Abstract
Background: Little is known about the relation between dietary intake and cerebral amyloid accumulation in aging.
Objective: We assessed the association of dietary glycemic measures with cerebral amyloid burden and cognitive performance in cognitively normal older adults.
Design: We performed cross-sectional analyses relating dietary glycemic measures [adherence to a high-glycemic-load diet (HGLDiet) pattern, intakes of sugar and carbohydrates, and glycemic load] with cerebral amyloid burden (measured by florbetapir F-18 positron emission tomography) and cognitive performance in 128 cognitively normal older adults who provided eligibility screening data for the University of Kansas’s Alzheimer’s Prevention through Exercise (APEX) Study. The study began in November 2013 and is currently ongoing.
Results: Amyloid was elevated in 26% (n = 33) of participants. HGLDiet pattern adherence (P = 0.01), sugar intake (P = 0.03), and carbohydrate intake (P = 0.05) were significantly higher in participants with elevated amyloid burden. The HGLDiet pattern was positively associated with amyloid burden both globally and in all regions of interest independently of age, sex, and education (all P ≤ 0.001). Individual dietary glycemic measures (sugar intake, carbohydrate intake, and glycemic load) were also positively associated with global amyloid load and nearly all regions of interest independently of age, sex, and educational level (P ≤ 0.05). Cognitive performance was associated only with daily sugar intake, with higher sugar consumption associated with poorer global cognitive performance (global composite measure and Mini-Mental State Examination) and performance on subtests of Digit Symbol, Trail Making Test B, and Block Design, controlling for age, sex, and education.
Conclusion: A high-glycemic diet was associated with greater cerebral amyloid burden, which suggests diet as a potential modifiable behavior for cerebral amyloid accumulation and subsequent Alzheimer disease risk. This trial was registered at clinicaltrials.gov as NCT02000583.
Keywords: Alzheimer disease, amyloid, PET imaging, carbohydrate, glycemic load, principal components analysis
INTRODUCTION
Alzheimer disease (AD), the most common form of dementia affecting >1 in 8 Americans aged >65 y (1), is marked by accumulation of amyloid-β plaque deposits in the brain. Molecular imaging techniques with the use of positron emission tomography (PET) with an amyloid-specific ligand allow for the detection of cerebral amyloid in those with AD (2). This technique is increasingly used as a research tool to detect the molecular pathology of AD in cognitively unimpaired individuals, of whom 20–40% have evidence of cerebral amyloid deposits and are thus considered to be at higher risk of developing AD (3). These individuals are the target of interventional trials to prevent or delay the onset of AD, with an increasing interest in lifestyle behaviors, including dietary approaches (4).
Current studies show that impaired glucose metabolism and peripheral hyperglycemia are associated with a higher risk of developing AD. For instance, individuals with type 2 diabetes (5, 6) and elevated blood glucose (7) are at higher risk of dementia and experience more rapid progression from mild cognitive impairment (MCI) to AD (8). Peripheral hyperglycemia and insulin resistance are also associated with low glucose metabolism in the brain, as evidenced by PET metabolism imaging studies (9–12), whereas cerebral hypometabolism itself has been found to correlate with cerebral amyloid deposition (13). Furthermore, a recent study showed that impaired fasting glucose was associated with increased regional cerebral amyloid burden in a cohort of cognitively normal adults (14).
Dietary factors are strongly linked to glucose metabolism. Postprandial glycemia and insulin secretion are highly affected by the amount and type of carbohydrate consumed in the diet (15). Dietary intake of high-glycemic-load foods, which can be characterized by a high intake of processed carbohydrate and sugar, elicits sharp increases in peripheral glucose and insulin secretion. Diets high in glycemic load have been strongly linked to impaired glucose metabolism and an increased risk of type 2 diabetes (16), which may also implicate diet as a modifiable behavioral factor that affects amyloid aggregation. The assessment of the association between dietary components and patterns known to affect glucose metabolism and Aβ deposition could help further elucidate their relation with AD risk.
Given the well-known relation between high dietary glycemic intake and glucose metabolism (16) and existing evidence that glucose metabolism may be related to cerebral amyloid (14), we examined the cross-sectional relation between dietary glycemic measures and cerebral amyloid in cognitively unimpaired older adults. A food-frequency questionnaire was used to identify dietary intakes of 4 indicators known to affect glycemia—high-glycemic-load diet pattern (HGLDiet) adherence and estimates of daily carbohydrate intake, sugar intake, and glycemic load—in individuals who had amyloid PET scans that measured global and regional cerebral amyloid deposition.
METHODS
Study design
We examined cross-sectional data collected from individuals screened for the University of Kansas’s Alzheimer’s Prevention through Exercise (APEX) trial to assess the association of dietary glycemic measures with regional cerebral amyloid burden. The APEX study is a randomized trial examining the effects of aerobic exercise on AD biomarkers (amyloid burden and MRI volumetrics) and cognitive decline in cognitively normal older adults aged ≥65 y (www.clinicaltrials.gov; NCT02000583) conducted at the University of Kansas Alzheimer’s Disease Center. All of the results presented are ancillary analyses of data collected at APEX eligibility screening visits before the initiation of the clinical trial. Informed consent was obtained before the collection of data at these visits. Dietary data were collected at eligibility screening visits for observational research purposes.
Participants
Data were available for 128 cognitively normal, sedentary participants aged ≥65 y who were screened for the APEX study. Participants were assessed by a trained clinician and performed a battery of cognitive tests (17) to exclude the presence of MCI or dementia syndromes. The trained clinician interviewed the participant and his or her study partner (usually a spouse or child) to assess for evidence of clinical decline and to complete a Clinical Dementia Rating (18). Clinical and cognitive data were reviewed at a consensus diagnosis conference. Eligible participants had a Clinical Dementia Rating score of 0 (no dementia) and also were without clinically meaningful deficits in their cognitive test performance. Participants were sedentary or underactive based on the Telephone Assessment of Physical Activity (19) (score of ≤4) and willing to participate in a 52-wk exercise intervention. Exclusion criteria for the APEX study included clinically meaningful depression or anxiety, insulin-dependent diabetes, uncontrolled hypertension, or recent history of major neuropsychiatric, musculoskeletal, or cardiorespiratory impairment in the past 2 y. The study protocol was approved by the Institutional Review Board at the University of Kansas Medical Center. Informed consent was obtained from all study participants according to institutional guidelines.
Dietary intake assessment
At the same time point as amyloid PET scans and neuropsychological testing, usual dietary intake was assessed by using the Web-based National Cancer Institute Diet History Questionnaire (DHQ) II (20), a semiquantitative food-frequency questionnaire with 134 food items. Participants were asked to report the average frequency of consumption and portion of food items for the previous year. Nutrient data were quantified by using the NCI Diet*Calc software (21).
Dietary glycemic measures
We examined 4 dietary glycemic measures derived from the DHQ II: daily intakes of carbohydrate and sugar, glycemic load, and adherence to an HGLDiet pattern. Three of these measures—daily intakes of carbohydrate and sugar, and glycemic load—represent output variables from the DHQ II, whereas the HGLDiet pattern and the pattern’s individual adherence scores were identified empirically via principal components analysis (PCA).
DHQ II output of the food variables in the My Pyramid Equivalents Database 2.0 (MPED 2.0) (22), which includes 32 food groups derived from 7752 different foods, was included in the PCA analysis and rotated with the varimax rotation method. Factor loading scores were computed for each individual food group, where higher factor loading values represent a stronger contribution to a specific dietary pattern. The first rotated factor from the PCA explained 14% of the total data variance and was kept for analysis. To better understand and name this dietary pattern, we analyzed the correlation of the dietary pattern with individual dietary intake components by using Pearson correlation coefficients. The pattern was most strongly correlated with glycemic load (r2 = 0.58, P < 0.001), carbohydrate intake (r2 = 0.53, P < 0.001), and sugar intake (r2 = 0.27, P < 0.001) and loaded highly on food groups associated with high glycemic load; therefore, it was named the HGLDiet pattern. We then calculated individual factor regression scores representing a quantitative value of how closely each participant’s dietary intake resembled the HGLDiet pattern. Higher scores indicate greater adherence to the HGLDiet pattern, whereas low and negative scores indicate the inverse.
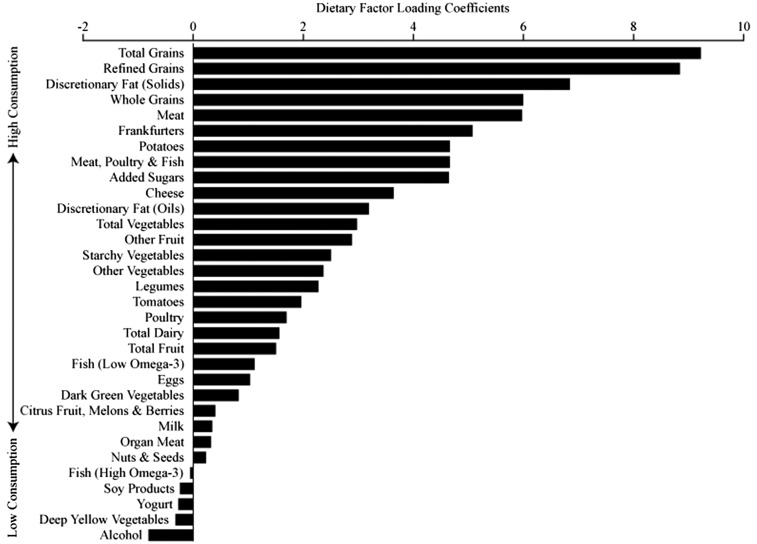
Rotated factor loadings for individual food groups and the HGLDiet pattern are shown in Figure 1. The HGLDiet pattern was characterized by intakes of high-glycemic-load foods, such as total grains, refined grains, potatoes, starchy vegetables, and added sugars. Whole grains, lean and fatty meat, and discretionary fats also loaded positively on this dietary pattern.
FIGURE 1.
Rotated factor loadings for the HGLDiet pattern. Greater intakes of foods with high-positive-loading coefficients resulted in higher HGLDiet pattern adherence scores (n = 128). Greater intakes of foods with low- or negative-loading coefficients resulted in low/negative HGLDiet pattern adherence scores. HGLDiet, high-glycemic-load diet.
Anthropometric assessments
Body weight and height were measured for all participants. Body weight was measured with a calibrated scale (±0.1 kg). Height was measured with a wall-mounted stadiometer. BMI (kg/m2) was calculated by using weight and height measurements.
Assessment of cerebral amyloid burden
PET images were obtained on a GE Discovery ST-16PET/computed tomography scanner after administration of intravenous florbetapir F-18 (370 MBq). Two PET brain frames of 5 min in duration were acquired continuously ∼50 min postinjection. Frames were then summed and attenuation corrected before interpretation. MIMneuro software (MiM Software Inc.) (14, 23, 24) quantitatively normalized the Aβ PET image to the entire cerebellum to calculate the standard uptake value ratio (SUVR; a marker of amyloid accumulation), with the use of a proprietary algorithm, for 6 regions of interest (ROIs): anterior cingulate, posterior cingulate, precuneus, inferior medial frontal, lateral temporal, and superior parietal cortex. The global SUVR was calculated by finding the mean of the SUVR values from all 6 ROIs. Three trained raters reviewed the visual images, the quantitative SUVR ROI data, and MIMneuro-generated cortical projections of amyloid burden [z scores comparing the SUVRs with an SUVR map of 74 individuals (48 men, 26 women) between the ages of 18 and 50 y] to assess the scans as “elevated” or “nonelevated.” The final determination of elevated or nonelevated was determined by majority (i.e., ≥2 raters in agreement).
Neuropsychological testing
All of the participants completed a standard battery of neuropsychological tests that included the Mini-Mental State Examination (MMSE), the Wechsler Adult Intelligence Scale–Revised (WAIS-R) Digit Symbol, Trail Making Test A, Trail Making Test B, Category Fluency (animals and vegetables), the Stroop Color-Word Interference Test, the WAIS-R Block Design, and the total free recall score from the Free and Cued Selective Reminding Test (17). Each neuropsychometric test score was converted to a z score by subtracting the study population mean from individual test scores and dividing by the study population SD. Higher scores on the Trail Making Test A and Trail Making Test B indicate greater cognitive impairment; thus, z scores for these tests were multiplied by −1 so that negative scores reflect greater impairment of cognition. Global cognition z scores were established by calculating the mean of the combined cognitive z scores.
Statistical analyses
The primary focus of this study was to investigate the relation between global cerebral amyloid burden (SUVR) and dietary glycemic measures: HGLDiet pattern adherence scores, carbohydrate intake, sugar intake, and glycemic load. The relations of intakes of the other macronutrients, as well as fat and sugar type, with amyloid burden were also considered and unrelated. We also investigated the relations of regional cerebral amyloid burden and cognitive performance with the dietary glycemic measures. Continuous variables were expressed as mean daily intakes ± SDs. Differences between elevated and nonelevated groups were assessed by using 1-factor ANCOVA including age and sex as covariates. Linear regression models were used to investigate the relations of the dietary measures with regional cerebral amyloid burden (SUVR) and cognitive performance. For all of the dependent variables, residual analyses were performed to assess applicability of linear regression and normality was assessed by using the Shapiro-Wilk test for normality. Dependent data with non-normally distributed residuals were log-transformed. Regression analyses were controlled for age, sex, and education. Energy intake, BMI, and oral diabetes medications were considered as regression covariates but had no effect on statistical outcomes. Statistical analyses were performed by using R (version 3.3.2; R Foundation). Significance was set at P < 0.05.
RESULTS
Data from 128 cognitively normal participants ranging in age from 65 to 90 y (mean ± SD: 71.3 ± 5.1 y) were included. Amyloid scans were interpreted as elevated in 26% (n = 33) of the participants, in line with the expected prevalence of elevated cerebral amyloid in cognitively normal older adults (25). Amyloid burden, anthropometric, neuropsychometric, dietary intake and cerebral amyloid characteristics are presented in Table 1.
TABLE 1.
Participant characteristics1
| Overall (n = 128) | Elevated (n = 33) | Nonelevated (n = 95) | P | |
| Age, y | 71.3 ± 5.12 | 72.5 ± 4.6 | 71.0 ± 5.2 | 0.15 |
| Sex (F/M), n | 84/44 | 19/14 | 65/30 | 0.50 |
| F/M, % | 66/34 | 59/41 | 68/32 | |
| BMI, kg/m2 | 28.9 ± 4.8 | 28.4 ± 4.2 | 29.0 ± 5.0 | 0.51 |
| Diagnosed diabetes, n (%) | 16 (13) | 6 (18) | 10 (11) | 0.38 |
| Taking oral diabetes medications | 15 (12) | 5 (15) | 10 (11) | 0.66 |
| Education, y | 16.5 ± 2.6 | 16.4 ± 3.1 | 16.6 ± 2.4 | 0.78 |
| Dietary intake3 | ||||
| Energy, kcal | 1590 ± 670 | 1700 ± 760 | 1550 ± 640 | 0.24 |
| Fat, g | 66 ± 31 | 68 ± 31 | 65 ± 31 | 0.53 |
| Protein, g | 65 ± 32 | 69 ± 29 | 64 ± 33 | 0.51 |
| Dietary glycemic measures3 | ||||
| Carbohydrate, g | 184 ± 85 | 208 ± 112 | 176 ± 72 | 0.05 |
| Sugar, g | 86 ± 49 | 102 ± 64 | 81 ± 42 | 0.03 |
| Glycemic load | 96 ± 47 | 106 ± 58 | 92 ± 43 | 0.11 |
| HGLDiet pattern | 0.1 ± 1.0 | 0.5 ± 1.1 | −0.1 ± 1.0 | 0.01 |
| Cognition scores | ||||
| MMSE | 29 ± 1 | 29 ± 1 | 29 ± 2 | 0.94 |
| WAIS-R Digit Symbol | 51 ± 10 | 49 ± 10 | 51 ± 10 | 0.18 |
| Trail Making Test A | 29 ± 10 | 31 ± 10 | 29 ± 10 | 0.30 |
| Trail Making Test B | 79 ± 38 | 78 ± 25 | 79 ± 41 | 0.88 |
| Category Fluency | 39 ± 8 | 38 ± 8 | 39 ± 8 | 0.54 |
| Block Design | 37 ± 11 | 36 ± 10 | 37 ± 11 | 0.41 |
| Stroop | 219 ± 32 | 216 ± 32 | 220 ± 32 | 0.45 |
| SRT–free total | 31 ± 5 | 29 ± 5 | 31 ± 5 | 0.05 |
| SUVR4 | ||||
| Global | 1.1 ± 0.2 | 1.3 ± 0.2 | 1.0 ± 0.1 | <0.001 |
| Anterior cingulate gyrus | 1.2 ± 0.2 | 1.4 ± 0.2 | 1.1 ± 0.1 | <0.001 |
| Inferior medial frontal gyrus | 1.0 ± 0.2 | 1.2 ± 0.2 | 0.9 ± 0.1 | <0.001 |
| Lateral temporal lobe | 1.1 ± 0.2 | 1.3 ± 0.2 | 1.0 ± 0.1 | <0.001 |
| Posterior cingulate gyrus | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.0 ± 0.1 | <0.001 |
| Precuneus | 1.1 ± 0.2 | 1.4 ± 0.2 | 1.0 ± 0.1 | <0.001 |
| Superior parietal lobule | 1.0 ± 0.2 | 1.2 ± 0.2 | 1.0 ± 0.1 | <0.001 |
Group differences were assessed by ANCOVA adjusted for age and sex. Significance was set at P ≤ 0.05. HGLDiet, high-glycemic-load diet; MMSE, Mini-Mental State Examination; SRT, Selective Reminding Test; Stroop, Stroop Color-Word Interference Test; SUVR, standard uptake value ratio; WAIS-R, Wechsler Adult Intelligence Scale–Revised.
Mean ± SD (all such values).
Derived by using a food-frequency questionnaire.
Derived by using florbetapir F-18 positron emission tomography imaging.
We first examined whether there were differences in dietary glycemic measures across amyloid-elevated and -nonelevated groups (Table 1). After age and sex were controlled for, the elevated amyloid group consumed more carbohydrates (P = 0.05) and sugar (P = 0.03) and had higher HGLDiet pattern adherence scores (P = 0.01) than did the nonelevated group. There were no differences in energy, fat, or protein consumption across amyloid groups.
We next examined the relation of dietary glycemic measures with regional and global measures of cerebral amyloid burden (SUVR) in the overall group by using linear regression controlling for age, sex, and education. All 4 measures of glycemic intake were positively associated with global amyloid and most regional measures of amyloid burden (Table 2). Figure 2 shows the relation between HGLDiet pattern adherence and global cerebral amyloid burden. Figure 3 shows the relation between the HGLDiet pattern and regional cerebral amyloid burden.
TABLE 2.
Relation of glycemic dietary intake measures with global and regional cerebral amyloid burden1
| HGLDiet2 pattern | Sugar intake | Glycemic load | Carbohydrate intake | |
| Global | 0.36*** | 0.21* | 0.22* | 0.24* |
| Anterior cingulate gyrus | 0.38*** | 0.20* | 0.23* | 0.25** |
| Inferior medial frontal gyrus | 0.33*** | 0.17 | 0.16 | 0.18 |
| Lateral temporal lobe | 0.34*** | 0.22* | 0.22* | 0.24* |
| Posterior cingulate gyrus | 0.31*** | 0.17 | 0.18* | 0.21* |
| Precuneus | 0.32*** | 0.22* | 0.21* | 0.24* |
| Superior parietal lobule | 0.30*** | 0.19* | 0.20* | 0.21* |
Values are standardized β values for the respective dietary glycemic measure determined by multivariate linear regression controlling for age, sex, and education; n = 128. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
HGLDiet, high-glycemic-load diet.
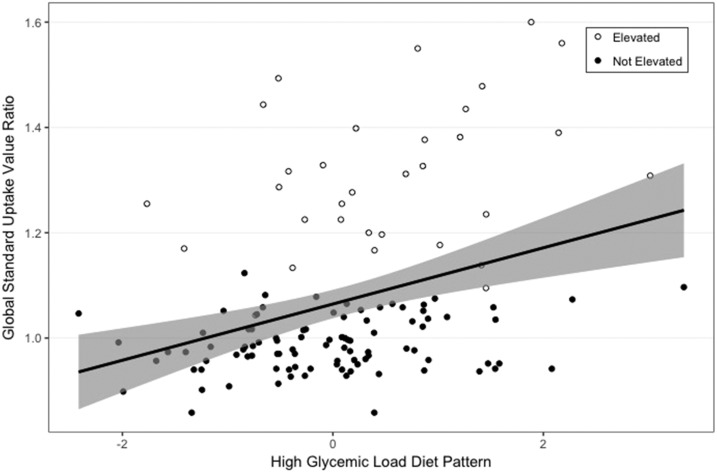
FIGURE 2.
Association of HGLDiet pattern adherence with global cerebral amyloid burden modeled using linear regression. The shaded area represents 95% CIs. HGLDiet pattern adherence explained 12% of the variability in global cerebral amyloid burden (r2 = 0.12, P ≤ 0.001) (n = 128). HGLDiet pattern adherence was also significantly correlated with amyloid burden in all regions of interest. HGLDiet, high-glycemic-load diet.
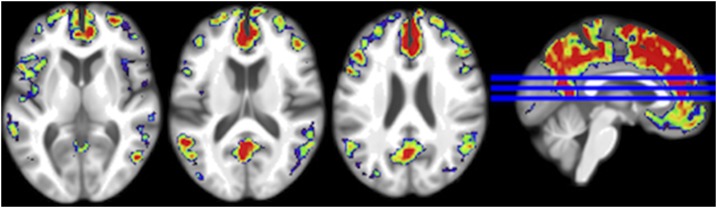
FIGURE 3.
Visualization of the relation between HGLDiet pattern adherence and cerebral amyloid burden. The HGLDiet pattern measure was regressed against standard uptake value ratios for all participants (n = 128). Standardized β values are projected on the MNI152 anatomical template, with warmer colors representing regions of greater association with HGLDiet. HGLDiet, high-glycemic-load diet.
We also examined how dietary glycemic measures related to cognitive performance. Cognitive analyses were controlled for age, sex, and education. Daily sugar intake negatively correlated with global cognition and cognitive performance on several individual neuropsychometric tests (Table 3), including the MMSE, Trail Making Test B, WAIS-R Digit Symbol, and Block Design. No other dietary measures were related to neuropsychometric test scores.
TABLE 3.
Relation of glycemic dietary intake measures with cognitive performance1
| HGLDiet pattern | Sugar intake | Glycemic load | Carbohydrate intake | |
| Global | −0.01 | −0.26** | −0.15 | −0.16 |
| MMSE | −0.07 | −0.18* | −0.16 | −0.11 |
| WAIS-R Digit Symbol | 0.07 | −0.23* | −0.13 | −0.13 |
| Trail Making Test A2 | 0.02 | −0.15 | −0.07 | −0.08 |
| Trail Making Test B2 | 0.02 | −0.29** | −0.16 | −0.17 |
| Category Fluency | −0.11 | −0.17 | −0.15 | −0.15 |
| Stroop | 0.05 | −0.01 | 0.04 | 0.03 |
| Block Design | 0.06 | −0.20* | −0.11 | −0.13 |
| SRT–free | −0.04 | −0.15 | −0.06 | −0.12 |
Values are standardized β values for the respective dietary glycemic measure determined by multivariate linear regression controlling for age, sex, and education; n = 128. *P ≤ 0.05, **P ≤ 0.01. HGLDiet, high-glycemic-load diet; MMSE, Mini-Mental State Examination; SRT, Selective Reminding Test; Stroop, Stroop Color-Word Interference Test; WAIS-R, Wechsler Adult Intelligence Scale–Revised.
For consistency with other measures, we multiplied z scores for Trail Making Tests A and B by −1 so that negative scores reflect cognitive decline.
DISCUSSION
The current study provides evidence that a high-glycemic diet is associated with increased global and regional cerebral amyloid burden in cognitively normal older adults. We calculated 4 different dietary glycemic measures derived from the DHQ II food-frequency questionnaire—daily intakes of sugar and carbohydrate, glycemic load, and adherence scores from a PCA-derived dietary pattern characterized by intakes of high-glycemic foods—and found strong relations with cerebral amyloid load for each measure. A modest negative association was found between sugar intake and cognitive performance but for no other dietary glycemic measures. These data suggest diet as a modifiable risk factor that may influence cerebral amyloid deposition, providing additional evidence that links glucose metabolism with AD pathophysiology.
Our data add to the growing body of evidence that links glucose metabolism with chronic disease. For instance, high carbohydrate intake and glycemic load have been linked to an increased risk of insulin resistance, type 2 diabetes, coronary artery disease, stroke, and multiple cancers (16, 26, 27). Our data extend this evidence by linking dietary glycemic measures directly with a primary AD biomarker in cognitively normal older adults. Individuals with cerebral amyloid plaques in the absence of cognitive symptoms are at higher risk of cognitive decline (28–30), brain atrophy (31, 32), and progression to AD than those without cerebral amyloid plaques (33). Imaging and pathological studies suggest that plaques may accumulate ≤10–15 y before the onset of clinically recognized dementia (3), although not all amyloid-positive individuals will develop dementia. Precise estimates of the magnitude and time frame for future risk of dementia are not yet available on an individual basis for cognitively unimpaired older adults with cerebral amyloid.
To date, only limited data address the relation between nutrition and cerebral amyloid. A previous longitudinal imaging initiative showed that nutrient intake patterns related to vitamin B-12, vitamin D, zinc, and omega-3 fatty acid consumption were associated with less amyloid deposition (34, 35). Another study found an inverse relation between serum DHA, an omega-3 fatty acid found abundantly in fatty fish, and cerebral amyloid burden in older adults with normal cognition and MCI (36). In addition to these findings related to cerebral amyloid, a higher intake of sugary beverages is associated with decreased brain volume and poorer episodic memory performance (37). We believe that our study extends these observations by being the first, to our knowledge, to link amyloid deposition with dietary glycemic measures. Our finding that a high-glycemic diet is associated with greater amyloid deposition is consistent with our previous work in a subset of the current cohort that found that impaired fasting glycemia was associated with increased amyloid burden in highly metabolic regions of the brain in cognitively normal older adults (14).
It is well established that diets with greater intakes of sugar, high-glycemic-index foods, and overall carbohydrate are linked to impaired glucose metabolism, including insulin resistance and type 2 diabetes (16), both of which are risk factors for AD and cognitive decline (5, 6). Emerging evidence indicates that glucose and insulin status may influence the modulation of cerebral amyloid accumulation (13) and that elevated glucose may evoke a state of decreased metabolism in the brain (9–12). Protein homeostasis requires a steady flux of energy; bioenergetic perturbations that arise due to elevated glycemia and insulin resistance may potentially contribute to altered amyloid precursor protein or β-amyloid processing (38). Relevant to this point, an AD mouse model study in which acute hyperglycemia was induced through the intravenous flow of dextrose increased amyloid amounts in the brain interstitial fluid compartment (39). Acute hyperglycemia from the consumption of processed carbohydrates and sugars could potentially mimic this effect.
To further validate our findings, we also looked for possible relations between dietary glycemic measures and cognitive performance. Although 3 of the dietary glycemic measures were not associated with cognitive performance, higher sugar intake was found to be inversely correlated with global cognitive performance (as measured by a global composite measure and the MMSE) and with individual tests in our battery (Digit Symbol, Trail Making B, and Block Design). Previous studies have shown detrimental cognitive effects of high intakes of sugar and high-glycemic-index foods in human and animal studies. Adults with type 2 diabetes who consumed a meal of high-glycemic-index, high-sugar foods exhibited poorer acute cognitive performance (40), and cognition changes have also been observed in rats fed high-sucrose and high-fructose corn syrup diets (41–43). Although cognitive changes are believed to be induced by poor glycemic control, it is unclear as to why cognitive performance in this study was only related to sugar intake and no other dietary glycemic measures.
Our study has several strengths. It featured a sample size of 128 well-screened, highly characterized, cognitively normal older adults who underwent amyloid PET imaging. The inclusion of only underactive or sedentary individuals controlled for any potential differences in physical activity. An additional strength of this study is the input of MPED 2.0 variables into PCA analysis to identify and characterize the HGLDiet pattern. MPED 2.0 translates dietary intake into quantitative food group variables, effectively reducing the number of dietary variables to consider for analysis and collinearity of foods with their corresponding nutrients. The use of PCA in this study allowed us to identify dietary patterns existing within the data collected, describe interpersonal dietary intake differences, and conduct a more comprehensive analysis of dietary intake than solely that of solitary nutrients (44). Finally, the finding that diet potentially influences an AD biomarker could have strong preclinical applications.
There are also limitations that should be considered. Cross-sectional studies are not designed to establish causal relations. Food-frequency questionnaires have been shown to be accurate, yet are potentially subject to under- or overreporting of some nutrients by individuals. The DHQ II estimates dietary intake over the past year, which leaves some question of chronic intake over the course of the participant’s lifetime. Other mechanisms could be considered. For example, a suggested increase in cerebral concentration of sugars in AD brains, inversely related to cerebral copper concentration, has a purported role in cerebral metabolism dysregulation and amyloid aggregation (45, 46). These changes may occur before AD onset, potentially influenced by the common intake of high-glycemic carbohydrates. Our data are unable to elucidate these possibilities. In addition, it is possible that amyloid status, contrary to our interpretation, influences dietary intake.
In conclusion, future studies are needed to further investigate the impact of carbohydrate intake on cerebral amyloid processing. Nevertheless, these findings in cognitively normal older adults suggest that dietary intake may influence amyloid accumulation before AD symptomology. Although the clinical relevance of amyloid burden in older adults is not precisely defined, the presence of brain amyloid aggregations does imply an elevated risk of future symptomatic dementia. Understanding the mechanisms through which dietary intake influences brain health and amyloid accumulation could have public health implications and suggest potential lifestyle-based AD-prevention strategies.
Acknowledgments
The authors’ responsibilities were as follows—MKT, DKS, EDV, JKM, and JMB: designed the research; MKT, EDV, JKM, and JMB: conducted the research; MKT and JDM: performed the statistical analyses; MKT and JMB: had primary responsibility for the final content; and all authors: wrote the manuscript, and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AD, Alzheimer disease; APEX, Alzheimer’s Prevention through Exercise; DHQ, Diet History Questionnaire; HGLDiet, high-glycemic-load diet; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MPED 2.0, My Pyramid Equivalents Database 2.0; PCA, principal components analysis; PET, positron emission tomography; ROI, region of interest; SUVR, standard uptake value ratio; WAIS-R, Wechsler Adult Intelligence Scale–Revised.
REFERENCES
- 1.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 2013;80:1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004;55:306–19. [DOI] [PubMed] [Google Scholar]
- 3.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otaegui-Arrazola A, Amiano P, Elbusto A, Urdaneta E, Martinez-Lage P. Diet, cognition, and Alzheimer’s disease: food for thought. Eur J Nutr 2014;53:1–23. [DOI] [PubMed] [Google Scholar]
- 5.Huang CC, Chung CM, Leu HB, Lin LY, Chiu CC, Hsu CY, Chiang CH, Huang PH, Chen TJ, Lin SJ, et al. Diabetes mellitus and the risk of Alzheimer’s disease: a nationwide population-based study. PLoS One 2014;9:e87095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, et al. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology 2010;75:764–70. [DOI] [PubMed] [Google Scholar]
- 7.Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE, et al. Glucose levels and risk of dementia. N Engl J Med 2013;369:540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris JK, Vidoni ED, Honea RA, Burns JM; Alzheimer’s Disease Neuroimaging Initiative. Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiol Aging 2014;35:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns CM, Chen K, Kaszniak AW, Lee W, Alexander GE, Bandy D, Fleisher AS, Caselli RJ, Reiman EM. Higher serum glucose levels are associated with cerebral hypometabolism in Alzheimer regions. Neurology 2013;80:1557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishibashi K, Kawasaki K, Ishiwata K, Ishii K. Reduced uptake of 18F-FDG and 15O-H2O in Alzheimer’s disease-related regions after glucose loading. J Cereb Blood Flow Metab 2015;35:1380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishibashi K, Onishi A, Fujiwara Y, Ishiwata K, Ishii K. Relationship between Alzheimer disease-like pattern of 18F-FDG and fasting plasma glucose levels in cognitively normal volunteers. J Nucl Med 2015;56:229–33. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki K, Ishii K, Saito Y, Oda K, Kimura Y, Ishiwata K. Influence of mild hyperglycemia on cerebral FDG distribution patterns calculated by statistical parametric mapping. Ann Nucl Med 2008;22:191–200. [DOI] [PubMed] [Google Scholar]
- 13.Sato N, Morishita R. The roles of lipid and glucose metabolism in modulation of beta-amyloid, tau, and neurodegeneration in the pathogenesis of Alzheimer disease. Front Aging Neurosci 2015;7:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris JK, Vidoni ED, Wilkins HM, Archer AE, Burns NC, Karcher RT, Graves RS, Swerdlow RH, Thyfault JP, Burns JM. Impaired fasting glucose is associated with increased regional cerebral amyloid. Neurobiol Aging 2016;44:138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002;76:5–56. [DOI] [PubMed] [Google Scholar]
- 16.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health—a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr 2008;87:258S–68S. [DOI] [PubMed] [Google Scholar]
- 17.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009;23:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–4. [DOI] [PubMed] [Google Scholar]
- 19.Mayer CJ, Steinman L, Williams B, Topolski TD, LoGerfo J. Developing a Telephone Assessment of Physical Activity (TAPA) questionnaire for older adults. Prev Chronic Dis 2008;5:A24. [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Institute. Diet History Questionnaire II and Canadian Diet History Questionnaire II (C-DHQII) [Internet]. [cited 2017 Aug 20]. Available from: https://epi.grants.cancer.gov/dhq2/.
- 21.National Cancer Institute. Diet History Questionnaire II & Canadian Diet History Questionnaire II: Diet*Calc Software [Internet]. [cited 2017 Aug 16]. Available from: https://epi.grants.cancer.gov/dhq2/dietcalc/.
- 22.Bowman SA, Friday JE, Moshfegh A. My Pyramid Equivalents Database, 2.0 for USDA survey foods, 2003-2004. Food Surveys Research Group [serial online] [Internet]. 2008. [cited 2017 Aug 16]. Available from: http://www.ars.usda.gov/ba/bhnrc/fsrg.
- 23.Burns JM, Johnson DK, Liebmann EP, Bothwell RJ, Morris JK, Vidoni ED. Safety of disclosing amyloid status in cognitively normal older adults. Alzheimers Dement 2017;13:1024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harn NR, Hunt SL, Hill J, Vidoni E, Perry M, Burns JM. Augmenting amyloid PET interpretations with quantitative information improves consistency of early amyloid detection. Clin Nucl Med 2017;42:577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen RC, Wiste HJ, Weigand SD, Rocca WA, Roberts RO, Mielke MM, Lowe VJ, Knopman DS, Pankratz VS, Machulda MM, et al. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol 2016;73:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melkonian SC, Daniel CR, Ye Y, Pierzynski JA, Roth JA, Wu X. Glycemic index, glycemic load, and lung cancer risk in non-Hispanic whites. Cancer Epidemiol Biomarkers Prev 2016;25:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu D, Zhang X, Shu XO, Cai H, Li H, Ding D, Hong Z, Xiang YB, Gao YT, Zheng W, et al. Dietary glycemic index, glycemic load, and refined carbohydrates are associated with risk of stroke: a prospective cohort study in urban Chinese women. Am J Clin Nutr 2016;104:1345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol 2009;66:1476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stomrud E, Hansson O, Zetterberg H, Blennow K, Minthon L, Londos E. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch Neurol 2010;67:217–23. [DOI] [PubMed] [Google Scholar]
- 30.Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, Dannals RF, Mathis CA, Klunk WE, Ferrucci L, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology 2010;74:807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jack CR Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain 2009;132:1355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Archer HA, Edison P, Brooks DJ, Barnes J, Frost C, Yeatman T, Fox NC, Rossor MN. Amyloid load and cerebral atrophy in Alzheimer’s disease: an 11C-PIB positron emission tomography study. Ann Neurol 2006;60:145–7. [DOI] [PubMed] [Google Scholar]
- 33.Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol 2009;66:1469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berti V, Murray J, Davies M, Spector N, Tsui WH, Li Y, Williams S, Pirraglia E, Vallabhajosula S, McHugh P, et al. Nutrient patterns and brain biomarkers of Alzheimer’s disease in cognitively normal individuals. J Nutr Health Aging 2015;19:413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosconi L, Murray J, Davies M, Williams S, Pirraglia E, Spector N, Tsui WH, Li Y, Butler T, Osorio RS, et al. Nutrient intake and brain biomarkers of Alzheimer’s disease in at-risk cognitively normal individuals: a cross-sectional neuroimaging pilot study. BMJ Open 2014;4:e004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yassine HN, Feng Q, Azizkhanian I, Rawat V, Castor K, Fonteh AN, Harrington MG, Zheng L, Reed BR, DeCarli C, et al. Association of serum docosahexaenoic acid with cerebral amyloidosis. JAMA Neurol 2016;73:1208–16. [DOI] [PubMed] [Google Scholar]
- 37.Pase MP, Himali JJ, Jacques PF, DeCarli C, Satizabal CL, Aparicio H, Vasan RS, Beiser AS, Seshadri S. Sugary beverage intake and preclinical Alzheimer’s disease in the community. Alzheimers Dement 2017;13:955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis 2010;20(Suppl 2):S265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macauley SL, Stanley M, Caesar EE, Yamada SA, Raichle ME, Perez R, Mahan TE, Sutphen CL, Holtzman DM. Hyperglycemia modulates extracellular amyloid-beta concentrations and neuronal activity in vivo. J Clin Invest 2015;125:2463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenwood CE, Kaplan RJ, Hebblethwaite S, Jenkins DJ. Carbohydrate-induced memory impairment in adults with type 2 diabetes. Diabetes Care 2003;26:1961–6. [DOI] [PubMed] [Google Scholar]
- 41.Agrawal R, Gomez-Pinilla F. ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol 2012;590:2485–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldbart AD, Row BW, Kheirandish-Gozal L, Cheng Y, Brittian KR, Gozal D. High fat/refined carbohydrate diet enhances the susceptibility to spatial learning deficits in rats exposed to intermittent hypoxia. Brain Res 2006;1090:190–6. [DOI] [PubMed] [Google Scholar]
- 43.Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008;18:1085–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev 2004;62:177–203. [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Begley P, Church SJ, Patassini S, McHarg S, Kureishy N, Hollywood KA, Waldvogel HJ, Liu H, Zhang S, et al. Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer’s disease: metabolic basis for dementia. Sci Rep 2016;6:27524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerber H, Wu F, Dimitrov M, Garcia Osuna GM, Fraering PC. Zinc and copper differentially modulate amyloid precursor protein processing by gamma-secretase and amyloid-beta peptide production. J Biol Chem 2017;292:3751–67. [DOI] [PMC free article] [PubMed] [Google Scholar]