Abstract
At least three transport systems function in targeting nuclear-encoded chloroplast proteins to the chloroplast thylakoid membrane. One of these systems requires a thylakoid pH gradient and is named the ΔpH-dependent protein transport system. A similar ΔpH export system of Escherichia coli contains four components, twin arginine translocation A (TatA), TatB, TatC, and TatE. TatC is a major component of the ΔpH-dependent protein transporter in E. coli and functions in the translocation of tightly folded proteins across membranes. We have isolated four transposon-inserted albino mutants named albino and pale green 2 (apg2) from Arabidopsis thaliana and showed that the transposons were inserted into different sites of a single gene. The APG2 gene product (named cpTatC) has sequence similarity with bacterial TatC and contains six putative transmembrane domains, including bacterial TatC proteins and a transit peptide in its N terminus. apg2 mutants showed albino phenotypes and could not grow in soil. The apg2 plastids were highly vacuolated, lacked internal membrane structures and lamellae of the thylakoid membrane, and contained many densely stained globule structures, like undifferentiated proplastids. Immunoblot analysis detected no thylakoid membrane proteins such as D1, light-harvesting complex, and OE23 in apg2 plastids, whereas soluble proteins such as rubisco large and small subunits were not decreased. These results indicate an essential role of cpTatC in chloroplast development, especially in thylakoid membrane formation.
Chloroplasts perform essential processes in photosynthesizing various primary metabolites, including carbohydrates, fatty acids, and amino acids. About 100 plastid proteins are encoded in the plastid genome (1–3). However, most of the plastid proteins are encoded by the nuclear genome and are synthesized as precursors in the cytosol, which are then transported into proper regions for their functions within chloroplasts. Precursor proteins have so-called “transit peptides” in their N terminus that ensure the proper targeting of the peptides into the plastids (4). Plastids are separated from the cytosol by outer and inner envelope membranes. Translocon protein complexes of the outer membrane (Toc complex; refs. 5, 6) and of the inner membrane (Tic complex; ref. 7) recognize transit sequences and import the precursors of plastid proteins into the stroma.
Thylakoid proteins in imported stromal proteins have a thylakoid-targeting domain of transit peptide and then direct transport across the thylakoid membrane (8). There are at least three transport pathways by which nuclear-encoded proteins are targeted to the thylakoid membrane. The thylakoid Sec pathway contains the cpSecA and cpSecY proteins, requires ATP, and is similar to the bacterial Sec system (9, 10). The LHC integration pathway shares some features with the GTP-dependent signal recognition particle systems of the endoplasmic reticulum and bacteria and requires cpSRP54, cpSRP43, cpFtsY, GTP, and soluble factors (11). The ΔpH-dependent pathway operates independently of ATP and soluble factors, requiring only a thylakoid pH gradient. Recently, a Sec-independent export system was discovered in Escherichia coli , which is related to the thylakoid ΔpH-dependent protein transport system of chloroplasts (12–14). The E. coli system involves the integral membrane proteins twin arginine translocation A (TatA), TatB, TatC, and TatE, and functions in the export of proteins that bind complex redox cofactors. All of the substrates transported by the ΔpH-dependent system possess a “twin arginine motif” in their signal peptides. TatC is a major component of the translocator complex in the ΔpH-dependent system. A central role of TatC-type proteins is the translocation of tightly folded proteins across biological membranes.
The maize mutants high-chlorophyll fluorescent 106 (hcf106) and thylakoid assembly 4 (tha4) were shown to be selectively defective in the ΔpH-dependent system (15, 16). The N subunit protein of photosystem I (PSI-N) and the oxygen-evolving complex OE23 and OE16/17 are translocated via the ΔpH-dependent system (17, 18). The Hcf106 protein is related in both structure and function to the E. coli tatA, tatB, and tatE gene products. The tha4 gene sequence resembles those of hcf106 and the E. coli tatA and tatB (16). Biological functions of plant homologues of the TatC protein have not previously been reported.
In this study, we describe an important function of a plant homologue of the TatC protein in chloroplast development. We isolated and analyzed Arabidopsis thaliana albino mutants in which a gene for TatC was tagged with a Dissociation (Ds) transposon. Disruption of the gene caused abnormal immature development of plastids. We precisely analyzed the albino mutants and their revertants and discuss essential roles of the TatC homologue of the ΔpH-dependent protein transporter in plastid development.
Materials and Methods
Construction of Ds Insertion Lines.
We made crosses between a transgenic line expressing Activator (Ac) transposase (Nae Ac380–16) as the female parent and Ds-GUS-T-DNA lines (Ds2 389–13 and Ds4 391–20) as pollen parents (19, 20). Selection of the transposed lines was described previously (21, 22).
Cloning of APG2 cDNA and DNA Gel Blot Analysis.
The thermal asymmetric interlaced (TAIL)-PCR fragments were used as probes to screen an Arabidopsis cDNA library for a full-length cDNA that encodes APG2. The isolation of genomic DNA of ecotypes Columbia, Nossen, and Landsberg erecta and DNA gel blot hybridization were performed as described by Ito et al. (21). The full-length APG2 cDNA fragment was used as a probe. The filter was washed twice with 0.1 × SSC/0.1% SDS at 65°C for 15 min (high-stringency condition) or at 42°C for 15 min (low-stringency condition).
Electron Microscopy.
The samples were fixed in 3% glutaraldehyde, which was buffered with 20 mM sodium cacodylate (pH 7.0) for 6 h at 4°C and washed with the same buffer for 16 h at 4°C. Samples were postfixed with 2% osmium tetroxide in 20 mM cacodylate buffer (pH 7.0) for 6 h at 4°C. The fixed samples were dehydrated through a series of alcohol solutions and embedded in Spurr's resin (Taab, Berkshire, UK). Ultrathin sections were obtained by cutting with a diamond knife on an Ultracut UCT ultramicrotome (Leica, Vienna, Austria) and were transferred to Formvar-coated grids. The sections were stained with 1% uranyl acetate for 15 min at 37°C and then with lead citrate solution for 10 min at room temperature. After being washed with distilled water, the samples were observed through a JEM-2000 FXII electron microscope (JEOL).
Transient Assay by Particle Bombardment.
The putative transit peptide region from APG2 (28-aa N-terminal region described as Fig. 3 A, arrow) was amplified by PCR and was cloned into the XbaI–BamHI sites of p35S-sGFP, consisting of a synthetic green fluorescent protein (GFP) under the control of cauliflower mosaic virus 35S promoter (23). The resulting fusion construct was named p35S∷APG2tp-sGFP. The p35S∷APG2tp-sGFP and 35S∷sGFP constructs were introduced into leaves of Nicotiana tabacum SR1 and onion epidermal cells by particle bombardment, as described (24). Cells expressing GFP were observed with a confocal laser-scanning microscope (LSM510, Zeiss). GFP signals were detected with a band-pass 505≈530-nm filter set (wavelength, 488-nm excitation), and chlorophyll autofluorescence was detected with a long-pass 560-nm filter set (wavelength, 543-nm excitation).
Figure 3.
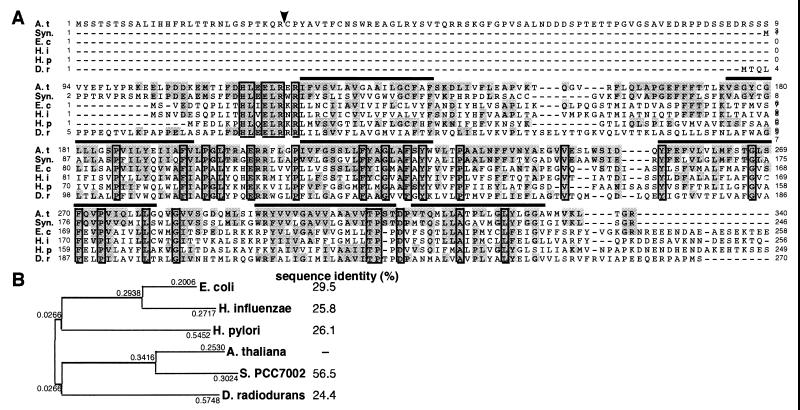
(A) Alignment of the deduced amino acid sequences from Arabidopsis APG2 (cpTatC) and TatC-like proteins. The arrowhead is the putative cleavage site of the transit peptide in the APG2 amino acid sequence. Over lines show transmembrane helix regions. A. t, cpTatC, encoded by APG2 from A. thaliana (GenBank/EMBL accession no. AB054096). Syn, YCF43 from Synechococcus PCC7002 (AAD26593). E. c, Sec-independent protein translocase TatC from E. coli (AJ005830; refs. 13, 40). H. i, Sec-independent protein translocase protein TatC from H. influenzae (P44560; ref. 41). H. p, hypothetical protein from Hemophilus pylori (D71942; ref. 42). D. r, putative Sec-independent protein translocase protein TatC from Deinococcus radiodurans (AE001935; ref. 43). Highly conserved amino acids are shaded, and identical amino acids are boxed. Dashes indicate gaps introduced to improve the alignment. (B) Phylogenetic analysis of the TatC-related proteins. The phylogenetic tree was constructed according to the neighbor-joining method (genetyx, Mac, Software Development, Tokyo) by using the full-length amino acid sequences from each protein. Sequence identity (%) indicates amino acid sequence identity with A. thaliana cpTatC protein.
RNA Gel Blot Analysis.
Total RNA was isolated from 3- to 4-week-old Arabidopsis plants that had been grown on germination medium plates (21, 25). Total RNA samples (20 μg) were denatured and fractionated by electrophoresis in a 1-M formaldehyde/1.25% agarose gel and then transferred to a nylon membrane (26). RNA gel blots were hybridized to random-primed [32P] cDNA or genomic DNA probes. The probes were generated by PCR amplification of the large (GenBank/EMBL accession nos. U91966, 471-1247) and small (X13611, 791-1614) subunits of rubisco, psbA (X79898, 281-1045), psbP (X98108, 290-1537), psaN (U32176, 31–529), 16S rRNA (AP000423, 136147–137637), APG2 (AB054096, 1–1023), and the chlorophyll a/b-binding protein (X03909, 201–934) of Arabidopsis. After hybridization, the filters were washed twice with 0.1 × SSC/0.1% SDS at 65°C for 15 min and autoradiographed.
Electrophoresis and Immunological Detection of Plastid Proteins.
To prepare soluble proteins for immunological detection of the rubisco large subunit and small subunit complex, leaves were homogenized in a microcentrifugal tube on ice in a solution containing 50 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 10% (vol/vol) glycerol, 1.4 mM 2-mercaptoethanol, and 1 mM DTT. Total proteins for immunological detection of thylakoid proteins D1, LHC, and OE23 were prepared as described previously (27).
Proteins were separated by SDS/PAGE (5% acrylamide stacking gel and 13% acrylamide separating gel), as described by Laemmli (28). After electrophoresis, proteins were stained with Coomassie brilliant blue or transferred electrophoretically to polyvinylidene difluoride filters. For Western blotting, for the procedures of enhanced chemiluminescence (Amersham Pharmacia), the manufacturer's instructions were followed. Polyclonal antibody against the tobacco rubisco L/S complex was provided by F. Sato of Kyoto University and T. Nakano of RIKEN, polyclonal antibody against spinach chlorophyll a/b-binding protein was provided by T. Masuda and K. Takamiya of the Tokyo Institute of Technology, polyclonal antibody against spinach D1 protein was provided by M. Ikeuchi of Tokyo University, and polyclonal antibody against pea OE23 protein was provided by T. Endo of Nagoya University.
Results
Identification of the Ds-Tagged apg2 Mutants.
To study the functions of nuclear genes involved in chloroplast development by a genetic approach, we constructed transposon (Ds)-inserted Arabidopsis mutants. We screened 2739 Ds-transposed lines carrying independent transposition events for mutants with the albino or pale green phenotype.
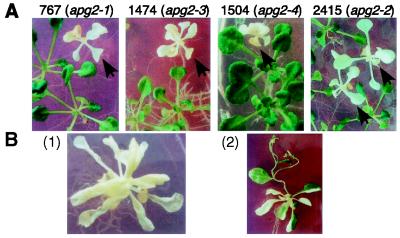
We carried out detailed analyses of four albino mutants, line numbers 767, 1474, 1504, and 2415. TAIL-PCR analysis (22) revealed that Ds was inserted into the same gene in all four lines (see below). When plated on agar medium, these mutants showed the albino phenotype (Fig. 1A). When sown on soil, they germinated but did not survive. To show that Ds insertion was responsible for the albino phenotype, we tried to isolate revertants from the albino phenotype to the wild-type phenotype. We monitored line 767 with a transposase gene driven by the cauliflower mosaic virus 35S promoter (35S∷TPase) and found somatic reversions (Fig. 1B). Revertant seeds were obtained from the green sector (Fig. 1B). The progeny plants obtained from revertants contained green seedlings, which are germinal revertants. Total DNA samples isolated from wild-type (ecotype Nossen), hygromycin-resistant heterozygous apg2 plant, apg2 mutant, and revertants were digested with HindIII and then subjected to DNA gel blot analysis by using the full-length APG2 cDNA probe. Ds does not contain a HindIII site. A 7-kb HindIII fragment was detected in the wild-type allele of apg2. The albino plants were homozygous for the Ds insertion and completely lacked a 7-kb fragment, but revertans had a 7-kb fragment (data not shown). These data indicate the linkage of Ds to the apg2 mutation. No revertants were obtained from the other three lines, probably because Ds was inserted irregularly (see below). These results indicate that the four lines had defects at the same locus. Therefore, we named the alleles apg2–1 (for albino and pale green mutant 2; line 767), apg2–2 (line 2415), apg2–3 (line 1475), and apg2–4 (line 1504). Most of the apg2–4 mutant seeds did not germinate, probably because of a large deletion in the Ds insertion site (see below).
Figure 1.
(A) Four Ds-tagged mutants (lines 767, 1474, 1504, and 2415) have an albino phenotype (arrows). Heterozygous seeds were plated on agar medium with hygromycin (10 μg/ml) for 21 days. Green siblings are heterozygous plants. (B) (1) Albino plant (line 767; apg2–1). (2) Revertant green sectors occurred in an albino plant (line 767) carrying the Ac transposase gene. The reversion is because of excision of Ds from the APG2 gene locus.
Isolation of the APG2 Gene.
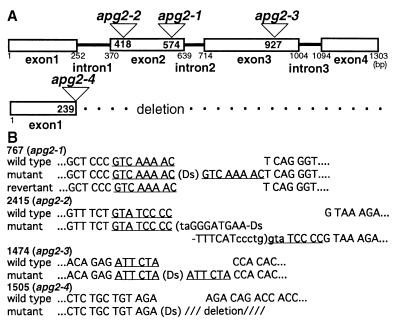
To isolate APG2, we used TAIL-PCR to obtain Ds-flanking regions in apg2 mutants and determined their nucleotide sequences. The four albino mutants contained Ds insertions in the APG2 gene. We isolated cDNA from a cDNA library by using the TAIL-PCR fragment as a probe and predicted the exon/intron structure by comparison with the genomic sequence database (Fig. 2A). All Ds insertion sites were located in various exons of APG2 (Fig. 2A).
Figure 2.
(A) Schematic representation of the genomic region flanked by Ds insertions. The Ds insertion sites of APG2 are shown by triangles in apg2 mutants. Boxes show exons of APG2. Numbers in the boxes show the insertion positions of Ds in APG2. Numbers under the boxes are nucleotides from the initiation codon (bp). (B) Sequences of flanking regions of Ds insertion sites in the apg2 mutant. The nucleotide sequences at the Ds insertion sites are shown for wild-type plants, Ds insertion mutants, and revertants. Nucleotides in lowercase letters were deleted during transposition. The underlined 8-bp sequence is the target site for duplication. PCR was performed by using primers on each side of the Ds element to amplify its flanking sites.
We determined the sequences around the Ds insertion sites of the four mutant alleles and around the Ds excision sites of the revertants from apg2–1 (Fig. 2B). The apg2–1 allele had an 8-bp target site duplication (TSD) sequence in the insertion site; its revertant perfectly recovered the wild-type sequence. Ds was inserted irregularly in the other three alleles, which is why no revertants were obtained (Fig. 2B). Part of an inverted repeat sequence of Ds and a TSD of the 3′ side were deleted in apg2–2. Allele apg2–3 had a 6-bp imperfect TSD, and allele apg2–4 had a long deletion from the 3′ side of APG2.
We carried out a DNA gel blot hybridization experiment by using the APG2 cDNA clone as a probe and showed that APG2 is a single-copy gene in the Arabidopsis genome (data not shown). When hybridization was carried out under low-stringency conditions, it revealed no additional bands, which indicates that there are no other sequences closely related to APG2 in Arabidopsis (data not shown). Moreover, polymorphism was not detected between ecotypes Columbia, Nossen, and Landsberg erecta when their total genomic DNA was digested with various restriction enzymes ( HindIII, KpnI, PstI, EcoRI, BglII, DraI, and XhoI) and DNA gel blot hybridization was carried out (data not shown). APG2 was mapped on a nuclear organizer region near marker rga at the end of the long arm of chromosome 2.
APG2 Encodes a TatC Homologue of E. coli ΔpH-Dependent Protein Transporter.
The APG2 cDNA contained a putative ORF. Its sequence shows a 340-aa ORF encoding a putative 37-kDa protein (Fig. 3A). A homology search by using the blastx program revealed high homology to the tatC gene from E. coli (Fig. 3A). We named the APG2 product cpTatC (chloroplast TatC homologue). In a ΔpH-dependent protein transport system, cpTatC is thought to be a major component of the translocator complex, like TatC proteins in E. coli. The tmpred program (http://www.ch.embnet.org/software/TMPRED_form.html) found transmembrane helices in amino acid sequences of cpTatC. The cpTatC protein is highly hydrophobic and probably contains six transmembrane helices, like E. coli TatC proteins (Fig. 3A).
In comparison with the amino acid sequences of TatC proteins from various organisms, the Arabidopsis TatC protein has the highest sequence homology with that of cyanobacteria (Fig. 3B). The chlorop program (http://www.cbs.dtu.dk/services/ChloroP) predicted the cleavage site of the transit peptide to be position 28–29 in Arabidopsis . These observations suggest that cpTatC is a chloroplast-targeted protein.
The Albino Tissue of apg2 Contains Abnormal Plastids Without Thylakoid Membranes.
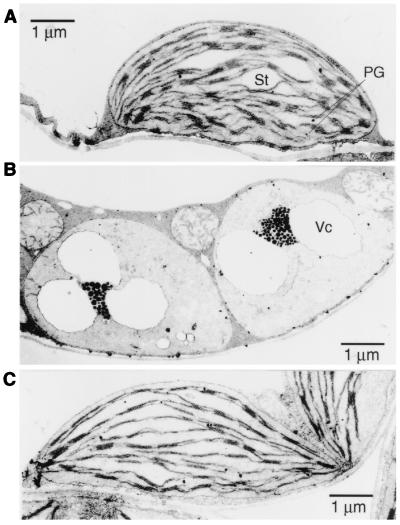
We analyzed morphological changes in plastids in the apg2 albino mutants by using electron microscopy (Fig. 4). We found abnormal plastids in albino tissue of the apg2–1 (Figs. 4B) and apg2–2 (data not shown) mutants. The mutant plastids had similar structures with similar size to those of undifferentiated wild-type proplastids, but the size of the mutant plastids was as that of the wild-type. They were highly vacuolated, lacked internal membrane structures, such as stromal and granal lamellae, and contained abnormal suborganelle structures. We detected a considerable number of densely stained globule structures, likely representing plastoglobuli (Figs. 4B). Thylakoids from chloroplasts of revertant tissue recovered the wild-type membrane structures (Fig. 4C). These results suggest an important role of the cpTatC in thylakoid membrane formation during plastid development.
Figure 4.
Transmission electron micrographs of plastids. (A) Chloroplast of Ds donor line Ds389–13. (B) Abnormal plastids of albino mutant apg2–1. (C) Chloroplast of revertant apg2–1. Vc, vacuolated structure; St, starch; PG, plastoglobule.
Role of the APG2 Transit Peptide in Plastid Targeting.
The N-terminal region of APG2 protein has typical structural features of transit peptides that are involved in chloroplast targeting. This structural feature of the APG2 suggests that the mature APG2 is localized in plastids. To analyze the role of its N-terminal region (28 aa) as a transit peptide, we constructed a chimeric gene, p35S∷APG2tp-sGFP, expressing a fusion protein of the N-terminal region of the APG2 (1–28 amino acids; Fig. 3A) and the sGFP (synthetic GFP gene of the jellyfish Aequorea victoria) coding region (23). When the p35S∷APG2tp-sGFP chimeric gene was introduced into epidermal cells of N. tabacum SR1 by particle bombardment method (24), GFP fluorescent signals were localized on chloroplast particles with the autofluorescence (Fig. 5 A and B). When a chimeric gene without the transit peptide was used, GFP signals were dispersed in cytosol and nuclei (data not shown). Similar results were obtained with onion epidermal cells (data not shown). These results suggest that APG2 encode cpTatC protein targeted into chloroplasts.
Figure 5.
Plastid targeting of the APG2tp-sGFP chimeric protein in tobacco epidermal cells. A tobacco epidermal cell in which the p35S∷APG2tp-sGFP construct was introduced by particle bombardment was observed. Fluorescence from GFP (A) and from chlorophyll autofluorescence (B) was detected by a laser confocal-scanning microscope.
Tissue-Specific Expression of APG2 in Arabidopsis Leaves.
We examined whether apg2 mutants accumulated APG2 mRNA, which we detected in wild-type plants but not in apg2 mutants by RNA gel blot analysis (Fig. 6A). This result indicates that APG2 mRNA did not accumulate in the apg2 mutants. We then analyzed the expression pattern of APG2 mRNA in various organs (Fig. 6B). The APG2 gene was strongly expressed mainly in the leaves, which contain many actively functioning chloroplasts (Fig. 6B). The expression level of the APG2 gene did not differ between young and old rosette leaves (data not shown). These results suggest that APG2 is expressed not only in the early stages of plastid development but also in all stages of chloroplast biogenesis.
Figure 6.
RNA expression analyses of APG2. (A) Gene expression of nuclear- and chloroplast-encoded genes. Total RNA samples were prepared from 21-day-old wild-type plants (lane 1, Columbia; lane 2, Nossen), Ds donor line Ds389–13 (lane 3), apg2–1 mutant (lane 4), and apg2–2 mutant (lane 5). The probes used for RNA blot hybridization were APG2, cab, rbcS, rbcL, psbA, psbP, psaN, and 16SrRNA; these are shown on the left. (B) Analysis of APG2 expression in various organs of normally grown Arabidopsis wild-type plants; flowers (1), roots (2), leaves (3), siliques (4), and stems (5).
Expression of Nuclear- and Chloroplast-Encoded Genes in apg2 Mutants.
We analyzed the expression of several chloroplast- and nuclear-encoded photosynthetic genes in both wild-type and apg2 plants. We used four nuclear genes and three chloroplast-encoded genes as probes. The nuclear genes were the small subunit of ribulose-bisphosphate carboxylase (rbcS; ref. 29), the light-harvesting chlorophyll a/b-binding protein (cab; ref. 30), the N subunit of photosystem I (psaN; ref. 31), and the 23-kDa polypeptide of the oxygen-evolving complex of photosystem II (psbP; ref. 32). The chloroplast-encoded genes were the D1 protein of photosystem II (psbA; ref. 33), the large subunit of ribulose-bisphosphate carboxylase (rbcL; ref. 34), and 16S rRNA (3). Transcripts of these genes were detected at similar levels in both wild-type and apg2 plants (Fig. 6A). These results indicate that the accumulation of mRNA of photosynthetic genes is not affected in the apg2 mutants.
Immunoblot Analysis of Chloroplast Proteins in the apg2 Mutants.
Next, we examined the effect of the apg2 mutations on the accumulation of photosynthetic proteins. The amounts of chloroplast proteins related to photosynthesis were examined by immunoblot analysis (Fig. 7). These proteins were the thylakoid membrane proteins D1 (a product of the chloroplast psbA gene), LHC (a product of the nuclear cab gene), and OE23 (a product of the nuclear psbP gene), and the rubisco small subunit (a product of the nuclear rbcS gene) and large subunit (a product of the chloroplast rbcL gene), which are soluble stroma proteins. D1, LHC, and OE23 were detected in neither apg2–1 nor apg2–2 (Fig. 7). The lack of thylakoid proteins in the apg2 mutants may be because of the lack of thylakoid membranes in the mutant plastids. Soluble proteins in stroma were not affected in the apg2 mutants (Fig. 7). These findings suggest an important role of the cpTatC protein in the development of thylakoid membranes.
Figure 7.
Immunoblot analysis of chloroplast proteins in apg2 mutants. Total protein samples were prepared from 21-day-old wild-type plants (lane 1, Columbia; lane 2, Nossen), Ds donor line Ds389–13 (lane 3), apg2–1 mutant (lane 4), and apg2–2 mutant (lane 5), and separated by SDS/PAGE. To detect D1, LHC, and OE23 proteins, 40 μg of total protein was loaded per lane. To detect Rubisco large subunit and small subunit proteins, 10 μg of total protein was loaded per lane. Immunoblot analysis was performed by using polyclonal antibodies for D1, LHC, OE23, and Rubisco LSU and SSU.
Discussion
Four apg2 Alleles Represent a Disrupted Gene for a TatC Homologue of the Bacterial ΔpH-Dependent Protein Transport System.
We analyzed four independent Ds-tagged mutants showing albino phenotypes (Fig. 1A). We recovered their Ds-flanking genomic sequences by using TAIL-PCR and showed that Ds was inserted in different regions of a single gene (Fig. 2). We named the mutants apg2. The reason we isolated as many as 4 apg2 alleles of 2,739 lines screened may be because of the localization of APG2 in the NOR2 region, a transposition hot spot, at the end of the long arm of chromosome 2 (35). Two transposition hot spots have been reported from narrow regions adjacent to nucleolus organizer regions NOR2 and NOR4 on Arabidopsis chromosomes 2 and 4, respectively (35).
The APG2 gene is a single-copy gene in the Arabidopsis genome and encodes a putative 37-kDa protein that has high sequence homology to the tatC gene from E. coli (Fig. 3). We named the APG2 product cpTatC (chloroplast TatC homologue). The APG protein has six transmembrane domains like bacterial TatC proteins. The N-terminal region of cpTatC has low homology with those of TatC from E. coli and Haemophilus influenzae, etc., and is thought to be a transit peptide involved in chloroplast targeting on the basis of its structural feature. We demonstrated that the N-terminal region of the cpTatC functions as a transit peptide involved in plastid targeting (Fig. 5). The amino acid sequence of Arabidopsis cpTatC shows the highest sequence similarity to that of cyanobacteria among bacterial TatC homologues (Fig. 3), which suggests that the ΔpH-dependent protein transport system of plants may be a cyanobacterial origin through endosymbiosis in evolution. Prokaryotes generally contain a ΔpH-dependent protein import system; in eukaryotes, plants have a similar system in chloroplast, whereas animals do not, possibly because of cyanobacterial origin of the system in plant.
An E. coli tatC mutant could not be complemented by APG2 cDNA (data not shown). This failure may be because of a difference in membrane structures or a difference in the functions of TatC proteins in protein transport between E. coli and plants.
Important Roles of the APG2 Gene in Chloroplast Development.
Maize mutants hcf106 and tha4 were shown to be defective in the ΔpH-dependent protein transport system (15, 16). Hcf106 and Tha4 are homologues of TatA, TatB, and TatE of E. coli. hcf106-mun1 mutant plants are pale green and die 3 weeks after germination in soil (15, 36). tha4 mutants are subtly chlorophyll-deficient and die after the development of three to four leaves (16). In our study, we identified an essential function of a plant homologue of the TatC protein in chloroplast development. The apg2 mutants were completely albino and could not survive after germination in soil. The mutant plastids lacked internal membrane structures and lamellae and contained abnormal suborganelle structures and many densely stained globule structures, similar to plastoglobuli (Fig. 4). The apg2 mutants showed a more severe phenotype than the maize null mutants of another component of the ΔpH-dependent transport system (16, 36). APG2 is a single-copy gene in the Arabidopsis genome and is essential for chloroplast development. These results suggest that the cpTatC protein, the APG2 product, is an important component in the ΔpH-dependent protein transport system in chloroplast development. cpTatC is thought to function as a channel protein with the main role in this protein transport system.
Thylakoid Peptides Are Defective in apg2 Mutants.
Some chloroplast proteins transported by the ΔpH-dependent protein transport system are known, such as the 23-kDa subunit of the oxygen-evolving complex (OE23, the psbP gene product), the 17-kDa subunit of the oxygen-evolving complex (OE17, the psbQ gene product), the T subunit of photosystem II (PSII-T, the psbT gene product), and the N subunit of photosystem I (PSI-N, the psaN gene product) (8). Transcripts of psbP and psaN were not affected in the apg2 mutants (Fig. 6), whereas no OE23 thylakoid protein was detected in the apg2 mutants (Fig. 7). Its precursor proteins were not detected as well. These results indicate that the psbP gene product (the OE23 precursor protein) is not correctly translated or that the translated OE23 precursor protein is not transported to the thylakoid and then degraded, whereas the nuclear psbP gene is normally transcribed in the apg2 mutants. Moreover, no thylakoid proteins such as D1 and LHC were detected in the apg2 mutants by immunoblot analysis (Fig. 7). By contrast, soluble stroma proteins like Rubisco were detected in the apg2 mutants (Fig. 7). Similar observations were reported in Arabidopsis var2 (yellow variegated) mutant (24). The VAR2 gene encodes a chloroplastic homologue of FtsH, an ATP-dependent metalloprotease. In yellow regions of the mutant leaves, photosynthetic protein components in the thylakoid membrane were reduced, whereas chloroplast mRNAs accumulated (24). If photosynthetic protein complexes are not assembled correctly or damaged in the thylakoid in albino or pale green mutants, the complexes together with unassembled subunit proteins are then rapidly degraded (37, 38). OE23, OE17, PSII-T, and PSI-N proteins are embedded on or bound to thylakoid membrane and function in assembling of PSI and PSII complexes. These proteins are not transported into the thylakoid membrane in apg2 mutants, which prevents assembly of subunits of PSI and PSII complexes. This may cause degradation of PSI and PSII components in apg2 mutants.
In summary, we first identified biological function of a plant tatC homologue of a bacteria-type ΔpH-dependent protein transport system in chloroplast development by using Arabidopsisapg2 albino mutant plants. The cpTatC gene is a single-copy gene in the Arabidopsis genome and has an essential role in chloroplast development. apg2 mutant plants with a disrupted cpTatC gene showed a severe albino phenotype; their plastids lacked an internal thylakoid membrane and had abnormal suborganelle structures. We could not detect thylakoid proteins and their precursors in the apg2 mutants, which causes abnormal and immature structures of thylakoid membrane. Now we are preparing transgenic plants with mild mutant phenotypes by using antisense or RNA interference constructs (39) to analyze the biochemical function of cpTatC in vivo . We also plan to isolate Arabidopsis mutants that have defects in TatA and TatB to examine how TatC interacts and cooperates with those proteins in the ΔpH-dependent protein transport system in higher plants.
Acknowledgments
We thank Mss. H. Kanahara, I. Furukawa, and S. Kawamura for their skillful technical assistance; Drs. R. Yoshida, Y. Narusaka, H. Abe, and H. Kuroda for their helpful comments; Drs. F. Sato and T. Nakano for the rubisco L/S complex antibody; Dr. M. Ikeuchi for the D1 antibody; Drs. T. Masuda and K. Takamiya for the LHC antibody; and Dr. T. Endo for the OE23 antibody. This work was supported by a grant for Genome Research from RIKEN, and in part by the Program for Promotion of Basic Research Activities for Innovative Biosciences, the Special Coordination Fund of the Science and Technology Agency, and a grant-in-aid from the Ministry of Education, Science, Sports and Culture to K.S.
Abbreviations
- GFP
green fluorescent protein
- TatC
twin arginine translocation C
- apg2
albino and pale green 2
- TAIL
thermal asymmetric interlaced
Note
While our manuscript was under review, cDNA related cpTatC was reported in rice (44).
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank/EMBL database (accession no. AB054096).
References
- 1.Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T, Zaita N, Chunwonges J, Obokata J, Yamaguchi-Shinozaki K, et al. EMBO J. 1986;5:2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugiura M. Plant Mol Biol. 1992;19:149–168. doi: 10.1007/BF00015612. [DOI] [PubMed] [Google Scholar]
- 3.Sato S, Nakamura Y, Kaneko T, Asamizu E, Tabata S. DNA Res. 1999;6:283–290. doi: 10.1093/dnares/6.5.283. [DOI] [PubMed] [Google Scholar]
- 4.von Heijne G, Steppuhn J, Herrmann R G. Eur J Biochem. 1989;180:535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]
- 5.Bauer J, Chen K, Hiltbunner A, Wehrii E, Eugster M, Schnell D, Kessler F. Nature (London) 2000;403:203–207. doi: 10.1038/35003214. [DOI] [PubMed] [Google Scholar]
- 6.Jarvis P, Chen L-J, Li H-M, Peto C A, Fankhauser C, Chory J. Science. 1998;282:100–103. doi: 10.1126/science.282.5386.100. [DOI] [PubMed] [Google Scholar]
- 7.Reumann S, Keegstra K. Trends Plant Sci. 1999;4:302–307. doi: 10.1016/s1360-1385(99)01449-1. [DOI] [PubMed] [Google Scholar]
- 8.Keegstra K, Cline K. Plant Cell. 1999;11:557–570. doi: 10.1105/tpc.11.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan J, Henry R, McCaffery M, Cline K. Science. 1994;266:796–798. doi: 10.1126/science.7973633. [DOI] [PubMed] [Google Scholar]
- 10.Roy L M, Barkan A. J Cell Biol. 1998;141:385–395. doi: 10.1083/jcb.141.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu C-J, Schuenemann D, Hoffman N E. J Biol Chem. 1999;274:27219–27224. doi: 10.1074/jbc.274.38.27219. [DOI] [PubMed] [Google Scholar]
- 12.Bogsch E G, Sargent F, Stanley N R, Berks B C, Robinson C, Palmer T. J Biol Chem. 1998;273:18003–18006. doi: 10.1074/jbc.273.29.18003. [DOI] [PubMed] [Google Scholar]
- 13.Sargent F, Bogsch E G, Stanley N R, Wexler M, Robinson C, Berks B C, Palmer T. EMBO J. 1998;17:3640–3650. doi: 10.1093/emboj/17.13.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sargent F, Stanley N R, Berks B C, Palmer T. J Biol Chem. 1999;274:36073–36082. doi: 10.1074/jbc.274.51.36073. [DOI] [PubMed] [Google Scholar]
- 15.Settles A M, Yonetani A, Baron A, Bush D R, Cline K, Martienssen R. Science. 1997;278:1467–1470. doi: 10.1126/science.278.5342.1467. [DOI] [PubMed] [Google Scholar]
- 16.Walker M B, Roy L M, Coleman E, Voelker R, Barkan A. J Cell Biol. 1999;147:267–275. doi: 10.1083/jcb.147.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaddock AM, Mant A, Karnauchov I, Brink S, Herrmann R G, Klösgen R B, Robinson C. EMBO J. 1995;14:2715–2722. doi: 10.1002/j.1460-2075.1995.tb07272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori H, Cline K. J Biol Chem. 1998;273:11405–11408. doi: 10.1074/jbc.273.19.11405. [DOI] [PubMed] [Google Scholar]
- 19.Fedoroff N V, Smith D L. Plant J. 1993;3:273–289. doi: 10.1111/j.1365-313x.1993.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith D, Yanai Y, Liu Y-G, Ishiguro S, Okada K, Shibata D, Whittier R F, Fedoroff N V. Plant J. 1996;10:721–732. doi: 10.1046/j.1365-313x.1996.10040721.x. [DOI] [PubMed] [Google Scholar]
- 21.Ito T, Kim G-T, Shinozaki K. Plant J. 2000;22:257–264. doi: 10.1046/j.1365-313x.2000.00728.x. [DOI] [PubMed] [Google Scholar]
- 22.Ito T, Seki M, Hayashida N, Shibata D, Shinozaki K. Plant J. 1999;17:433–444. doi: 10.1046/j.1365-313x.1999.00383.x. [DOI] [PubMed] [Google Scholar]
- 23.Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J. Curr Biol. 1996;6:325–330. doi: 10.1016/s0960-9822(02)00483-9. [DOI] [PubMed] [Google Scholar]
- 24.Takechi K, Sodmergen, Murata M, Motoyoshi F, Sakamoto W. Plant Cell Physiol. 2000;41:1334–1346. doi: 10.1093/pcp/pcd067. [DOI] [PubMed] [Google Scholar]
- 25.Valvekens D, Van Montagu M, Van Lijsebettens M. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 27.Douce R, Joyard J. In: Methods in Choroplast Molecular Biology. Edelman M, Hallick R B, Chua N H, editors. Amsterdam: Elsevier Biomedical; 1982. p. 239. [Google Scholar]
- 28.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Krebbers E, Seurinck J, Herdies L, Cashmore A R, Timko M P. Plant Mol Biol. 1988;11:745–759. doi: 10.1007/BF00019515. [DOI] [PubMed] [Google Scholar]
- 30.Leutwiler L S, Meyerowitz E M, Tobin E M. Nucleic Acids Res. 1986;14:4051–4064. doi: 10.1093/nar/14.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sehnke P C, Ferl R J. Plant Physiol. 1995;109:1126. [Google Scholar]
- 32.Kochhar A, Khurana J P, Tyagi A K. DNA Res. 1996;3:277–285. doi: 10.1093/dnares/3.5.277. [DOI] [PubMed] [Google Scholar]
- 33.Liere K, Kestermann M, Müller U, Link G. Curr Genet. 1995;28:128–130. doi: 10.1007/BF00315778. [DOI] [PubMed] [Google Scholar]
- 34.Zhu G, Jensen R G, Bohnert H J. Plant Physiol. 1997;114:395–396. doi: 10.1104/pp.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parinov S, Sevugan M, Yang W-C, Ye D, Kumaran M, Sundaresan V. Plant Cell. 1999;11:2263–2270. doi: 10.1105/tpc.11.12.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das L, Martienssen R. Plant Cell. 1995;7:287–294. doi: 10.1105/tpc.7.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vitry C, Olive J, Drapier D, Recouvreur M, Wollman F A. J Cell Biol. 1989;109:991–1006. doi: 10.1083/jcb.109.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi Y, Goldschmidt-Clermont M, Soen S-Y, Franzé L G, Rochaix J-D. EMBO J. 1991;10:2033–2040. doi: 10.1002/j.1460-2075.1991.tb07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chuang C-F, Meyerowitz E M. Proc Natl Acad Sci USA. 2000;97:4985–4990. doi: 10.1073/pnas.060034297. . (First Published April 18, 2000; 10.1073/pnas.060034297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blattner F, Plunkett G, Bloch C, Perna N, Burland V, Riley M, Collado-Vides J, Glasner J, Rode C, Mayhew G, et al. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 41.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, et al. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 42.Alm R A, Ling L S, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, et al. Nature (London) 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 43.White O, Eisen J A, Heidelberg J F, Hickey E K, Peterson J D, Dodson R J, Haft D H, Gwinn M L, Nelson W C, Richardson D L, et al. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agrawal G K, Yamazaki M, Kobayashi M, Hirochika R, Miyao A, Hirochika H. Plant Physiol. 2001;125:1248–1257. doi: 10.1104/pp.125.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]