Abstract
Hydrodistilled leaves of Chenopodium album yielded 0.64 % v/w of essential oil. GC and GC/MS analyses of the oil revealed that the bulk of the oil was constituted by aromatic compounds (60.1 %). The abundant constituents of the oil were: p- cymene (40.9 %), ascaridole (15.5 %), pinane-2-ol (9.9 %), α-pinene (7.0 %), β-pinene (6.2 %) and α-terpineol (6.2 %). The oil displayed strong anti-inflammatory activity against 12-O-tetradecanoylphorbol-13-acetate (TPA) - induced ear edema in mice.
Keywords: Chenopodium album, chenopodiaceae, p-cymene, ascaridole, pinane-2-ol, alpha-terpineol, anti-inflammatory
Introduction
Chenopodium album L. (family Chenopodiaceae) is an annual shrub widely grown in Europe, North America, Asia, and Africa. It is commonly known as pigweed, fat hen or lamb-quarters' (Bailey, 1977[2]; GRIN Database, 2005[15]). The plant is used in folk medicine in different parts of the world. As therapeutic agents, it is used as laxative, antihelmintic against round and hook worms and blood purifier. Its use for the treatments of hepatic disorders, spleen enlargement, intestinal ulcers and burns has also been documented (Sarma et al., 2008[24]).
Various bioactivities such as antifungal (Tahara et al., 1994[25]; Maruta et al., 1995[21]), antipruritic, antinociceptive (Dai et al., 2002[6]) and hypotensive (Gohar and Elmazar, 1997[13]) properties of crude and isolated compounds from the plant justified its uses in traditional medicine. Phytochemical analyses revealed the presence of alkaloids (Horio et al., 1993[16]; Cutillo et al., 2004[5]), apocarotenoids (DellaGreca et al., 2004[8]), flavonoids (Gohar and Elmazar, 1997[13]), phytoecdysteroids (Dinan, 1992[11]; Dinan et al., 1998[12]; DellaGreca et al., 2005[7]) and an unusual xyloside (DellaGreca et al., 2005[9]) in the plant. Earlier work on the leaf essential oil of East Mediterranean grown C. album revealed the abundance of Limonene (23.2 %), α-terpinyl acetate (13.7 %), α-terpinene (12.3 %) and cis-ascaridole (12.2 %) in the oil (Dembitsky et al., 2008[10]).
It has been established that geographical and agroclimatic conditions could affect the composition pattern of essential oil of a particular plant and therefore its biological activities (Lahlou, 2004[20]). Based on these factors, we investigate the leaf essential oil of Nigerian grown C. album and its anti-inflammatory activity.
Experimental
Plant materials
The fresh leaves of Chenopodium album were obtained in Ilorin, Kwara State, North Central, Nigera. Identification was carried out at the herbarium of the Department of Plant Biology, University of Ilorin, Nigeria, where voucher specimens were deposited.
Oil isolation
Pulverized leaves of Chenopodium album were hydrodistilled for 3 h in a Clevenger-type apparatus according to the British Pharmacopoeia (1980[4]) specification. The resulting oil was collected, preserved in a sealed sample tube and stored under refrigeration until analysis.
Gas chromatography
GC analysis were performed on an orion micromat 412 double focusing gas chromatography system fitted with two capillary columns coated with CP-Sil 5 and CP-Sil 19 (fused silica, 25 m×0.25 mm, 0.15 µm film thickness) and flame ionization detector (FID). The volume injected was 0.2 µL and the split ratio was 1:30. Oven temperature was programmed from 50-230° C, respectively. Qualitative data were obtained by electronic integration of FID area percents without the use of correction factors.
Gas chromatography/mass spectrometry
A Hewlett Packard (HP 5890A) GC interfaced with a VG Analytical 70-250S double focusing mass spectrometer was used. Helium was the carrier gas at 1.2 ml/min. The MS operating conditions were: ionization voltage 70 ev, ion source temperature 230° C. The GC was fitted with a 25 m×0.25 mm, fused silica capillary column coated with CP-Sil 5. The film thickness was 0.15 µm. The GC operating conditions were identical with those of GC analysis. The MS data were acquired and processed by online desktop computer equipped with disk memory. The percentage compositions of the oil were computed in each case from GC peak areas. The identification of the components was based on the retention indices (determined relative to the retention times of series of n-alkanes) and mass spectra with those of authentic samples and with data from literature (Jennings and Shibamito, 1980[17]; Adams 1995[1]; Joulain and Koenig, 1998[18]).
Anti-inflammatory activity of leaf essential oil of C. album
The animal experiment were approved by the Department of Biochemistry, University of Ilorin, Animal Care and Use Committee and conducted according to standard guidelines. Sixty-four, 21-day old male swiss Webster mice, weighing between 22 and 25 g were housed in groups of 8 in an NIH - approved facility. All groups were fed with standard rodent diet (Test diet 570B, Purina Mills, St. Louis, MO) and supplied water ad libitum, throughout the experimental period. The light in the facility were turned off between 1900 and 0700 h, with the environmental temperature maintained at 24 ± 1° C. All experimental procedures were conformed to the National Institute of Health, Public Health Service and Animal Welfare Act guidelines for the ethical treatment of laboratory animals.
Topical anti-inflammatory assay
A modification of the method of Young et al. (1984[26]) was used. The topical anti-inflammatory activity was evaluated as inhibition of the 12-O-tetradecanoylphorbol-13-acetate (TPA) induced ear edema in mice by the oils following standard procedure (Young et al., 1984[26]; Griffiths et al., 1988[14]; Bralley et al., 2008[3]). Edema was induced in ears of each mouse by the topical application of 2 µg TPA dissolved in 20 µl of acetone to both the inner and outer surfaces of the right ear (surface: about 1 cm2). Thirty minutes after the application of TPA, the inner and outer surfaces of each ear were treated (10 ul to each side) with:
50 % ethanolic solutions of the test essential oil (EO) in doses of 0.625 (CA4), 1.25 (CA3), 2.5 (CA2) and 5.0 (CA1) mg eo/ear (n = 8 at each dosage)
50 % ethanol (vehicle control)
Indomethacin (0.25 mg/ear dissolved in 50 % ethanol (as a standard anti-inflammatory drug).
The thickness of each ear was measured using a micrometer (Mitutoyo Series IP65, Mitutoyo America, Aurora, IL) before and at 4 h and 24 h after tetradecanoylphorobol-13-acetate administration. The micrometer was applied near the top of the ear distal to the cartilaginous ridges. At 24 h, each animal was sacrificed and a plug biopsies (6 mm diameter hole punch) were removed from both the treated (right) and the untreated (left) ears immediately, weighed, frozen and stored at 8° C. The edematous response was measured as the weight difference between the two plugs. The anti-inflammatory activity was expressed as percentage of the edema reduction in treated mice compared to the control mice. The pharmacological data were analyzed by the Student's t-test, and a probability level lower than 0.05 was considered as significant.
Results and Discussion
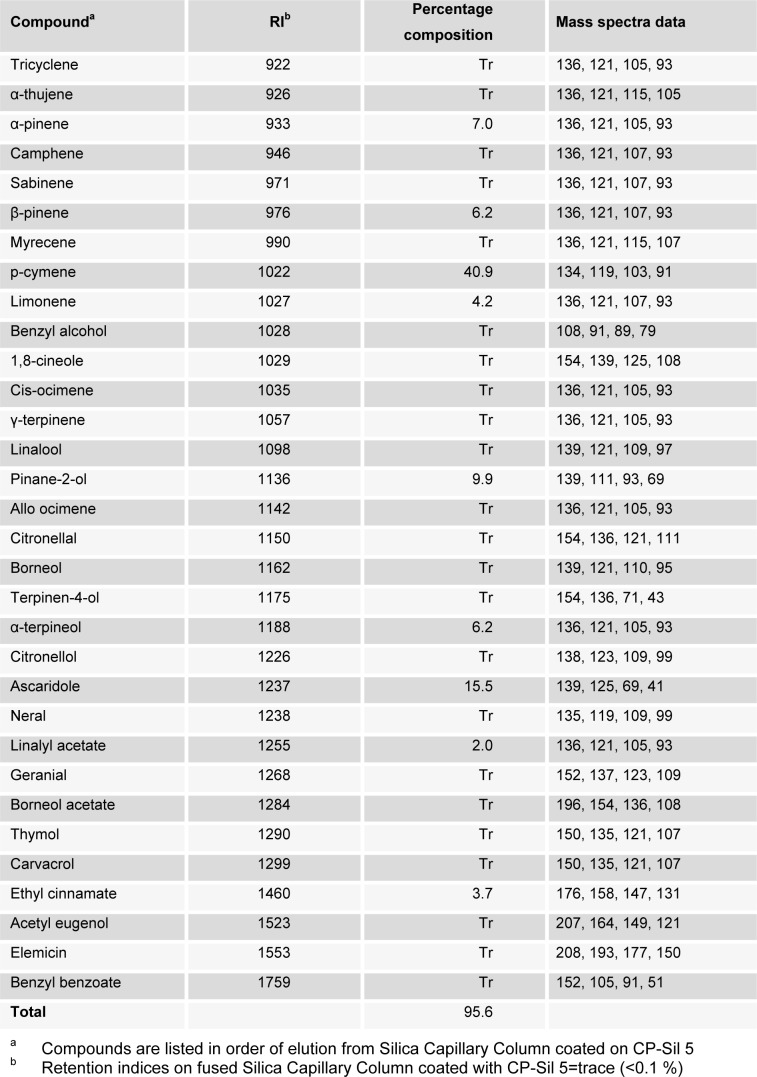
Pulverized leaves of Chenopodium album afforded oil in the yield of 0.64 % v/w. Table 1(Tab. 1) shows the retention indices, mass spectra data, identities and relative percentage of the constituents of the oil. A total of 37 compounds that represent 95.6 % of the oil were identified from their mass spectra. Hydrocarbon and oxygenated monoterpenes constituted 17.4 and 18.1 % of the oil. The percentage composition of aromatic compounds in the oil was 60.9 %.
Table 1. Chemical composition (%) of leaf oil of Chenopodium album.
The oil was characterized by the abundance of aromatic compounds. Bulk of the aromatic compounds was constituted by p-cymene (40.9 %). Other notable aromatic compounds in the oil were ascaridole (15.5 %) and ethyl cinnamate (3.7 %).
α-pinene was the most abundant hydrocarbon monoterpene in the oil. Other hydrocarbon monoterpenes that were found in significant proportions were β-pinene (6.2 %), limonene (4.2 %). The most abundant oxygenated monoterpene in the oil was pinane-2-ol (9.9 %). α-terpineol (6.2 %) and linalyl acetate (2.0 %) also occurred in appreciable quantities.
Qualitative and quantitative compositions of the oil were found to be different from the leaf essential oil of East Mediterranean grown C. album (Dembitsky et al., 2008[10]). For instance, the principal constituents in the leaf oil of the Nigerian grown species, α-terpineol, pinane-2-ol, linalyl acetate, p-cymene and ethyl cinnamate, were not identified in the oil of East Mediterranean C. album. The most abundant constituent of the oil was p-cymene; hence, the oil was of p-cymene chemotype. On the other hand, terpinenyl acetate and terpinen-1-ol that were found in appreciable amounts in the oil of East Mediterranean grown C. album were not identified in the oil of the Nigerian grown C. album. Meanwhile, α-pinene, β-pinene, limonene and ascaridole were identified in both oils. These constituents were of greater abundance in the oil of the Nigerian grown C. album except limonene that predominate the leaf oil of East Mediterranean grown C. album. Variations in composition patterns of the oils may be attributed to agroclimatic and geographical conditions.
It has been established that anti-inflammatory activities of essential oils are attributable to the presence of substituent such as; limonene, linalool, linalyl acetate and α-pinene (Peana et al., 2002[23]; Özbek and Sever, 2005[22]; Karaca et al., 2007[19]).
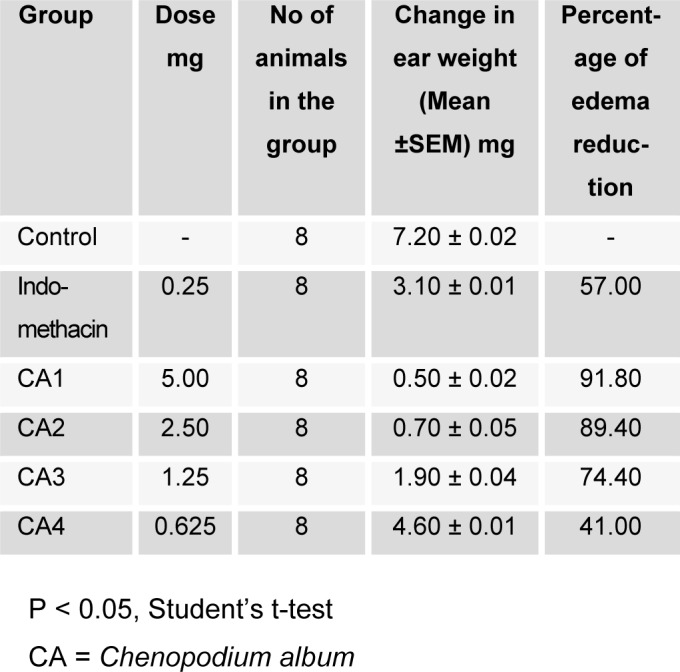
The result of anti-inflammatory activity of leaf essential oil of Chenopodium album is presented in Table 2(Tab. 2).The result revealed that the anti-inflammatory action of the oil is concentration dependent. Hence, the percentage reduction in the ear edema increases with increase in concentration of the oil. Furthermore, the oil caused significant reduction (p < 0.05) in the ear edema except at 0.625 mg concentration. The reduction may be due to the presence of α-pinene, linalool and linaly acetate in the oil. Hence, the oil can be use as anti-inflammatory agent.
Table 2. Anti-inflammatory activity of leaf essential oil of Chenopodium album.
References
- 1.Adams RP. Identification of essential oil components by gas chromatography and mass spectrometry. Carol Stream, IL.: Allured Publ; 1995. [Google Scholar]
- 2.Bailey LH. Manual of cultivated plants. Most commonly grown in the continental United States and Canada. Rev. ed., compl. restudied. NewYork: Macmillan; 1977. [Google Scholar]
- 3.Bralley EE, Greenspan P, Hargrove JL, Wicker L, Hartle DK. Topical anti-inflammatory activity of Polygonum cuspidatum extract in the TPA model mouse ear inflammation. J Inflamm. 2008;5:1. doi: 10.1186/1476-9255-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.British Pharmacopoeia. II. London: HMSO; 1980. p. 109. [Google Scholar]
- 5.Cutillo F, D’Abrosca B, DellaGreca M, Zarrelli A. Chenoalbicin, a novel cinnamic acid amide alkaloid from Chenopodium album. Chemistry and Biodiversity. 2004;1:1529–1583. doi: 10.1002/cbdv.200490118. [DOI] [PubMed] [Google Scholar]
- 6.Dai Y, Ye WC, Wang ZT, Matsuda H, Kubo M, But PPH. Antipruritic and antinociceptive effects of Chenopodium album L. in mice. J Ethnopharmacol. 2002;81:245–250. doi: 10.1016/s0378-8741(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 7.DellaGreca M, D’Abrosca B, Fiorentino A, Previtera L, Zarrelli A. Structure elucidation and phytotoxicity of ecdysteriods from Chenopodium album. Chemistry and Biodiversity. 2005;2:457–462. doi: 10.1002/cbdv.200590025. [DOI] [PubMed] [Google Scholar]
- 8.DellaGreca M, Di Mariono C, Zarrelli A, D’Abrosca B. Isolation and phytotoxicity of apocarotenoids from Chenopodium album. J Nat Prod. 2004;67:1492–1495. doi: 10.1021/np049857q. [DOI] [PubMed] [Google Scholar]
- 9.DellaGreca M, Previtera L, Zarrelli A. A new xyloside from Chenopodium album. Nat Prod Res. 2005;19:87–90. doi: 10.1080/14786410410001686391. [DOI] [PubMed] [Google Scholar]
- 10.Dembitsky V, Shkrob I, Hanus LO. Ascaridole and related peroxides from the genus Chenopodium. Biomed Pap Med Fac Univ Palacky Olomuc Czech Repub. 2008;152:209–215. doi: 10.5507/bp.2008.032. [DOI] [PubMed] [Google Scholar]
- 11.Dinan L. The analysis of phytoecdysteroids in single (preflowering stage) specimens of fat hen, Chenopodium album. Phytochem Anal. 1992;3:132–138. [Google Scholar]
- 12.Dinan L, Whiting P, Scott AJ. Taxonomic distribution of phytoecdysteroids in seeds of members of the chenopodiaceae. Biochem Syst Ecol. 1998;26:553–576. [Google Scholar]
- 13.Gohar AA, Elmazar MMA. Isolation of hypotensive flavonoids from Chenopodium species growing in Egypt. Phytother Res. 1997;11:564–567. [Google Scholar]
- 14.Griffiths RJ, Wood BE, Li S, Blackham A. Pharmacological modification of 12-O-tetradecanoylphorbol-13-acetate induced inflammation and epidermal cell proliferation in mouse skin. Agent Actions. 1988;25:344–51. doi: 10.1007/BF01965041. [DOI] [PubMed] [Google Scholar]
- 15.GRIN Database USDA, ARS. National Genetic Resources Program, Germplasm Resources Information Network (GRIN), National Germplasm Research Laboratory, Beltsville, Maryland. 2005. Available from: http://www.ars-grin.gov2/cgi-bin/npgs/html/genform.pl.
- 16.Horio T, Yoshida K, Kikuchi H, Kawabata J, Mizutani J. A phenolicamide from roots of Chenopodium album. Phytochemistry. 1993;33:807–808. [Google Scholar]
- 17.Jennings W, Shibamito I. Qualitative analysis of flavour volatiles by gas capillary chromatography. New York: Academic Press; 1980. [Google Scholar]
- 18.Joulain D, Koenig WA. The atlas of spectra data of sequiterpene hydrocarbons. Hamburg: E.B. Verlag; 1998. [Google Scholar]
- 19.Karaca M, Özbek H, Him A, Tütüncü M, Akkan HA, Kaplanoğlu V. Investigation of anti-inflammatory activity of Bergamot oil. Eur J Gen Med. 2007;4:176–9. [Google Scholar]
- 20.Lahlou M. Methods to study the phytochemistry and bioactivity of essential oils. Phytother Res. 2004;18:435–48. doi: 10.1002/ptr.1465. [DOI] [PubMed] [Google Scholar]
- 21.Maruta Y, Fukushi Y, Onkawa K, Nakanishi Y, Tahara S, Mizutani J. Antimicrobial stress compounds from Hypochoeris radicata. Phytochemistry. 1995;38:1169–73. doi: 10.1016/0031-9422(94)00792-r. [DOI] [PubMed] [Google Scholar]
- 22.Özbek H, Sever B. Investigation of lethal doses and anti-inflammatory effect of alpha-pinene on mice and rats. Turkish Pharmacological Society, 18th National Pharmacology Congress, September, 2005, Izmir-Turkey; 2005. p. 118. [Google Scholar]
- 23.Peana AT, D’Aquila PS, Panin F, Serra G, Pippia P, Moretti, MD Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 2002;9:721–6. doi: 10.1078/094471102321621322. [DOI] [PubMed] [Google Scholar]
- 24.Sarma H, Sarma A, Sarma CM. Traditional knowledge of weeds: a study of herbal medicines and vegetables used by the Assamese people (India) Kerba Polnica. 2008;54:80–8. [Google Scholar]
- 25.Tahara S, Kassai S, Innoue M, Kawabata J, Mizutani J. Identification of mucondialdehyde as a novel stress metabolite. Experientia. 1994;50:137–141. doi: 10.1007/BF01984952. [DOI] [PubMed] [Google Scholar]
- 26.Young JM, Spires DA, Bedford CJ, Wagner B, Ballaron SJ, De Young LM. The mouse ear inflammatory response to topical arachidonic acid. J Invest Dermatol. 1984;82:367–71. doi: 10.1111/1523-1747.ep12260709. [DOI] [PubMed] [Google Scholar]