Abstract
Background: Stunting affects ∼25% of children <5 y of age and is associated with impaired cognitive and motor development and increased morbidity and mortality. The pathogenesis of stunting is poorly understood.
Objective: The purpose of this study was to identify altered metabolic pathways associated with child stunting.
Design: We measured 677 serum metabolites using liquid chromatography–tandem mass spectrometry in a cross-sectional study of 400 Malawian children aged 12–59 mo, of whom 62% were stunted.
Results: A low height-for-age z score (HAZ) was associated with lower serum concentrations of 1) ω-3 (n–3) and ω-6 (n–6) polyunsaturated fatty acids (PUFAs), 2) sulfated neurosteroids, which play a role in brain development, 3) carnitine, a conditionally essential nutrient with an important role in the carnitine shuttle for the metabolism of fatty acids and energy production, and 4) γ-glutamyl amino acids, which represent an altered γ-glutamyl cycle of glutathione metabolism. A low HAZ was associated with significantly higher serum concentrations of 5 biomarkers related to cigarette smoke exposure.
Conclusions: This metabolomics study shows a cross-sectional association between stunting and low serum ω-3 and ω-6 long-chain PUFAs, which are essential for growth and development; low sulfated neurosteroids, which play a role in brain development; low carnitine, which is essential for β-oxidation of fatty acids; alterations in glutathione metabolism; and increased serum metabolites that are associated with secondhand tobacco smoke exposure. This trial was registered at www.controlled-trials.com as ISRCTN14597012.
Keywords: arachidonic acid, carnitine, dehydroepiandrosterone, docosahexaenoic acid, malnutrition, pregnenolone sulfate, stunting
INTRODUCTION
Stunting affects ∼25% of children <5 y of age worldwide. Stunting is defined as a height-for-age z score (HAZ) >2 SDs below the median (HAZ <−2) and is the best available summary measure of chronic malnutrition in children. There are an estimated 155 million stunted children aged <5 y worldwide (1). Stunting is associated with impaired cognitive and motor development and increased morbidity and mortality. Later in adulthood, those who were stunted have a higher risk of reduced economic productivity and a greater chance of being impoverished (2–6). The World Health Assembly and the UN have a global target to reduce by 40% the number of stunted <5-y-old children by 2025 (7, 8). Recent estimates based on proxy measures of stunting and poverty show that 250 million children <5 y old in low- and middle-income countries are at risk of not reaching their developmental potential (9).
Stunting is attributed to a host of factors, including poor prenatal nutrition, micronutrient deficiencies, insufficient breastfeeding, poor hygiene, and infectious diseases (10). Micronutrient-based supplementation or lipid-based supplements with micronutrients have had a limited impact on reducing stunting in children (11–14). Studies suggest that inadequate dietary intakes of essential amino acids (15, 16) and choline (16, 17) contribute to stunting. The richest dietary sources of essential amino acids and choline are animal-source foods, which are rarely consumed by children from poor families in low-income countries. The pathogenesis of stunting remains poorly understood. The biological mechanisms by which stunting could affect cognition and neurodevelopment of young children are also not well characterized.
We previously examined the relation between serum metabolites and stunting using an assay that was limited to 139 metabolites (16). To expand these investigations, we conducted a discovery phase metabolomics study by measuring serum metabolites using the Metabolon platform that can measure 600–700 serum metabolites.
METHODS
Study design and participants
This cross-sectional study involved children aged 12–59 mo from 6 villages (Masika, Makhwira, Mitondo, Mibiza, Chamba, and Mayaka) in rural southern Malawi in 2011 (Supplemental Figure 1). Eligibility criteria included no congenital or chronic disease or caretaker-reported diarrhea and no current treatment of acute malnutrition. Field staff measured weight to the nearest 5 g using a digital scale (Seca 344) and length to the nearest 0.1 cm using a rigid length board (Seca 417). Using a standardized questionnaire, the parent or guardian was asked about the primary source of water used by the household, i.e., wells, streams, or boreholes. Wells and streams were considered potentially contaminated, whereas boreholes were considered clean. However, no microbiological testing was done to corroborate these assumptions. The parent or guardian was also asked about animals in the household and various indicators of socioeconomic status. Written and oral informed consent from each child’s caretaker were obtained by Malawian research nurses before enrollment in the study. Consent for the study on the community level also was obtained from the village chief and local health officials. The study protocol was approved by the College of Medicine Research and Ethics Committee of the University of Malawi, the Human Research Protection Office of Washington University in St. Louis, and the Johns Hopkins School of Medicine Institutional Review Board. The study protocol was conducted in accordance with the 1964 Helsinki Declaration. The trial was registered at www.controlled-trials.com as ISRCTN14597012.
Measurement of serum metabolites
Venous blood was drawn by study nurses and doctors. Serum samples were processed, aliquoted, and snap-frozen in liquid nitrogen in cryovials within 4 h of blood drawing. Cryovials were transferred to storage at −80°C until time of analysis. The serum samples used in this study were previously subjected to 3 freeze-thaw cycles. Preanalytical studies show that 2–4 freeze-thaw cycles have minimal impact on serum metabolites (18, 19). All experimental samples were prepared and analyzed in a masked fashion. Samples were prepared at Metabolon Inc. with the use of their DiscoveryHD4 platform with methods as described elsewhere (20). The staff at Metabolon had no access to the clinical data. In brief, for quality control, recovery standards were added before the first step of the extraction process. Proteins were precipitated with methanol under vigorous shaking for 2 min by using a GenoGrinder 2000 (Glen Mills) followed by centrifugation. The resulting extract was divided into 5 fractions: 1) early- and 2) late-eluting compounds for analysis by ultra-HPLC–tandem mass spectrometry (UPLC-MS/MS) by using positive ionization, 3) for analysis by UPLC-MS/MS by using negative ionization, 4) for analysis by using a UPLC-MS/MS polar platform with negative ionization, and 5) a sample reserved for backup if needed.
Three types of controls were analyzed concomitantly with the experimental samples: 1) samples generated from a pool of human plasma that has been extensively characterized by Metabolon, 2) extracted water samples serving as process blanks, and 3) a cocktail of standards spiked into every analyzed sample to allow monitoring of instrument performance. Instrument variability was determined by calculating the median relative SD (RSD) for the standards that were added to each sample before injection into the mass spectrometers (median RSD typically = 4–6%; n ≥ 30 standards). Overall process variability was determined by calculating the median RSD for all endogenous metabolites (i.e., noninstrument standards) present in 100% of the pooled human plasma samples (median RSD = 10–14%; n = several hundred metabolites). Experimental samples and controls were randomized across the experimental runs.
Sample extracts were analyzed by using a standardized chromatographic UPLC-MS/MS method (20). All columns and solvents were obtained from a single manufacturer’s lot for the sample analysis of this study. For each sample, vacuum-dried samples were dissolved in injection solvent containing ≥8 injection standards at fixed concentrations, depending on the platform. The internal standards were used to ensure consistency of injections and chromatography. Instruments were tuned and calibrated for mass resolution and mass accuracy daily.
The UPLC-MS/MS platform consisted of an Acquity UPLC (Waters Corp.) and a Q-Exactive (Thermo Scientific) mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and an Orbitrap mass analyzer operated at 35,000 mass resolution. The sample extract was dried and then reconstituted in acidic or basic liquid chromatography–compatible solvents, each of which contained ≥8 injection standards at fixed concentrations. One aliquot was analyzed by using acidic, positive ion-optimized conditions. Another aliquot was analyzed by using basic, negative ion-optimized conditions. Two independent injections were done by using separate dedicated columns (Waters UPLC BEH C18–2.1 × 100 mm, 1.7 μm). Extracts reconstituted in acidic conditions were gradient-eluted by using water and methanol containing 0.1% formic acid, whereas the basic extracts, which also used water and methanol, contained 6.5 mmol ammonium bicarbonate/L. A third aliquot was analyzed via negative ionization after elution from an HILIC column (Waters UPLC BEH Amide 2.1 × 150 mm, 1.7 μm) by using a gradient consisting of water and acetonitrile with 10 mmol ammonium formate/L. The mass spectrometry (MS) analysis alternated between MS and data-dependent MS2 scans by using dynamic exclusion. The scan range was from 80 to 1000 m/z.
Metabolites were identified by an automated comparison of the ion features in the experimental samples against a reference library of chemical standard entries. The entries included molecular weight (m/z), retention time, preferred adducts, in-source fragment, and associated MS spectra. Entries have been curated by visual inspection for quality control by using software developed at Metabolon (21). The identification of known metabolites was based on comparison to metabolomic library entries of purified standards. Commercially available purified standard compounds were previously acquired and registered into the Laboratory Information Management System for determination of their detectable characteristics. Peaks were quantified by using AUC. Raw area counts for each metabolite in each sample were normalized to correct for variation resulting from instrument interday tuning differences by the median value for each run day and therefore setting the medians to 1.0 for each run. This normalization preserved the variation between samples but allowed metabolites of widely different raw peak areas to be compared on a similar graphical scale. Missing values were imputed with the observed minimum after normalization.
Statistical analysis
The distributions of serum metabolites and HAZ values were examined in exploratory data analyses by using histograms and boxplots. The primary analysis of this study was based on linear growth as represented by HAZ as a continuous variable. There were no a priori power calculations for this discovery-phase metabolomics study. Partial Spearman correlations of each metabolite with the HAZ were estimated with adjustment for age, sex, and village. A false-discovery rate approach was used to correct for multiple testing (22–24). Q values were computed at the 0.05 false-discovery rate level. Wilcoxon’s rank-sum test was used to compare serum metabolites between stunted and nonstunted children. This analysis was carried out in R software version 3.3.0. R package “ppcor” (25) and “corpcor” (26) were used for partial Spearman correlations. “Qvalue” was used for calculating the Q value (27).
RESULTS
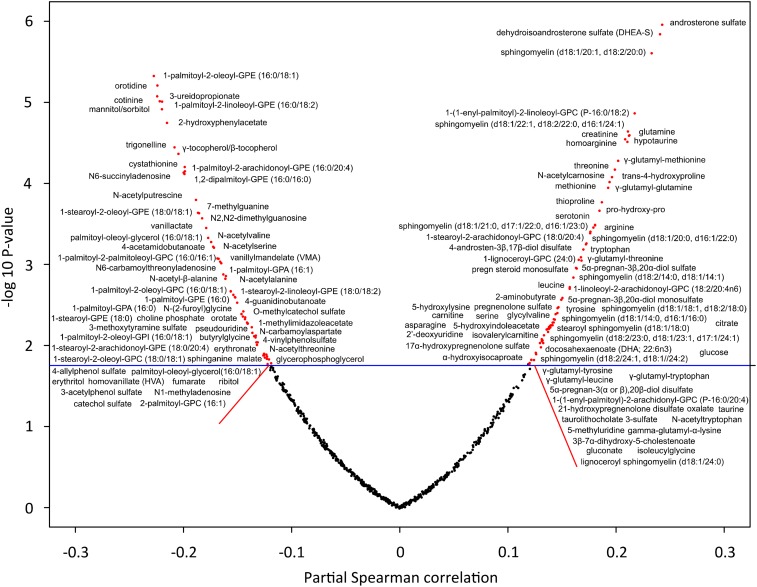
The demographic characteristics of the 400 children are shown in Table 1. Stunted children were older, less likely to have a father who was alive, and more likely to have a clean water source but less likely to have a pit latrine. There were differences in stunting between villages. There were 248 children (62%) who were stunted, as defined by HAZ <−2. The relation of HAZ with serum metabolites is summarized as a volcano plot in Figure 1. There were 136 metabolites that were significantly associated with HAZ. Sixty-two serum metabolites were negatively associated with HAZ, including 19 glycerophospholipids (notably 7 phosphatidylethanolamines) and 5 metabolites associated with cigarette smoking (Table 2). Seventy-four metabolites were positively associated with HAZ, including DHA, 5 glycerophospholipids with arachidonic acid (ARA) side chains [1-stearoyl-2-arachidonoyl-glycerophosphorylcholine (18:0/20:4), 1-stearoyl-2-arachidonoyl-glycerophosphorylcholine (18:0/20:4), 1-linoleoyl-2-arachidonoyl-glycerophosphorylcholine (18:2/20:4n–6), 1-(1-enyl-palmitoyl)-2-arachidonyl-glycerophosphorylcholine (P-16:0/20:4), and 1-arachindonyl-glycerophosphorylcholine (20:4n–6)], 12 sphingomyelins, 9 proteinogenic amino acids, 7 γ-glutamyl dipeptides, 6 glycerophosphocholines, and 4 neurosteroids (Table 3). Spearman correlations of serum metabolites, adjusted by age, sex, and village, with HAZ are shown in Supplemental Table 1. The medians and interquartile ranges of serum metabolites of children with and without stunting are shown in Supplemental Table 2.
TABLE 1.
Characteristics of the study population1
| Not stunted2 (n = 152) | Stunted2 (n = 248) | P3 | |
| Age, mo | 29.0 ± 12.5 | 36.9 ± 10.6 | <0.001 |
| Female | 80 (53) | 118 (48) | 0.33 |
| Weight-for-height z score | 0.1 ± 0.9 | 0.2 ± 1.0 | 0.50 |
| HAZ | −1.1 ± 0.9 | −3.1 ± 0.8 | <0.001 |
| Primary caretaker is mother | 148 (97) | 233 (94) | 0.12 |
| Father is alive | 152 (100) | 232 (94) | 0.001 |
| Siblings, n | 3.7 ± 1.8 | 3.8 ± 1.7 | 0.83 |
| Individuals that sleep in same room as child, n | 3.5 ± 1.2 | 3.2 ± 1.6 | 0.06 |
| Home with a metal roof | 28 (18) | 47 (19) | 0.90 |
| Family owns bicycle | 98 (64) | 146 (59) | 0.26 |
| Animals sleep in house | 46 (30) | 98 (40) | 0.06 |
| Water from a clean source | 89 (59) | 183 (74) | 0.002 |
| Child uses pit latrine | 127 (84) | 184 (74) | 0.03 |
| Village | <0.001 | ||
| Chamba | 6 (4) | 39 (16) | |
| Makwhira | 7 (5) | 20 (8) | |
| Masika | 97 (64) | 59 (24) | |
| Mayaka | 25 (16) | 71 (29) | |
| Mbiza | 11 (7) | 49 (20) | |
| Mitondo | 6 (4) | 10 (4) |
Values are means ± SDs or n (%). HAZ, height-for-age z score.
Stunted was defined as HAZ <−2.
Determined by Student’s t test for continuous variables or chi-square test for categorical variables.
FIGURE 1.
Volcano plot showing the relation of partial Spearman correlations, adjusted for age, sex, and village, between height-for-age z score and serum metabolites. The horizontal line indicates significance at P < 0.0176, which corresponds to a Q value <0.05. DHEA-S, dehyroepiandrosterone sulfate; GPA, glycerophosphate; GPC, glycerophosporylcholine; GPE, glycerophosphoethanolamine; GPI, glycerophosphatidylinositol.
TABLE 2.
Serum metabolites negatively associated with height-for-age z score1
| Metabolite | Description |
| 1-Palmitoyl-2-oleoyl-GPE (16:0/18:1) | Phosphatidylethanolamine, monoene |
| Orotidine | Intermediate in pyrimidine nucleotide biosynthesis |
| 3-Ureidopropionate | Intermediate in the metabolism of uracil |
| Cotinine | Major alkaloid in tobacco and marker of exposure to tobacco smoke |
| 1-Palmitoyl-2-linoleoyl-GPE (16:0/18:2) | Phosphatidylethanolamine, diene |
| Mannitol/sorbitol | Isomers, alcohol sugars |
| 2-Hydroxyphenylacetate | Substrate of the enzyme oxidoreductases in styrene degradation pathway |
| Trigonelline (N’-methylnicotinate) | Metabolite associated with cigarette smoking |
| γ-Tocopherol/β-tocopherol | Form of vitamin E |
| Cystathionine | Formed from transsulfuration of homocysteine |
| 1-Palmitoyl-2-arachidonoyl-GPE (16:0/20:4) | Phosphatidylethanolamine, polyene |
| N6-Succinyladenosine | Aspartic acid derivative |
| 1,2-Dipalmitoyl-GPE (16:0/16:0) | Phosphatidylethanolamine, saturate |
| N-Acetylputrescine | N-Acetylated form of putrescine, a common polyamine |
| 7-Methylguanine | Metabolite of DNA methylation and depurination |
| 1-stearoyl-2-oleoyl-GPE (18:0/18:1) | Phosphatidylethanolamine, monoene |
| N2,N2-Dimethylguanosine | Primary degradation product of transfer RNA |
| Vanillactate | Acidic catecholamine metabolite |
| N-Acetylvaline | N-Acetylated form of valine |
| Palmitoyl-oleoyl-glycerol (16:0/18:1) | Diglyceride, monoene |
| N-Acetylserine | N-Acetylated form of serine |
| 4-Acetamidobutanoate | In family of γ amino acids and derivatives, the conjugate base of 4-acetamidobenzoic acid |
| 1-Palmitoyl-2-palmitoleoyl-glycerophosphorylcholine (16:0/16:1) | Phosphatidylcholine, monoene |
| Vanillylmandelate | Product of catecholamine catabolism |
| N6-Carbamoylthreonyladenosine | Minor constituent of milk |
| 1-Palmitoleoyl-glycerophosphorylcholine (16:1) | Phosphatidic acid, monoene |
| N-Acetyl-β-alanine | β-Amino acid |
| N-Acetylalanine | N-Acyl-aliphatic-α amino acid |
| 1-Palmitoyl-2-oleoyl-glycerophosphorylcholine (16:0/18:1) | Phosphatidylcholine, monoene |
| 1-Stearoyl-2-linoleoyl-GPE (18:0/18:2) | Phosphatidylethanolamine, diene |
| 1-Palmitoyl-GPE (16:0) | Lyso-phosphatidylethanolamine, saturate |
| 1-Stearoyl-GPE (18:0) | Lyso-phosphatidylethanolamine, saturate |
| N-(2-Furoyl)glycine | Metabolite associated with cigarette smoking |
| O-Methylcatechol sulfate | Polyphenol metabolite |
| 1-Methylimidazoleacetate | Main metabolite of histamine |
| 1-Palmitoyl-GPA (16:0) | Lyso-phosphatidic acid, saturate |
| Orotate | Minor dietary constituent of dairy products, root vegetables |
| 4-Guanidinobutanoate | γ-Amino acid |
| Choline phosphate | Intermediate in synthesis of phosphatidylcholine |
| 3-Methoxytyramine sulfate | Dopamine metabolite |
| Pseudouridine | C-Glycoside isomer of the nucleoside uridine |
| N-Carbamoylaspartate | Intermediate product in pyrimidine biosynthesis |
| 4-Vinylphenol sulfate | Metabolite associated with cigarette smoking |
| 1-Palmitoyl-2-oleoyl-GPI (16:0/18:1) | Phosphatidylinositol, monoene |
| Butyrylglycine | Acyl glycine, minor metabolite of fatty acids |
| N-Acetylthreonine | N-Acetylated form of threonine |
| Erythronate | Oxidation product of N-acetyl-d-glucosamine |
| 1-Stearoyl-2-arachidonoyl-GPE (18:0/20:4) | Phosphatidylethanolamine, polyene |
| Malate | Intermediate in tricarboxylic acid cycle |
| Sphinganine | Blocker of postlysosomal cholesterol transport |
| 1-Stearoyl-2-oleoyl-glycerophosphorylcholine (18:0/18:1) | Phosphatidylcholine, monoene |
| Glycerophosphoglycerol | Diglyceride, monoene |
| Palmitoyl-oleoyl-glycerol (16:0/18:1) | Diglyceride, monoene |
| 4-Allylphenol sulfate | Phenolic found in fruit |
| Homovanillate | Dopamine metabolite |
| 3-Acetylphenol sulfate | Sulfated hydroxy-substituted alkyl phenyl ketone |
| Ribitol | Metabolic end product formed by the reduction of ribose |
| Fumarate | Dicarboxylic acid precursor to malate in tricarboxylic acid cycle |
| Erythritol | Small molecule found in foods |
| N1-Methyladenosine | Purine nucleoside |
| 2-Palmitoleoyl-glycerophosphorylcholine (16:1) | Lyso-phosphatidylcholine, monoene |
| Catechol sulfate | Metabolite associated with cigarette smoking |
GPA, glycerophosphate; GPE, glycerophosphoethanolamine; GPI, glycerophosphatidylinositol.
TABLE 3.
Serum metabolites positively associated with height-for-age z score
| Metabolite | Description |
| Androsterone sulfate | Sulfated neurosteroid |
| Dehydroisoandrosterone sulfate | Sulfated neurosteroid with memory enhancing, neuroprotective effects |
| Sphingomyelin (d18:1/20:1, d18:2/20:0) | Sphingomyelin |
| 1-(1-Enyl-palmitoyl)-2-linoleoyl-glycerophosphorylcholine (P-16:0/18:2) | Phosphatidylcholine plasmalogen, diene |
| Sphingomyelin (d18:1/22:1, d18:2/22:0, d16:1/24:1) | Sphingomyelin |
| Glutamine | Conditionally essential amino acid |
| Hypotaurine | Intermediate in biosynthesis of taurine |
| Creatinine | Breakdown product of creatine phosphate in muscle |
| Homoarginine | Nonproteinogenic amino acid formed from lysine, involved in vascular homeostasis |
| γ-Glutamyl-methionine | Dipeptide composed of γ-glutamate and methionine |
| Threonine | Essential amino acid |
| Trans-4-hydroxyproline | Major component of protein collagen (role in collagen stability) |
| N-acetylcarnosine | Related to carnosine but with extra acetyl group, found especially in muscle tissue |
| γ-Glutamyl-glutamine | Dipeptide composed of γ-glutamate and glutamine |
| Methionine | Essential amino acid |
| Thioproline | Metabolite of 5-hydroxytryptamine catabolism |
| Pro-hydroxy-pro | Major component of collagen |
| Serotonin (5-hydroxytryptamine) | Monoamine neurotransmitter, derived from tryptophan |
| Sphingomyelin (d18:1/21:0, d17:1/22:0, d16:1/23:0) | Sphingomyelin |
| Arginine | Conditionally essential amino acid |
| 1-Stearoyl-2-arachidonoyl-glycerophosphorylcholine (18:0/20:4) | Phosphatidylcholine, polyene |
| Sphingomyelin (d18:1/20:0, d16:1/22:0) | Sphingomyelin |
| Tryptophan | Essential amino acid |
| 4-Androsten-3β,17β-diol disulfate | Metabolite of dihydrotestosterone |
| 1-Lignoceroyl-glycerophosphorylcholine (24:0) | Lyso-phosphatidylcholine, long-chain saturate |
| γ-Glutamyl-threonine | Dipeptide composed of γ-glutamate and threonine |
| Pregn steroid monosulfate | Endogenous excitatory neurosteroid, with cognitive and memory-enhancing effects |
| 5α-Pregnan-3β,20α-diol sulfate | Sulfate of pregnanediol isomer, metabolite of plasma progesterone |
| Sphingomyelin (d18:2/14:0, d18:1/14:1) | Sphingomyelin |
| Leucine | Essential amino acid |
| 2-Aminobutyrate | Intermediate in biosynthesis of ophthalmic acid |
| 1-Linoleoyl-2-arachidonoyl-glycerophosphorylcholine (18:2/20:4n–6) | Phosphatidylcholine, polyene |
| 5α-Pregnan-3β, 20α-diol monosulfate | Sulfate of pregnanediol isomer, metabolite of plasma progesterone |
| 1-(1-Enyl-palmitoyl)-2-arachidonyl-glycerophosphorylcholine (P-16:0/20:4) | Phosphatidylcholine plasmalogen, polyene |
| Tyrosine | Nonessential amino acid |
| Sphingomyelin (d18:1/18:1, d18:2/18:0) | Sphingomyelin |
| Pregnenolone sulfate | Endogenous excitatory neurosteroid, with cognitive and memory-enhancing effects |
| Sphingomyelin (d18:1/14:0, d16:1/16:0) | Sphingomyelin |
| Glycylvaline | Dipeptide of glycine and valine |
| Serine | Nonessential amino acid |
| Carnitine | Conditionally essential nutrient, nonproteinogenic amino acid |
| Stearoyl sphingomyelin (d18:1/18:0) | Sphingomyelin |
| Sphingomyelin (d18:2/23:0, d18:1/23:1, d17:1/24:1) | Sphingomyelin long chain |
| 5-Hydroxyindoleacetate | Breakdown product of serotonin |
| Asparagine | Nonessential amino acid |
| Isovalerylcarnitine | C5 acylcarnitine |
| Citrate | Acid in the tricarboxylic acid cycle |
| DHA (22:6n–3) | Very-long-chain PUFA with a 22-carbon backbone and 6 double bonds, polyene |
| 5-Hydroxylysine | Hydroxylated derivative of lysine present in certain collagens |
| γ-Glutamyl-leucine | Dipeptide composed of γ-glutamate and leucine |
| Sphingomyelin (d18:2/16:0, d18:1/16:1) | Sphingomyelin |
| 1-(1-Enyl-stearoyl)-2-archidonoyl-glycerophosphoethanolamine (P-18:0/20:4) | Phosphatidylethanolamine, plasmalogen polyene |
| γ-Glutamyl-tryptophan | Dipeptide composed of γ-glutamate and tryptophan |
| 5-Bromotryptophan | Nonproteinogenic α-amino acid |
| 3-Hydroxy-5-cholestenoic acid | Found in the primary bile acid biosynthesis pathway |
| 1-Arachindonoyl-glycerophosphorylcholine (20:4n–6) | Lyso-phosphatidylcholine, polyene |
| 17α-Hydroxypregnenolone sulfate | Prohormone in the formation of dehydroepiandrosterone, itself a prohormone of the sex steroids |
| 2′-Deoxyuridine | Naturally occurring nucleoside |
| γ-Glutamyl-tyrosine | Dipeptide composed of γ-glutamate and tyrosine |
| Sphingomyelin (d18:2/24:1, d18:1//24:2) | Sphingomyelin, long-chain unsaturate |
| Glucose | Monosaccharide, primary source of energy |
| α-Hydroxyisocaproate | End product of leucine metabolism |
| 21-Hydroxypregnenolone disulfate | Essential intermediate in corticosterone synthesis |
| 5α-Pregnan-3(α or β),20β-diol disulfate | Sulfate of pregnanediol isomer, metabolite of plasma progesterone |
| N-Acetyltryptophan | Stabilizer |
| Oxalate (ethanedioate) | Dicarboxylic acid found in vegetables and produced endogenously by metabolism of glyoxylic acid or ascorbic acid |
| Taurine | Sulfonic acid with wide distribution in tissues |
| Taurolithocholate 3-sulfate | Sulfated bile acid |
| 5-Methyluridine (ribothymidine) | Endogenous methylated nucleoside |
| γ-Glutamyl-α-lysine | Dipeptide composed of γ-glutamate and lysine |
| 3β-7α-Dihydroxy-5-cholestenoate | Found in the primary bile acid biosynthesis pathway |
| Gluconate | Mild organic acid, abundant in plant foods, fruit, rice |
| Isoleucylglycine | Dipeptide composed of isoleucine and glycine |
| Lignoceroyl sphingomyelin (d18:1/24:0) | Sphingomyelin long chain saturate |
DISCUSSION
Stunted children have lower serum phospholipid DHA and ARA, sulfated neurosteroids, carnitine, and γ-glutamyl amino acids and higher serum biomarkers related to cigarette smoke exposure. Stunting was associated with lower creatinine and components of collagen. Many of these metabolites are related to the development of the nervous and musculoskeletal systems. To our knowledge, this is the first study to show an association of linear growth of young children with abnormalities in specific metabolites such as DHA, ARA, sulfated neurosteroids, carnitine, γ-glutamyl amino acids, creatinine, and amino acids involved in collagen assembly. The present study corroborates previous findings that child stunting is associated with lower serum proteinogenic amino acids and alterations in glycerophospholipids (16) and with tobacco use in poor families (28–30).
Stunted children had lower serum phospholipid DHA (22:6n–3), an ω-3 long-chain PUFA, and lower serum phospholipid arachidonic acid (20:4n–6), an ω-6 long-chain PUFA. DHA is essential for normal growth and development and is the most abundant ω-3 fatty acid in the brain (31). ARA content of phosphatidylcholine has been related to infant growth (32) and child stunting (33). Moreover, cognitive development is enhanced when infants receive formula containing ARA and DHA (34). DHA may be formed by biosynthesis from the precursor α-linoleic acid (18:3n–3). ARA is critical for infant growth, brain development, and health (35, 36). ARA may be formed by biosynthesis from linoleic acid (18:2n–6). Breast milk is a rich source of DHA and ARA, and the infant receives most of its supply of these long-chain PUFAs preformed because human metabolism from the 18-C precursors is very limited (37). During weaning and after cessation of breastfeeding, infants and young children in developing countries are at a high risk of insufficient DHA and ARA, because complementary foods contain inadequate amounts or are even devoid of DHA and ARA (31). Rich dietary sources of DHA and ARA include fish and eggs and meats and eggs, respectively.
Child stunting was associated with lower sulfated neurosteroids, pregnenolone sulfate, and dehydroepiandrosterone sulfate, which play an important role in brain development and are involved in cognition, learning, memory, neuronal development, and neuroprotection (38–40). Dehydroepiandrosterone sulfate is mainly produced by the adrenal glands (41) and exerts diverse effects in the brain through the modulation of various receptors. Stunting was also associated with lower serum 17α-hydroxypregnenolone sulfate, 21-hydroxypregnenolone disulfate, a metabolite formed from pregnenolone, and metabolites of progesterone (5α-pregnan-3β,20α-diol monosulfate, 5α-pregnan-3β,20α-diol disulfate), and metabolites of dihydrotestosterone (androsterone sulfate, 4-androsten-3β-diol disulfate). The sulfated steroids can act as circulating reservoirs for the peripheral formation of bioactive hormones (42). Lower serum sulfated steroids could potentially reflect lower availability of these steroids for brain development.
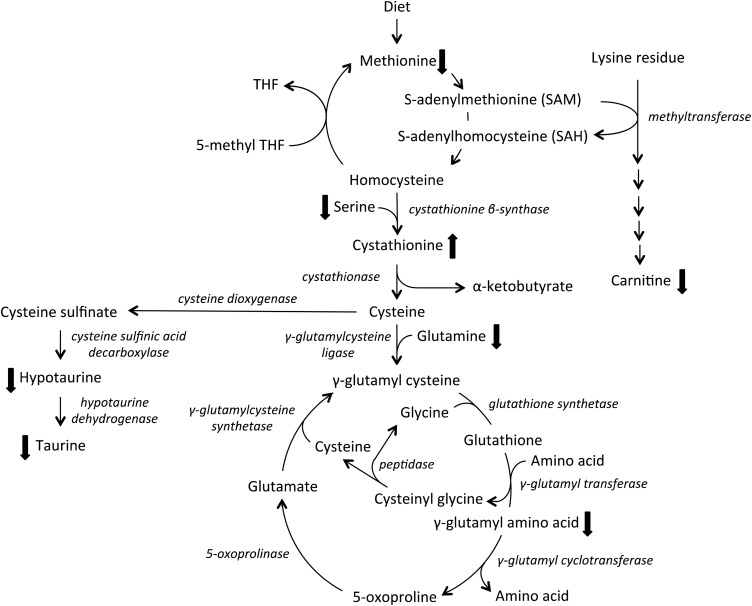
Stunted children had lower serum carnitine, a conditionally essential nutrient that plays a critical role in β-oxidation of fatty acids and energy production. Carnitine is found mainly in animal-source foods, such as red meat, chicken, fish, and dairy products. Plant foods contain negligible amounts of carnitine (43). Carnitine is also synthesized in the body from lysine and methionine (Figure 2). We recently showed that environmental enteric dysfunction is associated with secondary carnitine deficiency (44).
FIGURE 2.
Relation of the sulfur amino acid cycle, carnitine biosynthesis pathway, γ-glutamyl cycle and glutathione metabolism, and taurine pathway. The thick up and down arrows indicate higher or lower serum metabolite concentrations in children with stunting, respectively. THF, tetrahydrofolate.
Child stunting was associated with major abnormalities in the γ-glutamyl cycle of glutathione metabolism. Glutathione, a tripeptide consisting of γ-glutamine, cysteine, and glycine, is the major intracellular thiol that participates in cellular redox reactions and thioester formation. Glutathione protects cells against oxidative damage generated during energy production and immune response. Glutathione also plays a role in signal transduction, gene expression, and apoptosis (45). Stunting was associated with lower serum concentrations of 7 γ-glutamyl amino acids. Six of the dipeptides included essential amino acids whereas the seventh included glutamine, a conditionally essential amino acid. Glutathione in extracellular fluids is broken down at the cell membrane into cysteinyl glycine and γ-glutamyl amino acids (Figure 2). The amino acids can be used for synthesis of glutathione by the cells (46). It was previously believed that the γ-glutamyl cycle is used for transport of γ-glutamyl amino acids into cells; however, such dipeptide transport would require much higher energy compared with other known amino acid transporters (47).
Children with stunting had higher serum cystathionine, which suggests that homocysteine is diverted from the sulfur amino acid cycle by increased transsulfuration to cystathionine (Figure 2). The sulfur amino acid cycle plays a role in the biosynthesis of carnitine from lysine and methionine. The cysteine formed from cystathionine can be metabolized to cysteine sulfinate, hypotaurine, and taurine or can enter the γ-glutamyl cycle to produce γ-glutamyl cysteine through γ-glutamylcysteine ligase. Decreased γ-glutamyl amino acids may reflect less cysteine entering the γ-glutamyl cycle. Other metabolites in the sulfur amino acid pathway and taurine pathway that were significantly lower included methionine, serine, glutamine, hypotaurine, and taurine.
Serum creatinine is generated from creatine and creatinine phosphate, 95% of which is found in skeletal muscle (48). Stunted children had lower serum creatinine, a surrogate for skeletal muscle mass (49).
Stunting was associated with metabolic abnormalities suggestive of abnormal collagen prolyl and lysyl hydroxylation, 2 processes essential to the assembly of collagen, the main structural protein of the extracellular space of connective tissue. Stunted children had lower serum 5-hydroxylysine and trans-4-hydroxyproline, 2 posttranslationally modified amino acids that are found in collagen. Lysine in the α-chains of procollagen is converted to 5-hydroxylysine by lysyl hydroxylases. Proline is converted to 4-hydroxyproline on individual unfolded procollagen α-chains by prolyl 4-hydroxylases. 4-Hydroxylation of collagen proline residues is essential for the proper assembly and stabilization of collagen triple helices through intramolecular hydrogen bonding (50).
In the present study, stunted children had higher serum concentrations of cotinine, a well-known marker of exposure to tobacco smoke; catechol sulfate; 4-vinylphenol sulfate; trigonelline (N′-methylnicotinate); 3 serum metabolites of tobacco associated with smoking; and N-(2-furoyl)glycine, a metabolite found in tobacco smoke (51–53). Cotinine is the predominant metabolite of nicotine, which is found in tobacco leaves but not in firewood used for cooking fires. Paternal smoking was associated with child stunting among poor families in rural Indonesia and Bangladesh (28, 29). The diversion of family income from food to tobacco increases the risk of household food insecurity (30). The prevalence of smoking in Malawi is 25.9% among men and 2.9% among women (54). Although data on tobacco use was not collected in the present study, the elevated serum concentrations of 5 markers of exposure to tobacco smoke in stunted children raises concerns that scarce household resources are being diverted from food to tobacco, thus increasing the risk for children. Exposure to secondhand smoke also has detrimental effects on lung development and cardiovascular health in young children (55, 56).
The strengths of this study are the large, community-based sample size of children from an environment where stunting is highly prevalent and the use of an advanced metabolic platform. Findings from Malawi cannot necessarily be generalized to other settings because of environmental, genetic, and cultural differences. This cross-sectional study can show epidemiologic associations. Inferences of causality would be strengthened by longitudinal analyses and controlled trials.
Two potential interventions are identified for the reduction of child stunting: increasing the intake of animal-source foods (the main source of carnitine, essential amino acids, choline, and ω-3 and ω-6 long-chain PUFAs) and tobacco control. A recent trial shows increased egg consumption can reduce child stunting (57). Further efforts are underway to corroborate these encouraging findings.
Acknowledgments
The authors’ responsibilities were as follows—RDS, RM, IT, KMM, NS, and MJM: designed the research; IT, KMM, and MJM: conducted the research; RDS, KK, and MJM: provided essential reagents or materials; XL and MIO: analyzed the data; RDS, IT, NS, RM, and MJM: wrote the manuscript; RDS: had primary responsibility for the final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ARA, arachidonic acid; HAZ, height-for-age z score; RSD, relative SD; UPLC-MS/MS, ultra-HPLC–tandem mass spectrometry.
REFERENCES
- 1.UNICEF/WHO/World Bank Group. Levels and trends in child malnutrition: key findings of the 2017 edition [Internet]. c2017 [cited 2017 Jun 30]. Available from: http://www.who.int/nutgrowthdb/jme_brochure2017.pdf.
- 2.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B; International Child Development Steering Group. Developmental potential in the first 5 years for children in developing countries. Lancet 2007;369:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;371:340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, Caulfield LE, Danaei G. Nutrition Impact Model Study (anthropometry cohort pooling). Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS One 2013;8:e64636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoddinott J, Behrman JR, Maluccio JA, Melgar P, Quisumbing AR, Ramirez-Zea M, Stein AD, Yount KM, Martorell R. Adult consequences of growth failure in early childhood. Am J Clin Nutr 2013;98:1170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudfeld CR, McCoy DC, Danaei G, Fink G, Ezzati M, Andrews KG, Fawzi WW. Linear growth and child development in low- and middle-income countries: a meta-analysis. Pediatrics 2015;135:e1266–75. [DOI] [PubMed] [Google Scholar]
- 7.de Onis M, Dewey KG, Borghi E, Onyango AW, Blössner M, Daelmans B, Piwoz E, Branca F. The World Health Organization’s global target for reducing childhood stunting by 2025: rationale and proposed actions. Matern Child Nutr 2013;9(Suppl 2):6–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray CJ. Shifting to sustainable development goals–implications for global health. N Engl J Med 2015;373:1390–3. [DOI] [PubMed] [Google Scholar]
- 9.Black MM, Walker SP, Fernald LCH, Andersen CT, DiGirolamo AM, Lu C, McCoy DC, Fink G, Shawar YR, Shiffman J, et al. ; Lancet Early Childhood Development Series Steering Committee. Early childhood development coming of age: science through the life course. Lancet 2017;389:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, et al. ; Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 11.Mayo-Wilson E, Junior JA, Imdad A, Dean S, Chan XH, Chan ES, Jaswal A, Bhutta ZA. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age. Cochrane Database Syst Rev 2014:CD009384. [DOI] [PubMed] [Google Scholar]
- 12.Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Peerson JM, Arimond M, Ashorn U, Zeilani M, Vosti S, Dewey KG. Small-quantity, lipid-based nutrient supplements provided to women during pregnancy and 6 mo postpartum and to their infants from 6 mo of age increase the mean attained length of 18-mo-old children in semi-urban Ghana: a randomized controlled trial. Am J Clin Nutr 2016;104:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christian P, Shaikh S, Shamim AA, Mehra S, Wu L, Mitra M, Ali H, Merrill RD, Choudhury N, Parveen M, et al. . Effect of fortified complementary food supplementation on child growth in rural Bangladesh: a cluster-randomized trial. Int J Epidemiol 2015;44:1862–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, Webb P, Lartey A, Black RE; Lancet Nutrition Interventions Review Group, Maternal and Child Nutrition Study Group. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 2013;382:452–77. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S, Suri D, Uauy R. Assessment of protein adequacy in developing countries: quality matters. Br J Nutr 2012;108(Suppl 2):S77–87. [DOI] [PubMed] [Google Scholar]
- 16.Semba RD, Shardell M, Sakr Ashour FA, Moaddel R, Trehan I, Maleta KM, Ordiz MI, Kraemer K, Khadeer MA, Ferrucci L, et al. . Child stunting is associated with low circulating essential amino acids. EBioMedicine 2016;6:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semba RD, Zhang P, Gonzalez-Freire M, Moaddel R, Trehan I, Maleta KM, Ordiz MI, Ferrucci L, Manary MJ. The association of serum choline with linear growth failure in young children from rural Malawi. Am J Clin Nutr 2016;104:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breier M, Wahl S, Prehn C, Fugmann M, Ferrari U, Weise M, Banning F, Seissler J, Grallert H, Adamski J, Lechner A. Targeted metabolomics identifies reliable and stable metabolites in human serum and plasma samples. PLoS One 2014;9:e89728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin P, Peter A, Franken H, Zhao X, Neukamm SS, Rosenbaum L, Lucio M, Zell A, Häring HU, Xu G, et al. . Preanalytical aspects and sample quality assessment in metabolomics studies of human blood. Clin Chem 2013;59:833–45. [DOI] [PubMed] [Google Scholar]
- 20.Evans AM, Bridgewater BR, Liu Q, Mitchell MW, Robinson RJ, Dai H, Stewart SJ, DeHaven CD, Miller LAD. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics 2014;4:132. [Google Scholar]
- 21.Dehaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform 2010;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Storey JD. A direct approach to false discovery rates. J R Stat Soc B 2002;64:479–98. [Google Scholar]
- 23.Storey JD, Taylor JE, Siegmund D. Strong control, conservative point estimation, and simultaneous conservative consistency of false discovery rates: a unified approach. J R Stat Soc B 2004;66:187–205. [Google Scholar]
- 24.Rachakonda V, Gabbert C, Raina A, Bell LN, Cooper S, Malik S, Behari J. Serum metabolomic profiling in acute alcoholic hepatitis identifies multiple dysregulated pathways. PLoS One 2014;9:e113860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S. ppcor: partial and semi-partial (part) correlation. R package version 1.1 [Internet]. c2015 [cited 2017 Jun 30]. Available from: https://CRAN.R-project.org/package=ppcor.
- 26.Schafer J, Opgen-Rhein R, Zuber V, Ahdesmaki M, Duarte Silva AP, Strimmer K. corpcor: efficient estimation of covariance and (partial) correlation. R package version 1.6.8 [Internet]. c2015 [cited 2017 Jun 30]. Available from: https://CRAN.R-project.org/package=corpcor.
- 27.Storey JD, Bass AJ, Dabney A, Robinson D. qvalue: q-value estimation for false discovery rate control. R package version 2.6.0 [Internet]. c2015 [cited 2017 Jun 2017]. Available from: https://github.com/StoreyLab/qvalue.
- 28.Best CM, Sun K, de Pee S, Sari M, Bloem MW, Semba RD. Paternal smoking and increased risk of child malnutrition among families in rural Indonesia. Tob Control 2008;17:38–45. [DOI] [PubMed] [Google Scholar]
- 29.Best CM, Sun K, de Pee S, Bloem MW, Stallkamp G, Semba RD. Parental tobacco use is associated with increased risk of child malnutrition in Bangladesh. Nutrition 2007;23:731–8. [DOI] [PubMed] [Google Scholar]
- 30.Semba RD, Campbell AA, Sun K, de Pee S, Akhter N, Moench-Pfanner R, Rah JH, Badham J, Kraemer K, Bloem MW. Paternal smoking is associated with greater food insecurity among poor families in rural Indonesia. Asia Pac J Clin Nutr 2011;20:618–23. [PubMed] [Google Scholar]
- 31.Forsyth S, Gautier S, Salem N Jr.. Global estimates of dietary intake of docosahexaenoic acid and arachidonic acid in developing and developed countries. Ann Nutr Metab 2016;68:258–67. [DOI] [PubMed] [Google Scholar]
- 32.Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA. Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci USA 1993;90:1073–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsyth S, Gautier S, Salem N Jr.. Dietary intakes of arachidonic acid and docosahexaenoic acid in early life - with a special focus on complementary feeding in developing countries. Ann Nutr Metab 2017;70:217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colombo J, Carlson SE, Cheatham CL, Shaddy DJ, Kerling EH, Thodosoff JM, Gustafson KM, Brez C. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am J Clin Nutr 2013;98:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harauma A, Yasuda H, Hatanaka E, Nakamura MT, Salem N Jr., Moriguchi T. The essentiality of arachidonic acid in addition to docosahexaenoic acid for brain growth and function. Prostaglandins Leukot Essent Fatty Acids 2017;116:9–18. [DOI] [PubMed] [Google Scholar]
- 36.Hadley KB, Ryan AS, Forsyth S, Gautier S, Salem N Jr.. The essentiality of arachidonic acid in infant development. Nutrients 2016;8:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salem N Jr., Wegher B, Mena P, Uauy R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci USA 1996;93:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res 2010;186:113–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harteneck C. Pregnenolone sulfate: from steroid metabolite to TRP channel ligand. Molecules 2013;18:12012–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Menezes KJ, Peixoto C, Nardi AE, Carta MG, Machado S, Veras AB. Dehydroepiandrosterone, its sulfate and cognitive functions. Clin Pract Epidemiol Ment Health 2016;12:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stárka L, Dušková M, Hill M. Dehydroepiandrosterone: a neuroactive steroid. J Steroid Biochem Mol Biol 2015;145:254–60. [DOI] [PubMed] [Google Scholar]
- 42.Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA. The regulation of steroid action by sulfation and desulfation. Endocr Rev 2015;36:526–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demarquoy J, Georges B, Rigault C, Royer MC, Clairet A, Soty M, Lekounoungou S, Le Borgne F. Radioisotopic determination of L-carnitine content in foods commonly eaten in Western countries. Food Chem 2004;86:137–42. [Google Scholar]
- 44.Semba RD, Trehan I, Li X, Moaddel R, Ordiz MI, Maleta KM, Kraemer K, Shardell M, Ferrucci L, Manary M. Environmental enteric dysfunction is associated with carnitine deficiency and altered fatty acid oxidation. EBioMedicine 2017;17:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med 1999;27:916–21. [DOI] [PubMed] [Google Scholar]
- 46.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci 2001;38:263–355. [DOI] [PubMed] [Google Scholar]
- 47.Wu G. Amino acids: biochemistry and nutrition. Boca Raton (FL): CRC Press; 2013. [Google Scholar]
- 48.Balsom PD, Söderlund K, Ekblom B. Creatine in humans with special reference to creatine supplementation. Sports Med 1994;18:268–80. [DOI] [PubMed] [Google Scholar]
- 49.Schutte JE, Longhurst JC, Gaffney FA, Bastian BC, Blomqvist CG. Total plasma creatinine: an accurate measure of total striated muscle mass. J Appl Physiol 1981;51:762–6. [DOI] [PubMed] [Google Scholar]
- 50.Gjaltema RA, Bank RA. Molecular insights into prolyl and lysyl hydroxylation of fibrillar collagens in health and disease. Crit Rev Biochem Mol Biol 2017;52:74–95. [DOI] [PubMed] [Google Scholar]
- 51.Mattes W, Yang X, Orr MS, Richter P, Mendrick DL. Biomarkers of tobacco smoke exposure. Adv Clin Chem 2014;67:1–45. [DOI] [PubMed] [Google Scholar]
- 52.Cross AJ, Boca S, Freedman ND, Caporaso NE, Huang WY, Sinha R, Sampson JN, Moore SC. Metabolites of tobacco smoking and colorectal cancer risk. Carcinogenesis 2014;35:1516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zierer J, Kastenmüller G, Suhre K, Gieger C, Codd V, Tsai PC, Bell J, Peters A, Strauch K, Schulz H, et al. . Metabolomics profiling reveals novel markers for leukocyte telomere length. Aging (Albany NY) 2016;8:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brathwaite R, Addo J, Smeeth L, Lock K. A systematic review of tobacco smoking prevalence and description of tobacco control strategies in sub-Saharan African Countries; 2007 to 2014. PLoS One 2015;10:e0132401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibbs K, Collaco JM, McGrath-Morrow SA. Impact of tobacco smoke and nicotine exposure on lung development. Chest 2016;149:552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raghuveer G, White DA, Hayman LL, Woo JG, Villafane J, Celermajer D, Ward KD, de Ferranti SD, Zachariah J; American Heart Association Committee on Atherosclerosis, Hypertension, and Obesity in the Young of the Council on Cardiovascular Disease in the Young. Behavior change for improving health factors committee of the council on lifestyle and cardiometabolic health and council on epidemiology and prevention; and stroke council. Cardiovascular consequences of childhood secondhand tobacco smoke exposure: prevailing evidence, burden, and racial and socioeconomic disparities: a scientific statement from the American Heart Association. Circulation 2016;134:e336–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iannotti LL, Lutter CK, Stewart CP, Gallegos Riofrío CA, Malo C, Reinhart G, Palacios A, Karp C, Chapnick M, Cox K, et al. . Eggs in early complementary feeding and child growth: a randomized controlled trial. Pediatrics 2017;140:e20163459. [DOI] [PubMed] [Google Scholar]