubMed] [Google Scholar]
- 33.Slominski AT, Kim T-K, Shehabi HZ, Semak I, Tang EKY, Nguyen MN, Benson HAE, Korik E, Janjetovic Z, Chen J, et al. . In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J 2012;26:3901–15. [DOI] [PMC free article] [PubMed] [Google Scholar]