Abstract
Human paraoxonase 2 (PON2), which is a member of the paraoxonase family, possesses unique properties that distinguish it from PON1 and PON3. PON2 is ubiquitously expressed in many different tissue types and is highly expressed in the vital organs, such as the heart and lungs. Early research revealed that PON2 is exclusively intracellularly found, wherein it functions as an anti-oxidative protein by reducing intracellular and local oxidative stress. Studies in the last five years have demonstrated that PON2 protects against atherosclerosis by preventing low-density lipoprotein (LDL) oxidation, reversing the oxidation of mildly oxidised LDL, inhibiting monocyte chemotaxis, and increasing cholesterol efflux. Recently, emerging evidence has proposed that PON2 is an anti-atherosclerotic and may be associated with cardiovascular disease (CVD). The number of investigations concerning the relationship between two common PON2 polymorphisms and CVD among different ethnic groups and regions is rapidly growing. Here, we briefly review the developments in PON2 research by focusing on past and recent findings.
Keywords: antioxidant, atherosclerosis, paraoxonase 2, oxidative stress
Introduction
The paraoxonase (PON) gene family consists of three highly conserved genes (PON1, PON2, and PON3) that are located in a cluster on chromosome 7q21.3-22.1 and share a high degree of identity (La Du 1996[27]; Primo-Parmo et al., 1996[50]). Studies in the last five years have focused on PON2 because of its unique expression and localisation. In contrast to PON1 and PON3, which are exclusively found in the liver and are associated with HDL in the blood stream following secretion (Mackness et al., 1996[36]), PON2 is an intracellular protein that is widely expressed in many tissues, including the liver, kidney, lung, heart, placenta, testis, stomach, spleen, pancreas, small intestine, skeletal muscle, artery wall cell, and macrophages (Ng et al., 2001[44], Levy et al., 2007[30]). Although PON2 has a similar structure to that of PON1 based on high amino acid sequence homology, PON2 possesses biological functions that are distinct from PON1. PON2 cannot hydrolyse organophosphates, such as paraoxon, but, instead, possesses hydrolase and lactonase activities (Draganov et al., 2005[10]). Due to its anti-oxidative and anti-atherosclerotic properties, which are similar to those of PON1, PON2 has become the subject of intense investigation. Both in vivo and in vitro models have revealed that PON2 prevents low-density lipoprotein (LDL) oxidation, reverses the oxidation of mildly oxidised LDL, inhibits oxidised LDL-induced monocyte chemotaxis (Ng et al., 2001[44]), increases cholesterol efflux (Ng et al., 2006[43]), and decreases the size of atherosclerotic lesions (Ng et al., 2006[43][42]). Furthermore, the anti-apoptotic capability of PON2 has been found to play a role in atherosclerotic protection (Horke et al., 2007[20]).
The human PON2 gene has two common polymorphisms, which result in amino acid substitutions of an alanine (A) or glycine (G) at codon 148 (A148G) and a cysteine (C) or serine (S) at codon 311 (C311S) (Mochizuki et al., 1998[41]). As with PON1, inconsistent data have obfuscated the relationship between these two PON2 polymorphisms and numerous pathophysiological conditions.
PON2 Expression
The human PON2 gene is located on the long arm of chromosome 7q21.3 and is adjacent to PON gene family members PON1 and PON3 (Primo-Parmo et al., 1996[50]). Based on a phylogenetic analysis, PON2 is the oldest member of this gene family (Hassett et al., 1991[15]; Draganov and La Du, 2004[9]). Approximately 70% and 65% of PON2 nucleotide and amino acid sequences, respectively, are similar to those of PON1 and PON3 (Primo-Parmo et al., 1996[50]). PON2 consists of nine exons that encode a 355-amino-acid protein of approximately 43 kDa in size. The PON2 gene contains numerous transcription start sites and may be alternatively spliced, resulting in several mRNA forms of PON2 (Mochizuki et al., 1998[41]); however, data regarding the cell types that express these various PON2 isoforms are lacking. Until now, only two PON2 protein isoforms of 40 and 43 kDa in size have been commonly observed in a variety of cell types via immunoblotting with a specific anti-PON2 antibody. These two isoforms are believed to be non-glycosylated and glycosylated, respectively, although the different functions of these isoforms have not been reported (Horke et al., 2007[20]).
The expression profile of PON2 demonstrates two unique differences in comparison to those of PON1 and PON3. PON2 is widely expressed in many different tissues, and it was first detected in the brain, liver, kidney, and testis (Mochizuki et al., 1998[41]). Ng and colleagues have detected higher levels of PON2 transcripts in the heart, lung, liver, placenta, and testis in comparison to other examined tissue types. PON2 expression has also been observed in primary and immortalised human endothelial cells and in human arterial smooth muscle cells (Ng et al., 2001[44]); however, the subcellular localisation of PON2 remains ambiguous. Although early studies have claimed that PON2 is an intracellular protein, evidence obtained via confocal microscopy, biochemical cell fractionation, and the digestion of outer membrane proteins has revealed that PON2 is located in the nuclear envelope and the endoplasmic reticulum (ER) of EA.hy 926 cells (Horke et al., 2007[20]); however, PON2 mRNA and protein has recently been detected in the human gastrointestinal tract with decreasing expression levels from the upper to the lower levels of the tract (Levy et al., 2007[30]). Moreover, PON2 localisation to the ER and release from the brush-border membrane in Caco-2 and HT-29 cells have been observed (Shamir et al., 2005[61]; Rothem et al., 2007[58]). These inconsistent findings imply that the cell type in which PON2 is expressed may influence the localisation of PON2 protein; Caco-2 and HT-29 cells are human adenocarcinomas of the colon, whereas EA.hy 926 cells are human vascular endothelial cells.
Modulation of PON2
Most studies concerning the induction of PON2 expression by various stimuli have focused on oxidative stress because PON2 plays a role as an intracellular antioxidant. Both in vitro and in vivo studies have demonstrated that PON2 expression and enzymatic activity increase during oxidative stress. PON2 is up-regulated in response to oxidative stress in different cell types (HepG2 cells and macrophages), animal models (mice fed high fat diets and apoE knockout mice), and in hypercholesterolemic patients (Shih et al., 1996[63], 1998[64]; Forte et al., 2002[11]; Rosenblat et al., 2003[55], 2004[56]). Mouse peritoneal macrophages (MPMs) that were treated with various agents that induce oxidative stress have demonstrated increased PON2 expression and lactonase activity (Rosenblat et al., 2003[55]). Shiner et al. have observed an approximate seven-fold increase in PON2 expression during monocyte/macrophage differentiation that was dependent on the presence of nicotinamide adenine dinucleotide phosphate (NADPH), and this phenomenon was observed to correlate with an increase in cellular oxidative stress (Shiner et al., 2004[69]). In addition, PON2 has been found to be inactivated during low levels of oxidative stress. Therefore, high levels of oxidative stress may induce a cellular compensatory mechanism that up-regulates PON2 expression in macrophages (Shiner et al., 2006[66]).
PON2 expression has also been investigated in the context of common pathological conditions, such as metabolic disorders that are characterised by an increase in oxidative stress due to high cholesterol or glucose levels. An in vivo chronic exposure of mice to high-cholesterol has been observed to cause an increase in hepatic PON2 mRNA levels (Forte et al., 2002[11]). Studies in J774A.1 mouse macrophages have provided similar results. Lysophosphatidylcholine (LPC) has been observed to increase PON2 lactonase activity in J774A.1 cells (Rosenblat et al., 2006[57]). In another in vivo study, human monocyte-derived macrophages (HMDMs) from hypercholesterolemic patients, which were subjected to oxidative stress, were observed to demonstrate less than half of the PON2 expression levels that were detected in HMDMs that had been isolated from control patients (Rosenblat et al., 2004[56]). A similar result was obtained in diabetic mice, which demonstrated that the up-regulation of cellular PON2 in macrophages was associated with an increase in oxidative stress (Hayek et al., 2007[16]). Moreover, increased PON2 expression has also been observed in response to pharmaceutical compounds, such as the lipid lowering agent atorvastatin and the anti-diabetic drug rosiglitazone, among other agents. Atorvastatin or rosiglitazone can up-regulate PON2 expression, and elevated PON2 activity has been observed to result in the reduction of cellular oxidative stress in patient HMDMs and mouse macrophages, respectively (Rosenblat et al., 2004[56]; Shiner et al., 2007[68]). In contrast, proinflammatory agents, such as lipopolysaccharide (LPS), have been found to decrease PON2 expression in Caco-2/15 cells and in the human intestine (Precourt et al., 2009[49]).
Studies investigating dietary components that modulate PON2 expression are currently underway. Pomegranate juice (PJ) and extracts from different parts of the plant significantly increase PON2 expression and lactonase activity in J774A.1 cells and in MPMs that have been isolated from atherosclerotic apolipoprotein E-deficient mice (Shiner et al., 2007[67]; Aviram et al., 2008[2]). Supplementation with quercetin or the methylated quercetin derivative isohamnetin has been found to up-regulate PON2 mRNA and protein levels in the RAW264.7 murine macrophage cell line; however, studies in humans have provided contrasting results. Quercetin supplementation in human volunteers had no affect on PON2 mRNA levels in human monocytes (Boesch-Saadatmandi et al., 2009[3]).
Elucidation of the mechanism that is responsible for the modulation of PON2 requires further investigation, although recent findings have demonstrated that unfolded proteins in the endoplasmic reticulum (ER) induce PON2 expression at both the promoter and protein levels in the three assessed cell lines (human umbilical vein endothelial cell-derived EA.hy 926 cells, primary human coronary artery smooth muscle cells [SMCs], and primary human aortic adventitial fibroblasts [AoAFs]) (Horke et al., 2007[20]). Investigations of the signalling pathways of PON2 modulation have revealed that it may involve the urokinase plasminogen activator (uPA), extracellular signal-regulated kinase (ERK1/2), NADPH oxidase (NOX), phosphatidylinositol 3-kinase (PI3K), platelet-derived growth factor receptor-β (PDGFR-β), the tyrosine kinase cascade, the NF-kappa B pathway and lipid peroxidation, PPARγ, and AP-1 (Shiner et al., 2007[67][68]; Fuhrman et al., 2008[13], 2009[12]; Precourt et al., 2009[49]). Recently, a functional study of the PON2 promoter revealed a putative sterol regulatory binding protein-2 (SREBP-2) DNA element that is located between -593 bp and -575 bp of the PON2 promoter that may play a major role in PON2 regulation (Fuhrman et al., 2009[12]; Lim and Kim, 2009[33]).
Role of PON2 in Human Diseases
As with other PON family members, when PON2 was first discovered, the specific substrates and exact physiological functions of this protein were uncertain; however, the high structural similarity of PON2 to other members of the PON family have indicated that it might possess an activity that is analogous to those of PON1 and PON3. Like PON1 and PON3, PON2 very efficiently metabolises 5-hydroxy-eicosatetraenoic acid 1,5-lactone and 4-hydroxy-docosahexaenoic acid, which are the oxidation products of arachidonic acid and docosahexaenoic acid, respectively, and may also be endogenous substrates for PON2 (Draganov et al., 2005[10]); however, unlike PON1, PON2 is incapable of hydrolysing organophosphates, such as paraoxon, although it has been demonstrated to possess a greater lactonase activity in comparison to those of PON1 and PON3 when dihydrocoumarin and some arylesters are used as substrates (Rosenblat et al., 2003[55]; Draganov et al., 2005[10]). Although PON2 has a lower anti-oxidative capacity than PON1 (Draganov et al., 2005[10]), several recent investigations of this protein have been conducted due to its high expression level and widespread localisation. Studies using either purified PON2 or PON2-overexpressing cells have provided similar results. Exposure of PON2-overexpressing Hela cells to hydrogen peroxide or oxidised phospholipids has been observed to decrease intracellular oxidative stress by reducing the levels of reactive oxygen species (ROS), preventing LDL lipid peroxidation, reversing the oxidation of mildly oxidised LDL (MM-LDL), and inhibiting the ability of MM-LDL to induce monocyte chemotaxis (Ng et al., 2001[44]). Treatment of mouse macrophages with purified recombinant PON2 has been shown to prevent LDL oxidation (Rosenblat et al., 2003[55]).
Studies in animals have also provided similar results. PON2 has been shown to provide protection against HDL and LDL oxidation and inhibit monocyte transmigration in response to LDL oxidation in PON2-knockout (PON2KO) mice (Ng et al., 2006[43]). Interestingly, these PON2 knockout mice demonstrate an increased number of foam cells and lipid droplets and develop significantly larger atherosclerotic lesions in comparison to their wild-type counterparts (Ng et al., 2006[42]). In contrast, the atherosclerotic lesions in AdPON2-overexpressing apoE-deficient mice (apoE-/-) are significantly smaller than those in their wild-type counterparts. Furthermore, serum obtained from AdPON2-treated mice contains significantly lower levels of lipid hydroperoxides and demonstrates an enhanced capacity to induce cholesterol efflux from cholesterol-loaded macrophages. In addition to increasing cholesterol efflux, PON2 has been found to inhibit triglyceride (TG) biosynthesis and microsomal diacylglycerol acyltransferase 1 (DAGT1) activity, resulting in a decreased accumulation of TG in macrophages of PON2-deficient mice (Meilin et al., 2009[40]; Rosenblat et al., 2009[54]). PON2 has been proposed to function as an antiapoptotic factor in atherosclerosis. PON2 overexpression in EA.hy 926 cells was found to protect the endoplasmic reticulum against oxidative stress-induced apoptosis by specifically preventing the generation of superoxide radicals at the inner mitochondrial membrane (Horke et al., 2008[19]; Altenhöfer et al., 2010[1]). Currently, based on several lines of evidence, the anti-atherosclerotic effects of PON2 have been clearly delineated.
Association of PON2 Polymorphisms with Human Diseases
Several polymorphisms in the PON2 gene have been reported to date; however, only two common PON2 polymorphisms play a prominent role in pathophysiological conditions: codon 148 is either an alanine or a glycine (A148G), and codon 311 is either a cysteine or a serine (C311S) (Mochizuki et al., 1998[41]). Many studies have investigated the PON2 gene polymorphism frequencies, and the results therein have revealed variations in different ethnic groups as shown in Table 1(Tab. 1) (References in Table 1: Oliveira et al., 2004[45]; McKeown-Eyssen et al., 2004[39]; Wang et al., 2003[76]; Pasdar et al., 2006[47]; Karban et al., 2007[26]; Martinelli et al., 2004[38]; Yamada et al., 2003[82]; Shin, 2009[65]; Saeed et al., 2007[59]; Slowik et al., 2007[71]; Ranade et al., 2005[52]). Based on current evidence, most populations carry the A allele at codon 148 and the S allele at codon 311.
Table 1. Gene distributions of PON2 at codons 148 and 311.
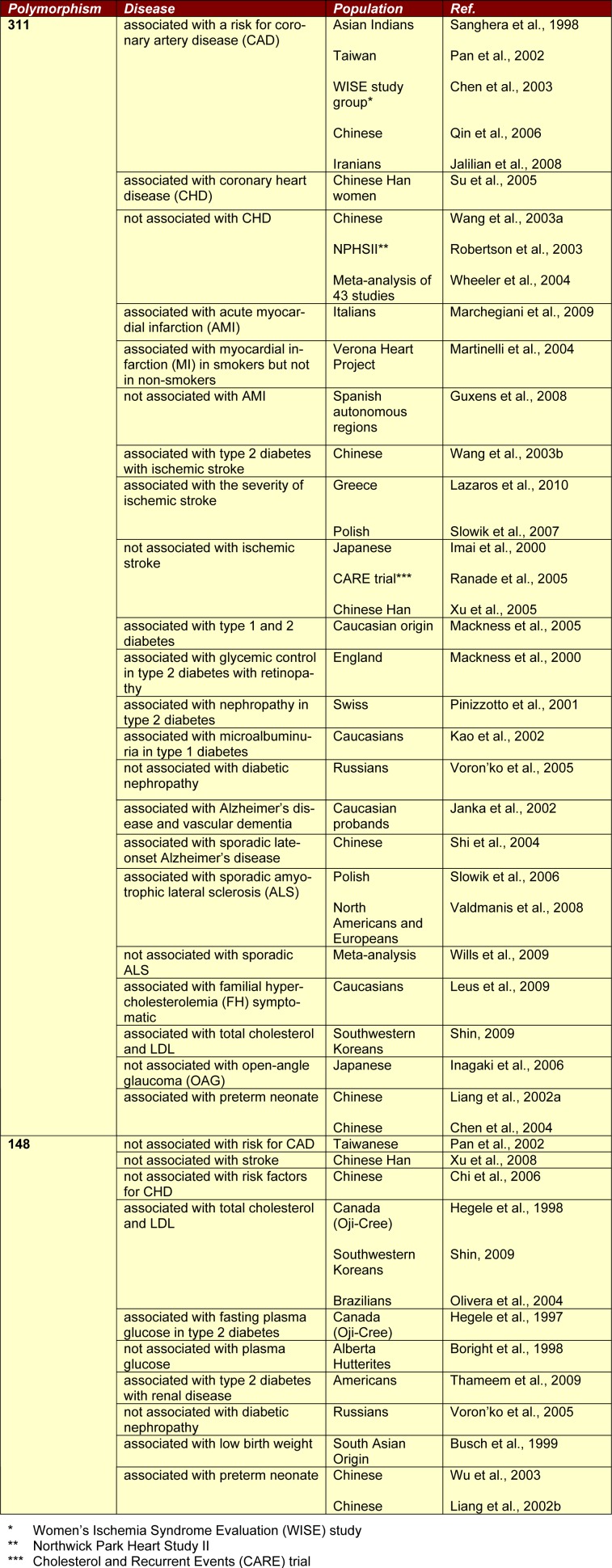
Oxidative stress is believed to be involved in human diseases. Because of the anti-oxidative and anti-atherosclerotic potential of PON2, there has been interest in examining the association of PON2 with CVD and other pathological disorders, such as diabetic mellitus, Alzheimer's disease, and sporadic amyotrophic lateral sclerosis (ALS); however, most studies have investigated the associations of PON2 polymorphisms with these diseases rather than the relationship between PON2 activity and disease. This may be because the true biological substrate of PON2 remains unknown. Currently, the activity of PON2 is determined from the hydrolysis of dihydrocoumarin (DHC), and the appropriateness of this substrate is still subject to debate. Therefore, studies investigating the association of PON2 activity with diseases have been scarce because of the lack of well-established methods. Instead, most studies emphasise the association of PON2 polymorphisms with various pathologies and diseases in different ethnic groups as shown in Table 2(Tab. 2) (References in Table 2: Sanghera et al., 1998[60]; Pan et al., 2002[46]; Chen et al., 2003[7]; Qin et al., 2006[51]; Jalilian et al., 2008[23]; Su et al., 2005[72]; Wang et al., 2003[76]; Robertson et al., 2003[53]; Wheeler et al., 2004[78]; Marchegiani et al., 2009[37]; Martinelli et al., 2004[38]; Guxens et al., 2008[14]; Wang et al., 2003[77]; Lazaros et al., 2010[28]; Slowik et al., 2007[71]; Imai et al., 2000[21]; Ranade et al., 2005[52]; Xu et al., 2005[81]; Mackness et al., 2005[35]; Mackness et al., 2000[34]; Pinizzotto et al., 2001[48]; Kao et al., 2002[25]; Janka et al., 2002[24]; Shi et al., 2004[62]; Slowik et al., 2006[70]; Valdmanis et al., 2008[74]; Wills et al., 2009[79]; Leus et al., 2009[29]; Shin, 2009[65]; Inagaki et al., 2006[22]; Liang et al., 2002[31]; Chen et al., 2004[6]; Pan et al., 2002[46]; Xu et al., 2008[81]; Chi et al., 2006[8]; Hegele et al., 1998[18]; Shin, 2009[65]; Olivera et al., 2004[45]; Hegele et al., 1997[17]; Boright et al., 1998[4]; Thameem et al., 2009[73]; Voron'ko et al., 2005[75]; Busch et al., 1999[5]; Wu et al., 2003[80]; Liang et al., 2002[32]; Voron'ko et al., 2005[75]). The resulting data have been inconsistent because of the variations in different experimental conditions, ethnicities, and geographical locations. Hence, further investigations are required.
Table 2. Association between PON2 polymorphisms and various diseases.
Conclusion
The anti-oxidative and antiatherogenic activities of PON2 have been well documented over the past five years; however, an identification of the location, biological functions, and activity of PON2 is required for further investigation. It would be of interest to explore PON2 within the context of other disorders, such as cancer or abnormalities in metabolic consumption, because of its anti-oxidative role, intracellular location, and expression in most organs at high metabolic rates and incidence in relation to distinct types of cancers, such as those associated with the lung, liver, testis, heart, and placenta. An investigation of the exogenous modulation of PON2, especially by nutrition and pharmacological means, has only recently been initiated; hence, many issues and questions still remain. Future studies should provide substantial evidence on both PON2 modulation and the mechanisms by which chemicals may modulate PON2 expression. Most studies have attempted to relate DNA sequence variants in the PON2 locus to the risk for CVD and other diseases. The controversial findings that have been obtained for the two common PON2 polymorphisms that are associated with CVD have led to equivocal conclusions. The differences in these findings may derive from individual susceptibilities that are related to many other factors that are associated with ethnic background and life style.
References
- 1.Altenhöfer S, Witte I, Teiber JF, Wilgenbus P, Pautz A, Li H, et al. One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J Biol Chem. 2010;285:24398–24403. doi: 10.1074/jbc.M110.118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aviram M, Volkova N, Coleman R, Dreher M, Reddy MK, Ferreira D, et al. Pomegranate phenolics from the peels, arils, and flowers are antiatherogenic: studies in vivo in atherosclerotic apolipoprotein E-deficient (E0) mice and in vitro in cultured macrophages and lipoproteins. J Agric Food Chem. 2008;56:1148–1157. doi: 10.1021/jf071811q. [DOI] [PubMed] [Google Scholar]
- 3.Boesch-Saadatmandi C, Pospissil RT, Graeser AC, Canali R, Boomgaarden I, Doering F, et al. Effect of quercetin on paraoxopnase 2 levels in RAW264.7 macrophages and in human monocytes-role of quercetin metabolism. Int J Mol Sci. 2009;10:4168–4177. doi: 10.3390/ijms10094168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boright AP, Connelly PW, Brunt JH, Scherer SW, Tsui LC, Hegele RA. Genetic variation in paraoxonase-1 and paraoxonase-2 is associated with variation in plasma lipoproteins in Alberta Hutterites. Atherosclerosis. 1998;139:131–136. doi: 10.1016/s0021-9150(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 5.Busch CP, Ramdath DD, Ramsewak S, Hegele RA. Association of PON2 variation with birth weight in Trinidadian neonates of South Asian ancestry. Pharmacogenetics. 1999;9:351–356. doi: 10.1097/00008571-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Chen D, Hu Y, Chen C, Yang F, Fang Z, Wang L, et al. Polymorphisms of the paraoxonase gene and risk of preterm delivery. Epidemiology. 2004;15:466–470. doi: 10.1097/01.ede.0000129509.59912.b2. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Reis SE, Kammerer CM, McNamara DM, Holubkov R, Sharaf BL, et al. Association between the severity of angiographic coronary artery disease and paraoxonase gene polymorphisms in the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) study. Am J Hum Genet. 2003;72:13–22. doi: 10.1086/345312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi DS, Liang WH, Ma J, Xia M, Hou MJ, Wang Q, et al. Study of the association between paraoxonase1 55 Met/Leu, paraoxonase2 148/Gly and manganese superoxide dismutase (MnDOD) 9 Ala/Val genetic polymorphisms and coronary heart disease. Zhonghua Xin Xue Guan Bing Za Zhi. 2006;27:808–813. [PubMed] [Google Scholar]
- 9.Draganov DI, La Du BN. Pharmacogenetics of paraoxonases: a brief review. Naunyn Schmiedeberg's Arch Pharmacol. 2004;369:78–88. doi: 10.1007/s00210-003-0833-1. [DOI] [PubMed] [Google Scholar]
- 10.Draganov DI, Teiber JE, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Forte TM, Subbanagounder G, Berliner JA, Blanche PJ, Clermont AO, Jia Z, et al. Altered activities of anti-atherogenic enzymes LCAT, paraoxonase, and platelet-activating factor acetylhydrolase in atherosclerosis-susceptible mice. J Lipid Res. 2002;43:477–485. [PubMed] [Google Scholar]
- 12.Fuhrman B, Gantman A, Khateeb J, Volkova N, Horke S, Kiyan J, et al. Urokinase activates macrophage PON2 gene transcription via the PI3K/ROS/MEK/SREBP-2 signalling cascade mediated by the PDGFR-beta. Cardiovasc Res. 2009;84:145–154. doi: 10.1093/cvr/cvp184. [DOI] [PubMed] [Google Scholar]
- 13.Fuhrman B, Khateeb J, Shiner M, Nitzan O, Karry R, Volkova N, et al. Urokinase plasminogen activator upregulates paraoxonase2 expression in macrophages via an NADPH oxidase-dependent mechanism. Arterioscler Thromb Vasc Biol. 2008;28:1361–1367. doi: 10.1161/ATVBAHA.108.166041. [DOI] [PubMed] [Google Scholar]
- 14.Guxens M, Tomás M, Elosua R, Aldasoro E, Segura A, Fiol M, et al. Association between paraoxonase-1 and paraoxonase-2 polymorphisms and the risk of acute myocardial infarction. Rev Esp Cardiol. 2008;61:269–275. [PubMed] [Google Scholar]
- 15.Hassett C, Richter RJ, Humbert R, Chapline C, Crabb JW, Omiecinski CJ, et al. Characterisation of cDNA clones encoding rabbit and human serum paraoxonase: the mature protein retains its signal sequence. Biochemistry. 1991;30:10141–10149. doi: 10.1021/bi00106a010. [DOI] [PubMed] [Google Scholar]
- 16.Hayek T, Kaplan M, Kerry R, Aviram M. Macrophage NADPH oxidase activation, impaired cholesterol fluxes, and increased cholesterol biosynthesis in diabetic mice: a stimulatory role for D-glucose. Atherosclerosis. 2007;195:277–286. doi: 10.1016/j.atherosclerosis.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Hegele RA, Connelly PW, Scherer SW, Hanley AJ, Harris SB, Tsui LC, et al. Paraoxonase-2 gene (PON2) G148 variant associated with elevated fasting plasma glucose in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997;82:3373–3377. doi: 10.1210/jcem.82.10.4289. [DOI] [PubMed] [Google Scholar]
- 18.Hegele RA, Harris SB, Connelly PW, Hanley AJ, Tsui LC, Zinman B, et al. Genetic variation in paraoxonase-2 is associated with variation in plasma lipoproteins in Canadian Oji-Cree. Clin Genet. 1998;54:394–399. doi: 10.1111/j.1399-0004.1998.tb03752.x. [DOI] [PubMed] [Google Scholar]
- 19.Horke S, Witte I, Wilgenbus P, Altenhöfer S, Krüger M, Li H, et al. Protective effect of paraoxonase-2 against endoplasmic reticulum stress-induced apoptosis is lost upon disturbance of calcium homoeostasis. Biochem J. 2008;416:395–405. doi: 10.1042/BJ20080775. [DOI] [PubMed] [Google Scholar]
- 20.Horke S, Witte I, Wilgenbus P, Krüger M, Strand D, Förstermann U. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation. 2007;115:2055–2064. doi: 10.1161/CIRCULATIONAHA.106.681700. [DOI] [PubMed] [Google Scholar]
- 21.Imai Y, Morita H, Kurihara H, Sugiyama T, Kato N, Ebihara A, et al. Evidence for association between paraoxonase gene polymorphisms and atherosclerotic diseases. Atherosclerosis. 2000;149:435–442. doi: 10.1016/s0021-9150(99)00340-8. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki Y, Mashima Y, Funayama T, Ohtake Y, Fuse N, Yasuda N, et al. Paraoxonase 1 gene polymorphisms influence clinical features of open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2006;244:984–990. doi: 10.1007/s00417-005-0200-7. [DOI] [PubMed] [Google Scholar]
- 23.Jalilian A, Javadi E, Akrami M, Fakhrzadeh H, Heshmat R, Rahmani M, et al. Association of Cys311Ser polymorphism of paraoxonase-2 gene with the risk of coronary artery disease. Arch Iranian Med. 2008;11:544–549. [PubMed] [Google Scholar]
- 24.Janka Z, Juhász A, Rimanóczy AA, Boda K, Márki-Zay J, Kálmán J. Codon 311 (Cys Ser) polymorphism of paraoxonase-2 gene is associated with apolipoprotein E4 allele in both Alzheimer's and vascular dementias. Mol Psychiatry. 2002;7:110–112. doi: 10.1038/sj.mp.4000916. [DOI] [PubMed] [Google Scholar]
- 25.Kao Y, Donaghue KC, Chan A, Bennetts BH, Knight J, Silink M. Paraoxonase gene cluster is a genetic marker for early microvascular complications in type 1 diabetes. Diabet Med. 2002;19:212–215. doi: 10.1046/j.1464-5491.2002.00660.x. [DOI] [PubMed] [Google Scholar]
- 26.Karban A, Hartman C, Eliakim R, Waterman M, Nesher S, Barnett-Griness O, et al. Paraoxonase (PON)1. 192R allele carriage is associated with reduced risk of inflammatory bowel disease. Dig Dis Sci. 2007;52:2707–2715. doi: 10.1007/s10620-006-9700-5. [DOI] [PubMed] [Google Scholar]
- 27.La Du BN. Structural and functional diversity of paraoxonase. Nat Med. 1996;2:1186–1187. doi: 10.1038/nm1196-1186. [DOI] [PubMed] [Google Scholar]
- 28.Lazaros L, Markoula S, Kyritsis A, Georgiou I. Paraoxonase gene polymorphisms and stroke severity. Eur J Neurol. 2010;17:757–759. doi: 10.1111/j.1468-1331.2009.02860.x. [DOI] [PubMed] [Google Scholar]
- 29.Leus FR, Zwart M, Kastelein JJ, Voorbij HA. PON2 gene variants are associated with clinical manifestations of cardiovascular disease in familial hypercholesterolemia patients. Atherosclerosis. 2001;154:641–649. doi: 10.1016/s0021-9150(00)00440-8. [DOI] [PubMed] [Google Scholar]
- 30.Levy E, Trudel K, Bendayan M, Seidman E, Delvin E, Elchebly M, et al. Biological role, protein expression, subcellular localization, and oxidative stress response of paraoxonase 2 in the intestine of humans and rats. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1252–G1261. doi: 10.1152/ajpgi.00369.2007. [DOI] [PubMed] [Google Scholar]
- 31.Liang HY, Wu BY, Chen DF, Yang F, Hu HY, Chen L, et al. Association of CYP2E1 of PON2 311 polymorphisms in neonates with preterm. Yi Chuan Xue Bao. 2002;29:847–853. [PubMed] [Google Scholar]
- 32.Liang HY, Wu BY, Chen DF, Yang F, Hu HY, Chen L, et al. Association of PON2 gene polymorphisms in neonates with preterm. Yi Chuan Xue Bao. 2002;24:515–518. [PubMed] [Google Scholar]
- 33.Lim JA, Kim SH. Transcriptional activation of an anti-oxidant mouse Pon2 gene by dexamethasone. BMB Rep. 2009;42:421–426. doi: 10.5483/bmbrep.2009.42.7.421. [DOI] [PubMed] [Google Scholar]
- 34.Mackness B, Durrington PN, Abuashia B, Boulton AJ, Mackness MI. Low paraoxonase activity in type II diabetes mellitus complicated by retinopathy. Clin Sci (Lond) 2000;98:355–363. [PubMed] [Google Scholar]
- 35.Mackness B, McElduff P, Mackness MI. The paraoxonase-2-310 polymorphism is associated with the presence of microvascular complications in diabetes mellitus. J Intern Med. 2005;258:363–368. doi: 10.1111/j.1365-2796.2005.01554.x. [DOI] [PubMed] [Google Scholar]
- 36.Mackness MI, Mackness B, Durrington PN, Connelly PW, Hegele RA. Paraoxonase: biochemistry, genetics and relationship to plasma lipoproteins. Curr Opin Lipidol. 1996;7:69–76. doi: 10.1097/00041433-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Marchegiani F, Spazzafumo L, Provinciali M, Cardelli M, Olivieri F, Franceschi C, et al. Paraoxonase2 C311S polymorphism and low levels of HDL contribute to a higher mortality risk after acute myocardial infarction in elderly patients. Mol Genet Metab. 2009;98:314–318. doi: 10.1016/j.ymgme.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Martinelli N, Girelli D, Olivieri O, Stranieri C, Trabetti E, Pizzolo F, et al. Interaction between smoking and PON2 Ser311Cys polymorphism as a determinant of the risk of myocardial infarction. Eur J Clin Invest. 2004;34:14–20. doi: 10.1111/j.1365-2362.2004.01292.x. [DOI] [PubMed] [Google Scholar]
- 39.McKeown-Eyssen G, Baines C, Cole D, Riley N, Tyndale RF, Marshall L, et al. Case-control study of genotypes in multiple chemical sensitivity: CYP2D6, NAT1, NAT2, PON1, PON2 and MTHFR. Int J Epidemiol. 2004;33:971–978. doi: 10.1093/ije/dyh251. [DOI] [PubMed] [Google Scholar]
- 40.Meilin E, Aviram M, Hayek T. Paraoxonase 2 (PON2) decreases high glucose-induced macrophage triglycerides (TG) accumulation, via inhibition of NADPH-oxidase and DGAT1 activity: Studies in PON2-deficient mice. Atherosclerosis. 2009;208:390–395. doi: 10.1016/j.atherosclerosis.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 41.Mochizuki H, Scherer SW, Xi T, Nickle DC, Majer M, Huizenga JJ, et al. Human PON2 gene at 7q21.3: cloning, multiple mRNA forms, and missense polymorphisms in the coding sequence. Gene. 1998;213:149–157. doi: 10.1016/s0378-1119(98)00193-0. [DOI] [PubMed] [Google Scholar]
- 42.Ng CJ, Bourquard N, Grijalva V, Hama S, Shih DM, Navab M, et al. Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoproteinB-containing lipoproteins: anti-atherogenic role for paraoxonase-2. J Biol Chem. 2006;281:29491–29500. doi: 10.1074/jbc.M605379200. [DOI] [PubMed] [Google Scholar]
- 43.Ng CJ, Hama SY, Bourquard N, Navab M, Reddy ST. Adenovirus mediated expression of human paraoxonase 2 protects against the development of atherosclerosis in apolipoprotein E-deficient mice. Mol Genet Metab. 2006;89:368–373. doi: 10.1016/j.ymgme.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, et al. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem. 2001;276:44444–44449. doi: 10.1074/jbc.M105660200. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira SA, Mansur AP, Ribeiro CC, Ramires JA, Annichino-Bizzacchi JM. PON1 M/L55 mutation protects high-risk patients against coronary artery disease. Int J Cardiol. 2004;94:73–77. doi: 10.1016/j.ijcard.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Pan JP, Lai ST, Chiang SC, Chou SC, Chiang AN. The risk of coronary artery disease in population of Taiwan is associated with Cys-Ser 311 polymorphism of human paraoxonase (PON)-2 gene. Zhonghua Yi Xue Za Zhi (Taipei) 2002;65:411–412. [PubMed] [Google Scholar]
- 47.Pasdar A, Ross-Adams H, Cumming A, Cheung J, Whalley L, St Clair D, et al. Paraoxonase gene polymorphisms and haplotype analysis in a stroke population. BMC Med Genet. 2006;7:28. doi: 10.1186/1471-2350-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinizzotto M, Castillo E, Fiaux M, Temler E, Gaillard RC, Ruiz J. Paraoxonase2 polymorphisms are associated with nephropathy in type II diabetes. Diabetologia. 2001;44:104–107. doi: 10.1007/s001250051586. [DOI] [PubMed] [Google Scholar]
- 49.Precourt LP, Seidman E, Delvin E, Amre D, Deslandres C, Dominguez M, et al. Comparative expression analysis reveals differences in the regulation of intestinal paraoxonase family members. Int J Biochem Cell Biol. 2009;41:1628–1637. doi: 10.1016/j.biocel.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/ arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33:498–507. doi: 10.1006/geno.1996.0225. [DOI] [PubMed] [Google Scholar]
- 51.Qin Q, Li YL, Zhao FM, Wang H, Li Y, Cui RZ, et al. Association of paraoxonase polymorphisms and serum homocysteine thiolactone complex with coronary heart disease. Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34:803–807. [PubMed] [Google Scholar]
- 52.Ranade K, Kirchgessner TG, Iakoubova OA, Devlin JJ, DelMonte T, Vishnupad P, et al. Evaluation of the paraoxonases as candidate genes for stroke: Gln192Arg polymorphism in the paraoxonase 1 gene is associated with increased risk of stroke. Stroke. 2005;36:2346–2350. doi: 10.1161/01.STR.0000185703.88944.7d. [DOI] [PubMed] [Google Scholar]
- 53.Robertson KS, Hawe E, Miller GJ, Talmud PJ, Humphries SE. Northwick Park Heart Study II. Human paraoxonase gene cluster polymorphisms as predictors of coronary heart disease risk in the prospective Northwick Park Heart Study II. Biochim Biophys Acta. 2003;1639:203–212. doi: 10.1016/j.bbadis.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Rosenblat M, Coleman R, Reddy ST, Aviram M. Paraoxonase 2 attenuates macrophage triglyceride accumulation via inhibition of diacylglycerol acyltransferase 1 the rate limiting enzyme in triglycerides biosynthesis. J Lipid Res. 2009;50:870–879. doi: 10.1194/jlr.M800550-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenblat M, Draganov D, Watson CE, Bisgaier CL, La Du BN, Aviram M. Mouse macrophage paraoxonase 2 activity is increased whereas cellular paraoxonase 3 activity is decreased under oxidative stress. Arterioscler Thromb Vasc Biol. 2003;23:468–474. doi: 10.1161/01.ATV.0000059385.95664.4D. [DOI] [PubMed] [Google Scholar]
- 56.Rosenblat M, Hayek T, Hussein K, Aviram M. Decreased macrophage paraoxonase 2 expression in patients with hypercholesterolemia is the result of their increased cellular cholesterol content: effect of atorvastatin therapy. Arterioscler Thromb Vasc Biol. 2004;24:175–180. doi: 10.1161/01.ATV.0000104011.88939.06. [DOI] [PubMed] [Google Scholar]
- 57.Rosenblat M, Oren R, Aviram M. Lysophosphatidylcholine (LPC) attenuates macrophage-mediated oxidation of LDL. Biochem Biophys Res Commun. 2006;344:1271–1277. doi: 10.1016/j.bbrc.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 58.Rothem L, Hartman C, Dahan A, Lachter J, Eliakim R, Shamir R. Paraoxonases are associated with intestinal inflammatory diseases and intracellularly localized to the endoplasmic reticulum. Free Radic Biol Med. 2007;43:730–739. doi: 10.1016/j.freeradbiomed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Saeed M, Iqbal P, Yousuf FA, Perveen S, Shafiq M, Sajid J, et al. Interactions and associations of paraoxonase gene cluster polymorphisms with myocardial infarction in a Pakistani population. Clin Genet. 2007;71:238–247. doi: 10.1111/j.1399-0004.2007.00753.x. [DOI] [PubMed] [Google Scholar]
- 60.Sanghera DK, Aston CE, Saha N, Kamboh MI. DNA polymorphisms in two paraoxonase genes (PON1 and PON2) are associated with the risk of coronary heart disease. Am J Hum Genet. 1998;62:36–44. doi: 10.1086/301669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shamir R, Hartman C, Karry R, Pavlotzky E, Eliakim R, Lachter J, et al. Paraoxonases (PONs) 1, 2, and 3 are expressed in human and mouse gastrointestinal tract and in Caco-2 cell line: selective secretion of PON1 and PON2. Free Radic Biol Med. 2005;39:336–344. doi: 10.1016/j.freeradbiomed.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 62.Shi J, Zhang S, Tang M, Liu X, Li T, Han H, et al. Possible association between Cys311Ser polymorphism of paraoxonase 2 gene and late-onset Alzheimer's disease in Chinese. Mol Brain Res. 2004;120:201–204. doi: 10.1016/j.molbrainres.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 63.Shih DM, Gu L, Hama S, Xia Y-R, Navab M, Fogelman AM, et al. Genetic-dietary regulation of serum paraoxonase expression and its role in atherogenesis in a mouse model. J Clin Invest. 1996;97:1630–9. doi: 10.1172/JCI118589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shih DM, Gu LY, Xia R, Navab M, Li W, Hama S, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 65.Shin BS. Paraoxonase gene polymorphism in south-western Korean population. J Korean Med Sci. 2009;24:561–566. doi: 10.3346/jkms.2009.24.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shiner M, Fuhrman B, Aviram M. A biphasic U-shape effect of cellular oxidative stress on the macrophage anti-oxidant paraoxonase 2 (PON2) enzymatic activity. Biochem Biophys Res Commun. 2006;349:1094–1099. doi: 10.1016/j.bbrc.2006.08.150. [DOI] [PubMed] [Google Scholar]
- 67.Shiner M, Fuhrman B, Aviram M. Macrophage paraoxonase 2 (PON2) expression is up-regulated by pomegranate juice phenolic anti-oxidants via PPAR gamma and AP-1 pathway activation. Atherosclerosis. 2007;195:313–321. doi: 10.1016/j.atherosclerosis.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Shiner M, Fuhrman B, Aviram M. Macrophage paraoxonase2 (PON2) expression is upregulated by unesterified cholesterol through activation of the phosphatidylinositol 3-kinase (PI3K) pathway. Biol Chem. 2007;388:1353–1358. doi: 10.1515/BC.2007.145. [DOI] [PubMed] [Google Scholar]
- 69.Shiner M, Fuhrman B, Aviram M. Paraoxonase 2 (PON2) expression is upregulated via a reduced-nicotinamide-adenine-dinucleotide-phosphate (NADPH)-oxidase-dependent mechanism during monocytes differentiation into macrophages. Free Radic Biol Med. 2004;37:2052–2063. doi: 10.1016/j.freeradbiomed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Slowik A, Tomik B, Wolkow PP, Partyka D, Turaj W, Marecki MT, et al. Paraoxonase gene polymorphisms and sporadic ALS. Neurology. 2006;67:766–770. doi: 10.1212/01.wnl.0000219565.32247.11. [DOI] [PubMed] [Google Scholar]
- 71.Slowik A, Wloch D, Szermer P, Wolkow P, Malecki M, Pera J, et al. Paraoxonase 2 gene C311S polymorphism is associated with a risk of large vessel disease stroke in a Polish population. Cerebrovasc Dis. 2007;23:395–400. doi: 10.1159/000101462. [DOI] [PubMed] [Google Scholar]
- 72.Su SY, Chen JH, Huang JF, Wang XL, Zhao JG, Shen Y, et al. Paraoxonase gene cluster variations associated with coronary heart disease in Chinese Han women. Chin Med J (Engl) 2005;118:1167–1174. [PubMed] [Google Scholar]
- 73.Thameem F, He X, Voruganti VS, Nath SD, Fanti P, Blangero J, et al. Evaluation of polymorphisms in paraoxonase 2 (PON2) gene and their association with cardiovascular-renal disease risk in Mexican Americans. Kidney Blood Press Res. 2009;32:200–204. doi: 10.1159/000225943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valdmanis PN, Kabashi E, Dyck A, Hince P, Lee J, Dion P, et al. Association of paraoxonase gene cluster polymorphisms with ALS in France, Quebec, and Sweden. Neurology. 2008;71:514–520. doi: 10.1212/01.wnl.0000324997.21272.0c. [DOI] [PubMed] [Google Scholar]
- 75.Voron'ko OE, Iakunina N, Shestakova MV, Zotova EV, Chugunova EV, Shamkhalova M, et al. A search for association between the polymorphic markers of PON1 and PON2 genes and diabetic nephropathy in patients with type I diabetes mellitus. Genetika. 2005;41:844–849. [PubMed] [Google Scholar]
- 76.Wang X, Fan Z, Huang J, Su S, Yu Q, Zhao J, et al. Extensive association analysis between polymorphisms of PON gene cluster with coronary heart disease in Chinese Han population. Arterioscler Thromb Vasc Biol. 2003;23:328–334. doi: 10.1161/01.atv.0000051702.38086.c1. [DOI] [PubMed] [Google Scholar]
- 77.Wang XY, Xue YM, Wen SJ, Zhang NL, Ji Z, Pan SY. The association of paraoxonase 2 gene C311S variant with ischemic stroke in Chinese type 2 diabetes mellitus patients. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2003;20:215–219. [PubMed] [Google Scholar]
- 78.Wheeler JG, Keavney BD, Watkins H, Collins R, Danesh J. Four paraoxonase gene polymorphisms in 11212 cases of coronary heart disease and 12786 controls: meta-analysis of 43 studies. Lancet. 2004;363:689–695. doi: 10.1016/S0140-6736(04)15642-0. [DOI] [PubMed] [Google Scholar]
- 79.Wills AM, Cronin S, Slowik A, Kasperaviciute D, Van Es MA, Morahan JM, et al. A large-scale international meta-analysis of paraoxonase gene polymorphisms in sporadic ALS. Neurology. 2009;73:16–24. doi: 10.1212/WNL.0b013e3181a18674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu BY, Liang HY, Chen DF, Liu L, Yang F, Hu HY, et al. Associations of Rsa I polymorphism at the 5' flanking region of CYP2E1 and PON2 148 polymorphism in neonates with preterm delivery. Chuan Xue Bao. 2003;30:577–583. [PubMed] [Google Scholar]
- 81.Xu HW, Yuan N, Zhao Z, Zhang L, Xia J, Zeng KM, et al. Study of the relationship between gene polymorphisms of paraoxonase 2 and stroke in a Chinese population. Cerebrovasc Dis. 2008;25:87–94. doi: 10.1159/000111996. [DOI] [PubMed] [Google Scholar]
- 82.Yamada Y, Ando F, Niino N, Miki T, Shimokata H. Association of polymorphisms of paraoxonase 1 and 2 genes, alone or in combination, with bone mineral density in community-dwelling Japanese. J Hum Genet. 2003;48:469–475. doi: 10.1007/s10038-003-0063-x. [DOI] [PubMed] [Google Scholar]