Abstract
Anaemia and immunosuppresion have been shown to be a cardinal feature in African trypanosomosis. In this study, we have evaluated and compared the capacity of two registered veterinary trypanocides Novidium® (homidium chloride) and Berenil® (diminazene aceturate) to reduce haematological and biochemical lesions in rats experimentally infected with T. brucei. Packed cell volume (PCV), lymphocyte and eosinophil values in infected negative control group were significantly different and lower compared to positive control group as well as infected animals treated with homidium chloride and diminazene aceturate (P<0.05). Also the white blood cell (WBC) and neutrophil counts in the negative control group were lower and significantly different from the other groups indicating evidence of infection-induced immunosuppresion. Haematological indices in infected rats treated with homidium chloride and diminazene aceturate were higher (P<0.05) than obtained in infected negative control group and significantly different from positive control (P<0.05). Total protein and albumin concentrations in infected negative control group were higher and significantly different from control and treated animals (P<0.05). In contrast, significantly lower values were obtained for albumin concentrations in treated animals compared to both negative and positive control groups (P<0.05). Results suggest that drugs administered have capacity to improved blood components as well as reverse immunosuppressive action of infecting trypanosomes.
Keywords: anaemia, immunosuppression, T. brucei, biochemical lesions, trypanocides
Introduction
For several decades trypanosomosis has continued to contribute adversely to the economic and social well being of sub-Saharan Africans (WHO, 2006[25]; Adeyemi et al., 2009[3]). This scourge remains a pressing challenge especially to African medical scientists for possible action plan that would include both preventive measures and treatment modalities (Okochi et al., 2003[20]). African trypanosomes cause trypanosomosis, known as sleeping sickness and nagana in man and animals respectively. The trypanosome species affecting man and his domestic animals have been subdivided into two groups, the haematinic group (Trypanosoma congolense, T. vivax) which remains in the plasma and the tissue invading group (T. brucei, T. evansi, T. gambiense, T. rhodesiense and T. equiperdum) found in extra and intra vascular spaces (Abubakar et al., 2005[1]; Chretien and Smoak, 2005[6]; Ngure et al., 2008[18]). Because of their presence in the blood, these invading parasites produce numerous changes in the cellular and biochemical constituents of blood (Igbokwe and Mohammed, 1992[15]; Taiwo et al., 2003[23]). Trypanosoma brucei infection like other trypanosome infections precipitate increased red blood cell destruction which results in anaemia as well as tissue damage (Ekanem and Yusuf, 2008[12]; Akanji et al., 2009[4]). These changes together with the need by the host to destroy the parasite are presumably responsible for the symptoms of African sleeping sickness. The application of antitrypanosomal drugs has been the most widely practised means of controlling trypanosomosis in domestic livestock since the early 1950s, either as curative or prophylactic drugs. Thus, control of trypanosomosis may depend in the nearest future on the use of the existing trypanocides. The challenge, therefore, remains to make optimal use of the three relatively old compounds until new methods of treatment emerge (Adamu et al., 2009[2]). In this study, we have investigated the effects of two veterinary drugs; novidium® (homidium chloride) and berenil® (diminazene aceturate) on haematological parameters and protein concentrations in rats experimentally infected with T. brucei..
Materials and Methods
24 male albino rats of average weight between 200-220 g were obtained from the Animal Laboratory Unit of the Department of Biochemistry, University of Ilorin, Nigeria. The rats were randomly distributed into four (4) groups of six (6) animals each. They were kept in clean cages with access to clean water and commercial rat pellets ad libitum. The principles governing the use of laboratory animals as laid out by the University of Ilorin Committee on Ethics for Medical and Scientific Research were duly observed.
Parasite
Trypanosoma brucei was obtained from the Nigerian Institute for Trypanosoma Research (NITR), Vom - Jos, Nigeria. The parasite was injected into uninfected rats intraperitoneally and maintained in other rats by repeated passaging. Parasitaemia was monitored by preparing a thin film of blood obtained from animal tail and viewing under light microscope at x100 magnification according to the method of Adeyemi et al. (2009[3]).
Drugs
Drugs were dissolved and reconstituted in distilled water according to manufacturer's instructions, and administered to animals intraperitoneally (I.P.) in the following concentrations: Homidium chloride (Novidium® 17 rue Bourgelat 69002 Lyon-France): 1.0 mg/kg and Diminazene aceturate (Berenil®, Hoechst, Germany): 3.5 mg/kg.
Animals were inoculated with parasites and infection was allowed to develop for 72 hours before treatment was initiated on day 4. Animals were checked daily (one day before therapy and thereafter for 12 days) for parasitaemia in blood collected from tail vein.
Treatment of animals
The animals were divided as follows and received appropriate dosages of the drug that corresponded to their body weight:
Group 1 - Positive Control received appropriate dosages of the vehicle for drug administration
Group 2 - Negative Control were infected with an inoculum containing T. brucei parasites and received no drug treatment
Group 3 - Infected with an inoculum containing T. brucei parasites and treated with homidium chloride
Group 4 - Infected with an inoculum containing T. brucei parasites and treated with diminazene aceturate.
The treatment with drugs started on the first day the parasites were sighted in the blood. All animals were sacrificed on the 12th day post infection.
Inoculation of rats with parasite
Parasite infected blood was obtained from the tail of infected rats at high parasitaemia and used to maintain parasite suspension in 0.90 % saline solution which was inoculated into the peritoneal cavity of uninfected rats weighing approximately between 200-220 g. The suspension - as described by Ekanem et al. (2005[11]) and Adeyemi et al. (2009[3]) - contained 3 or 4 trypanosomes per view at x100 magnification.
Tissue collection and preparation
Collection of sample for biochemical analyses was as described previously (Yakubu et al., 2005[26]). Rats were anaesthetized in slight chloroform and blood samples collected into clean, dry centrifuge tubes. The blood samples were allowed to stand for 10 minutes at room temperature and then centrifuged at 1000 rpm for 15 minutes on laboratory centrifuge (SM 800B, Surgifriend Medicals, England). The supernatant (serum) was carefully removed with Pasteur pipette, and stored frozen until needed for further analyses. The tissues (liver, kidney and brain) were excised and transferred into ice-cold 0.25 M sucrose solution. The tissues which were weighed and finely cut with clean sterile blade were homogenized in ice-cold 0.25 M sucrose solution [1:5w/v] using mortar and pestle. The homogenates were kept frozen overnight before analyses. Blood used for haematological analysis was collected into heparinized sample bottles and taken for analyses within 24 hours of collection.
Biochemical analysis
Serum and tissue total protein as well as albumin were analysed using the biuret and bromocresol green methods, respectively. In both cases, methods according to Gornall et al., (1949[13]) and Doumas et al., (1971[8]) were employed respectively.
Haematological studies
Blood parameters including packed cell volume (PCV), white blood cell (WBC), neutrophil, lymphocytes and eosinophil counts were determined using the Automated Haematologic Analyzer, Sysmex, KX-21 (Japan) as described by Dacie and Lewis, (1991[7]).
Statistical analysis
The group mean ± S.E.M. was calculated for each analyte and significant difference between means evaluated by analysis of variance (ANOVA). Post test analysis was done using the Tukey multiple comparison tests. Values of P<0.05 were considered as statistically significant.
Results
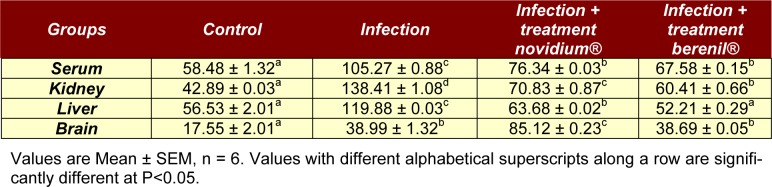
Table 1(Tab. 1) presents results for haematological indices. Haematological observations of animals in the negative control group revealed low counts of PCV, WBC, neutrophil, lymphocytes and eosinophil which were significantly different (P<0.05) from the positive control and treatment groups. In contrast, increased protein and albumin concentrations (Table 2(Tab. 2) and 3(Tab. 3) respectively) were observed in the negative control group compared to positive control and treatment groups (P<0.05). Albumin concentrations in the animals treated with both drugs (novidium® and berenil®) however resulted in decreased concentrations compared to positive control group (P<0.05).
Table 1. Effects of daily administration of homidium chloride (novidium®) and diminazene aceturate (berenil®) on haematological indices in rat serum.
Table 2. Effects of daily administration of novidium® and berenil® on protein concentration in rat serum and tissues.
Table 3. Effects of daily administration of novidium® and berenil® on albumin concentration in rat serum and tissues.
Discussion
It has been established that the measurement of anaemia gives a reliable indication of the disease status and productive performance of trypanosome infected animals (Ekanem et al., 2005[11], 2006[10]). Trypanosome infection may cause anaemia as a result of massive erythrophagocytosis by an expanded and active mononuclear phagocytic system (MPS) of the host (Igbokwe and Nwosu, 1997[16]). Haematological results obtained in this study agree with earlier studies (Anosa, 1988[5]; Igbokwe et al., 1994[14]; Ekanem et al., 2008[9]). The low PCV observed in the infected group may be as a result of acute haemolysis due to growing infection. Previous studies have shown that infection with trypanosomes resulted in increased susceptibility of red blood cell membrane to oxidative damage probably as a result of depletion of reduced glutathione on the surface of the red blood cell (Igbokwe et al., 1994[14], 1996[17]; Taiwo et al., 2003[23]; Akanji et al., 2009[4]). Severity of anaemia usually reflects the intensity and duration of parasitaemia. Several reports (Ogunsanmi and Taiwo, 2001[19]; Umar et al., 2007[24]; Ekanem et al., 2008[9]; Saleh et al., 2009[22]) have also ascribed acute anaemia in trypanosomosis to proliferating parasites. The lower counts of WBC, lymphocytes and neutrophil observed in the infected negative control group may be attributed to the immunosuppressive actions of trypanosome infection (Abubakar et al., 2005[1]; Ekanem and Yusuf, 2008[12]). Leucocytosis which may be due to lymphocytosis has been implicated in trypanosomosis and these conditions are usually as a result of wax and wear syndrome on the animal immune system caused by the ever changing variable surface glycoprotein of the infecting trypanosomes (Abubakar et al., 2005[1]). In contrast these lesions were reduced significantly in the infected animals treated with novidium® and berenil®. Results suggest that administered drugs improved blood components possibly by depletion of proliferating parasites. High protein concentrations observed in the infected animals agree with previous reports (Orhue et al., 2005[21]; Ekanem and Yusuf, 2008[12]). Increase in total protein and albumin concentrations in the serum of infected negative control animals could be as a result of cell derived proteins due to haemolysis which is secondary to infection. It may also be attributed to increase in parasite mass proteins and/or released intracellular enzymes due to increasing parasitaemia or lysis of parasites by host immune system respectively. Synthesis of immunoglobulin in response to infection may have also contributed to the observed increases in protein concentration. Progressive anaemia is a common pathogenic characteristic of T. brucei infection in mammals (Anosa, 1988[5]) thus release of erythrocytes-derived enzymes and proteins may be one possible source of plasma protein. Protein loss from tissues may also have contributed to the increased protein concentrations observed. High protein and albumin concentrations in tissues may indicate increased protein synthesis due to increased protein need secondary to infection and or symptom of cytolysis as the infection progressed. These changes however were reduced (P<0.05) in the treatment group suggesting attempts by the administered drugs to restore cellular functions to the pre-infection state. The increased WBC, lymphocyte and neutrophil counts (Table 1(Tab. 1)) are indicative of increased host action in the presence of administered drugs against the infection. These boosts will contribute to the development of phagocytes and antibodies against the recognisable antigens of parasite origin. In conclusion, we have provided evidence that the administered drugs (novidium® and berenil®) have potentials for modulating the state of anaemia and immunosuppressive conditions in trypanosome infected rats.
References
- 1.Abubakar A, Iliyasu B, Yusuf AB, Igweh AC, Onyekwelu NA, Shamaki BA, Afolayan DO, Ogbadoyi EO. Antitrypanosomal and haematological effects of selected Nigerian medicinal plants in Wistar rats. Biokemistri. 2005;17:95–99. [Google Scholar]
- 2.Adamu S, Barde N, Abenga JN, Useh NM, Ibrahim NDG, Esievo KAN. Experimental Trypanosoma brucei infection-induced changes in the serum profiles of lipids and cholesterol and the clinical implications in pigs. J Cell Animal Biol. 2009;3(2):15–20. [Google Scholar]
- 3.Adeyemi OS, Akanji MA, Oguntoye S. Ethanolic leaf extract of Psidium guajava: Phytochemical and trypanocidal activity in rats infected with Trypanosoma brucei brucei. J Medicinal Plants Res. 2009;3:420–423. [Google Scholar]
- 4.Akanji MA, Adeyemi OS, Oguntoye SO, Sulyman F. Psidium guajava extract reduces trypanosomosis associated lipid peroxidation and raises glutathione concentrations in infected animals. EXCLI J. 2009;8:148–154. [Google Scholar]
- 5.Anosa VO. Haematological and biochemical changes in human and animal trypanosomiasis. Part I. Rev Elev Med Vet Pays Trop. 1988;41:65–78. [PubMed] [Google Scholar]
- 6.Chretien JPL, Smoak BL. African Trypanosomiasis: Changing epidemiology and consequences. Curr Infect Dis Rep. 2005;7:54–60. doi: 10.1007/s11908-005-0024-y. [DOI] [PubMed] [Google Scholar]
- 7.Dacie JV, Lewis SM. Practical haematology. 7th ed. Edingburgh: Churchill Livingston; 1991. [Google Scholar]
- 8.Doumas BT, Watson W, Biggs HG. Albumin standards and the measurement serum albumin with bromocresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 9.Ekanem JT, Kolawole OM, Abbah OC. Trypanocidal potential of methanolic extract of Bridelia ferruginea benth bark in Rattus novergicus. Afr J Biochem Res. 2008;2:45–50. [Google Scholar]
- 10.Ekanem JT, Majolagbe OR, Sulaiman FA, Muhammad NO. Effects of honey supplemented diet on the parasitaemia and some enzymes of Trypanosoma brucei-infected rats. Afr J Biotechnol. 2006;5:1557–1561. [Google Scholar]
- 11.Ekanem JT, Sulyman FA, Adeyemi OS. Therapeutic properties and serum iron in T. brucei infected rats treated with amodiaquine and mefloquine. Biokemistri. 2005;17:115–121. [Google Scholar]
- 12.Ekanem JT, Yusuf OK. Some biochemical and haematological effects of black seed (Nigella sativa) oil on T.brucei-infected rats. Afr J Biomed Res. 2008;11:79–85. [Google Scholar]
- 13.Gornall AG, Bardawill CJ, David MM. Determination of serum protein by biuret method. J Biol Chem. 1949;117:751–766. [PubMed] [Google Scholar]
- 14.Igbokwe IO, Esievo KA, Saror DI, Obagaiye OK. Increased susceptibility of erythrocytes to in vitro peroxidation in acute Trypanosoma brucei infection in mice. Vet Parasitol. 1994;55:279–286. doi: 10.1016/0304-4017(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 15.Igbokwe IO, Mohammed A. Some plasma biochemical changes in experimental Trypanosoma brucei infection in Sokoto red goats. Rev Elev Med Pays Trop. 1992;45(3-40):287–290. [PubMed] [Google Scholar]
- 16.Igbokwe IO, Nwosu CO. Lack of correlation of anaemia with splenomegaly and hepatomegaly in Trypanosoma brucei and Trypanosoma congolense infections of rats. J Comp Pathol. 1997;117:261–265. doi: 10.1016/s0021-9975(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 17.Igbokwe IO, Umar IA, Omage JJ, Ibrahim NDG, Kadima KB, Obagaiye OK, Saror DI, Esievo KAN. Effect of acute Trypanosoma vivax infection on cattle erythrocyte glutathione and susceptibility to in vitro peroxidation. Vet Parasitol. 1996;63:215–224. doi: 10.1016/0304-4017(95)00887-x. [DOI] [PubMed] [Google Scholar]
- 18.Ngure RM, Ndungu JM, Ngotho JM, Nancy MK, Maathai RG, Gateri LM. Biochemical changes in the plasma of vervet monkeys (Chlorocebus aethiops) experimentally infected with Trypanosoma brucei rhodesiense. J Cell Animal Biol. 2008;2:150–157. [Google Scholar]
- 19.Ogunsanmi AO, Taiwo VO. Pathobiochemical mechanisms involved in the control of the disease caused by Trypanosoma congolense in African grey duiker (Sylvicapra grimmia) Vet Parasitol. 2001;96:51–63. doi: 10.1016/s0304-4017(00)00410-6. [DOI] [PubMed] [Google Scholar]
- 20.Okochi VI, Okpuzor J, Okubena MO, Awoyemi AK. The influence of African Herbal Formula on the haematological parameters of trypanosome infected rats. Afr J Biotechnol. 2003;2:312–316. [Google Scholar]
- 21.Orhue N, Nwanze E, Okafor A. Serum total protein, albumin and globulin levels in Trypanosoma brucei-infected rabbits: effect of orally administered Scoparia dulcis. Afr J Biotechnol. 2005;4:1152–1155. [Google Scholar]
- 22.Saleh MA, Bassam MA, Sanousi SA. Oxidative stress in blood of camels (Camelus dromedaries) naturally infected with Trypanosoma evansi. Vet Parasitol. 2009;162:192–9. doi: 10.1016/j.vetpar.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 23.Taiwo VO, Olaniyi MO, Ogunsanmi AO. Comparative plasma biochemical changes and susceptibility of erythrocytes to in vitro peroxidation during experimental Trypanosome congolense and T brucei infections in sheep. Israel J Vet Med. 2003;58(4) [Google Scholar]
- 24.Umar IA, Ogenyi E, Okodaso D, Kimeng E, Stancheva GI, Omage JJ, Isah S, Ibrahim MA. Amelioration of anaemia and organ damage by combined intraperitoneal administration of vitamins A and C to Trypanosoma brucei brucei-infected rats. Afr J Biotechnol. 2007;6:2083–6. [Google Scholar]
- 25.WHO, World Health Organization. African Trypanosomosis (sleeping sickness) Geneva: WHO; 2006. (Fact sheet 259). [Google Scholar]
- 26.Yakubu MT, Adebayo OJ, Egwim EC, Owoyele VB. Increased liver alkaline phosphatase and aminotransferase activities following administration of ethanolic extract of Khaya senegalensis stem bark to rats. Biokemistri. 2005;17:27–32. [Google Scholar]