Abstract

An Ugi multicomponent reaction based two-step strategy was applied to generate medium-sized rings. In the first linear expansion phase, a series of diamines reacted with cyclic anhydrides to produce different lengths of terminal synthetic amino acids as the starting material for the second phase. The Ugi-4-center 3-component reaction was utilized to construct complex medium-sized rings (8–11) by the addition of isocyanides and oxo components. This method features mild conditions and a broad substrate scope.
Medium-sized rings are molecules which contain between 8 and 11 ring atoms.1,2 Surprisingly, they are highly underrepresented in screening libraries and in general in the synthetic organic chemistry world. Examples of bioactive medium-sized cycles, however, exist and include taxol (8-membered), griseoviridin (9-membered),3 crotalanian alkaloids (10-membered), and diplodialide natural products (11-membered).4 As opposed to small rings or macrocycles, medium-sized rings are a challenging class of synthetic targets.5 While small rings can often be closed based on favorable enthalpy, macrocyclic ring closure needs a favorable entropic component. Medium cycles, however, often show an unfavorable entropy and enthalpy component, including Pitzer ring strains and transannular interactions, for their ring closure and thus are highly demanding synthetic targets.6 Different synthetic methodologies have been applied for the ring closure of medium-sized cycles, including ring-closing metathesis (RCM), macro-lactonization, metal mediated C–C coupling reactions, and ring expansion.7 However, these methods mostly suffer from low yields and limited substrate scope. Therefore, the discovery of general methodologies for the fast and efficient construction of various medium rings in good yields and with useful levels of diversity is of considerable importance. Our design toward the synthesis of complex medium ring structures is based on two simple but diverse reactions steps. Moreover, it is based on commercially available starting materials. The first step involves a ring opening reaction of cyclic carboxylic acid anhydrides with unprotected diamines to afford α,ω-amino acids. In the next step, α,ω-amino acids are used in an Ugi reaction with oxo components and isocyanides to close medium rings of 8–11 membered size (Figure 1). This method closely follows our recently described strategy to create a manifold of artificial macrocycles by a short sequence involving an initial linear diversification, followed by an exponential diversification step of macrocyclization using an Ugi and Passerini multicomponent reaction (MCR) .8,9
Figure 1.

Described macrocyclization strategy.
We initially started our study by the synthesis of α-amino ω-carboxylic acid by reacting symmetrical and unsymmetrical cyclic anhydride with diamines followed by an Ugi reaction. We reacted a total of nine cyclic anhydrides with five diamines providing 11 amino acids of different linker length (Scheme 1).
Scheme 1. Starting Materials for the Synthesis of α-Amino ω-Carboxylic Acids.
All starting materials are commercially available.
For the synthesis of medium-sized ring compounds, we focused on the small diamines, such as ethylene diamine, propane diamine, butane diamine, and pentane diamine derivatives (Scheme 1). The ring opening reaction of cyclic anhydrides was carried out by slowly dropping anhydride to the unprotected alkyl diamine solution in THF as an aprotic polar solvent. This reaction proceeded under diluted conditions (0.1 M), on a 10 mmol scale, at room temperature, and the product α-amino ω-carboxylic acid was isolated in good yields (59–80%). Next, the macrocyclic ring closure was performed by Ugi MCR under optimized conditions using 1 equiv of oxo component and isocyanide (Scheme 1). We extensively screened different conditions by varying temperature, solvent, and time. By using methanol as a solvent at 0.01 M concentrations of reactants and after 48 h at room temperature, a range of medium-sized rings were obtained in optimal yields. To investigate substrate scope and limitations, we synthesized 23 examples, as shown in Scheme 2. Several commercially available aliphatic, aromatic, and heteroaromatic aldehyde and ketone building blocks reacted as oxo-components in the Ugi reaction to afford the medium-sized cycles in acceptable good to excellent (21–65%) yields after purification by column chromatography. Variously functionalized isocyanides, such as aliphatic, aromatic, and benzylic, including indole-derived isocyanides, reacted well to give the desired products.
Scheme 2. Ugi-4CR Synthesis of Medium-Sized Rings and the Product Structures with Isolated Yields.
In light of our efforts to find an effective method of synthesizing medium-sized rings in a one-pot reaction, including amino acid synthesis and also in situ isocyanide synthesis, we used some unpurified synthetic amino acids in the ring closure step by simply removing the THF solvent from the anhydride ring opening reaction and reacting the crude amino acid with the oxo and isocyanide components in methanol. With this one-pot procedure, we were able to produce medium-sized rings (for examples, 6a, 6b, 6h, 6k, and 6u) in 27% to 65% yields. In a similar way, we also simplified and extensively studied the in situ isocyanide formation and ring closure reactions in one pot. Thus, we used our recently described in situ isocyanide synthesis protocol from formamides to produce the complex IMCR products in one pot, without tedious synthesis and isolation of the foul smelling and toxic isocyanides.10 Triphosgene (0.4 equiv) with Et3N (2.4 equiv) in dichloromethane (DCM) (0.5 M) proved to be the best dehydrating system for the isocyanide formation. N-Benzylformamide was treated with 2.4 equiv of Et3N and 0.4 equiv of triphosgene in DCM (0.5 M) as solvent at 0 °C. After 15 min, the crude mixture was passed through a small bed of silica gel into a round-bottom flask, which already contained amino acid 3i and 2-azidobenzaldehyde, as shown in Scheme 3. The reaction mixture was stirred for another 48 h, and the 11-membered medium-sized ring product (6p) was obtained in 31% yield after column purification. These in situ methods will increase the value of MCR chemistry, even though the in situ isocyanide process gives a lower yield compared to the procedure involving isolation and purification of the isocyanide and amino acid (6p, 40% yield, Scheme 3).
Scheme 3. One-Pot in Situ Isocyanide Synthesis Followed by Ugi-MCR.

A determinant of passive membrane permeation is the potential of macro- and medium-sized cycles to form intramolecular hydrogen bonds which improve their chameleonic behavior by switching between conformations in aqueous solution and while passing through lipid cell membranes by exposing polar atoms and hydrophobic residues, respectively.11 Thus, we were interested in oxidizing the sulfur medium-sized cycle 6q to the sulfoxide and the sulfone, respectively. The chemistry to the sulfoxide and sulfones was accomplished by m-chloroperbenzoic acid (mCPBA) in DCM (Scheme 4). The compound 6q was reacted with 1.0 and 4.0 equiv of mCPBA in DCM for 4 h to afford sulfoxide 7qa and sulfone 7qb in excellent yields (70% and 90%, respectively). Our future plan is to investigate how this sulfoxide and sulfone can participate in the formation of amide–sulfoxide and amide–sulfone intramolecular hydrogen bonds and thus determine the 3D conformations.
Scheme 4. Selective Oxidative Modifications on Refractory Sulfur Contained Medium Cycles.
Diastereomer formation was also investigated by reacting 3j, chlorobenzaldehyde, and benzyl isocyanide. Not surprisingly, a moderate dr of 7:3 was observed by 1H NMR of the crude reaction mixture (Scheme 5).
Scheme 5. Example of Reaction Diasteroselectivity.
In order to confirm the product structure and to gain insight into the ring conformation and intra- vs intermolecular hydrogen bonding, we crystallized compound 6i and determined its solid-state structure by X-ray crystallography (Figure 2). Interestingly, in the 6i structure, the exocyclic isocyanide derived amide group is bending back over the macrocycle to form an intramolecular hydrogen bonding with the medium-sized cycle amide group. Moreover, the medium cycle secondary amide undergoes a hydrogen bonding with the same amide group of a neighboring medium cycle. This kind of intramolecular hydrogen bonding in related medium or macrocyclic systems has been determined to facilitate passive membrane diffusion, an important determinant of cellular activity and oral bioavailability.12,13
Figure 2.

Representative MCR-derived 10-membered ring 6i in solid state featuring intramolecular and intermolecular hydrogen bonding contacts.
Physicochemical properties are of uttermost importance for the usefulness of compounds as chemical probes or development candidates. What is the profile of our medium-sized cycles? To answer this question, we constructed a random virtual 1000 medium ring library (Supporting Information). Then, we calculated the properties of the library, including molecular weight, lipophilicity, number of hydrogen bond donors and acceptors, number of rotatable bonds, polar surface area, and moment of inertia. Interestingly, an analysis of the library shows 30% obey the Lipinski rule of five (RO5). Even more stringent central nervous system multiparameter optimization (CNS MPO) desirability can be reached for a percentage of the compound.14 The cLogP versus MW distribution is favorably drug-like with an average MW and cLogP of 538 and 3.3, respectively (Figure 3). Moreover, punctual analysis of 3D modeled representatives and X-ray structures underline the nonflat shapes of the medium-sized rings.
Figure 3.
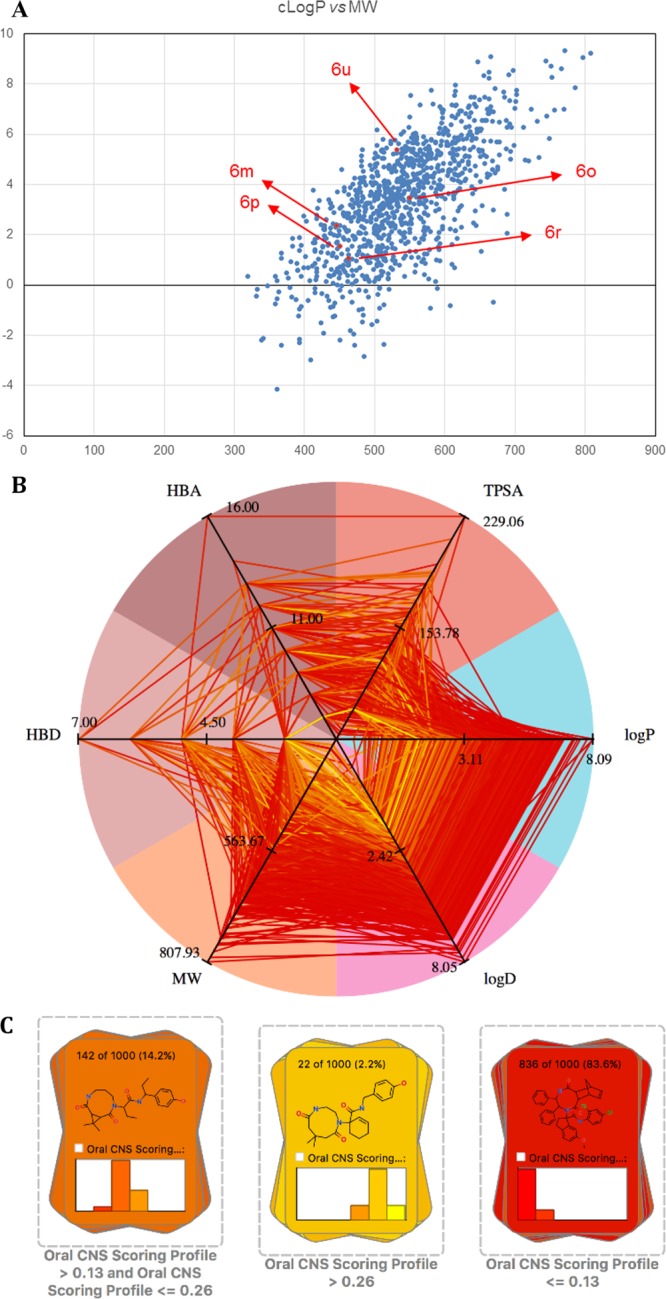
Some calculated physicochemical properties of the chemical space of medium-sized rings. (A) cLogP over MW scatter plot. (B) Oral CNS scoring radar plot. (C) Compound distribution based on oral CNS scoring.14
In conclusion, we introduced a very mild, straightforward two-step, rapid, and highly diverse medium-sized cycle (8–11 membered) synthesis pathway via MCRs. The strategy using an Ugi reaction in the cyclization is particularly appealing for several reasons. First, it offers outstanding diversity. Second, it potentially provides various medicinally and pharmaceutically important products containing an amide bond that can serve as conformationally constrained peptidomimetics. Finally, this strategy will allow a unique route for the synthesis of medium-sized cyclic nonpeptidic molecules. We are currently synthesizing and screening extensive medium-ring libraries and will report on their biological activity in due course.
Acknowledgments
The work was financially supported by NIH 2R01GM097082-05, the Innovative Medicines Initiative (Grant Agreement No. 115489), also European Union’s Seventh Framework Programme (FP7/2007-2013), and EFPIA companies’ in-kind contribution. Funding came also from the European Union’s Horizon 2020 research and innovation programme under MSC ITN “Accelerated Early stage drug dIScovery” (No. 675555), CoFund ALERT (No 665250), and the Dutch Cancer Society (KWF Grant Agreement No. 10504). The work was supported by the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (Contract No. POIG.02.01.00-12-023/08). Eman M. M. Abdelraheem was supported by the Egyptian government.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.7b03094.
General procedures, characterization data of all the compounds, crystal structure determination and computational chemistry (PDF)
Author Contributions
∥ E.M.M.A. and R.M. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Brown H. C.; Fletcher R. S.; Johannesen R. B. J. Am. Chem. Soc. 1951, 73, 212. 10.1021/ja01145a072. [DOI] [Google Scholar]
- Prelog V. Pure Appl. Chem. 1963, 6, 545. 10.1351/pac196306040545. [DOI] [PubMed] [Google Scholar]
- Appukkuttan P.; Dehaen W.; Van Der Eycken E. Chem. - Eur. J. 2007, 13, 6452. 10.1002/chem.200700177. [DOI] [PubMed] [Google Scholar]
- Ferraz H. M. C.; Bombonato F. I.; Sano M. K.; Longo L. S. Quim. Nova 2008, 31, 885. 10.1590/S0100-40422008000400029. [DOI] [Google Scholar]
- Hussain A.; Yousuf S. K.; Mukherjee D. RSC Adv. 2014, 4, 43241. 10.1039/C4RA07434C. [DOI] [Google Scholar]
- a Hendrickson J. B. J. Am. Chem. Soc. 1964, 86, 4854. 10.1021/ja01076a027. [DOI] [Google Scholar]; b Anslyn E. V., Dougherty D. A., Eds. Modern Physical Organic Chemistry; Higher Education Press: Beijing, 2009. [Google Scholar]
- a Molander G. A. Acc. Chem. Res. 1998, 31, 603. 10.1021/ar960101v. [DOI] [Google Scholar]; b Yet L. Chem. Rev. 2000, 100, 2963. 10.1021/cr990407q. [DOI] [PubMed] [Google Scholar]; c Bauer R. A.; Wenderski T. A.; Tan D. S. Nat. Chem. Biol. 2012, 9, 21. 10.1038/nchembio.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Vo C.-V. T.; Luescher M. U.; Bode J. W. Nat. Chem. 2014, 6, 310. 10.1038/nchem.1878. [DOI] [PubMed] [Google Scholar]; e Hall J. E.; Matlock J. V.; Ward J. W.; Gray K. V.; Clayden J. Angew. Chem., Int. Ed. 2016, 55, 11153. 10.1002/anie.201605714. [DOI] [PubMed] [Google Scholar]
- a Liao G. P.; Abdelraheem E. M. M.; Neochoritis C. G.; Kurpiewska K.; Kalinowska-Tluscik J.; McGowan D. C.; Domling A. Org. Lett. 2015, 17, 4980. 10.1021/acs.orglett.5b02419. [DOI] [PubMed] [Google Scholar]; b Madhavachary R.; Abdelraheem E. M. M.; Rossetti A.; Twarda-Clapa A.; Musielak B.; Kurpiewska K.; Kalinowska-Tluscik J.; Holak T. A.; Domling A. Angew. Chem., Int. Ed. 2017, 56, 10725. 10.1002/anie.201704426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelraheem E. M. M.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. J. Org. Chem. 2016, 81, 8789. 10.1021/acs.joc.6b01430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neochoritis C. G.; Stotani S.; Mishra B.; Dömling A. Org. Lett. 2015, 17, 2002. 10.1021/acs.orglett.5b00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitty A.; Zhong M.; Viarengo L.; Beglov D.; Hall D. R.; Vajda S. Drug Discovery Today 2016, 21, 712. 10.1016/j.drudis.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsson P.; Kihlberg J. J. Med. Chem. 2017, 60, 1662. 10.1021/acs.jmedchem.7b00237. [DOI] [PubMed] [Google Scholar]
- Villar E. A.; Beglov D.; Chennamadhavuni S.; Porco J. A. Jr; Kozakov D.; Vajda S.; Whitty A. Nat. Chem. Biol. 2014, 10, 723. 10.1038/nchembio.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T. T.; Hou X.; Verhoest P. R.; Villalobos A. ACS Chem. Neurosci. 2016, 7, 767–775. 10.1021/acschemneuro.6b00029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






