Abstract
This study aims to identify the types of proteins in malted and dry Bambara groundnut seeds and through a comparative analysis, identify similarities and their known uses. Dry viable bambara seed was stored for five days to malt. The proteins in the dry and malted seed were subsequently extracted in potassium phosphate buffer pH 7.0 and precipitated with saturated ammonium sulphate. MudPit (multidimensional protein identification technology) and LC-MALDI TOF-TOF (liquid chromatography - matrix-assisted laser desorption ionization tandem time-of-flight) mass spectrometry were thereafter used to identify the different types of proteins. A total of ten and twelve different types of proteins present in other legume species were identified in the malted and dry seeds respectively from the 214 peptides isolated after searching 586 proteins of the genus Vigna. Seed storage protein B and vicilin were observed to be the major proteins common to both malted and dry seeds and are similar to Vigna luteola. Some of the other proteins observed showed amino acid sequence homology with Vigna radiata and Vigna unguiculata species. The following proteins BV1, Heat shock and Bowman-Birk Inhibitor (a protease), were observed only in the malted state. This information may enhance the appreciation of the nutritional and health benefits of the seed.
Keywords: Voandzeia subterranea L. Verdc., Malted Seed, MudPit, LC-MALDI TOF-TOF mass spectrometry, Vigna species, Peptides
Introduction
Bambara groundnut (Voandzeia subterranea L. Verdc) is a seed crop of African origin used locally as a vegetable or snack. It is cultivated principally by farmers as a "famine culture" crop because it has several natural agronomic advantages including high nutritional value, drought tolerance and the ability to produce in soils considered insufficiently fertile for cultivation of other more favoured species such as common beans and groundnuts (Arachid hypogea) (Anchirinah et al., 2001[3]; Azam-Ali et al., 2001[6]). Despite these numerous advantages, it is unfortunately, one of the neglected and under-utilized crops in sub Saharan Africa. The seed is regarded as a balanced food because when compared to most food legumes, it is rich in iron and the protein contains high lysine and methionine (Adu-Dapaah and Sangwan, 2004[2]). In addition, bambara groundnut is known to contain 63 % carbohydrates, 18 % oil and the fatty acid content is predominantly linoleic, palmitic and linolenic acids (Minka and Bruneteau, 2000[13]).
In view of world food shortages caused by drought, wars and inadequate government attention to agriculture especially in developing countries, it becomes expedient to harness proteins from all available food sources to minimise food and nutritional crises. As observed by Padulosi et al. (2002[15]), neglected and underutilized crops could play prominent roles in sustaining the impoverished rural African populations by increasing their available food and protein basket.
Bambara groundnut is eaten in several ways and at different stages of maturation. The young fresh seeds may be boiled and eaten as a snack in a manner similar to boiled peanuts and could be made into pudding locally called Moi Moi or Okpa (bean porridge) in the some parts of Nigeria. It has been reported that in Zambia, bambara groundnut is used for bread making (Brough et al., 1993[8]) while Poulter and Caygill (2006[16]) also reported that it could be used for milk making.
Wazael (2004[21]) described the great genetic diversity potential of bambara groundnut. Although, the types of proteins available in other species have been studied and reported, to the best of our knowledge, there has been no documented information on the types of protein in either the dry or the malted bambara groundnut (Voandzeia subterranea L. Verdc).
The goal of this investigation is to identify the different types of proteins in the malted and dry seeds of Voandzeia subterranea L. Verdc, their similarities to other known vigna species and the various uses which are expected to enhance the appreciation of the nutritional and health values of this seed.
Materials and Methods
Source of bambara groundnut
Bambara groundnuts (V. subterranea) were purchased in April, 2008 from a local market in Lagos metropolis and identified in the Department of Botany & Microbiology, University of Lagos, Akoka, Yaba, Lagos, Nigeria.
Malting of bambara groundnut
Fifty grams of bambara groundnuts were washed with distilled water and spread in a tray lined with three layers of Whatman filter paper No 3. The seeds were then covered completely with the filter paper and allowed to stand on the laboratory bench for five days at room temperature (37 ± 2 °C) for the seed to malt.
Ammonium sulphate precipitation
Twenty grams of malted bambara groundnuts were macerated and extracted in 50 ml potassium phosphate buffer, pH 7.0 using an electric blender, original millennium Nakai (blender 248) special. The macerate was centrifuged at 10,000 rpm for 10 mins and the supernatant decanted. The resulting suspension which is the crude protein extract was subjected to saturated ammonium sulphate precipitation. The precipitate was dialyzed overnight in phosphate buffer pH 7.0 at 4 °C.
Sample preparation for LC-MALDI
Digestion and sample preparation
A 50 µL aliquot of the sample was centrifuged at 4000 rpm for 5 mins. The supernatant was extracted and the pellet washed with digestion buffer (50 mM ammonium bicarbonate). The digestion buffer wash was combined with the original supernatant and subjected to denaturation and proteolytic digestion using trypsin. Samples collected were lyophilized and dissolved in 100 µL of 50 mM ammonium bicarbonate in 20 % acetonitrile for trypsin digestion. The samples were then reduced and alkylated with 10 µL of 250 mM DTT (60 min / 55 °C) and 10 µL of 625 mM iodoacetamide (60 min/room temperature / in the dark). Proteolytic digestion was performed in 50 mM ammonium bicarbonate buffer using a trypsin to protein ratio of 1:100. The digestion was carried out overnight at 37 °C. The digests were cleaned by repeated lyophilizing and reconstituting in a 0.1 M acetic acid solution. After final lyophilization, the digests were reconstituted in strong cation exchange (SCX) loading buffer (5 mM ammonium formate in 20 % acetonitrile, pH 3).
Strong cation exchange fractionation
After digestion, the protein samples were fractionated using SCX ProteaTip spin Tips. The tips were first washed to wet the packing material by adding 50 µL of strong cation exchange loading buffer and centrifuging the system at 4000rpm for 3 mins. The sample was then loaded in the Spin Tip and centrifuged at 4000rpm for 3 min after which it was washed to elute salts and other non-retained components by adding 50 µL of the rinse solution (5 mM ammonium format in 20 % acetonitrile) to the top of the Spin Tip. The Spin Tip was transferred to a new clean centrifuge tube to collect the sample during elution with 200 µL of elution solution. Eight different elution solutions were used to fractionate the peptides and these were 20, 60, 100, 150, 200, 250, 400, 500 mM ammonium formate in 20 % acetonitrile. The collected fractions were cleaned by repeated lyophilizing and reconstituting in a 0.1 M acetic acid solution. After final lyophilization, the digests were reconstituted in LC run buffer.
LC-MALDI spotting
Lyophilized digested samples were reconstituted in 20 µL of 0.1 % TFA in de-ionized water and 5 µL corresponding to 25 % of the sample (5 µL) was injected into ABI Tempo LC MALDI (Proteabio Inc.) with a Separating column - Merck Chromolith CapRod monolith column - 150 X 0.1 mm with separation gradient of 15 mins.
Mass spectrometry and data analysis
Mass spectrometric analysis was performed using an ABI 4800 MALDI TOF/TOF analyzer in positive ion reflector mode. Data was collected over a mass range of m/z = 850 - 4000. For MS acquisition, a total of 400 laser shots per spectrum with an S/N threshold of 10. For MS/MS acquisition, the 15 strongest precursors chosen for MS/MS with S/N threshold were set at 30 for MSMS precursors. Each MALDI spot was interrogated until at least 4 peaks in the MSMS spectra achieved an S/N of 70. The MS/MS data acquired data was processed with Protein ProteinPilot software 2.0 (Applied Biosystems) using the Paragon and Pro Group search algorithms. The search parameter for sample type was set for identification, trypsin was set as the digestion enzyme and the search database was the latest NCBInr database. Vigna was selected as the species and other special factors were cysteine alkylation and I.D focus on biological modifications. The search mode was set to thorough identification, and detected protein confidence threshold was set at 66 %.
Results
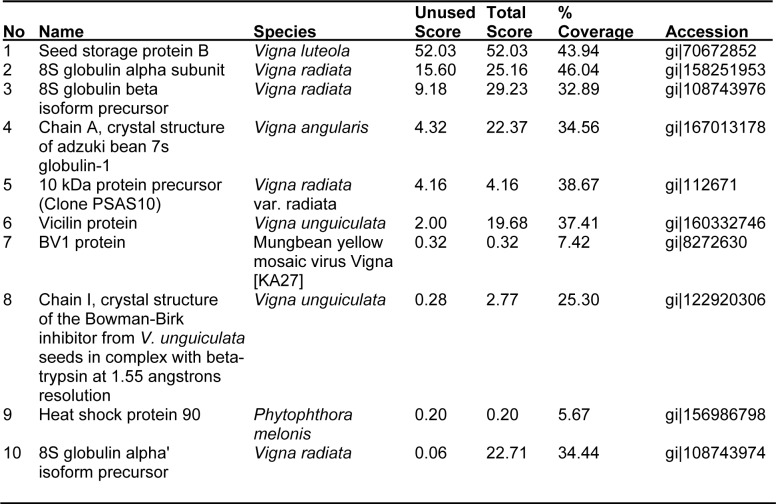
A total of ten and twelve different proteins were identified in the malted and dry seeds of bambara groundnut respectively from 214 peptides after searching 586 proteins of the genus Vigna. Of the twenty two different proteins identified, B storage protein and vicilin were the proteins most common to the malted and dry seeds.
The Swissprot protein database containing 586 proteins of the genus vigna which was used for this investigation did not contain any V. subterranea proteins. The latest NCBInr database also contained only but a few. Most of the proteins identified in both conditions of the seed had amino acid homology to other known species of Vigna.
Malted seed
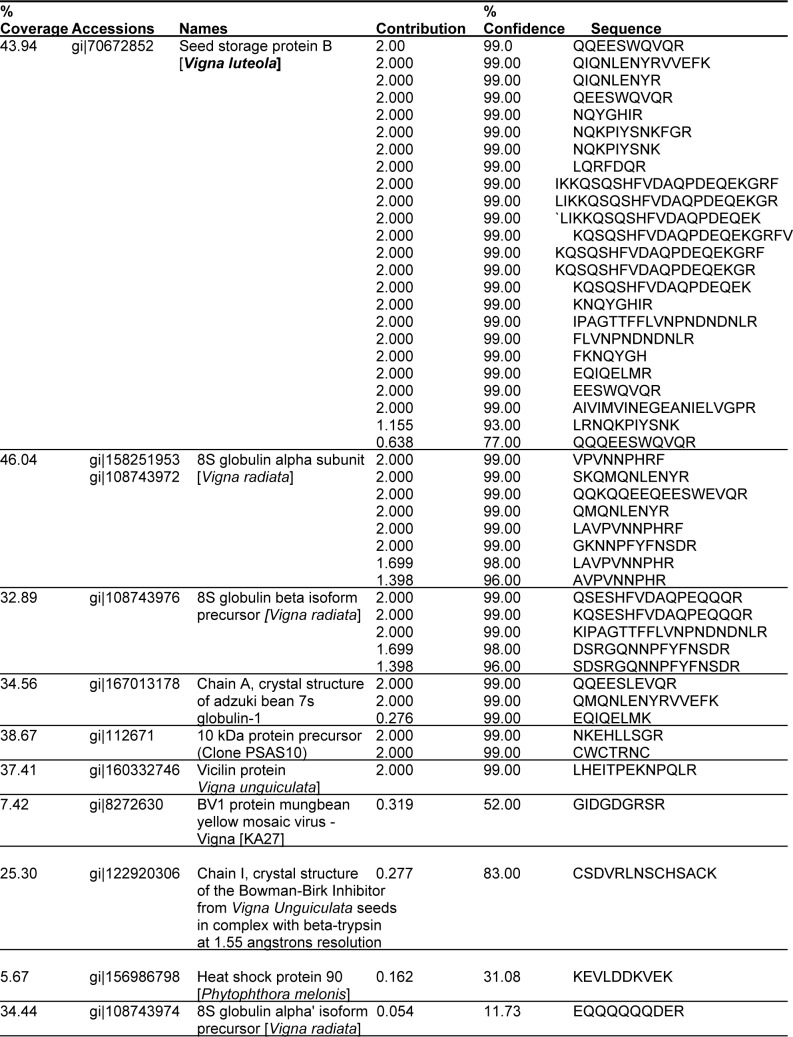
Ten proteins were identified in the malted state of the seed (Table 1(Tab. 1)). The major protein observed is the Seed storage protein B which exhibited a total protscore of 52.03 and percentage coverage of approx 43 that was found to be similar to that of Vigna luteola. Forty percent of the ten proteins identified showed amino acid sequence homology to Vigna radiata species, twenty percent is similar to Vigna unguiculata and only ten percent of the proteins were identical to Vigna angularis and Vigna luteola. The B storage protein of the latter incidentally, possesses the highest unused protscore (measure of the protein confidence for a detected protein, calculated from the peptide confidence for peptides from spectra that have not already been completely “used” by higher scoring proteins) in malted seed. However, the other proteins BV1, Heat shock protein, 8S globulin alpha isoform and Chain I, Crystal Structure of The Bowman-Birk Inhibitor protein had protscore (measure of the total amount of evidence for a detected protein) lower than the cut-off threshold of 0.47 used at 66 % confidence (the confidence for the peptide identification, expressed as a percentage), but presented fairly good percentage coverage (the number of amino acids matching at least one identified peptide divided by the total number of amino acids in the protein sequence, expressed as a percentage (Table 2(Tab. 2))). Interestingly, the peptide sequences of Chain I, Crystal Structure of The Bowman-Birk Inhibitor protein had low percentage coverage of only 25 % but exhibited high confidence level of 83 % indicating the accuracy of protein identification process.
Table 1. Proteins identified in malted bambara groundnut (Vigna subterranea).
Table 2. Peptide sequence of malted Vigna subterranea.
Dry seed
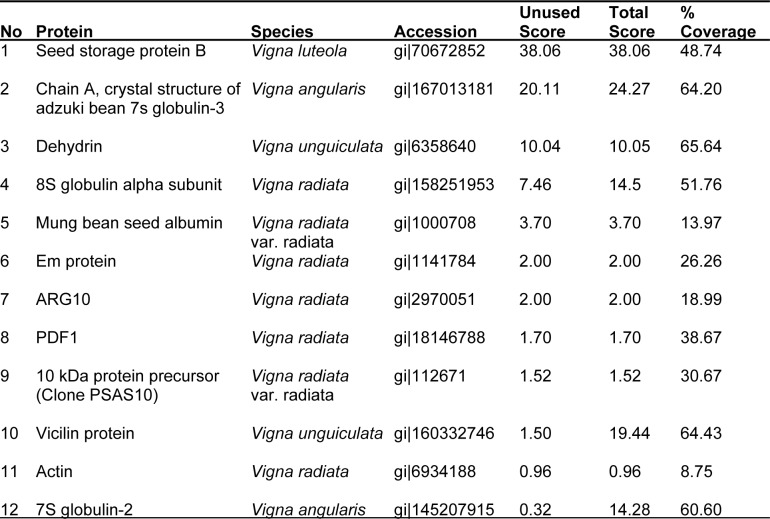
In the dry seeds, twelve proteins were identified (Table 3(Tab. 3)). Three had protscore above 19 at 66 % confidence level with percentage coverage above 32. The other types of protein however, had lower protscores ranging between 0.96-14.0 and percentage coverage of between 8 and 38.
Table 3. Proteins identified in dry bambara groundnut (Vigna subterranea).
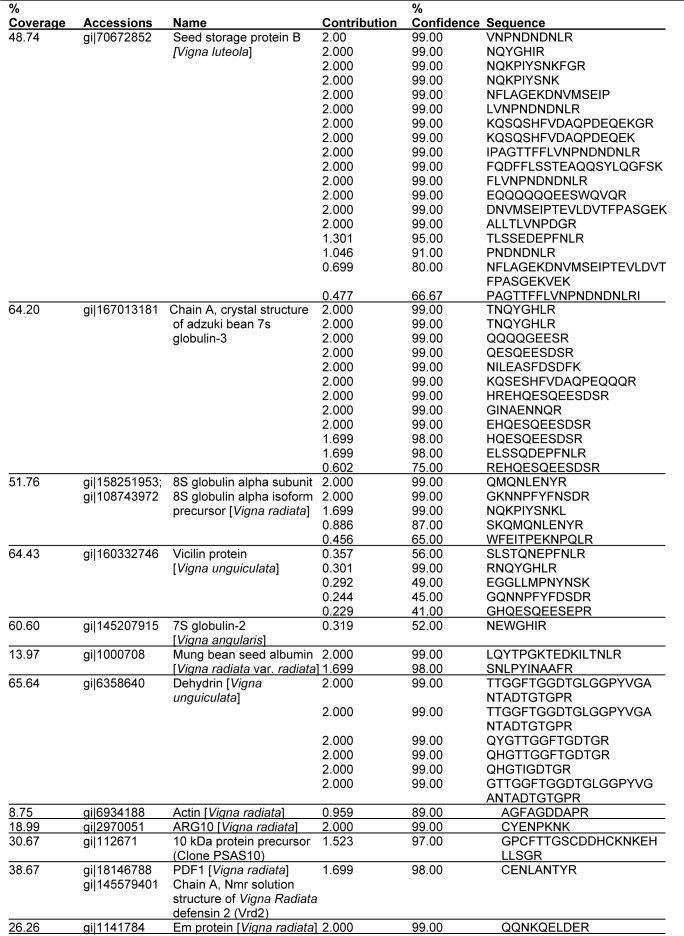
Seven identified proteins of V. subterranea, 8S globulin alpha subunit, Mung bean seed albumin, Em protein, ARG10, PDF1, 10 kDa protein precursor (Clone PSAS10) and Actin had sequence homology to Vigna radiata specie. Two proteins, Chain A, Crystal Structure of Adzuki Bean 7s Globulin-3 and 7S globulin-2 had amino acid sequence similar to Vigna angularis while another two, Dehydrin and vicilin was similar to Vigna unguiculata. Only one protein type (Seed storage protein B) was similar to Vigna luteola specie. The identified peptide sequences showed that some proteins with low protscores had high percentage confidence level (Table 4(Tab. 4)).
Table 4. Peptide sequence of dry Vigna subterranea.
Discussion
In this study, we investigated the types of proteins present in the mature malted and dry seeds of Vigna subterrenea. Bambara groundnut is an important legume that could become a major source of dietary protein in developing countries whose staple foods are predominantly carbohydrates. Unfortunately however, bambara nut is eaten mostly by a small proportion of the population especially, in the Eastern part of Nigeria.
Ten different types of proteins were identified from malted while twelve were found in the dry seeds. The difference in the number of proteins observed in the two states of the bambara seed may suggest that the dryseeds require additional proteins to cope with biological processes. In both conditions of the seed however, only five of the proteins were identical and these are Seed storage protein B, vicilin, beta and alpha isoforms of 8S globulin, and 10kDa protein precursors. The other proteins observed varied according to the condition of the seeds. Østergaard et al. (2002[14]) observed that there were cultivar differences in the 2-D gel spot patterns of proteins at the mature barley seeds and the malt. Only five protein types were identical in the two conditions emphasising that each state is unique and develops the requirements that enables it to cope with biological demands.
A viral protein with homology to Mung bean yellow mosaic virus (KA27), identified in the malted sample might be from a virus known to infect seeds of the vigna species (Garrido-Ramirez et al., 2000[11]). The BV1 gene of the bipartite Begomovirus genome which encodes a nuclear shuttle protein (NSP) is also an avirulence determinant present in common bean (Zhou et al., 2007[24]). The virus infection might have occurred during the malting process of Vigna subterranea. Heat shock proteins identified in this study had amino acid homology with that found in Phytophthora melonis. Heat shock proteins (HSPs) are expressed in response to heat and other forms of stress (Queitsch et al., 2000[17]) and are known to be responsible for protein folding, assembly, translocation and degradation in many normal cellular processes. They also assist in stabilizing proteins and membranes and under stress conditions, can assist in protein refolding (Wang et al., 2004[20]). It is our thinking that during the malting process of bambara seeds, there was a form of stress which was expressed by the appearance of heat shock proteins. This stress could be in form of excess or shortage of water or infection. Infection is likely to be the case; therefore, this protein may be transient because it has a very small percentage coverage and low confidence level.
It is likely that the malted sample rather than increase or decrease its seed nitrogen, produced other protein types like Chain A, crystal structure of adzuki bean 7s globulin-1 and Chain I, crystal structure of the Bowman-Birk Inhibitor. Dehydrin present in the dry seeds may assist the seed in withstanding stress due to lack of water in that state.
There are reports that seeds of higher plants accumulate large quantities of seed proteins including storage proteins and lectins (Adachi et al., 2003[1]). Seed storage proteins are deposited in protein bodies, specialized vacuoles known as protein storage vacuoles (PSVs) (Shimada et al., 2003[18]). They serve as nitrogen, sulphur and carbon source for the developing embryo during seed germination (Adachi et al., 2003[1]). They are also major sources of dietary protein for humans. Shimada et al., (2003[18]), demonstrated that unique vesicles which are precursor-accumulating vesicles, mediate the mass transport of storage proteins to PSVs in maturing pumpkin seeds. They also showed that isolated precursor-accumulating vesicles contain large amount of the precursors of major storage proteins of seeds. These types of precursor proteins are 8S globulin beta and alpha isoforms and 10kDa protein precursors. Vicilins also sequenced in this study, is a globulin storage protein found in plants. The importance of vicilin proteins was reported in soyabeans where 7S globulin subunit of soyabeans was observed to lower plasma lipids and upregulates liver (ß)-VLDL receptors in rats fed with hypercholesterolomic diet (Duranti et al., 2004[10]).
Vicilins of various legume seeds show very high degree of sequence homology but present different properties when treated with proteases (Deshpande and Damodaran, 1989[9]). We observed that the condition of the seed (dry or malted), determined the type of proteins identified. Swire-Clark and Marcotte (1999[19]) had observed that late embryogenesis-abundant (LEA) proteins are capable of binding large amounts of water and it is possible that this is the function of Em protein found in the dry bambara seed. Chain I, crystal structure of the Bowman-Birk Inhibitor from Vigna unguiculata widespread in leguminous plants are involved in diverse cellular processes such as cell cycle, apoptosis, intracellular protein breakdown (Barbosa et al., 2007[7]). The reorganization of cellular processes in plants has been linked to Actin cytoskeleton (Aon et al., 1999[5]). Although the protscore and sequence coverage of Actin protein is low, it is important in maintaining the cytoskeleton. The presence of ARG10 protein which down regulates auxin showed that the dry bambara seed is in a dormant state and the cells are not actively dividing.
These proteins, PDF1 and Em may be important for the seeds to fulfil other biological processes apart from storing protein. Em proteins function in the embryo as storage protein for nutrients that are necessary in the early period of the germination process. It has been suggested that Em protein may be a cryptobiotic protein analogue found in Artemia. It therefore provides a form of desiccation tolerance to the cytoplasm while it is dehydrated at seed maturation (McCubbin et al., 1985[12]). PDF1 plays a defensive role as shown in Arabidopsis plant.
The study of this legume is important because of its potential nutritional and health benefits to the populations of the developing world. Seed proteins have been implicated in anti-hypertensive activities (Yamada et al., 2008[22]), regulation of food intake and body weight in humans (Anderson and Moore, 2004[4]) and the improvement of plasma and liver lipid profile in rats fed with high cholesterol diet (Yang et al., 2007[23]).
This study reports the different types of proteins of bambara groundnut (Vigna subterranea) present in the malted and dry seeds, their similarities and various uses which may enhance the appreciation of the food, nutritional and health values of the seed. Bambara groundnut belongs to the group of seeds labelled under-utilized and neglected, it is therefore strongly recommended for mass production to fill the current food gaps. Furthermore, extensive research is suggested to fully exploit the nutritional and health benefits derivable from it for the overall benefit of the starving populations of the third world.
References
- 1.Adachi M, Kanamori J, Masuda T, Yagasaki K, Kitamura K, Mikani B, Utsumi S. Crystal structure of soybean 11S globulin: glycin A3B4 homohexamer. Proc Natl Acad Sci USA. 2003;100:7395–7400. doi: 10.1073/pnas.0832158100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adu-Dapaah HK, Sangwan RS. Improving bambara groundnut productivity using gamma irradiation and in vitro techniques. Afr J Biotechnol. 2004;3:260–265. [Google Scholar]
- 3.Anchirinah VM, Yiridoe EK, Bennett-Lartey SO. Enhancing sustainable production and genetic resource conservation of Bambara groundnut: A survey of indigenous agricultural knowledge systems. Outlook on Agric. 2001;30:281–288. [Google Scholar]
- 4.Anderson GH, Moore SE. Dietary proteins in the regulation of food intake and body weight in humans. J Nutr. 2004;134:974S–979S. doi: 10.1093/jn/134.4.974S. [DOI] [PubMed] [Google Scholar]
- 5.Aon MA, Cortassa S, Gomez CDF, Iglesias AA. Effects of stress on cellular infrastructure and metabolic organization in plant cells. Int Rev Cytol. 1999;194:239–73. doi: 10.1016/s0074-7696(08)62398-0. [DOI] [PubMed] [Google Scholar]
- 6.Azam-Ali SN, Sesay A, Karikari KS, Massawe FJ, Aguilar-Manjarrez J, Bannayan M, Hampson KJ. Assessing the potential of an underutilized crop - a case study using bambara groundnut. Exp Agric. 2001;37:433–72. [Google Scholar]
- 7.Barbosa JARG, Silva LP, Teles CL, Esteves GF, Azevedo FB, Ventura MM, deFreitas SM. Crystal structure of the Bowman-Birk Inhibitor from Vigna unguiculata seeds in complex with β-trypsin at 1.55 Å resolution and its structural properties in association with proteinases. Biophys J. 2007;92:1638–50. doi: 10.1529/biophysj.106.090555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brough SH, Azam-Ali SN, Taylor AJ. The potential of bambara groundnut (Vigna subterranea) in vegetable milk production and basic protein functionality systems. Food Chem. 1993;47:277–283. [Google Scholar]
- 9.Deshpande SS, Damodaran S. Structure-digestibility relationships of legume 7S storage proteins. J Food Sci. 1989;54:108–113. [Google Scholar]
- 10.Duranti M, Lovati MR, Dani V, Barbiroli A, Scarafoni A, Castiglioni S, Ponzone C, Morazzoni P. The alpha' subunit from soybean 7S globulin lowers plasma lipids and upregulates liver ß-VLDL receptors in rats fed a hypercholesterolemic diet. J Nutr. 2004;134:1334–1339. doi: 10.1093/jn/134.6.1334. [DOI] [PubMed] [Google Scholar]
- 11.Garrido-Ramirez ER, Sudarshana MR, Lucas WJ, Gilbertson RL. Bean dwarf mosaic virus BV1 protein is a determinant of the hypersensitive response and avirulence in Phaseolus vulgaris. Mol Plant Microbe Interact. 2000;13:1184–1194. doi: 10.1094/MPMI.2000.13.11.1184. [DOI] [PubMed] [Google Scholar]
- 12.McCubbin WD, Kay CM, Lane BG. Hydrodynamic and optical properties of the wheat germ Em protein. Can J Biochem Cell Biol. 1985;63:803–811. [Google Scholar]
- 13.Minka SR, Bruneteau M. Partial chemical composition of bambara pea (Vigna subterranea (L. Verde) Food Chem. 2000;68:273–276. [Google Scholar]
- 14.Østergaard O, Melchior S, Roepstorff P, Svensson B. Initial proteome analysis of mature barley seeds and malt. Proteomics. 2002;2:733–739. doi: 10.1002/1615-9861(200206)2:6<733::AID-PROT733>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 15.Padulosi S, Hodgkin T, Williams JT, Haq N. Underutilized crops: trends, challenges and opportunities in the 21st century. In: Engels JMM, et al., editors. Managing plant genetic diversity. UK and Rome: CABI-IPGRI; 2002. pp. 323–338. [Google Scholar]
- 16.Poulter NH, Caygill JC. Vegetable milk processing and rehydration characteristics of bambara groundnut [Voandzeia subterranea (L.) thouars] J Sci Food Agric. 2006;31:1158–1163. [Google Scholar]
- 17.Queitsch C, Hong S, Vierling E, Lindquist S. Heat shock protein 101 plays a crucial role in thermotolerance in arabidopsis. Plant Cell. 2000;12:479–492. doi: 10.1105/tpc.12.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I. Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2003;100:16095–16100. doi: 10.1073/pnas.2530568100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swire-Clark GA, Marcotte Jr. WR. The wheat LEA protein Em functions as an osmoprotective molecule in Saccharomyces cerevisiae. Plant Mol Biol. 1999;39:117–128. doi: 10.1023/a:1006106906345. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Vinocur B, Shoseyov O, Altmar A. Role of plant heat-shock protein and molecular chaperons in the abiotic stress response. Trends Plant Sci. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Wazael HN, Inga CB, Jorgen LC, Sven BA. Analysis of genetic diversity in bambara groundnut [Vigna subterranea (L.) Verdc] landraces using amplified fragment length polymorphism (AFLP) markers. Afri J Biotech. 2004;3:220–225. [Google Scholar]
- 22.Yamada Y, Nishizawa K, Yokoo M, Hui Z, Onishi K, Teraishi M, Utsumi S, Ishimoto M, Yoshikawa M. Anti-hypertensive activity of genetically modified soybean seeds accumulating novokinin. Peptides. 2008;29:331–337. doi: 10.1016/j.peptides.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Yang S-C, Liu S-M, Yang H-Y, Lin Y-H, Chen J-R. Soybean protein hydrolysate improves plasma and liver lipid profiles in rats fed high-cholesterol diet. J Amer Coll Nutr. 2007;26:416–423. doi: 10.1080/07315724.2007.10719631. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y-C, Garrido-Ramirez ER, Sudarshana MR, Yendluri S, Gilbertson RL. The N-terminus of the Begomovirus nuclear shuttle protein (BV1) determines virulence or avirulence in Phaseolus vulgaris. Mol Plant Microbe Interact. 2007;20:1523–1534. doi: 10.1094/MPMI-20-12-1523. [DOI] [PubMed] [Google Scholar]