Abstract
Methanolic extract and fractions, ethylacetate (EtF) and butanol (BuF) of leaves of African mistletoe (Tapinanthus bangwensis, Engl. & K. Krause) were evaluated for their hepatoprotective potential using CCl4-induced hepatotoxicity in Wistar albino rats. The activities of the marker enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and bilirubin were highest in rats treated with CCl4 alone. Oral administration at a fixed dose of 400 mg/kg body weight (BW) of the extract and fractions of T. bangwensis for seven days significantly (p ≤ 0.05) decreased the activity of marker enzymes and bilirubin. Total protein concentration increased significantly (p ≤ 0.05). These extracts also decreased the concentration of thiobarbituric acid reactive substances (TBARS) which indicated a reduction in lipid peroxidation. Histopathological examination of hepatocytes of rats administered methanolic extract (MeE) and fractions (EtF and BuF) showed normal architecture whereas rats treated with CCl4 alone was characterized by necrosis of the liver. Generally, among the three extracts, the BuF and EtF showed more hepatoprotective effect. The crude methanolic extract did not show any mortality up to a dose of 2000 g/kg BW. These findings suggest that T. bangwensis possesses strong antioxidant properties and hepatoprotective potentials against CCl4-induced hepatotoxicity in rats.
Keywords: African mistletoe, carbon tetrachloride, biochemical parameters, fractions, histopathology, lipid peroxidation
Introduction
The liver has great capacity to detoxicate toxic substances and synthesize useful principles (Shahani, 1999[25]; Subramoniam and Pushpagandan, 1999[26]). An antioxidant may serve as a hepatoprotective agent. Therefore, the antioxidant mechanism is a major defense system, which converts active oxygen molecules into non-toxic compounds (Halliwell and Gutteridge, 1984[5]; Hochstein and Atallah, 1988[8]) and finally revert cellular damage.
African mistletoe is a semi-parasitic plant found growing on a host of evergreen and deciduous trees all year round. It is an obligate parasite, obtaining part of its food from the host plant. It depends on its host for minerals and water only, but obtains carbohydrate by the process of photosynthesis (Osadebe and Uzochukwu, 2006[21]). In Nigeria, extracts from the leaves are used by traditional medical practitioners to treat various ailments such as liver disorders. They are used as anticancer agents (Grossarth-Maticek and Ziegler, 2007[4]) and in the management of diabetes mellitus (Kafaru, 1993[9]; Obatomi et al., 1994[17]; Osadebe et al., 2004[20]) as well as anti-hypertensive agent (Kafaru, 1993[9]). Kafaru (1993[9]) described the mistletoe plant as “an all purpose herb” because of its rich folkloric uses. Some of these uses have been reported (Obatomi et al., 1994[17], Fischer et al., 1997[2]; Osadebe et al., 2004[20]). However, information is scanty on the hepatoprotective effect of the plant in experimental animals. The present study was therefore designed to evaluate the hepatoprotective effect of methanolic extract (MeE) and its fractions (EtF and BuF) of the leaves of the African mistletoe (T. bangwensis).
Materials and Methods
Plant materials
Collection and authentication of plant materials
The leaves of African mistletoe (T. bangwensis) were collected from the Orchard near the Vice Chancellor's Lodge, Delta Park, University of Port Harcourt, Rivers State, Nigeria. The plant was identified by a curator Mr. N.L Edwin-Wosu of the Department of Plant Science and Biotechnology, University of Port Harcourt.
Extraction of plant
The sun-dried leaves of T. bangwensis were pulverized into a fluffy mass. Five hundred grams of the powdered leaves were extracted with 8 litres of 80 % MeOH using Soxhlet extractor for 24 h. The extract was evaporated to dryness under reduced pressure (below 40 °C). The yield was 115.58 g (23.12 %, w/v) of powdered methanol extract, which was stored in the refrigerator for further use. This methanolic extract was used for the toxicity test.
Phytochemical screening
Freshly prepared crude methanolic extract of the leaves of T. bangwensis was subjected to preliminary phytochemical screening (Harborne, 1984[6]).
Fractionation of the extract
The dried crude methanolic extract (40 g) was dissolved in distilled water and made up to 200 ml. It was further fractionated by successive solvent extraction with ethyl acetate (EtOAc) (2 x 100 ml) and n-butanol saturated with H20 (3 x 100 ml). Each extract was evaporated to dryness under reduced pressure to yield EtOAc fraction (6.69 g), butanol fraction (8.78 g) and remaining H2O fraction (5.86 g).
Experimental animals
Healthy male Wistar albino rats weighing 120-180 g from the Central Animal House of HEJ Research Institute of Chemistry, University of Karachi, Pakistan were used throughout the study. They were kept under standard environmental conditions at 25 °C with 12:12 h light-dark cycle in ventilated plastic cages. The rats were fed with a standard rat feed and water ad libitium. The experiment was performed in accordance with the guidelines established by the European Community for the Care and Use of Laboratory Animals and were approved by Institutional Animal Ethical Committee (IAEC) of University of Karachi.
Acute toxicity test
Healthy male Wistar albino rats weighing 120-180 g maintained under standard laboratory conditions were used for acute toxicity test according to Organisation for Economic Cooperation and Development (OECD) guideline 423 (OECD Guideline, 2000[18]). A total of five rats were used which received a single oral-dose of 2000 mg/kg BW of crude methanolic extract (MeE) of T. bangwensis. The rats were kept overnight fasting prior to administration of crude extracts by oral gavage. Food was withheld for further 3-4 h. The rats were observed individually at least once during the first 30 min after dosing, then periodically during first 24 h (with special attention during the first 4 h) and daily thereafter for a period of 14 days. Daily observations on the changes in skin and fur, eyes and mucus membrane (nasal), respiratory rate, circulatory signs (heart rate and blood pressure), autonomic effects (salivation, lacrimation, perspiration, piloerection, urinary incontinence and defecation) and central nervous system (ptosis, drowsiness, gait, tremors and convulsion) changes were noted (OECD Guideline, 2000[18]).
Hepatoprotective study
Healthy and mature male Wistar albino rats weighing 170-200 g were equalized with respect to body weight and randomly divided into six groups of six rats each.
Group I (Control) received normal saline (5 ml/kg, po) daily for 7 days.
Group II received normal saline (5 ml/kg, po) daily and CCl4: olive oil (1:1 v/v) (1 ml, ip) on alternate days for seven days.
Group III was administered methanolic extract (400 mg/kg) daily and CCl4: olive oil (1:1 v/v) (1 ml, ip) on alternate days for seven days.
Group IV was administered EtOAc fraction (400 mg/kg) daily and CCl4: olive oil (1:1 v/v) (1 ml, ip) on alternate days for seven days.
Group V was administered BuOH fraction (400 mg/kg po) daily and CCl4: olive oil (1:1 v/v) (1 ml, ip) on alternate days for seven days while
Group VI was administered vitamin E (100 mg/kg, po) daily and CCl4: olive oil (1:1 v/v) (1 ml, ip) on alternate days for 7 days.
The animals were sacrificed under light ether anaesthesia 24 h after the last treatment of CCl4. On the 8th day, blood was collected by cardiac puncture, allowed to coagulate at 37 °C for 30 min and serum separated by centrifugation at 2500 rpm for 10 min and stored at 4 °C. The serum was used to estimate serum ALT, AST, ALP, total and direct bilirubin and total protein concentration using a Hitachi-902-Roche-Japan Auto Analyzer. After collection of blood, the liver was immediately excised and rinsed in ice cold normal saline and divided into two parts. The first was stored at -20 °C for TBARS analysis, while the second part was used for histopathological studies.
Histopathological study
A portion of the liver of all the rat groups was fixed in 10 % buffered neutral formalin for 48 h followed by bovine solution for 6 h and then processed for paraffin embedding. By using a microtome, sections of 5 µm thickness were taken, processed in alcohol-xylene series and were stained with alum-haematoxylin and eosin (Galigher and Kayloff, 1971[3]) and subjected to histopathological examination.
Estimation of thiobarbituric acid reactive substances (TBARS)
The product of the reaction between malondialdehyde (MDA) and thiobarbituric acid reactive substances (TBARS) were measured by the method of Ohkawa et al., (1979[19]). The reaction mixture consisted of 0.2 ml of 8.1 % sodium lauryl sulphate, 1.5 ml of 20 % acetic acid solution adjusted to pH 3.50 with sodium hydroxide and 1.5 ml of 0.8 % aqueous solution of thiobarbituric acid was added to 0.2 ml of 10 % (w/v) of liver homogenate. The mixture was brought to 4.0 ml with distilled water and heated at 95 °C for 60 min. After cooling with tap water, 1.0 ml distilled water and 5.0 ml of the mixture of n-butanol and pyridine (15:1 v/v) was added and centrifuged at 3000 rpm for 10 min. The organic layer was discarded and absorbance of the clear upper (n-butanol) layer was measured using Spectra Max Plus-384 (Molecular Devices, USA) Spectrophotometer at 532 nm. TBARS were quantified using an extinction coefficient of 1.56 x 105 cm-1 M-1 and expressed as nmol of TBARS/mg protein.
Statistical analysis
Values are expressed as means ± standard error of mean (S.E.M). The results were analyzed statistically by Analysis of Variance (ANOVA) followed by Turkey Multiple Comparison Test. Significance was accepted at a p-value of 0.05.
Results
The results of the preliminary screening of crude methanolic extract (MeE) of T. bangwensis showed the presence of secondary metabolites such as tannins, phlobatannins, alkaloids, anthraquinones, flavonoids and cardiac glycosides. The results of acute toxicity studies showed no mortality or physical changes in skin and fur, eyes and mucus membrane (nasal), respiratory rate, circulatory signs (heart rate and blood pressure), autonomic effects (salivation, perspiration, piloerection, urinary incontinence and defecation) and central nervous system (ptosis, drowsiness, gait, tremors and convulsion) among the rats administered 2000 mg/kg BW of crude methanolic extract (MeE) of T. bangwensis. The results of hepatoprotective effect of methanolic extract (MeE) and fractions (EtF and BuF) on CCl4-intoxicated rats are shown in Tables 1(Tab. 1) and 2(Tab. 2). The CCl4-treated group showed significant (p ≤ 0.05) increase in serum hepatic enzyme levels (AST, ALT and ALP), total and direct bilirubin; and a significant (p ≤ 0.05) decrease in total protein levels when compared to control group (Table 2(Tab. 2)). Administration of methanolic extract and fractions at a fixed dose of 400 mg/kg BW and Vit. E (100 mg/kg BW) decreased the activities of serum hepatic enzymes, total and direct bilirubin while total protein was significantly (p ≤ 0.05) increased when compared to the group treated with CCl4 alone. Results obtained from the study showed an increase in the level of thiobarbituric acid reactive substances (TBARS) in CCl4-intoxicated group when compared to control group. Treatment with methanolic extract (MeE) and fractions (EtF and BuF) at 400 mg/kg BW and Vit. E (100 mg/kg BW) significantly (p ≤ 0.05) decreased the concentration of TBARS (Table 3(Tab. 3)). Histopathological examination of the liver tissues of control group showed normal hepatic cells with well-preserved cytoplasm and prominent nucleus (Figure 1A(Fig. 1)). CCl4-treated group showed severe hepatic lesions characterized by necrosis of hepatocytes around the central vein, collapsed central vein with signs of necrosis such as karyolysis, karyorrhexis, pyknosis and eosinophilia of the cytoplasm (Figure 1B(Fig. 1)). Administration of a fixed dose of 400 mg/kg BW of methanolic extract and fractions of leaves of T. bangwensis followed by CCl4-treatment showed a normal appearance of hepatocytes and sinusoids with well defined central veins and no evidence of granuloma or malignancy (Figure 1C-E(Fig. 1)). Section of liver taken from group administered Vit. E showed a near normal architecture (Figure 1F(Fig. 1)).
Table 1. Effect of methanol extract and fractions of T. bangwensis on biochemical parameters in CCl4-induced hepatotoxicity in rats.
Table 2. Effect of methanolic extract and fractions (EtOAc and BuOH) of T. bangwensis on total protein concentration in Wistar albino rats.
Table 3. Effect of methanolic extract and fractions (EtOAc and BuOH) of leaves of T. bangwensis on thiobarbituric acid reactive substances (TBARS).
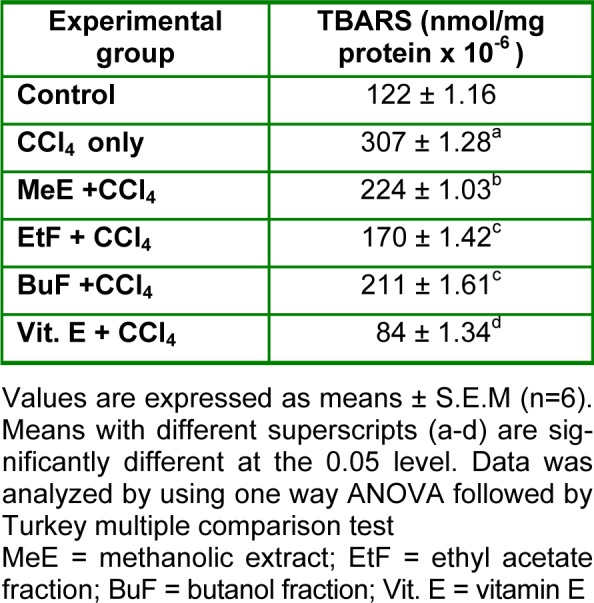
Figure 1. Photomicrographs of haematoxylin and eosin-stained histological sections of the liver.
Control (1A);
rats treated with CCl4 only (1B);
rats administered 400 mg/kg BW of BuF and CCl4 (1C);
rats administered 400 mg/kg BW of EtF and CCl4 (1D);
rats administered 400 mg/kg BW of MeE and CCl4 (1E);
rats administered 100 mg/kg BW of Vit. E and CCl4 (1F).
MeE = methanolic extract; EtF = ethyl acetate fraction; BuF = butanol fraction; Vit. E = vitamin E
Discussion
Preliminary phytochemical studies showed the presence of flavonoids in the methanolic extract of leaves of T. bangwensis. Flavonoids have been shown to be hepatoprotective (Seevola et al., 1984[24]; Wegner and Fintelmann, 1999[29]). The observed antioxidant and hepatoprotective activity of leaves of T. bangwensis may therefore be due to the presence of flavonoids.
The result of the acute toxicity test suggests that the crude extract of the plant was non-toxic to rats.
Serum ALT, AST, ALP, total and direct bilirubin in plasma have been reported to be a sensitive indicator in liver injury (Molander et al., 1955[13]). The decrease in ALT and AST activities by the methanolic extract (MeE) and fractions (EtF and BuF) of T. bangwensis may be an indication of regeneration process and repair of hepatic tissue damage induced by CC14 as earlier reported (Suresh Kumar and Mishra, 2008[27]; Moselhy and Ali, 2009[14]). This result corroborates the findings of Thabrew et al. (1987[28]), who reported that serum transaminase activities were restored with the healing of hepatic parenchyma and regeneration of hepatocytes. The elevated level of serum ALP observed in rats administered with BuF may be due to increased production of the enzyme. Increase in serum ALP level has been reported to be due to increased synthesis, in the presence of increasing biliary pressure (Muriel and Garcipiana, 1992[15]).
The changes in albumin and globulin levels observed in CCl4-treated rats and subsequent reversal caused by the administration of these extracts towards normalization is suggestive of the stabilization of endoplasmic reticulum leading to the repair of impaired protein synthesis caused by CCl4 with concurrent improvement of Kupffer cells (Suresh Kumar and Mishra, 2008[27]). Thus, administration of methanolic extract and fractions of T. bangwensis showed hepatoprotective activity against the toxic effect of CCl4. Estimation of TBARS is commonly used as an index of lipid peroxidation. The elevation of lipid peroxidation produced by CCl4 was reversed in rats administered with methanolic extract (MeE) and fractions (EtF and BuF) of leaves of T. bangwensis. Lipid peroxidation is postulated as the mechanism of free radical-induced tissue injury; hence free radical scavenging is established as the means by which antioxidants inhibit lipid peroxidation. Lipid peroxidation is probably the most extensively investigated process induced by free radicals and reactive oxygen species (ROS). This process and subsequent sub-organelle (mitochondria/ lysosomes) membrane damage are regarded as an important mechanism underlying the toxicity of several oxidative xenobiotics (Nakagawa et al., 1992[16]).
The ability of a hepatoprotective drug to reduce the injurious effects or to preserve the normal hepatic physiological mechanisms which have been disturbed by a hepatotoxin is the index of its protective effect (Krishna et al., 2010[11]). Therefore, CCl4-induced hepatic injuries are commonly used models for the screening of hepatic drugs and the extent of damage is assessed by the level of released cytoplasmic transaminases (ALT and AST) and alkaline phosphatase (ALP) in circulation (Patrick-Iwuanyanwu et al., 2007[23]; Patrick-Iwuanyanwu and Wegwu, 2008[22]; Hegde and Joshi, 2009[7]). The mechanism of CCl4-induced liver damage is considered to be due to the enzymatic activation of CCl4 by cytochrome P450 to produce trichloromethyl free radicals (• CCl3) within the membrane of the endoplasmic reticulum (Kyung et al., 2007[12]). These free radicals bind covalently to unsaturated membrane lipids resulting in increased lipid peroxides followed by pathological changes such as, elevated levels of serum marker enzymes like ALT, AST and ALP, depletion of glutathione (GSH), decreased protein synthesis, triglycerides accumulation, increased lipid peroxidation, destruction of Ca2+ homeostatis and finally hepatocyte damage (Kaplowitz et al., 1986[10]). Thus, antioxidation or inhibition of free radical generation is important in protection against CCl4-induced liver lesion (Castro et al., 1974[1]).
Our observation in the histological examination of hepatic tissues further validates the result of the biochemical studies. The near normal architecture of liver tissues of rats treated with CCl4 followed by the administration of 400 mg/kg BW of methanolic extract and fractions of the plant is suggestive of the hepatoprotective nature of the extract and fractions. This result was comparable to that administered with a known antioxidant (Vit. E).
In conclusion, data obtained from our study may suggest that methanolic extract (MeE) and fractions (EtF and BuF) of leaves of T. bangwensis have significant antioxidant and hepatoprotective effects on CCl4-induced hepatic damage in rats.
Acknowledgement
The corresponding author wishes to acknowledge the Academy of Sciences for the Developing World (TWAS) for the award of the TWAS-ICCBS Postgraduate Fellowship and the International Center for Chemical and Biological Sciences (ICCBS) University of Karachi, Pakistan for providing the necessary facilities.
References
- 1.Castro JA, D’Acosta N, De Castro CR, De Ferreyra EC, De Castro CR, Diaz Gomez, MI, De Fenos OM. Studies on thioacetamide-induced liver necrosis. Toxicol Appl Pharmacol. 1974;30:79–86. [Google Scholar]
- 2.Fischer S, Scheffler A, Kabelitz D. Oligoclonal in vitro response of CD4 T cells to vesicles of mistletoe extracts in mistletoe-treated cancer patients. Cancer Immunol Immunother. 1997;44:150–6. doi: 10.1007/s002620050367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galigher AE, Kayloff EN. Essentials of practical microtechniques. Philadelphia: Lea and Febiger; 1971. [Google Scholar]
- 4.Grossarth-Maticek R, Ziegler R. Prospective controlled cohort studies on long-term therapy of ovarian cancer patients with mistletoe (Viscum album L.) extracts iscador. Arzneimittelforschung. 2007;57:665–678. doi: 10.1055/s-0031-1296666. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B, Gutteridge JMC. Lipid peroxidation, oxygen radicals, cell damage and antioxidant therapy. Lancet. 1984;23:1396–1397. doi: 10.1016/s0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- 6.Harborne JB. Phytochemical methods. A guide to modern techniques of plant analysis. 2nd ed. London: Chapmann and Hall; 1984. p. 192. [Google Scholar]
- 7.Hegde K, Joshi AB. Hepatoprotective effect of Carissa carandas Linn root extract against CCl4 and paracetamol induced hepatic oxidative stress. Indian J Exp Biol. 2009;47:660–667. [PubMed] [Google Scholar]
- 8.Hochstein P, Atallah AS. The nature of oxidants and antioxidant systems in the inhibition of mutation and cancer. Mutat Res. 1988;202:363–375. doi: 10.1016/0027-5107(88)90198-4. [DOI] [PubMed] [Google Scholar]
- 9.Kafaru E. “Mistletoe—an example of an all-purpose herb” herbal remedies. Guardian Newspaper. 1993 Jun 3;:11. [Google Scholar]
- 10.Kaplowitz N, Aw TY, Simon FR, Stolz A. Drug-induced hepatotoxicity. Ann Intern Med. 1986;104:826. doi: 10.7326/0003-4819-104-6-826. [DOI] [PubMed] [Google Scholar]
- 11.Krishna KL, Mruthunjaya K, Patel JA. Antioxidant and hepatoprotective potential of stem methanolic extract of Justicia gendarussa Burm. Int J Pharmacol. 2010;6:78–80. [Google Scholar]
- 12.Kyung JL, Jea HC, Hye GJ. Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol. 2007;45:2118–2125. doi: 10.1016/j.fct.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Molander DW, Wroblewski F, La-Due JS. Transaminase compared with cholesterase and alkaline phosphatase an index of hepatocellular integrity. Clin Res Proc. 1955;3:20–24. [Google Scholar]
- 14.Moselhy SS, Ali HK. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol Res. 2009;42:93–98. [PubMed] [Google Scholar]
- 15.Muriel P, Garcipiana T. Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J Appl Toxicol. 1992;12:439–442. doi: 10.1002/jat.2550120613. [DOI] [PubMed] [Google Scholar]
- 16.Nakagawa Y, Moldeus P, Moore GA. Cytotoxicity of orthophenylphenol in isolated rat hepatocytes. Biochem Pharmacol. 1992;43:159–65. [PubMed] [Google Scholar]
- 17.Obatomi DK, Bikomo FO, Temple VJ. Antidiabetic properties of the African mistletoe in Streptozotocin-induced diabetic rats. J Ethnopharmacol. 1994;43:13–17. doi: 10.1016/0378-8741(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 18.OECD (Organisation for Economic Co- Operation and Development) Acute oral toxic class method. Guideline 423, adopted 23.03.1996. Eleventh Addendum to the OECD guidelines for the Testing of Chemicals, Paris, June 2000. 2000.
- 19.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Osadebe PO, Okide GB, Akabogu IC. Studies on antidiabetic activities of crude methanolic extract of loranthus micranthus (Linn) sourced from five different host trees. J Ethnopharmacol. 2004;95:133–138. doi: 10.1016/j.jep.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Osadebe PO, Uzochukwu IC. Chromatographic and antimotility studies on extracts of Loranthus micranthus Linn. J Pharmaceut Allied Sci. 2006;3:263–268. [Google Scholar]
- 22.Patrick-Iwuanyanwu KC, Wegwu MO. Prevention of carbon tetrachloride (CCl4)–induced liver damage in rats by Acanthus montanus. Asian J Biochem. 2008;3:213–220. [Google Scholar]
- 23.Patrick-Iwuanyanwu KC, Wegwu MO, Ayalogu EO. Prevention of CCl4–induced liver damage by ginger, garlic and Vitamin E. Pak J Biol Sci. 2007;10:617–621. doi: 10.3923/pjbs.2007.617.621. [DOI] [PubMed] [Google Scholar]
- 24.Seevola D, Baebacini GM, Bona S. Flavonoids and hepatic cyclic monophosphates in liver injury. Boll Ins Sieroter Milan. 1984;63:777–82. [PubMed] [Google Scholar]
- 25.Shahani S. Evaluation of hepatoprotective efficacy of APCL-A polyherbal formulation in vivo in rats. Indian Drugs. 1999;36:628–31. [Google Scholar]
- 26.Subramoniam A, Pushpangadan P. Development of phytomedicine for liver diseases. Indian J Pharmacol. 1999;31:166–75. [Google Scholar]
- 27.Suresh Kumar SV, Mishra SH. Hepatoprotective effect of Pergularia daemia (Forsk.) ethanol extract and its fraction. Indian J Exp Biol. 2008;46:447–452. [PubMed] [Google Scholar]
- 28.Thabrew MI, Joice PDTM, Rajatissa WA. Comparative study of efficacy of Paetta indica and Osbeckia octandra in the treatment of liver dysfunction. Planta Medica. 1987;53:239–241. doi: 10.1055/s-2006-962691. [DOI] [PubMed] [Google Scholar]
- 29.Wegner T, Fintelmann V. Flavonoids and bioactivity. Wien Med Wochenschr. 1999;149:241–7. [PubMed] [Google Scholar]





