Abstract
Dietary exposure to cadmium, even at lower doses, can lead to free radical induced neurotoxicity, neurobehavioral changes and alteration in neurotransmitters. Such changes are likely to be more pronounced in the developing brain due to incompleteness of blood brain barrier (BBB). Hippocampus being the seat of intelligence has a role in learning and cognitive behavior and any damage to hippocampus during developmental stage is likely to result in neurodegenerative changes in later life. To this end, fetal and neonatal exposure to cadmium was induced by exposing pregnant dams of Swiss albino strain throughout the period of gestation and following parturition up till 5th day post partum (pp) through drinking water (3ppm/animal/day). The neonates were sacrificed on day 6 pp and indices of oxidative stress, levels of trace elements and changes in cholinergic system were evaluated in the hippocampus. Increased lipid peroxidation, surge in reactive oxygen species (ROS), depressed antioxidant defense, increased accumulation of cadmium, differential alterations in trace elements and decreased activity of AChE were the features of cadmium toxicity. Simultaneous administration of melatonin to cadmium challenged animals offset these detrimental changes. The results suggest that melatonin co-administration can effectively protect against the adverse effects of cadmium on endogenous antioxidant status, changes in trace metal concentrations and compromised hippocampal cholinergic system.
Keywords: cadmium, melatonin, hippocampus, acetylcholinesterase, trace metals
Introduction
Food, including vegetables, is an important source of entry of cadmium (Cd) and, populations such as subsistence farmers that consume locally grown produce are at particular risk (Millis et al., 2004[48]; Chary et al., 2008[16]). An earlier study from our laboratory had found seven times higher than the recommended levels of Cd in cereals and vegetables grown along the Baroda effluent channel and irrigated with channel water (Ramachandran, 2003[55]). Several investigators have demonstrated Cd induced neurotoxicity and hippocampal damage (Kumar et al., 1996[37]; Cheung, 2003[19]; Manda and Reiter, 2010[42]). The brain is especially vulnerable during its phase of growth spurt and, the post natal day 7 of rat brain is claimed to be anatomically, biochemically and physiologically equivalent to the full term human infant (Johnston, 1983[33]). Several multifaceted functions like fear, memory, shock experience, temporal processing of objects and appetite and anxiety have all been ascribed to hippocampus (Tracy et al., 2001[65]; McEown and Treit, 2009[46]). Cholinergic neurons and their projections are widely distributed throughout CNS, exerting regulation over many vital functions such as learning, memory, cortical organization of movement and cerebral blood flow (Mesulam, 2004[47]). Alterations in cholinergic neurotransmission and consequent behavioral impairment have been observed in both animals and humans exposed to cadmium (Pari and Murugavel, 2007[53]). However, there are no reports on the effects of gestational and lactational exposure to cadmium on the effects of hippocampal cholinergic system and oxidative stress.
Melatonin (N-acetyl-5-methoxytriptamine) is synthesized mainly in the pineal gland of all mammals including humans and also by many extra-pineal tissues (Reiter et al., 2003[57]). Compelling evidence exists to suggest the efficacy of melatonin to scavenge several ROS and RNS (Reiter et al., 2003[57]). In addition, melatonin has been reported to protect fetal rat brain against oxidative mitochondrial damage (Wakatsuki et al., 2001[68]). In this background of cereals, vegetables and cigarette smoke contributing to low dose Cd exposure and, the greater vulnerability of neonates to metals, the relevance of Cd toxicity to brain in the critical phases of development and the role of any protectant become very relevant.
To the best of our knowledge, there are no reports on cholinergic perturbations due to cadmium exposure in the hippocampus of neonates. Hence, the present study was envisaged essentially to assess the protective role of melatonin against Cd induced oxidative stress and alterations in the status of antioxidants, trace elements and hippocampal cholinergic system of mice.
Materials and Methods
Female Swiss albino mice (body weight 35-40 gm approximately 8-9 weeks of age) were procured from the Central Animal House of Zoology Department, University School of Science, M.L.S. University, Udaipur, India. The animals were acclimatized under hygienic conditions and were fed on standard pelleted rat diet (Pranav Agro, Baroda) and water ad libitum. The diet had adequate quantities of micro nutrients as well as macro nutrients and the animals were kept and cared at all stages in compliance with the applicable CPCSEA guidelines.
The animals were bred by keeping males and females in the ratio of 1:2 in polypropylene cages. Mating was confirmed by the presence of vaginal copulation plug (day = 0 of gestation). One group of mothers served as control and the other group was exposed to CdCl2 through drinking water at a dose of 3 ppm/animal/day. [Stock solution of 10 ppm was kept in 250 ml drinking bottles and the rate of water consumption was monitored on a daily basis. The average intake of cadmium was found to be 3 ppm/animal/day]. In order to study the protective effect of melatonin, yet another group of mothers was administered with melatonin (10 mg/Kg body weight) through oral gavage at 16:00 hours in addition to exposure to cadmium. The pups were sacrificed on day 6 pp and the pups of 2 litters for each group were mixed to attain the experimental size of 8 each.
The summary of the various experimental groups are as below:
Control: Animals provided with RO (Reverse Osmosis) grade water.
Melatonin (Mel): Animals provided with RO grade water together with melatonin (10 mg/Kg body weight) at 16:00 hours.
Cadmium (Cd): Animals were provided with cadmium chloride at a dose of 3 ppm /animal/day through drinking water.
Cadmium + Melatonin (Cd + Mel): Animals provided with cadmium chloride at a dose of 3 ppm/animal/day through drinking water plus melatonin (10 mg/Kg body weight) at 16:00 hours.
Preparation of melatonin
Melatonin (Mel) was dissolved in absolute ethanol and then diluted with RO grade water; the final concentration of alcohol was < 1 %. The same volume of ethanol was added to all homogenates regardless of treatment.
For different biochemical measurements, the animals of different groups were sacrificed by cervical decapitation. Their brains were dissected out and hippocampus was isolated and rinsed in ice cold 0.9 % saline and blotted dry. All processes were carried under cold conditions. The hippocampus was homogenized in 10 mM/L phosphate buffer saline (10 % w/v) at pH 7.4.
All chemicals used in the study were of highest purity and of analytical grade.
Lipid peroxidation and reactive oxygen species
Hippocampal LPO was estimated according to the procedure of Beuge and Aust (1978[8]). Malondialdehyde produced during peroxidation of lipids served as an index of LPO.
Hydrogen peroxide production was assessed by the spectrophotometric method of Holland and Storey (1981[31]) and expressed as mol/min/mg protein. Hydroxyl radical production was quantified by the method of Puntarulo and Cederbaum (1988[54]) and expressed as mol/min/mg protein.
Total GSH in the hippocampus
Total GSH in the hippocampus was determined by the method of Beutler et al. (1969[9]). DTNB, a disulfide compound is readily reduced by sulfhydryl compounds forming a yellow colored chromophore whose absorbance was measured at 412 nm. The results were expressed as μg GSH/mg protein.
Assay of antioxidant enzymes
Superoxide dismutase (EC 1.15.1.1, SOD)
Superoxide dismutase activity was assayed according to the method of Marklund and Marklund (1974[43]). The rate of oxidation of pyrogallal was immediately read at 470 nm against blank containing all components except the enzyme and pyrogallol at 1 min interval for 3 min on a spectrophotometer. The enzyme activity was expressed as units/mg protein.
Catalase (EC 1.11.1.6, CAT)
Catalase activity was assayed by the method of Sinha (1972[63]). The activity of catalase was expressed as units/mg protein (1 unit is the amount of enzyme that utilizes 1 μmol of H2O2/min).
Glutathione peroxidase (EC 1.11.1.9, GPx)
The activity of glutathione peroxidase was determined by the method of Rotruck et al. (1973[58]). The enzyme activity was expressed as units/mg protein (1 U is the amount of enzyme that converts 1 mol GSH to GSSG in the presence of hydrogen peroxide/min).
Concentrations of cadmium and trace elements in hippocampus
Samples of known weight (whole hippocampus) were subjected to dry mineralization in an electric oven as per Zmudzki (1977[72]). The ash was dissolved in a known volume of 1 N HNO3. The concentrations of cadmium and the levels of trace elements in hippocampus (after appropriate dilution) were assessed by atomic absorption spectrophotometry (Thermo S series) with electrothermal atomization in a graphite cuvette (cadmium) or flame atomization in an air-acetylene burner (trace elements).The cathode lamps of respective elements were operated under standard conditions using their respective resonance lines: Cd, 228.8 nm; Zn, 213.9 nm; Cu, 324.75 nm; Fe, 248.3 nm; Mn, 279.5 nm. The concentrations of metals were expressed as μg/g wet tissue.
Determination of ACh levels
ACh levels were determined as described by Augustinssion (1963[3]). The intensity of the color developed was read at 540 nm against a reagent blank in a spectrophotometer.
Determination of AChE activity
The specific activity of AChE was determined as per the method of Ellman et al. (1961[22]). The color absorbance was measured at 412 nm in a spectrophotometer (Perkin Elmer, Model U-2000). For the determination of pseudocholinesterase activity, butyrylthiocholine iodide was added instead of acetylthiocholine iodide as the substrate and the activity of butyrylcholinesterase (BuChE) was assayed and subtracted from the total cholinesterase activity to obtain the specific activity of AChE. The enzyme activity was expressed as mmol of ACh hydrolyzed/mg protein/h.
Estimation of protein content
Protein content of hippocampus was estimated by the method of Lowry et al. (1951[40]).
Histochemistry of AChE
AChE histochemistry was performed as described by Hedreen et al. (1985[30]). Mice were anesthetized with sodium pentobarbital and perfused with 50 mM phosphate buffered saline (PBS, pH 7.4, 4 °C) followed by 4 % paraformaldehyde (4 ºC) through cardiac catheter. Brains were removed and post-fixed in paraformaldehyde for 2 h. This was followed by cryoprotection in 10 %, 20 %, and 30 % serially. Eight micron thick sections were cut in a cryostat. The sections were rinsed in 0.1 M phosphate buffer (pH 6.0) and incubated in 50 ml medium containing 32.5 ml of 0.1 M phosphate buffer (pH 6.0); 2.0 ml of 0.1 M sodium citrate; 5 ml of 0.03 M cupric sulfate; 1.0 ml of 0.0005 M potassium ferricyanide; 25 mg of acetylthiocholine iodide and 9.5 ml of distilled water. Sections were also incubated in 10 %, 20 %, and 30 % sucrose serially. The sections were incubated for 20 min at room temperature and then dehydrated in ethanol series, cleared in xylene and mounted in permount.
Results
Lipid peroxidation (LPO) and reactive oxygen species (ROS)
The levels of hippocampal LPO and reactive oxygen species, such as hydrogen peroxide and hydroxyl radical are shown in Figure 1(Fig. 1).
Figure 1. Effect of cadmium exposure and simultaneous administration of melatonin on mouse hippocampal lipid peroxidation (A), hydrogen peroxide (B) and hydroxyl radical (C) on day 6 pp. Each value is mean ± S.E.M. of 8 animals. a and b represent statistical significance at p <0.05 compared to control and cadmium, respectively.

The levels of LPO and reactive oxygen species were significantly elevated in cadmium treated rat hippocampus as compared to control. Further, simultaneous administration of melatonin controlled Cd-induced lipid peroxidation and significantly modulated the levels of MDA and ROS.
Hippocampal antioxidant enzymes
The effects of cadmium and cadmium + melatonin on the specific activities of hippocampal antioxidant enzymes such as SOD, CAT and GPx are shown in Figure 2(Fig. 2).
Figure 2. Effect of cadmium and co-treatment with melatonin on hippocampal antioxidant enzymes (A): SOD, (B): CAT, (C): GPx of control and experimental groups of mice. All values are expressed as mean ± S.E.M (n = 8). a and b represent statistical significance at p <0.05 compared to control and cadmium, respectively.

The cadmium-exposed mice showed a marked reduction (P <0.05) in the activities of SOD, CAT and GPx as compared to control. Melatonin treatment along with cadmium exposure showed maintenance of activity levels of all the three enzymes in the normal range.
Hippocampal non-enzymatic antioxidant (GSH)
The data obtained on hippocampal non-enzymatic antioxidant GSH is presented in Figure 3(Fig. 3). The concentration of GSH was significantly decreased (P <0.05) in the hippocampus of cadmium administered mice while, in Cd + Mel group, there was significant protective effect on GSH content.
Figure 3. Effect of simultaneous administration of melatonin on the status of GSH in the hippocampus of cadmium exposed mice. Values are expressed as mean ± S.E.M. of 8 animals. a and b represent statistical significance at p <0.05 compared to control and cadmium, respectively.

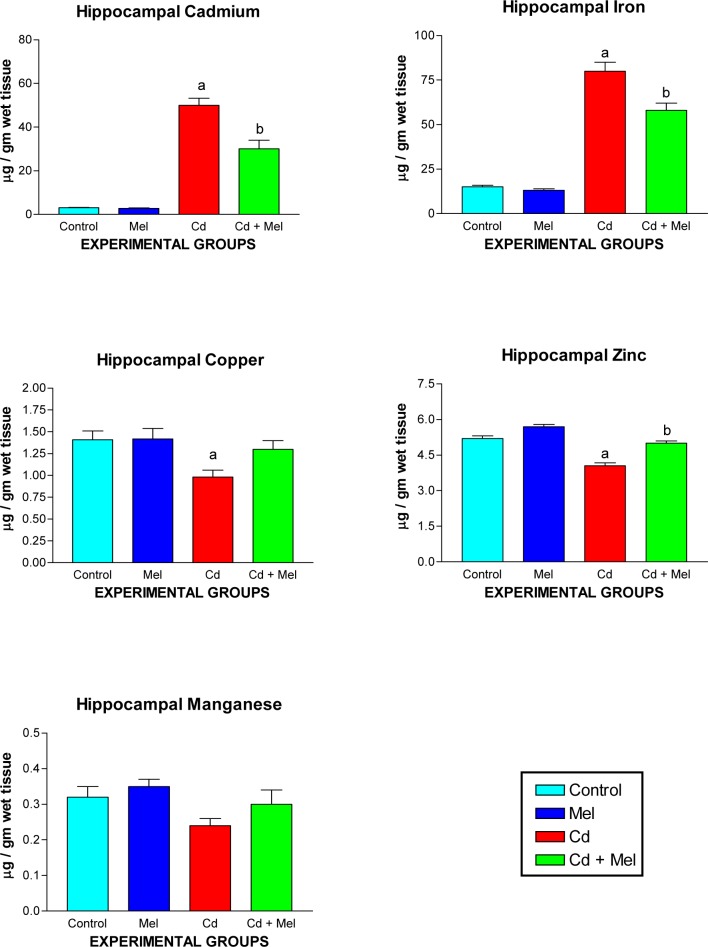
Levels of cadmium and trace elements
Contents of hippocampal cadmium and trace elements in the control and experimental groups are presented in Figure 4(Fig. 4). Administration of cadmium resulted in its accumulation in the hippocampus. Amongst the bioelements, cadmium led to an increase in hippocampal iron while there was significant decrease in Cu, Zn and Mn. Simultaneous administration of melatonin offset these cadmium induced alterations.
Figure 4. Concentration of cadmium and other trace elements in the hippocampus of cadmium treated mice. Values are expressed as mean ± S.E.M. of 8 animals. a and b represent statistical significance at p <0.05 compared to control and cadmium, respectively.
ACh level, AChE activity and AChE histochemistry
Cadmium-exposure caused significant decrease in ACh content and specific activity of AChE (Figure 5(Fig. 5)). Histochemically, AChE activity showed significant decrease in the brain of mice exposed to cadmium. Co-administration of melatonin offset these detrimental changes and maintained the AchE level (Figure 6(Fig. 6)).
Figure 5. Effect of melatonin on ACh content (A) and AChE activity (B) in Cd exposed mice. Values are expressed as mean ± S.E.M. of 8 animals. a and b represent statistical significance at P <0.05 compared to control and cadmium.

Figure 6. Histochemical staining for AChE in hippocampal region of the brain of control and Cd - exposed mice. Arrows indicate intense staining and areas marked with asterisk (*) in Cd-exposed brain show loss of AChE activity. A= Control, B= Melatonin, C = Cadmium, D= Cadmium + Melatonin, Magnification (200X).

Discussion
Cadmium induces oxidative damage in different tissues by enhancing the peroxidation of membrane lipids and by inhibiting endogenous antioxidants and enzymes involved in the utilization of reactive oxygen species (Manca et al.,1991[41]). The results of our study on hippocampus of neonatal mice indicate oxidative stress as evidenced by the surge in hydroxyl radicals and peroxide production. It has been estimated that, in excess of 50 % of molecular damage sustained as a consequence of free radicals is attributable to •OH (Hassan, 1997[29]). This highly reactive species has an estimated half-life within organisms in the order of 10-9 s and it travels hardly a few Ångstroms before it interacts with another molecule. Thus, the molecular mutilation carried out by •OH is in the immediate proximity of its generation and, the damage as a consequence, is often referred to as being site specific. The entire area encompassed by the •OH has been referred to as its 'reaction cage' (Borg, 1993[12]). With several multifaceted functions ascribed to hippocampus, such as mediation of unconditioned and conditioned fear, memory and shock experience (McEown and Treit, 2009[46]), temporal processing of objects (Tracy et al., 2001[65]) and control of appetite and anxiety, the presently observed overproduction of hydroxyl radical in Cd exposed hippocampus implies serious damage to the organ. Our data also display diminished levels of LPO and of ROS in the hippocampus of Cd + Mel animals. The ability of melatonin to scavenge free radicals has been reported in several studies (Shen et al., 2002[60]; Ananth et al., 2003[2]; Baydas et al., 2003[7]; Carretero et al., 2009[14]) and hence it is possible to assume that, lipid peroxidation triggered by ROS and transition metals can be effectively countered by melatonin as in the present study.
Subcellular membranes and associated thiol-bearing enzymes (GPx) represent sensitive sites of action for cadmium, causing perturbation in cellular functions (Chavez et al., 1985[18]). Reactive oxygen species can themselves also reduce the activity of these enzymes (Searle and Willson, 1980[59]). Significantly elevated level of MDA during metal exposure could be partly attributed to the compromised status of free radical scavenging enzymes. Enzymatic scavengers of oxygen free radicals (OFRs) like SOD, CAT, GPx, GST, GR and G6PDH may protect the system from deleterious effect of OFRs (Banerjee et al., 1999[5]) and decreased levels of SOD and CAT as seen herein can result in accumulation of superoxides and peroxides. In the present study, the recorded decrease in the activities of enzymatic antioxidants in cadmium exposed hippocampus could be construed to tilt the balance between pro-oxidants and antioxidants in favour of the former and the resultant higher levels of ROS can be implicated in augmented LPO. SOD is responsible for the dismutation of superoxide radical to hydrogen peroxide, which in turn gets neutralized by the combined action of CAT and GPx in all vertebrates (Sies, 1993[62]; Mates, 2000[45]). These enzymes act in coordination to contain oxidative stress and, compromised activities of these enzymes can portend increased oxidative stress. In keeping with the known tissue specific differential activity of CAT, central nervous system is known to have poor catalase activity (Marklund et al., 1982[44]). In the present study, reduced activities of catalase and GPx in the hippocampus of cadmium-treated mice suggest heightened free radical injury. In order to partly explain the observation on GPx, it may be worthwhile to recapitulate on the role of reduced glutathione. Both GPx and GST, are enzymes which depend on effective availability of GSH for their catalysis (Chasseaud, 1979[17]) and the reduced availability of GSH as in the present study on Cd exposure is likely to compromise the functional ability of these enzymes as has been also been reported by Mates (2000[45]). Apparently, the decreased activity of GPx can be explained as either due to a direct impairment of functional groups, or an indirect effect due to the compromised supply of reduced glutathione (GSH) and NADPH.
Cadmium is shown to preferentially bind to membrane-bound sulfhydryl groups and Ochi et al. (1988[50]) have stated that GSH can function as first line of defense against Cd even before the induction of metallothionein. The observed depletion of GSH in the present study tends to support this role of GSH and the concurrent presence of both glutathione and melatonin in the hippocampal neurons may be responsible for the noted attenuation in the level of lipid peroxidation in the Cd exposed animals. Melatonin endowed with the capacity to pass thorough both lipid and aqueous media, has the potential for direct action against free radicals by way of its non genomic mediation.
The observed increase in tissue burden of cadmium needs to be discussed in the light of the fact that, toxic manifestation in the current experimental schedule occurs by two independent mechanisms. In the gestational period, the neural uptake of metals could be attributed to an increased uptake of this toxicant through a breach in blood placental barrier while, in the suckling neonates, it is delivered through milk. In other words, day 0 pp marks the transition phase wherein fetal maternal transfer gives way to lactational transfer. Interestingly, metal uptake has been reported to be more in the sucklings with greater retention in the brain more readily during infancy than during adulthood as has been reported in rats for lead, cadmium and mercury (Kostial et al., 1978[36]; Jamall and Smith, 1985[32]; Limaye and Shaikh, 1999[38]). Thus the manifold increase in cadmium in the hippocampus in the present study can be attributed to an increased uptake of this toxicant in the developmental period. Apart from cadmium, other bioelements analyzed in our study are constituents of various metalloenzymes. Iron (Fe), a constituent of catalase, is vital to life while, its potential ability to generate ROS by Fenton reaction is a detrimental aspect. Several studies have shown that, Fe distribution in the body is affected by Cd exposure (Jurczuk et al., 1997[34]; Stojanović et al., 1999[64]; Oishi et al., 2000[51]; Djukic-Cosic et al., 2008[20]) and that, cadmium can cause redistribution of Fe and replacement of Fe in iron-dependent enzymes and proteins by (Casalino et al., 1997[15]). In our study, the Fe content is significantly elevated in the cadmium exposed group of neonates when compared to the other three groups. The present findings also demonstrate decreased activity of catalase concurrent to Fe elevation suggesting possible displacement of iron. In similar lines, decrease in Cu, Zn and Mn can also be correlated with the status of SOD. It is possible that, the lack of these metal ions can distort SOD1 structure, exposing the Cys residues and promoting protein aggregation (Banci et al., 2008[4]). The loss of Cu and Zn from SOD1 also facilitates the reduction of the intrasubunit disulfide bond between Cys57 at the Zn loop and Cys146 at the β-barrel, thus leading to the dissociation of SOD1 subunits, a fact that greatly increases the formation of insoluble aggregates (Doucette et al., 2004[21]; Lindberg et al., 2004[39]). From the above inferred relation between trace elements and antioxidant enzymes and, the results of our study, it can be deduced that, any significant alteration in the level of the trace elements can result in compromised activity of antioxidant enzymes and consequent susceptibility of tissues to oxidative stress as has also been suggested by Alturfan and Zengin (2007[1]).
Guan et al. (2000[27], 2001[28]) in their recent studies, on cultured cell lines treated with free radical inducers, have shown low expression of nAChRs and in fact a decrease in this enzyme, a membrane localized one, can bear a direct correlation with increased LPO induced membrane damage. In this behest, it is possible that, cadmium induced oxidative stress may play an important role in the mechanism of down-regulation of nAChRs and as such, a marked decrease in hippocampal AChE activity, a key enzyme of cholinergic transmission in the central and peripheral nervous system, has been recorded in cadmium exposed mice. The observations of Bhatnagar et al. (2006[10]), Reddy et al. (2007[56]) and Bouaziz et al. (2010[13]) of decreased AchE activity in the brain of rodents exposed to lead and fluoride provide support to our present findings on Cd induced decrease in AchE activity. Thus it can be presumed that, decrease in AChE activity can severely impair synaptic transmission in the brain and is in accordance with previous report of Bhatnagar et al. (2006[10]) on reduced activities of hippocampal AchE and BchE on excessive intake of fluoride. Histochemical findings of our study (Figure 6(Fig. 6)) suggest possible loss of cholinergic neurons in cadmium exposed mice. Our results corroborate the several reports indicating loss of hippocampal neurons either by necrosis or apoptosis (Mullenix et al., 1995[49]; Varner et al., 1998[67]; Shivarajashankara et al., 2001[61]; Bhatnagar et al., 2002[11]; Ge et al., 2005[24]; Valko et al., 2005[66]). Loss of synaptic structure (Zhang et al., 2001[70]; Bhatnagar et al., 2002[11]) or even mere inhibition of enzyme activity (Zhao and Wa, 1998[71]; Zhai et al., 2003[69]). A similar decrease in the activity of hepatic and muscle cholinesterase has been related to an inhibition of the enzyme activity or to loss of synaptic structure (Bhatnagar et al., 2006[10]). Overall, the current findings suggest that, gestational and neonatal exposures to cadmium are fraught with consequential effects on hippocampal cholinergic system.
Simultaneous administration of melatonin could successfully counteract the deleterious effects of cadmium and prevent the loss of hippocampal cholinergic neurons. In our study, this was evident from maintenance of normal levels of GSH, AChE and Ach. The results are in agreement with the reported increase in the activities of GPx and SOD in fetal brain on administration of melatonin to pregnant rats (Baydas et al., 2007[6]). A stimulatory effect of melatonin on brain GPx activity has been reported by Baydas et al. (2007[6]) in the form of a two-fold-rise within 30 minutes of melatonin administration in the brain of female rats. The importance of melatonin as an antioxidant depends on several characteristics; its lipophilic and hydrophilic nature, its ability to cross all barriers with ease and its availability to all tissues and cells. It distributes equally well in all cellular compartments with a predominantly higher load maintained in the nucleus and mitochondria. In addition, protective effects of melatonin against metal induced oxidative damage have also been reported in many in vitro studies (Omurtag et al., 2008[52]; Escames and Acuna-Castroviejo, 2009[23]). The ability of melatonin to scavenge free radicals is undoubtedly an important property in its protective role against oxidative stress. The efficacy of melatonin in antioxidative defense is related with its ability to scavange the highly toxic hydroxyl radical to form cyclic 3-hydroxymelatonin (3-OHM), a stable metabolite of melatonin under both in vitro and in vivo conditions (see Karbownik and Reiter, 2000[35]). Melatonin in the present study has also shown efficacy in quenching cadmium induced hydroxyl radicals. This hydroxyl radical scavenging action of melatonin can also be related with the consequent ability to prevent loss of cholinergic neurons. The ability of melatonin to modulate hippocampal cadmium and other trace metals which serve as cofactors of antioxidant enzymes needs to be interpreted with caution. With the exact mechanism of action still unclear, most of the explanations can only be at the realms of speculation. In this context, our study is on neonatal animals wherein the blood brain barrier is yet incompletely developed and thereby permits free access to cadmium (Kostial et al., 1978[36]). Incompletely formed BBB in neonates can be considered as a summation of areas completely devoid of BBB and areas where BBB is partially formed. Melatonin can apparently stabilize these partially formed BBB, restricting sites of entry of cadmium. Thus the decrease in hippocampal cadmium load observed in Cd + Mel hippocampus can be attributed to the ability of melatonin to stabilize BBB. Moreover, in all conditions of oxidative stress induced-neurodegenerative diseases, there is need for antioxidants like melatonin that can penetrate the blood brain barrier (Gilgun-Sherki et al., 2001[25], 2002[26]). Hence, the action of melatonin in preventing accumulation of hippocampal cadmium and modulation of other trace metals, at least in part, can be attributed to the role of this pineal product in maintaining the BBB.
In conclusion, this study shows that, a low dose Cd exposure results in profound oxidative damage to hippocampus leading to disruption in cholinergic system. There is growing evidence that, exposure to toxicants in early life may cause later life health effects. The observed phenomenon of ''fetal origins of disease'' suggests that, early environmental exposures such as metals, program later life gene expression. In this context, our results may be of special relevance to infant subjects dwelling in polluted environment. Despite population explosion, industrialization, urbanization, increasing pollution and life style changes with sedentary work pattern, higher intellectual attainments demand a healthy brain devoid of any degenerative changes and, this necessitates insulation against toxic insults during the early developmental stages. Though pollution load can be contained but not prevented in toto, the encouraging results of melatonin in preventing toxic manifestation in the early developmental stages pave way for greater clinical trials in human subjects during the neonatal stage whence, the blood brain barrier is not fully developed. Antioxidant coverage with melatonin and, a cocktail of various other natural antioxidants during the neonatal period is worth exploring.
References
- 1.Alturfan AA, Zengin EN. Investigation of zinc and copper levels in methimazole - induced hypothyroidism: relation with the oxidant - antioxidant status. Folia Biol (Praha) 2007;53:183–188. [PubMed] [Google Scholar]
- 2.Ananth C, Gopalakrishnakone P, Kaur C. Protective role of melatonin in domoic acid-induced neuronal damage in the hippocampus of adult rats. Hippocampus. 2003;13:375–387. doi: 10.1002/hipo.10090. [DOI] [PubMed] [Google Scholar]
- 3.Augustinssion K. Cholinesterase and anticholinesterase agents. Berlin: Springer Verlag; 1963. [Google Scholar]
- 4.Banci L, Bertini I, Boca M, Girotto S, Martinelli M, Valentine, JS, Vieru M. SOD1 and amyotrophic lateral sclerosis. Mutations and oligomerization. PLoS One. 2008;3:e1677. doi: 10.1371/journal.pone.0001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee BD, Seth V, Bhattacharya A, Pasha ST, Chakraborty AK. Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol Lett. 1999;107:33–47. doi: 10.1016/s0378-4274(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 6.Baydas G, Koz ST, Tuzcu M, Etem E, Nedzvetsky VS. Melatonin inhibits oxidative stress and apoptosis in fetal brains of hyperhomocysteinemic rat dams. J Pineal Res. 2007;43:225–231. doi: 10.1111/j.1600-079X.2007.00465.x. [DOI] [PubMed] [Google Scholar]
- 7.Baydas G, Reiter RJ, Nedzvetskii VS, Yasar A, Tuzcu M, Ozveren F, Canatan H. Melatonin protects the central nervous system of rats against toluene-containing thinner intoxication by reducing reactive gliosis. Toxicol Lett. 2003;137:169–174. doi: 10.1016/s0378-4274(02)00400-9. [DOI] [PubMed] [Google Scholar]
- 8.Beuge JA, Aust SD. Microsomal lipid peroxidation. Meth Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 9.Beutler E, Duron O, Kelly BM. Improved method for the reduced glutathione. J Lab Clin Med. 1969;61:882–888. [PubMed] [Google Scholar]
- 10.Bhatnagar M, Rao P, Saxena R, Bhatnagar AR, Meena P, Barbar S, Chouhan A, Vimal S. Biochemical changes in brain and other tissues of young adult female mice from fluoride in their drinking water. Fluoride. 2006;39:280–4. [Google Scholar]
- 11.Bhatnagar M, Rao P, Shukla S, Jain S. Neurotoxicity of fluoride: evidence of neurodegeneration in hippocampus of female mice. Ind J Exp Biol. 2002;40:546–54. [PubMed] [Google Scholar]
- 12.Borg DC. Oxygen free radicals and tissue injury. In: Tan M, Samson F, editors. Oxygen free radicals and tissue injury. Boston: Birkhauser; 1993. pp. 12–53. [Google Scholar]
- 13.Bouaziz H, Amara IB, Essefi M, Croute F, Zeghal N. Fluoride-induced brain damages in suckling mice. Pesticide Biochem Physiol. 2010;96:24–29. [Google Scholar]
- 14.Carretero M, Escames G, Lopez LC, Venegas C, Dayoub JC, Garcia L, Acuña-Castroviejo D. Long-term melatonin administration protects brain mitochondria from aging. J Pineal Res. 2009;47:192–200. doi: 10.1111/j.1600-079X.2009.00700.x. [DOI] [PubMed] [Google Scholar]
- 15.Casalino E, Sblano C, Landriscina C. Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Arch Biochem Biophys. 1997;346:171–179. doi: 10.1006/abbi.1997.0197. [DOI] [PubMed] [Google Scholar]
- 16.Chary NS, Kamala CT, Raj DS. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol Environ Saf. 2008;69:513–524. doi: 10.1016/j.ecoenv.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Chasseaud LF. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- 18.Chavez E, Briones R, Michel B, Bravo C, Jay D. Evidence for the involvement of dithiol groups in mitochondrial calcium transport: studies with cadmium. Arch Biochem Biophys. 1985;242:493–497. doi: 10.1016/0003-9861(85)90235-8. [DOI] [PubMed] [Google Scholar]
- 19.Cheung RT. The utility of melatonin in reducing cerebral damage resulting from ischemia and reperfusion. J Pineal Res. 2003;34:153–160. doi: 10.1034/j.1600-079x.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 20.Djukic-Cosic D, Curcic Jovanovic M, Plamenac Bulat Z, Ninkovic M, Malicevic Z, Matovic V. Relation between lipid peroxidation and iron concentration in mouse liver after acute and subacute cadmium intoxication. J Trace Elem Med Biol. 2008;22:66–72. doi: 10.1016/j.jtemb.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Doucette PA, Whitson LJ, Cao X, Schirf V, Demeler B, Valentine JS, Hansen JC, Hart PJ. Dissociation of human copper-zinc superoxide dismutase dimers using chaotrope and reductant. Insights into the molecular basis for dimer stability. J Biol Chem. 2004;279:54558–54566. doi: 10.1074/jbc.M409744200. [DOI] [PubMed] [Google Scholar]
- 22.Ellman GL, Courtney KD, Andres V, Jr., Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 23.Escames G, Acuna-Castroviejo D. Melatonin, synthetic analogs, and the sleep/wake rhythm. Rev Neurol. 2009;48:245–254. [PubMed] [Google Scholar]
- 24.Ge Y, Ning H, Wang S, Wang J. Effect of high fluoride and low iodine on brain effects of high fluoride and low iodine on brain histopathology in offspring rats. Fluoride. 2005;38:127–32. [Google Scholar]
- 25.Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40:959–975. doi: 10.1016/s0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 26.Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002;54:271–284. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- 27.Guan Z, Zhang X, Nordberg A. Influence of lipid peroxidation on the nicotinic acetylcholine receptors in PC12 cells. Neurosci Lett. 2000;286:163–166. doi: 10.1016/s0304-3940(00)01127-7. [DOI] [PubMed] [Google Scholar]
- 28.Guan ZZ, Zhang X, Mousavi M, Tian C, Unger C, Nordberg A. Reduced expression of neuronal nicotinic acetylcholine receptors during the early stages of damage by oxidative stress in PC 12 cells. J Neurosci Res. 2001;66:551–8. doi: 10.1002/jnr.1245. [DOI] [PubMed] [Google Scholar]
- 29.Hassan HM. Cytotoxicity of oxyradicals and the evolution of superoxide dismutases. In: Clerch LB, Massaro DJ, editors. Oxygen, gene expression and cellular function. New York: Marcel Dekker; 1997. pp. 27–47. [Google Scholar]
- 30.Hedreen JC, Bacon SJ, Price DL. A modified histochemical technique to visualize acetylcholinesterase-containing axons. J Histochem Cytochem. 1985;33:134–140. doi: 10.1177/33.2.2578498. [DOI] [PubMed] [Google Scholar]
- 31.Holland MK, Storey BT. Oxygen metabolism of mammalian spermatozoa. Generation of hydrogen peroxide by rabbit epididymal spermatozoa. Biochem J. 1981;198:273–280. doi: 10.1042/bj1980273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamall IS, Smith JC. Effects of cadmium on glutathione peroxidase, superoxide dismutase, and lipid peroxidation in the rat heart: a possible mechanism of cadmium cardiotoxicity. Toxicol Appl Pharmacol. 1985;80:33–42. doi: 10.1016/0041-008x(85)90098-5. [DOI] [PubMed] [Google Scholar]
- 33.Johnston MV. Neurotransmitter alterations in a model of perinatal hypoxic-ischemic brain injury. Ann Neurol. 1983;13:511–518. doi: 10.1002/ana.410130507. [DOI] [PubMed] [Google Scholar]
- 34.Jurczuk M, Brzóska MM, Galazyn-Sidorczuk M, Moniuszko-Jakoniuk J, Rogowski F. Distribution of the 59Fe radioisotope in the organs of a rat after a subacute exposure to cadmium. Polish J Environ Studies. 1997;6(Suppl):70–3. [Google Scholar]
- 35.Karbownik M, Reiter RJ. Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proc Soc Exp Biol Med. 2000;225:9–22. doi: 10.1177/153537020022500102. [DOI] [PubMed] [Google Scholar]
- 36.Kostial K, Kello D, Jugo S, Rabar I, Maljkovic T. Influence of age on metal metabolism and toxicity. Environ Health Perspect. 1978;25:81–86. doi: 10.1289/ehp.782581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar R, Agarwal AK, Seth PK. Oxidative stress-mediated neurotoxicity of cadmium. Toxicol Lett. 1996;89:65–69. doi: 10.1016/s0378-4274(96)03780-0. [DOI] [PubMed] [Google Scholar]
- 38.Limaye DA, Shaikh ZA. Cytotoxicity of cadmium and characteristics of its transport in cardiomyocytes. Toxicol Appl Pharmacol. 1999;154:59–66. doi: 10.1006/taap.1998.8575. [DOI] [PubMed] [Google Scholar]
- 39.Lindberg MJ, Normark J, Holmgren A, Oliveberg M. Folding of human superoxide dismutase: disulfide reduction prevents dimerization and produces marginally stable monomers. Proc Natl Acad Sci U S A. 2004;101:15893–15898. doi: 10.1073/pnas.0403979101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 41.Manca D, Ricard AC, Trottier B, Chevalier G. Studies on lipid peroxidation in rat tissues following administration of low and moderate doses of cadmium chloride. Toxicology. 1991;67:303–323. doi: 10.1016/0300-483x(91)90030-5. [DOI] [PubMed] [Google Scholar]
- 42.Manda K, Reiter RJ. Melatonin maintains adult hippocampal neurogenesis and cognitive functions after irradiation. Progr Neurobiol. 2010;90:60–68. doi: 10.1016/j.pneurobio.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 44.Marklund SL, Westman N, Lundgren E, Roos G. Copper- and zinc-containing superoxide dismutase, mananese-containing superoxide dismutase, catalase and glutathione peroxidase in normal and neoplastic cell lines and normal human tissues. Cancer Res. 1982;42:1955–1961. [PubMed] [Google Scholar]
- 45.Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 46.McEown K, Treit D. The role of the dorsal and ventral hippocampus in fear and memory of a shock-probe experience. Brain Res. 2009;1251:185–194. doi: 10.1016/j.brainres.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 47.Mesulam MM. The cholinergic innervation of the human cerebral cortex. Progr Brain Res. 2004;145:67–78. doi: 10.1016/S0079-6123(03)45004-8. [DOI] [PubMed] [Google Scholar]
- 48.Millis PR, Ramsey MH, John EA. Heterogeneity of cadmium concentration in soil as a source of uncertainty in plant uptake and its implications for human health risk assessment. Sci Total Environ. 2004;326:49–53. doi: 10.1016/j.scitotenv.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Mullenix PJ, Denbesten PK, Schunior A, Kernan WJ. Neurotoxicity of sodium fluoride in rats. Neurotoxicol Teratol. 1995;17:169–77. doi: 10.1016/0892-0362(94)00070-t. [DOI] [PubMed] [Google Scholar]
- 50.Ochi T, Otsuka F, Takahashi K, Ohsawa M. Glutathione and metallothioneins as cellular defense against cadmium toxicity in cultured Chinese hamster cells. Chem Biol Interact. 1988;65:1–14. doi: 10.1016/0009-2797(88)90026-9. [DOI] [PubMed] [Google Scholar]
- 51.Oishi S, Nakagawa J, Ando M. Effects of cadmium administration on the endogenous metal balance in rats. Biol Trace Elem Res. 2000;76:257–278. doi: 10.1385/bter:76:3:256. [DOI] [PubMed] [Google Scholar]
- 52.Omurtag GZ, Tozan A, Sehirli AO, Sener G. Melatonin protects against endosulfan-induced oxidative tissue damage in rats. J Pineal Res. 2008;44:432–438. doi: 10.1111/j.1600-079X.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 53.Pari L, Murugavel P. Diallyl tetrasulfide improves cadmium induced alterations of acetylcholinesterase, ATPases and oxidative stress in brain of rats. Toxicology. 2007;234:44–50. doi: 10.1016/j.tox.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 54.Puntarulo S, Cederbaum AI. Effect of oxygen concentration on microsomal oxidation of ethanol and generation of oxygen radicals. Biochem J. 1988;251:787–794. doi: 10.1042/bj2510787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramachandran AV. Environment global changes and challenges. Jaipur: ABD Publishers; 2003. Aftermath of Baroda Effluent Channel: Impact assessment along the channel and the Mahi estuary with reference to heavy metals; pp. 15–49. [Google Scholar]
- 56.Reddy GR, Devi BC, Chetty CS. Developmental lead neurotoxicity: alterations in brain cholinergic system. Neurotoxicology. 2007;28:402–407. doi: 10.1016/j.neuro.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 57.Reiter RJ, Tan DX, Manchester LC, Lopez-Burillo S, Sainz RM, Mayo JC. Melatonin: detoxification of oxygen and nitrogen-based toxic reactants. Adv Exp Med Biol. 2003;527:539–548. doi: 10.1007/978-1-4615-0135-0_62. [DOI] [PubMed] [Google Scholar]
- 58.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 59.Searle AJ, Willson RL. Glutathione peroxidase: effect of superoxide, hydroxyl and bromine free radicals on enzyme activity. Int J Radiat Biol Relat Stud Phys Chem Med. 1980;37:213–217. doi: 10.1080/09553008014550261. [DOI] [PubMed] [Google Scholar]
- 60.Shen YX, Xu SY, Wei W, Wang XL, Wang H, Sun X. Melatonin blocks rat hippocampal neuronal apoptosis induced by amyloid beta-peptide 25-35. J Pineal Res. 2002;32:163–167. doi: 10.1034/j.1600-079x.2002.1o839.x. [DOI] [PubMed] [Google Scholar]
- 61.Shivarajashankara YM, Shivarashankara AR, Bhat PG, Rao SH. Effect of fluoride intoxication on lipid peroxidation and antioxidant systems in rats. Fluoride. 2001;34:108–13. [Google Scholar]
- 62.Sies H. Strategies of antioxidative defense. Eur J Biochem. 1993;215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 63.Sinha AK. Calorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 64.Stojanović Z, Matović V, Vujanović D, Soldatović D. Effect of acute and prolonged cadmium intoxication on zinc, copper, iron and magnesium content in rabbit blood. Fund Clin Pharmacol. 1999;13:364. [Google Scholar]
- 65.Tracy AL, Jarrard LE, Davidson TL. The hippocampus and motivation revisited: appetite and activity. Behav Brain Res. 2001;127:13–23. doi: 10.1016/s0166-4328(01)00364-3. [DOI] [PubMed] [Google Scholar]
- 66.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 67.Varner JA, Jensen KF, Horvath W, Isaacson RL. Chronic administration of aluminum-fluoride or sodium-fluoride to rats in drinking water: alterations in neuronal and cerebrovascular integrity. Brain Res. 1998;784:284–298. doi: 10.1016/s0006-8993(97)01336-x. [DOI] [PubMed] [Google Scholar]
- 68.Wakatsuki A, Okatani Y, Ikenoue N, Kaneda C, Fukaya T. Effects of short-term melatonin administration on lipoprotein metabolism in normolipidemic postmenopausal women. Maturitas. 2001;38:171–177. doi: 10.1016/s0378-5122(00)00221-8. [DOI] [PubMed] [Google Scholar]
- 69.Zhai JX, Guo ZY, Hu CL, Wang QN, Zhu QX. Studies on fluoride concentration and cholinesterase activity in rat hippocampus. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2003;21:102–4. [PubMed] [Google Scholar]
- 70.Zhang Z, Shen X, Xu X. Effects of selenium on the damage of learning memory ability of mice induced by fluoride. Wei Sheng Yan Jiu. 2001;30:144–6. [PubMed] [Google Scholar]
- 71.Zhao X, Wa JH. Actions of sodium fluoride on acetylcholinesterase activities in rats. Biomed Environ Sci. 1998;11:1–6. [PubMed] [Google Scholar]
- 72.Zmudzki J. Determination of lead in biological material by atomic absorption spectrophotometry (AAS) Medycyna Weterynaryjna. 1977;33:179–181. [Google Scholar]