Abstract
Background: Obesity is associated with reduced activation in the left dorsolateral prefrontal cortex (DLPFC), a region of the brain that plays a key role in the support of self-regulatory aspects of eating behavior and inhibitory control. Transcranial direct current stimulation (tDCS) is a noninvasive technique used to modulate brain activity.
Objectives: We tested whether repeated anodal tDCS targeted at the left DLPFC (compared with sham tDCS) has an immediate effect on eating behavior during ad libitum food intake, resulting in weight change, and whether it might influence longer-term food intake–related appetite ratings in individuals with obesity.
Design: In a randomized parallel-design study combining inpatient and outpatient assessments over 31 d, 23 individuals with obesity [12 men; mean ± SD body mass index (BMI; in kg/m2): 39.3 ± 8.42] received 15 sessions of anodal (i.e., enhancing cortical activity) or sham tDCS aimed at the left DLPFC. Ad libitum food intake was assessed through the use of a vending machine paradigm and snack food taste tests (SFTTs). Appetite was evaluated with a visual analog scale (VAS). Body weight was measured. We examined the effect of short-term (i.e., 3 sessions) and long-term (i.e., 15 sessions) tDCS on these variables.
Results: Relative to sham tDCS, short-term anodal tDCS did not influence ad libitum intake of food from the vending machines. Accordingly, no effect on short-term or 4-wk weight change was observed. In the anodal tDCS group, compared with the sham group, VAS ratings for hunger and the urge to eat declined significantly more (P = 0.01 and P = 0.05, respectively), and total energy intake during an SFTT was relatively lower in satiated individuals (P = 0.01), after long-term tDCS.
Conclusions: Short-term anodal tDCS of the left DLPFC did not have an immediate effect on ad libitum food intake or thereby weight change, relative to sham tDCS. Hunger and snack food intake were reduced only after a longer period of anodal tDCS in individuals with obesity. This trial was registered at clinicaltrials.gov as NCT00739362.
Keywords: transcranial direct current stimulation (tDCS), neuromodulation, dorsolateral prefrontal cortex, obesity, ad libitum food intake, weight change, snack food intake, hunger, eating in the absence of hunger
See corresponding editorial on page 1331.
INTRODUCTION
The interaction between the limbic system and the prefrontal cortex (PFC) is likely an important pathway that influences behavioral control of food intake (1). Poor executive functioning of the PFC may result in compromised self-control, potentially leading to consistently unhealthy eating behaviors (2, 3). Negative feedback may also impair executive function (2), a trait linked with obesity and nonhomeostatic food intake (4, 5). By including perceptive input into the decision-making process, the dorsolateral PFC (DLPFC) takes part in the supervision of eating behavior (5, 6). In line with the implication of the PFC in making healthy eating decisions, obese individuals have less left DLPFC activation after a meal compared with lean individuals, indicating that deregulated inhibitory mechanisms influence eating behavior and food choice (5, 7). The exact role of DLPFC activation in such regulatory mechanisms is still unclear, as is whether adiposity leads to this dysregulation or, as more evidence indicates, is a result of this lower activity (4–6). In subjects with obesity, reduced postprandial activity of the left DLPFC may be restored by weight loss or indicate the ability of certain individuals to achieve sustained weight loss (8, 9).
Neuromodulation through the use of transcranial direct current stimulation (tDCS) is thought to result in polarization of brain tissue by applying low-intensity currents through electrodes placed on the scalp (10). Based on this theory, anodal tDCS would increase the excitability of cortical (11, 12) and subcortical (13) neurons, augmenting their response to neuronal input and facilitating neuronal discharge (10, 14). Repeated sessions of tDCS may also induce long-term effects, possibly mediated by synaptic plasticity mechanisms (12). Although noninvasive, the safety and tolerability of tDCS are still under study.
tDCS aimed at enhancing DLPFC activity led to decreased food consumption (15, 16), with effects on behaviors associated with excess calorie intake (17–19). Specifically, in obese subjects, repeated anodal compared with cathodal tDCS of the left DLPFC led to lower ad libitum food intake and thus weight loss (7).
To test the effect of repeated anodal compared with sham tDCS of the left DLPFC on eating behavior in individuals with obesity, we primarily investigated 1) whether anodal (compared with sham) tDCS targeted to the left DLPFC leads to immediate decreases in ad libitum intake of food from vending machines and 2) whether such an effect on food intake would lead to weight loss. To investigate whether anodal (compared with sham) tDCS of the left DLPFC influences food intake in a different setting over a longer period, and to elucidate possible affected mechanisms involved in nutrient intake, our secondary aims were to 1) detect a difference in ad libitum snack food intake and 2) assess appetite-related ratings, such as satiety (5, 17–19) and taste and liking (20).
METHODS
Subjects
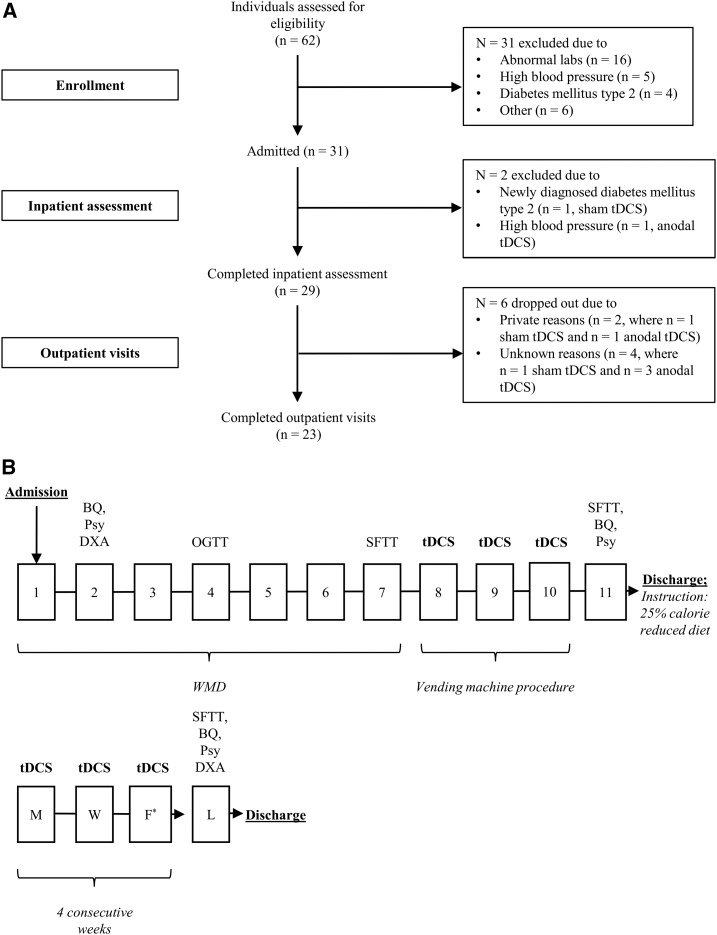
After obtaining written informed consent and after assessing medical history, physical status, and basic laboratory measurements, 31 healthy participants with obesity [BMI (in kg/m2): ≥30; ethnicities: African American, Asian, Hispanic, Native American, white] were admitted to the clinical research unit to participate in our randomized parallel-design study. Somatic and psychiatric diseases (21), as well as medication and illicit drug use, possibly influencing neurocognitive processes were ruled out during the screening and admission process. No individuals had diabetes (22) per a 2-h, 75-g oral-glucose-tolerance test [Beckman Instruments; or AutoAnalyzer (Technicon)]; all were right-handed and had a stable weight (±5%) for the past 3 mo. Before the study, no participant had ever received tDCS. Of those individuals who entered the study, 23 completed the entire clinical trial (i.e., both the inpatient assessment and outpatient visits), whereas 29 participants completed the inpatient study only (Figure 1A, Table 1). The study cohort was recruited from February 2013 to August 2016. The study is registered at clinicaltrials.gov (NCT00739362) and was approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases.
FIGURE 1.
Overview of study design. (A) Flowchart showing the number of individuals enrolled and admitted and the number of participants who completed the inpatient assessment and outpatient visits. Reasons for dropping out are listed on the right. For the inpatient assessment, 14 participants receiving anodal tDCS and 17 individuals receiving sham tDCS were analyzed to determine differences between the groups, whereas for the outpatient visits, 9 and 14 individuals in each group, respectively, were analyzed. In panel B, squares with numbers indicate the days of inpatient assessment, whereas squares with letters indicate weekdays when outpatient visits occurred. After receiving informed consent, the study physician enrolled the participants. Upon admission, before the vending machine paradigm (days 8, 9, and 10), subjects were administered an individualized WMD adjusted for body weight and sex (20% protein, 30% fat, 50% carbohydrate) (23). On day 2, eating-related behaviors and psychopathology were investigated through the administration of a Psy and a BQ, and fat-mass, fat-free mass, and percentage of body fat were estimated by DXA (DPX-1; Lunar Radiation Corp) (24). Glucose tolerance was determined on day 4. As a measure of ad libitum food intake and to assess taste preferences, an SFTT was performed on day 7 (25, 26). Study participants received anodal or sham tDCS of the left dorsolateral prefrontal cortex on days 8, 9, and 10. On these days, food intake was measured through the use of the vending machine paradigm. The SFTT, Psy, and BQ were repeated on day 11, and weight was measured at discharge. After the inpatient period, individuals were given instructions for a 25% calorie-reduced diet. Outpatient visits began the week after inpatient assessment. tDCS was performed for 4 consecutive weeks, 3 sessions/wk, each on a separate day. On the last day of the study, 1 d after the last outpatient stimulation, participants were fed breakfast on the clinical research unit and the Psy, BQ, and DXA were repeated. After lunch, participants completed the follow-up SFTT. *Except for the last week of outpatient visits, when a final tDCS was performed on a Thursday. BQ, behavioral questionnaire; DXA, dual X-ray absorptiometry; F, Friday; L, last day of the study (day after final transcranial direct current stimulation); M, Monday; OGTT, oral-glucose-tolerance test; Psy, psychological performance test; SFTT, snack food taste test; tDCS, transcranial direct current stimulation; W, Wednesday; WMD, weight-maintaining diet.
TABLE 1.
Population characteristics1
| Anodal tDCS |
Sham tDCS |
|||
| Male (n = 6) | Female (n = 7) | Male (n = 6) | Female (n = 10) | |
| Race, n | ||||
| Asian | 1 | 0 | 0 | 0 |
| Hispanic | 2 | 3 | 0 | 0 |
| African American | 0 | 0 | 2 | 2 |
| Native American | 1 | 4 | 3 | 7 |
| White | 1 | 0 | 0 | 1 |
| Mixed | 12 | 0 | 13 | 0 |
| Age, y | 43.9 ± 5.63 | 32.8 ± 9.33 | 32.7 ± 12.0 | 32.8 ± 9.33 |
| Weight at admission, kg | 110 ± 14.2 | 106 ± 17.5 | 122 ± 15.3 | 106 ± 17.5 |
| Weight measured by DXA, kg | ||||
| At admission | 110 ± 13.8 | 105 ± 17.6 | 121 ± 15.6 | 102 ± 25.3 |
| At last visit | 105 ± 15.2 | 107 ± 20.5 | 128 ± 9.68 | 102 ± 25.3 |
| BMI, kg/m2 | ||||
| At admission | 35.2 ± 3.89 | 42.5 ± 7.98 | 39.9 ± 4.88 | 38.1 ± 9.98 |
| At inpatient assessment | 34.9 ± 3.59 | 41.9 ± 7.88 | 39.4 ± 4.89 | 37.7 ± 9.83 |
| At last visit | 33.6 ± 2.74 | 42.9 ± 9.38 | 41.3 ± 2.50 | 38.0 ± 9.93 |
| Body fat, % | ||||
| At admission | 33.2 ± 4.05 | 42.8 ± 3.54 | 33.9 ± 2.72 | 53.9 ± 31.4 |
| At last visit | 34.6 ± 2.49 | 42.0 ± 4.03 | 35.5 ± 1.79 | 43.4 ± 3.68 |
| Fat mass, kg | ||||
| At admission | 36.4 ± 5.61 | 45.2 ± 9.35 | 41.3 ± 7.66 | 48.1 ± 20.2 |
| At last visit | 36.0 ± 3.55 | 45.1 ± 10.6 | 45.6 ± 5.20 | 44.8 ± 13.7 |
| Fat-free mass, kg | ||||
| At admission | 73.7 ± 11.2 | 59.8 ± 9.45 | 79.8 ± 8.69 | 53.3 ± 7.98 |
| At last visit | 68.8 ± 12.3 | 61.9 ± 11.5 | 82.7 ± 5.17 | 57.3 ± 12.2 |
| Plasma glucose, mg/dL | ||||
| Fasting | 98.7 ± 13.2 | 96.5 ± 5.18 | 97.2 ± 6.20 | 95.7 ± 7.46 |
| 2-h OGTT | 120 ± 47.6 | 135 ± 35.1 | 142 ± 29.7 | 131 ± 20.7 |
Values are means ± SDs, unless otherwise indicated. Weight at admission was measured on entrance to the study. DXA, used to estimate body composition and calculate body weight, was performed on day 2 of the inpatient assessment (at admission) and at the last outpatient visit (at last visit). Plasma glucose concentrations were measured with an OGTT on day 4. No differences regarding population characteristics were found between the tDCS subgroups (all P > 0.05). DXA, dual X-ray absorptiometry; OGTT, oral-glucose-tolerance test; tDCS, transcranial direct current stimulation.
Participant had both Native American and Hispanic ethnicity.
Participant was of both African American and white race.
Study protocol
Inpatient assessment
The inpatient assessment of the study consisted of 11 d on the clinical research unit (Figure 1B). During the inpatient stay, staff did not discuss diet, exercise, or weight loss with the participants. Except for days on which tDCS was received (see tDCS Protocol and Vending Machine Paradigm), participants consumed a weight-maintaining diet (WMD), with calories adjusted to maintain their weight within 1% (23). Physical activity was limited. Because weight loss interventions are commonly examined in conjunction with lifestyle modification (27), on the last day of the inpatient assessment, instructions for reduced energy intake, with 25% fewer calories based on the individual’s calculated WMD, were given by either the principal investigator (MEG) or a study physician (SH, MR).
Outpatient visits
For the next 4 wk, 12 outpatient tDCS sessions (3 sessions/wk, each on a separate day; Figure 1B) took place in accordance with the inpatient tDCS protocol. In line with the inpatient assessment, study staff refrained from discussing diet, exercise, or weight loss with the participants during the outpatient visits. Individuals participated in follow-up assessment from March 2013 to September 2016.
tDCS protocol
tDCS of the left DLPFC was performed on 3 consecutive days (days 8–10) during the inpatient assessment and in 3 sessions/wk (each on a separate day; Figure 1B) for 4 wk during the outpatient visits (i.e., a total of 12 outpatient tDCS sessions). Participants were randomized by sex, in groups of 2–6 subjects, to anodal or sham stimulation (computerized randomization was performed by JK, then results were passed on to one of the study physicians, SH or MR) and were assigned to anodal compared with sham tDCS, respectively, by the study physician. Treatment assignment was blinded except for the physician administering tDCS, who was not involved in scoring any study-related questionnaires or measurements (single-blinded study design). Blinding success was assessed by asking participants whether they thought they had received anodal or sham tDCS after the last inpatient tDCS session and after the last outpatient tDCS session. No differences regarding population characteristics were found between the tDCS subgroups (all P > 0.05; Table 1). tDCS took place in the morning, after awakening, while participants were in a fasted state, following previously described standards (28). In accordance with the 10:20 system for electroencephalography (29), a sponge electrode (width × length = 35 cm2) dampened with 0.9% sodium chloride solution was placed at F3 over the left DLPFC. Computational models provided additional evidence that placing the electrode at F3 would best target the left DLPFC (30). A reference cathode was placed on the right supraorbital region. A constant current of 2 mA was applied for 40 min to participants receiving anodal tDCS (neuroConn DC-STIMULATOR; neuroConn GmbH), whereas the sham group received 10 s of 2-mA stimulation and no tDCS at all for the remaining 39 min 50 s. This montage and the stimulation parameters, informed by computational models of tDCS currents in obesity (30), were selected to optimally engage the target area (left DLPFC). Participants were seated during stimulation and were asked to stay awake and relaxed. To minimize confounders such as food- or eating-related topics, participants watched nature documentaries in a private room accessed only by the study physician.
Food intake–related appetite and mood ratings were assessed before and after tDCS: using a visual analog scale (VAS), participants rated hunger, the urge to eat, fullness, and mood states (i.e., stress, anger, sleepiness, anxiousness). Scores ranged from 0 (“not at all”) to 100 (“extremely”).
Vending machine paradigm
The vending machine (VM) paradigm measures ad libitum food consumption in a highly reproducible manner (31). On day 2 during the inpatient assessment, a food preferences questionnaire was completed by the participants to evaluate hedonic ratings of 80 food items (32). Items given an intermediate rating were placed into the automated VM along with soda, juice, milk, and condiments. A participant’s use of the VM was recorded through the use of individualized codes needed to access the food. On days 8, 9, and 10, after tDCS in the morning, participants had access to the VM for 23 h each day, and ad libitum food intake was assessed (31, 33). All food was consumed in the VM room. Subjects were instructed to return food wrappings and unconsumed, leftover food selected from the VMs in order to correct for noningested nutrients. To reduce confounders interfering with eating behavior, food selection, and food consumption, participants were not allowed to use cell phones or watch television while selecting and eating food. Consumed calories and macronutrients were assessed through the use of the Food Processor nutritional analysis software (version 10.0.0; ESHA Research) (31). For the 3 d of ad libitum intake of food from the VM, daily, mean, and total energy intake (kilocalories) from meals, soda, and condiments, and their respective macronutrient contents (i.e., fat, carbohydrate, protein, in grams), were calculated. Nutrient intake relative to weight maintenance needs was assessed by dividing daily energy intake measurements by the individual WMD (percentage).
Snack food taste test
A snack food taste test (SFTT) was administered within 1 h after lunch on days 5 and 9 during the inpatient assessment and on the last day of the outpatient visits. The SFTT served as an additional measure of ad libitum food intake and thus as an indirect measure of eating behavior. It was also used to evaluate taste preferences (25). VAS ratings for hunger, fullness, and the urge to eat were taken before the test. In addition, VAS ratings of craving, boredom, and stress were assessed before the SFTT, and ratings of taste and liking were obtained during the test. The rating scales were 0 (“not at all”) to 100 (“extremely”).
During the test, participants were exposed to high-calorie snacks (potato chips, peanuts, cookies, and candy) and asked to provide VAS ratings for each type of food [e.g., liking, salty, sweet; scale: 0 (“not at all”) to 100 (“extremely”)]. They were instructed to consume as much food as they desired and were left alone in the room for 10 min. Participants were not told that overall energy intake was being measured. Leftover snacks were weighed to assess snack food intake (grams) and energy intake (kilocalories).
Of the 23 participants who took the outpatient SFTT, 21 had data recorded for energy intake (kilocalories) and snack food intake (grams) (Table 2). Twenty of 23 individuals who completed the outpatient visits had complete VAS ratings (n = 12 receiving sham tDCS; n = 8 receiving anodal tDCS). In both cases, sensitivity analyses showed no differences for the 2 subgroups and the overall study population (inpatient assessment: total n = 29, n = 16 receiving sham tDCS, n = 13 receiving anodal tDCS; outpatient visits: total n = 23, n = 14 receiving sham tDCS, n = 9 receiving anodal tDCS; all P > 0.05) (Figure 1A).
TABLE 2.
Total energy intake and candy consumption during SFTT1
| Total energy intake, kcal |
Candy consumed, g |
|||
| SFTT | Anodal tDCS | Sham tDCS | Anodal tDCS | Sham tDCS |
| 1 | 408 ± 252 (n = 13) | 481 ± 268 (n = 16) | 20.8 ± 20.9 (n = 13) | 22.9 ± 23.5 (n = 16) |
| 2 | 354 ± 416 (n = 13) | 415 ± 320 (n = 16) | 10.2 ± 8.20 (n = 13) | 16.5 ± 21.4 (n = 16) |
| 3 | 226 ± 153 (n = 8) | 577 ± 394 (n = 13) | 7.38 ± 5.34 (n = 8) | 34.2 ± 28.7 (n = 13) |
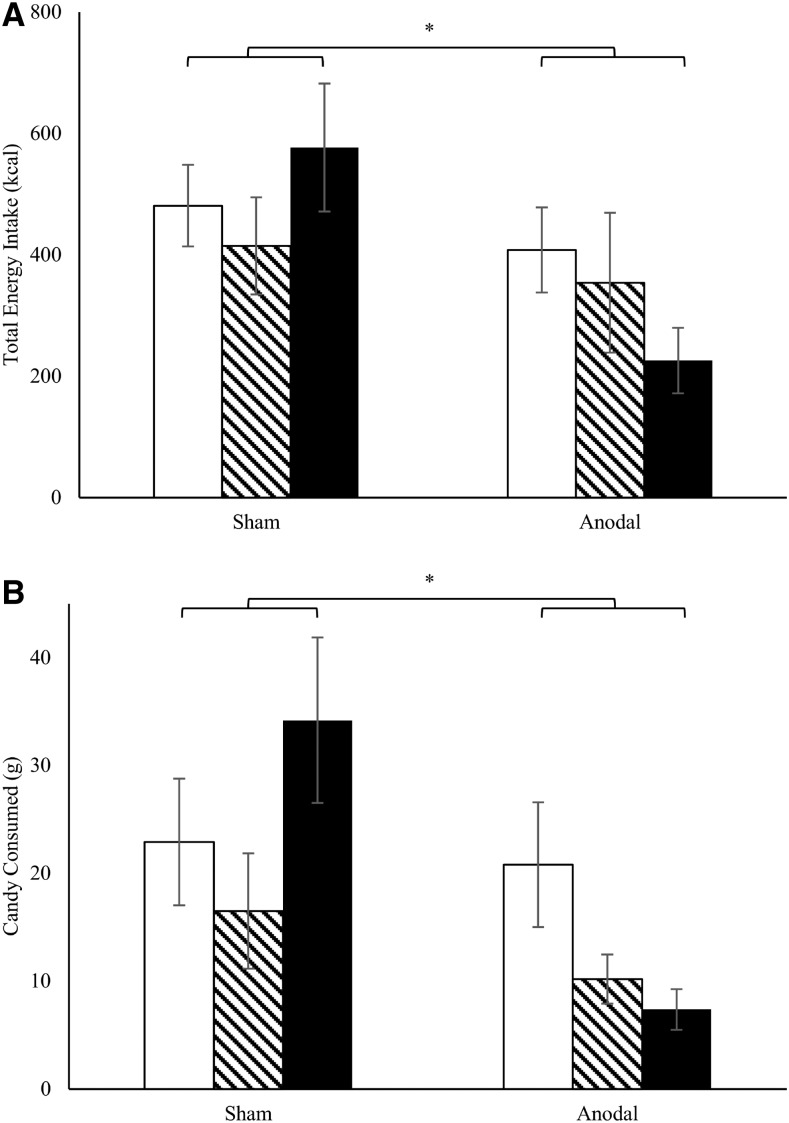
Values are means ± SDs (number of participants). Total energy intake and candy consumption during the SFTTs for baseline assessment (SFTT1, on day 7), on the last day of the inpatient period (SFTT2, on day 11), and on the last day of the study (i.e., the last day of the outpatient visits; SFTT3). Total energy intake (kilocalories) and candy consumed (grams) on SFTT3, relative to SFTT1 and compared with the sham group, were significantly lower in the anodal tDCS group, as assessed with ANCOVA (P = 0.01; β: −376 kcal; 95% CI: 92.0, 661 kcal; df: 1; t value: 2.77; and P = 0.01; β: −28.6 g; 95% CI: 8.33, 48.8 g; df: 1; t value: 2.95, respectively). In a mixed model, the time-by-treatment interaction for total energy intake and for candy consumed is P = 0.05. SFTT, snack food taste test; tDCS, transcranial direct current stimulation.
Behavioral questionnaires
Behavioral questionnaires were administered on days 2 and 11 of the inpatient assessment and on the last day of the outpatient period. Participants completed the Binge Eating Scale developed by Gormally et al. (34) to assess binge-eating behavior and the Three-Factor Eating Questionnaire (35) to measure restraint (i.e., cognitive control of eating), disinhibition (tendency to have an uninhibited response to food), and perceived hunger (susceptibility of eating in response to subjective feelings of hunger).
Assessment of tDCS side effects
Immediately after each tDCS session, a physician examined the participant’s scalp for skin irritation. Side effects were evaluated with a standard questionnaire (19) (Table 3).
TABLE 3.
Assessment of side effects: anodal and sham tDCS1
| Anodal tDCS (n = 9) | Sham tDCS (n = 14) | |
| Headache | ||
| Absent | 8 (88.9%) | 10 (71.4%) |
| Present | 1 (11.1%) | 4 (28.6%) |
| Neck pain | ||
| Absent | 9 (100%) | 13 (92.9%) |
| Present | 0 (0%) | 1 (7.14%) |
| Scalp pain | ||
| Absent | 6 (66.7%) | 9 (64.3%) |
| Present | 3 (33.3%) | 5 (35.7%) |
| Scalp burn | ||
| Absent | 6 (66.7%) | 10 (71.4%) |
| Present | 3 (33.3%) | 4 (28.6%) |
| Tingling | ||
| Absent | 7 (77.8%) | 6 (42.9%) |
| Present | 2 (22.2%) | 8 (57.1%) |
| Skin redness2 | ||
| Absent | 2 (22.2%) | 10 (71.4%) |
| Present | 7 (77.8%) | 4 (28.6%) |
| Sleepiness | ||
| Absent | 6 (66.7%) | 5 (35.7%) |
| Present | 3 (33.3%) | 9 (64.3%) |
| Trouble concentrating | ||
| Absent | 7 (77.8%) | 12 (85.7%) |
| Present | 2 (22.2%) | 2 (14.3%) |
| Mood changes | ||
| Absent | 8 (88.9%) | 11 (78.6%) |
| Present | 1 (11.1%) | 3 (21.4%) |
Absolute and relative (%, in parentheses) numbers of individuals who reported side effects for ≥1 visit, assessed after anodal compared with sham tDCS. Side effects were self-reported through the use of a standard questionnaire (19). Therefore, frequencies reflect the subjective interpretation of a participant. tDCS, transcranial direct current stimulation.
Relative to the group receiving sham stimulation, the group receiving anodal tDCS had a higher prevalence of skin redness (P < 0.05, chi-square test). Only individuals who participated in both the inpatient assessment and outpatient visits were analyzed.
Statistical analyses
Statistical analyses were performed with SAS Enterprise Guide 7.1 (SAS Institute Inc.). Data analyzed were normally distributed and α was set to 0.05. The Student t test was used to discern differences in energy intake. In addition, mixed models were used to test for a longitudinal effect of anodal stimulation on food intake, allowing adjustment for age, sex, and fat-free mass (36). ANCOVA was used to investigate the influence of anodal tDCS on weight change and body composition after adjusting for values measured before randomization. The same model was used to evaluate differences in food intake during the SFTT. VAS scores given during tDCS sessions and before the SFTT were evaluated with the use of repeated-measures mixed models adjusting for age and sex. A chi-square test was used to compare tDCS side effects by group. Results from ANCOVA are reported as estimates with 95% CIs, whereas mixed model results are reported as β coefficients with SEs. Descriptive statistics are reported as means ± SDs. Analyses were performed by original assigned group.
Based on the initial power calculation for this protocol, the recruitment goal was set at 27 individuals/group (a total of 54 participants, assuming equal variances). This was based on the assumption of a mean ± SD ad libitum food intake of 3476 ± 1106 kcal/d (37) and to detect a 25% decrease in food intake (869 kcal/d) in the anodal compared with sham groups at a 2-sided α of 0.05 with a power of 0.80. Because recruitment for the study progressed extremely slowly, we performed an interim data analysis when we had recruited 31 participants in order to determine whether the effect size of the intervention on early measurement of ad libitum food intake, and thus short-term and 4-wk weight loss, warranted continuing the study. Upon determining that the mean ± SD difference in food intake actually observed between the 2 groups (−129 ± 930 kcal/d) was far lower than hypothesized (described in the Results), we calculated that, in order to detect a significant difference in ad libitum food intake at this magnitude, we would require a parallel-design study with 1632 subjects to achieve a power of 0.80 at an α of 0.05. Thus, we stopped the study because of futility and analyzed our primary and secondary hypothesized end points.
RESULTS
Effect of anodal tDCS on measures of energy intake from VMs and weight change
Blinding throughout the study, as assessed after the last inpatient and outpatient tDCS sessions, was successful (P > 0.05, chi-square test).
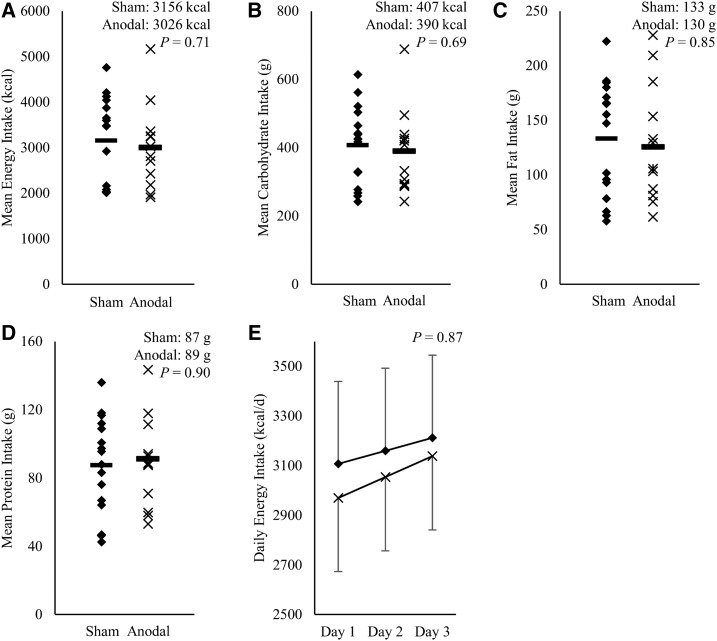
Measures of food intake during the VM paradigm are given in Supplemental Table 1. Ad libitum food intake from meals, soda, or macronutrients during the VM paradigm was not different between the sham and anodal stimulation groups with respect to daily (all P ≥ 0.30), mean (all P ≥ 0.69; Figure 2), and total energy (all P ≥ 0.69) intakes. Daily, mean, and total nutrient intakes relative to weight maintenance needs (all P ≥ 0.39) and daily, mean, and total energy intakes from condiments (all P ≥ 0.19) were also not different between the groups. In a mixed model, anodal tDCS (relative to sham stimulation) had no effect on daily energy intake [P = 0.87; β: 31.6 kcal/d (SE: 203.2 kcal/d); Figure 2E] or energy intake from fat, carbohydrate, protein, or soda (all P ≥ 0.26). Also in this model, energy intake from condiments and nutrient intake relative to weight maintenance needs (all P ≥ 0.72) were not different between the groups.
FIGURE 2.
Effect of anodal compared with sham tDCS on food intake from vending machines. Mean energy intake (A) and mean total macronutrient intake [carbohydrates (B), fat (C), and protein (D), all in grams] for anodal (× n = 13) compared with sham (◆ n = 16) tDCS during a 3-d vending machine paradigm. The Student t test was used to discern differences in energy intake. Mixed models were used to test for a longitudinal effect of anodal stimulation on food intake. For mean energy intake (t value: 0.37), mean total carbohydrate intake (t value: 0.40), mean total fat intake (t value: 0.19), and mean total protein intake (t value: −0.13), no differences between the groups were observed (all P > 0.05; df: 27). Outliers in the anodal group for total mean energy intake (A) and mean total carbohydrate intake (B) were considered in the analyses and did not influence the significance of results. During the vending machine paradigm, no effect of anodal tDCS on mean food intake was observed compared with sham stimulation (all P > 0.05) (A–D). Daily energy intake (kilocalories per day) was determined through an assessment of ad libitum intake during the vending machine paradigm on days 1, 2, and 3 (E), adjusted for fat-free mass, with SEs indicated. No effect of anodal tDCS was observed on food intake from vending machines compared with sham stimulation (P > 0.05, df 56; F value: 0.02). tDCS, transcranial direct current stimulation.
Including only the inpatient period and, in a separate analysis, also the outpatient period, we saw no effect of anodal stimulation of the left DLPFC on weight change relative to the sham group (all P ≥ 0.46). Measurements of fat mass, fat-free mass, body fat, and BMI on the last study day, adjusted for baseline anthropometric measures, were not different between the groups (all P ≥ 0.18).
Effect of anodal tDCS on snack food intake
Comparing the SFTTs before and at the end of the inpatient period, no significant differences occurred in total energy intake (grams consumed from candy, potato chips, cookies, or nuts) between anodal and sham tDCS (all P ≥ 0.27). However, adjusted for baseline snack food consumption before the onset of the tDCS protocol and relative to the sham group, total energy intake during the last (outpatient) SFTT was lower in participants who had received anodal tDCS [P = 0.01; β: −376 kcal (95% CI: 92.0, 661 kcal); df: 1; t value: 2.77] (Table 2, Figure 3). Relative to sham stimulation, the anodal group ate significantly less candy [P = 0.01; β: −28.6 g (95% CI: 8.33, 48.8 g); df: 1; t value: 2.95] (Table 2, Figure 3) and trended toward a lower consumption of nuts [P = 0.11; β: −15.0 g (95% CI: −3.46, 33.4 g); df: 1; t value: 1.70] and cookies [P = 0.18; β: −33.3 g (95% CI: −16.2, 82.7 g); df: 1; t value, 1.41].
FIGURE 3.
Effect of anodal compared with sham tDCS on food intake during SFTT. Total energy intake (kilocalories; A) and candy consumed (grams; B) during SFTT 1 (first test during the baseline assessment; white columns), SFTT 2 (last test during the baseline assessment; striped columns), and SFTT 3 (test on the last day of the study during the outpatient visits; black columns). For baseline assessment (11 d), anodal and sham tDCS was performed in 13 and 16 subjects, respectively. Snack food intake could be analyzed in 8 and 13 individuals in the anodal and sham tDCS groups, respectively, during the outpatient assessment (4 consecutive weeks). SFTT1 and SFTT3 were compared and, relative to those who received sham tDCS, individuals who received anodal stimulation had significantly lower total energy intake (df: 1; t value: 2.77) and ate less candy (df: 1; t value: 2.95), as assessed with ANCOVA; this indicates an effect of anodal (compared with sham) tDCS on energy intake and thus indirectly on eating behavior in this setting. In a mixed model, time-by-treatment interaction for total energy intake and for candy consumed is P = 0.05. SFTT, snack food taste test; tDCS, transcranial direct current stimulation. *P < 0.05.
Effect of anodal tDCS on appetite and mood ratings
VAS ratings before and after tDCS
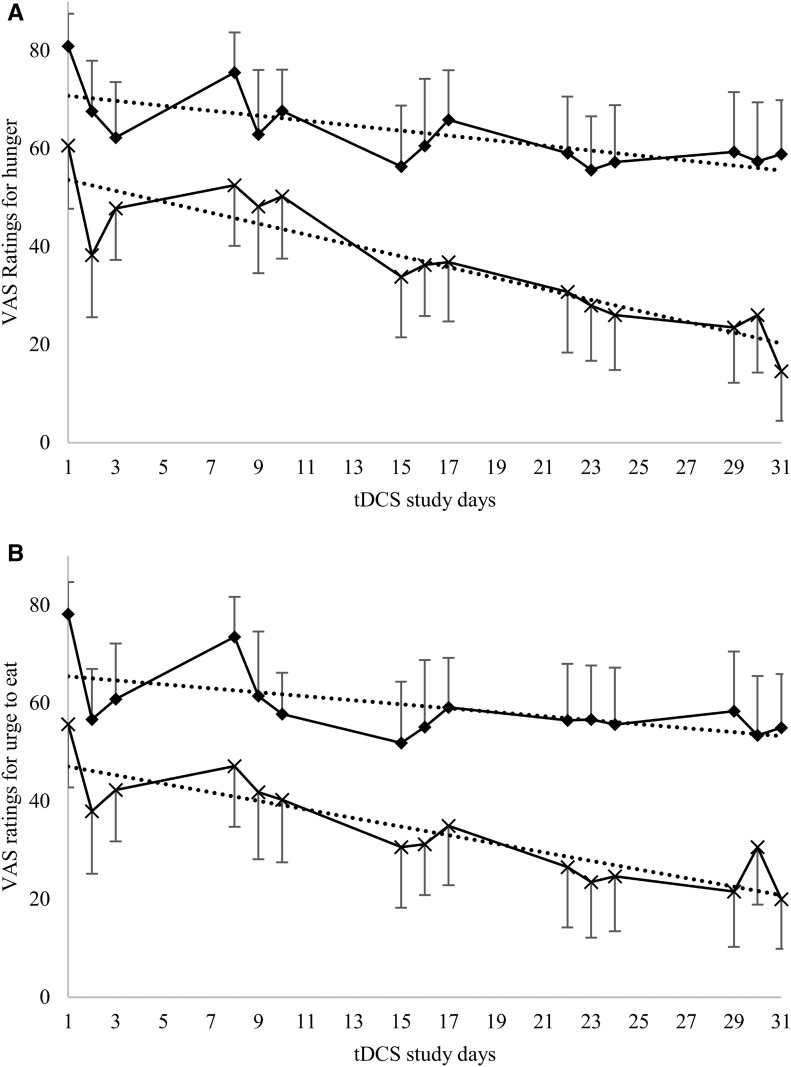
VAS ratings for hunger, the urge to eat, fullness, and mood assessed before and after tDCS were not different between the sham and anodal stimulation groups during the inpatient assessment (all P > 0.05). Including all outpatient tDCS sessions, relative to the sham group and adjusted for age and sex, individuals who received anodal stimulation showed a greater daily reduction in VAS ratings for hunger [β: −0.61 VAS score/d (SE: 0.22 VAS score/d); df: 610; t value: 2.69] (Figure 4A) and urge to eat [β: −0.46 VAS score/d (SE: 0.23 VAS score/d); df: 610; t value: 1.96] (Figure 4B), as indicated by the significant difference in the slope for these ratings comparing both groups over time (P = 0.01 and P = 0.05, respectively).
FIGURE 4.
Effect of anodal compared with sham tDCS on VAS ratings during tDCS sessions. VAS ratings of hunger (A) and the urge to eat (B) during the inpatient assessment (tDCS study days 1–3) and outpatient visits (tDCS study days 8–31), comparing anodal (×) and sham (◆) stimulation, relative to study days after the first stimulation session. Means of ratings before and after tDCS are shown for all individuals within 1 study group. A total of 29 individuals completed the inpatient assessment (anodal tDCS, n = 13; sham tDCS, n = 16), and 23 completed the outpatient assessment (anodal tDCS, n = 9; sham tDCS, n = 14). Relative to the sham group, individuals receiving anodal tDCS had a greater decrease in VAS ratings for hunger and the urge to eat (P = 0.01; β: −0.61 VAS score/d; SE: 0.22 VAS score/d; df: 610; t value: 2.69; and P = 0.05; β: −0.46 VAS score/d; SE: 0.23 VAS score/d; df: 610; t value: 1.96, respectively) in a mixed model adjusted for age and sex, as indicated by the significant difference in the slope for these ratings comparing both groups over time (P = 0.01 and P = 0.05, respectively). Dotted lines represent trendlines for the decrease in ratings. For hunger ratings (A), the slopes of the trendlines for sham compared with anodal tDCS are −0.51 (P = 0.003) and −1.11 (P < 0.0001), respectively. VAS ratings for the urge to eat (B) display a steeper decrease in the anodal group (slope = −0.87; P < 0.0001) compared with the sham group (slope = −0.41; P = 0.02). Absolute VAS ratings of hunger and the urge to eat were consistently lower in the anodal group, including those ratings given before the first tDCS session. Because no identifiable baseline differences were found in the anodal and sham groups (all P > 0.05), consistently lower VAS ratings of hunger and the urge to eat in the anodal compared with the sham group is likely because of the relatively small study cohort. Of note, however, assessment of the greater declines in hunger and the urge to eat in mixed models accounted for the baseline ratings. tDCS, transcranial direct current stimulation; VAS, visual analog scale.
VAS ratings and the SFTTs
During the inpatient assessment period, after adjusting for baseline ratings, VAS ratings for hunger, fullness, the urge to eat, craving, boredom, and stress before the second SFTT, as well as taste and liking of snacks, did not differ between the anodal and sham tDCS groups (all P > 0.05). After the outpatient SFTT and compared with the first SFTT, subjects receiving anodal tDCS reported lower VAS ratings for taste perception of the saltiness of chips [P = 0.02; β: −28 VAS score (95% CI: 5, 52 VAS score)] and the sweetness of cookies [P = 0.01; β: −36 VAS score (95% CI: 12, 60 VAS score)]. Appetite-related VAS ratings before the second SFTT during the inpatient assessment were not different between groups (all P > 0.05). VAS ratings before the outpatient SFTT, when relatively lower snack food intake was observed, were as follows: In the anodal and sham stimulation groups, respectively, individuals expressed little hunger [13 VAS score (95% CI: −2, 27 VAS score) and 12 VAS score (95% CI: 2, 23 VAS score), respectively], a low desire to eat [13 VAS score (95% CI: −0.5, 27 VAS score) and 24 VAS score (95% CI: 10, 38 VAS score), respectively], and low craving [8 VAS score (95% CI: −6, 23 VAS score) and 14 VAS score (95% CI: 3, 24 VAS score), respectively], as well as high to moderate levels of fullness [55 VAS score (95% CI: 34, 76 VAS score) and 69 VAS score (95% CI: 3, 24 VAS score), respectively]. These scores did not differ significantly between the 2 groups (all P > 0.05).
Effect of anodal tDCS on ratings from behavioral questionnaires and side effect assessment
No differences were found between the anodal and sham stimulation groups on the Binge Eating Scale or the Three-Factor Eating Questionnaire at any time points (all P > 0.05). Participants receiving anodal stimulation had a relatively higher prevalence of skin redness than those receiving sham tDCS (P = 0.02). No differences were seen for other side effects (Table 3).
DISCUSSION
In a randomized parallel-design study of individuals with obesity, we investigated the effect of anodal and sham tDCS of the left DLPFC on 1) early measurement of ad libitum energy intake through the use of a 3-d VM paradigm and early and later measurements of ad libitum energy intake through the use of SFTTs after meals, 2) changes in body weight as an effect of tDCS on energy intake, and 3) appetite-related ratings. Comparing study groups, energy intake during the period of ad libitum intake of food from VMs and body weight after the inpatient or outpatient intervention were not different. Ratings for hunger and the urge to eat declined significantly more in the anodal tDCS group than in the sham tDCS group over the extended study period. Consistent with an overall decrease in these ratings, relative to baseline snack food intake, participants receiving anodal tDCS consumed fewer kilocalories during their outpatient SFTT.
Relatively lower activity of the left DLPFC has been attributed to feelings of satiety and the urge to eat, promoting behaviors that favor weight gain (5, 8, 17–19, 38). In line with reports of lower left DLPFC activity as a potentially acquired feature of obesity (5, 8, 38), we previously observed a decrease of ad libitum energy intake and greater weight change after short-term (3 sessions) stimulatory anodal tDCS to the left DLPFC in 8 individuals with obesity relative to inhibitory cathodal tDCS (7, 14). To date, only a few small studies including a small number of subjects have investigated the effect of repeated tDCS to the DLPFC on food intake and measures of eating behavior (39). Following up on our previous study, which used a crossover design and demonstrated an effect of tDCS of the left DLPFC on food intake, we present here, to our knowledge, the first randomized parallel-design study in a larger cohort of subjects with obesity undergoing inpatient assessment and prolonged outpatient follow-up. Our study included detailed assessment of ad libitum food intake and appetite-related ratings and attempted to further define tDCS as an intervention technique for obesity. We administered a total of 15 tDCS sessions, each lasting 40 min, which is longer than previously reported durations (7, 15–19). We did not find an effect of anodal tDCS on ad libitum food intake with the use of the VM paradigm, indicating that proposed activation of the left DLPFC area did not influence eating behavior early (after only 3 d of treatment). Given the lack of a more immediate effect on energy intake, we were not surprised that we did not demonstrate an influence on weight during the inpatient or outpatient assessment. In comparison to our previous study evaluating stimulatory and inhibitory tDCS (7, 14), it is possible that the opposing effect of these stimulation modes on the left DLPFC led to a divergence in energy intake. Thus, compared with sham tDCS, short-term or medium-term anodal tDCS of the left DLPFC might not sufficiently modulate neuronal excitability, leading to a lack of influence on ad libitum energy intake or weight change within the time frame of our study. Yet this study provides evidence for a consistent decrease in ratings of hunger and the urge to eat and, after 6 wk of repeated stimulations, a reduction in energy intake during the SFTT in the anodal compared with the sham group. This indicates that repeated, long-term anodal stimulation of the left DLPFC (relative to sham tDCS) is needed to demonstrate an effect on ad libitum food intake.
Hunger and the urge to eat are eating-related determinants of nonhomeostatic eating behavior in individuals with obesity (17–19). The relative decrease in hunger and the urge to eat with anodal tDCS of the left DLPFC are consistent with the role of the DLPFC in central regulatory control and the differential activity patterns found after meals in obese compared with nonobese individuals (5, 6, 17–19, 40). Activation of this area is thought to be involved in the integration of taste perception into cognitive executive control, possibly modifying behavior (40). The ability to control perceptions of hunger and the urge to eat after tasting food (particularly snack food items) might be advantageous to supporting or maintaining weight loss (5, 17–19, 40) and may indicate a role for longer or more intensive (such as daily) tDCS in this regard.
The reason for the decline in ad libitum food intake during the outpatient SFTT could be interpreted as a result of the generally lower overall perception of hunger and the reduced urge to eat. VAS ratings obtained immediately before the final SFTT (within 1 h of lunch) indicated that study participants were satiated to an equal degree in the sham and anodal stimulation groups. Thus another possible effect of anodal tDCS may be on eating in the absence of hunger (EAH), a disinhibited eating behavior that promotes obesity (41) and is closely linked to central reward mechanisms (42). Considering the implication of lower activity of the left DLPFC after a meal in obesity (5, 8, 38) and of central inhibition in excess nutrient uptake (42), it is possible that stimulation of the left DLPFC could affect EAH. Because food cues may deactivate homeostatic satiety mechanisms, leading to EAH (42, 43), the after-meal SFTT seems to be useful in measuring this overeating behavior (26).
Of note, no consensus exists on which neuromodulation technique (transcranial magnetic stimulation or tDCS) to use or the frequency or intensity of administration necessary to influence behavioral mechanisms driving food intake (17, 39). Our study addresses this by investigating the effect of multiple sessions of tDCS to modify eating behavior (39). Compared with our previous trial, we kept the stimulation frequency consistent (i.e., 3 sessions/wk during the inpatient and outpatient portions of the study), allowing for comparability between excitatory only and combined excitatory and inhibitory tDCS modes. In the inpatient setting, tDCS was performed each day that participants ate food obtained through the validated VM paradigm (31).
Imbalance between a central “go” system, involving the DLPFC, and the opposed “stop” system (ventromedial PFC) may favor compulsive eating behavior (44). It was suggested that tDCS of the DLPFC affects the interplay between these systems (17, 45). Therefore, studies investigating the implication of the DLPFC in eating-related behavior could benefit from a parallel assessment of ventromedial PFC activity.
As previously reported (46, 47), no serious adverse effects occurred as a result of tDCS in our study. However, other adverse effects were reported (some frequently), although equally so in each group. It is unclear to what extent these effects may have influenced outcome measures. Limitations of our study include that it was stopped because of futility; interim analyses demonstrated no early effect of tDCS. Although we previously detected tDCS-induced weight loss after only 1 wk when comparing anodal and cathodal stimulation (7), the current results indicate a lack of an early effect and a need for longer-term studies to investigate whether tDCS-targeting of brain areas governing food intake can lead to weight change. Participant dropout, leading to fewer follow-up data for outpatient visits, might have affected the detection of differences in study outcomes when comparing anodal and sham tDCS. Notably, where possible, analysis methods were used to account for any missing data until the last follow-up visit (e.g., mixed models for analysis of tDCS VAS ratings). Furthermore, we did not use other measures of food intake (e.g., food diaries) in the outpatient setting or assess activity of the DLPFC with neuroimaging.
In conclusion, in individuals with obesity and relative to sham treatment, repeated anodal tDCS of the left DLPFC reduced ad libitum snack food consumption in satiated individuals and coincided with decreases in ratings of hunger and the urge to eat. Ad libitum energy intake was not reduced through the use of a VM paradigm, reflecting no early effect of anodal tDCS on eating behavior in this setting; accordingly, no differences in weight change were detected. Nevertheless, we have confirmed that repeated sessions of tDCS to the left DLPFC reduced food intake in a manner consistent with a reduction of EAH in subjects with obesity. This observation indicates an important effect on inhibitory control, but one that may require repeated, intensive, and longer-term treatment to result in weight loss.
Acknowledgments
The authors’ responsibilities were as follows—SH: conducted the research, analyzed the data, and wrote the manuscript; MR, CMW, CV, and ED: conducted the research; PP and EJS: analyzed the data; MA-A and EMW: provided essential help with the project conception; SBV: provided significant advice and consultation on the food intake measurements used in the study; MEG and JK: designed the research; MEG: oversaw the study and had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: DLPFC, dorsolateral prefrontal cortex; EAH, eating in the absence of hunger; PFC, prefrontal cortex; SFTT, snack food taste test; tDCS, transcranial direct current stimulation; VAS, visual analog scale; VM, vending machine; WMD, weight-maintaining diet.
REFERENCES
- 1.Stoeckel LE, Birch LL, Heatherton T, Mann T, Hunter C, Czajkowski S, Onken L, Berger PK, Savage CR. Psychological and neural contributions to appetite self‐regulation. Obesity (Silver Spring) 2017;25(Suppl 1)S17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall PA, Marteau TM. Executive function in the context of chronic disease prevention: theory, research and practice. Prev Med 2014;68:44–50. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Papies EK, Barsalou LW. A core eating network and its modulations underlie diverse eating phenomena. Brain Cogn 2016;110:20–42. [DOI] [PubMed] [Google Scholar]
- 4.Hall PA, Lowe C, Vincent C. Executive control resources and snack food consumption in the presence of restraining versus facilitating cues. J Behav Med 2014;37:587–94. [DOI] [PubMed] [Google Scholar]
- 5.Le DSN, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, Krakoff J. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr 2006;84:725–31. [DOI] [PubMed] [Google Scholar]
- 6.Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision-making in the human brain. Nature 2004;431:859–62. [DOI] [PubMed] [Google Scholar]
- 7.Gluck ME, Alonso‐Alonso M, Piaggi P, Weise CM, Jumpertz‐von Schwartzenberg R, Reinhardt M, Wassermann EM, Venti CA, Votruba SB, Krakoff J. Neuromodulation targeted to the prefrontal cortex induces changes in energy intake and weight loss in obesity. Obesity (Silver Spring) 2015;23:2149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DSN, Pannacciulli N, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Krakoff J. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am J Clin Nutr 2007;86:573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weygandt M, Mai K, Dommes E, Ritter K, Leupelt V, Spranger J, Haynes J-D. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage 2015;109:318–27. [DOI] [PubMed] [Google Scholar]
- 10.Wassermann EM, Grafman J. Recharging cognition with DC brain polarization. Trends Cogn Sci 2005;9:503–5. [DOI] [PubMed] [Google Scholar]
- 11.Morrell F. Effect of anodal polarization on the firing pattern of single cortical cells. Ann N Y Acad Sci 1961;92:860–76. [DOI] [PubMed] [Google Scholar]
- 12.Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol 1964;18:166–85. [DOI] [PubMed] [Google Scholar]
- 13.Bolzoni F, Pettersson LG, Jankowska E. Evidence for long-lasting subcortical facilitation by transcranial direct current stimulation in the cat. J Physiol 2013;591:3381–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wassermann E, Epstein C, Ziemann U, Walsh V, Paus T, Lisanby SH, editors. Oxford handbook of transcranial stimulation. Oxford (United Kingdom): Oxford University Press; 2008. [Google Scholar]
- 15.Jauch-Chara K, Kistenmacher A, Herzog N, Schwarz M, Schweiger U, Oltmanns KM. Repetitive electric brain stimulation reduces food intake in humans. Am J Clin Nutr 2014;100:1003–9. [DOI] [PubMed] [Google Scholar]
- 16.Lapenta OM, Di Sierve K, de Macedo EC, Fregni F, Boggio PS. Transcranial direct current stimulation modulates ERP-indexed inhibitory control and reduces food consumption. Appetite 2014;83:42–8. [DOI] [PubMed] [Google Scholar]
- 17.Kekic M, McClelland J, Campbell I, Nestler S, Rubia K, David AS, Schmidt U. The effects of prefrontal cortex transcranial direct current stimulation (tDCS) on food craving and temporal discounting in women with frequent food cravings. Appetite 2014;78:55–62. [DOI] [PubMed] [Google Scholar]
- 18.Goldman RL, Borckardt JJ, Frohman HA, O’Neil PM, Madan A, Campbell LK, Budak A, George MS. Prefrontal cortex transcranial direct current stimulation (tDCS) temporarily reduces food cravings and increases the self-reported ability to resist food in adults with frequent food craving. Appetite 2011;56:741–6. [DOI] [PubMed] [Google Scholar]
- 19.Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FA, Nitsche MA, Mecca T, Macedo EC, Pascual-Leone A, Boggio PS. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite 2008;51:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel L, Alonso-Alonso M, Audette M, Malbert C-H, Stice E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin 2015;8:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis I disorders. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 22.Genuth S, Alberti K, Bennett P, Buse J, DeFronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–7. [DOI] [PubMed] [Google Scholar]
- 23.Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr 1991;53:1368–71. [DOI] [PubMed] [Google Scholar]
- 24.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 1990;51:1106–12. [DOI] [PubMed] [Google Scholar]
- 25.Houben K. Overcoming the urge to splurge: Influencing eating behavior by manipulating inhibitory control. J Behav Ther Exp Psychiatry 2011;42:384–8. [DOI] [PubMed] [Google Scholar]
- 26.Shomaker LB, Tanofsky‐Kraff M, Mooreville M, Reina SA, Courville AB, Field SE, Matheson BE, Brady SM, Yanovski SZ, Yanovski JA. Links of adolescent‐and parent‐reported eating in the absence of hunger with observed eating in the absence of hunger. Obesity (Silver Spring) 2013;21:1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers M, Day WW, Bowden CH. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr 2012;95:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 2005;64:872–5. [DOI] [PubMed] [Google Scholar]
- 29.Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr Clin Neurophysiol 1957;10:370–5. [Google Scholar]
- 30.Truong DQ, Magerowski G, Blackburn GL, Bikson M, Alonso-Alonso M. Computational modeling of transcranial direct current stimulation (tDCS) in obesity: impact of head fat and dose guidelines. Neuroimage Clin 2013;2:759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venti CA, Votruba SB, Franks PW, Krakoff J, Salbe AD. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. Am J Clin Nutr 2010;91:343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geiselman PJ, Anderson AM, Dowdy ML, West DB, Redmann SM, Smith SR. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiol Behav 1998;63:919–28. [DOI] [PubMed] [Google Scholar]
- 33.Rising R, Alger S, Boyce V, Seagle H, Ferraro R, Fontvieille AM, Ravussin E. Food intake measured by an automated food-selection system: relationship to energy expenditure. Am J Clin Nutr 1992;55:343–9. [DOI] [PubMed] [Google Scholar]
- 34.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav 1982;7:47–55. [DOI] [PubMed] [Google Scholar]
- 35.Geliebter A, Aversa A. Emotional eating in overweight, normal weight, and underweight individuals. Eat Behav 2003;3:341–7. [DOI] [PubMed] [Google Scholar]
- 36.Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher daily energy expenditure and respiratory quotient, rather than fat-free mass, independently determine greater ad libitum overeating. J Clin Endocrinol Metab 2015;100:3011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell JE, Gosnell BA, Roerig JL, Zwaan M, Wonderlich SA, Crosby RD, Burgard MA, Wambach BN. Effects of sibutramine on binge eating, hunger, and fullness in a laboratory human feeding paradigm. Obes Res 2003;11:599–602. [DOI] [PubMed] [Google Scholar]
- 38.Le DSNT, Chen K, Pannacciulli N, Gluck M, Reiman EM, Krakoff J. Reanalysis of the obesity-related attenuation in the left dorsolateral prefrontal cortex response to a satiating meal using gyral regions-of-interest. J Am Coll Nutr 2009;28:667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowe CJ, Vincent C, Hall PA. Effects of noninvasive brain stimulation on food cravings and consumption: a meta-analytic review. Psychosom Med 2017;79:2–13. [DOI] [PubMed] [Google Scholar]
- 40.Kringelbach ML, de Araujo IE, Rolls ET. Taste-related activity in the human dorsolateral prefrontal cortex. Neuroimage 2004;21:781–8. [DOI] [PubMed] [Google Scholar]
- 41.Kral TV, Faith MS. Influences on child eating and weight development from a behavioral genetics perspective. J Pediatr Psychol 2009;34:596–605. [DOI] [PubMed] [Google Scholar]
- 42.Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity (Silver Spring) 2011;19:2175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng H, Lenard N, Shin A, Berthoud H-R. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33(Suppl 2):S8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore CF, Sabino V, Koob GF, Cottone P. Pathological overeating: emerging evidence for a compulsivity construct. Neuropsychopharmacology 2017;42:1375–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hare TA, Hakimi S, Rangel A. Activity in dlPFC and its effective connectivity to vmPFC are associated with temporal discounting. Front Neurosci 2014;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lefaucheur J-P, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 2017;128:56–92. [DOI] [PubMed] [Google Scholar]
- 47.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, Mourdoukoutas AP, Kronberg G, Truong D, Boggio P. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul 2016;9:641–61. [DOI] [PMC free article] [PubMed] [Google Scholar]