Abstract
The effects of spironolactone, a non-selective aldosterone antagonist, were examined on thermally-induced pain using the hot-plate and tail-flick tests, on chemogenic pain induced by intraplantar capsaicin, on electrically-induced pain, on visceral nociception induced by intraperitoneal acetic acid injection and on haloperidol-induced catalepsy in mice. Spironolactone significantly shortened response latency in the mouse tail-flick test but produced modest decreases in response latencies in the mouse hot plate test. The drug reduced the antinociceptive effect of tramadol in the hot plate test. Spironolactone in addition decreased nociceptive thresholds of electrically-induced pain in mice. In contrast, spironolactone elicited significant antinociceptive actions in the mouse acetic-acid-induced writhing assay and at doses of 20-160 mg/kg decreased capsaicin-induced chemogenic pain. Spironolactone at doses of 40 or 80 mg/kg reduced spontaneous activity and produced a significant impairment on the rotarod test in mice. The drug (10-80 mg/kg) increased the duration of catalepsy induced by haloperidol by 56.3-188.5 %. In conclusion, spironolactone increased pain behavior in a dose-dependent manner in models of thermal and electrical pain, but decreased inflammatory visceral pain due to intraperitoneal acetic acid and chemogenic pain due to intraplantar capsaicin. The effect of spironolactone on various types of pain needs further evaluation.
Keywords: spironolactone, aldosterone antagonism, acute nociceptive pain, mice
Introduction
Spironolactone, a synthetic steroid with an aldosterone-like structure acts as non-specific antagonist of aldosterone receptors. The drug is used for blocking aldosterone-dependent sodium transport in the distal tubule of the kidney in order to reduce oedema and to treat essential hypertension and primary hyperaldosteronism (Conn and Hinerman, 1977[8]). Spironolactone is also used in the treatment of secondary hyperaldosteronism occurring in such conditions as liver cirrhosis and congestive heart failure (Mantero and Lucarelli, 2000[29]; Tang et al., 2005[41]; Dib et al., 2006[10]). The drug has in addition weak anti-androgenic action due to androgen receptor blockade (Carmina, 2002[6]) and recently has been demonstrated to possess anti-inflammatory and immune modifying properties (Francis et al., 2003[14]; Hansen et al., 2004[20]; Sonder et al., 2006[39]).
The mineralocorticoid receptor (MR) is mainly localized in the classical peripheral target tissues such as the kidney and in some areas within the brain (Krozowski and Funder, 1983[27]; Geerling et al., 2006[16]). In addition to their prime physiologic role in the control of electrolyte homeostasis and blood pressure, studies have shown that mineralocorticoid receptors (MRs) and glucocorticoid receptors (GRs) are involved in the long-term corticosterone modulation of the anxiety response induced by restraint (Calvo and Volosin, 2001[5]), while forebrain mineralocorticoid receptor overexpression enhances memory, reduces anxiety and attenuates neuronal loss in cerebral ischemia (Lai et al., 2007[28]). Excessive stimulation of MRs in the brain increases sympathetic excitation (Gomez-Sanchez, 1997[17]). In the amygdala, MR-mediated mechanisms are likely to be involved in descending pathways onto lumbosacral spinal neurons that induce colorectal hypersensitivity to luminal distension (Qin et al., 2003[35]).
The present study was designed to investigate for a possible pain modulating effect of aldosterone receptor blockade with spironolactone. In the present study, the effect of the non-selective aldosterone antagonist, spironolactone, was evaluated in the acute nociceptive models of thermal, chemogenic, electrical and visceral pain in mice. The effect of spironolactone was in addition examined on haloperidol-induced catalepsy in mice, a model of Parkinsonism, caused about by D2 receptor blockade in the striatum (Farde et al., 1992[11]; Hoffman and Donovan, 1995[23]).
Materials and Methods
Male Swiss albino mice (22-25 g) were used. Mice were housed under standardized conditions with free access to food and water. Animal procedures were performed in accordance with the Ethics Committee of the National Research Centre and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985). Equal groups of 6 mice each were used in all experiments.
Tail-flick assay
The tail-flick test was used in mice to elicit a spinal tail flick response to noxious thermal stimuli. The test was performed with the tail-flick analgesia meter (Ugo Basile, Italy). Groups of mice (n=6/group) were given spironolactone (5, 10, 20, 40 or 80 mg/kg, s.c., 0.2 ml) or saline (control). Each mouse was gently held with one hand and its tail positioned on the source of radiant heat. The tail-flick response was elicited by applying radiant heat to the dorsal surface of the tail. The time elapsed till the animal flicked its tail was determined. The test was done at 60 and 120 min after drug injection.
Hot-plate assay
The hot-plate test was performed using an electronically controlled hotplate (Ugo Basile, Italy) heated to 53 °C (± 0.1 °C). Each mouse was placed unrestrained on hot plate for the baseline measurement just prior to saline or drug administration. Different groups of mice (n=6/group) were given spironolactone (10, 20, 40 or 80 mg/kg, s.c.) or saline (control). Measurements were then taken 30 and 60 min after drug administration. Latency to lick a hind paw or jump out of the apparatus was recorded for the control and drug-treated groups. The cut-off time was 30s. In another experiment, the effect of spironolactone (40 or 80 mg/kg, s.c.) on antinociception caused by tramadol (10 or 20 mg/kg, s.c.) was studied. Drugs were co-administered 30 min prior to testing.
Tail electric stimulation test
Groups of mice (n=6/group) were given spironolactone (10, 20, 40 or 80 mg/kg, s.c.) or saline (control). The minimum current required to elicit vocalization upon electrical stimulation of the tail was determined for the control and drug-treated groups (Michael-Titus and Costentin, 1987[30]). Electrical stimulation of the tail was applied by means of Pulse generator 57800-001 (Ugo Basile EXT Unit) (Frequency 50 pulse/sec, shock duration 2 sec). Spironolactone was administered 30 min prior to testing.
Acetic acid-induced writhing
Separate groups of 6 mice each were administered vehicle (saline) or spironolactone 5, 10, 20, 40 or 80 mg/kg; 0.2 ml, orally. After 60 min of administration drug administration, an intraperitoneal (i.p.) injection of 0.6 % acetic acid (0.2 ml) was administered (Koster et al., 1959[26]). In other experiments, the effect of the alpha-2 adrenoceptor antagonist yohimbine (5 mg/kg, s.c.), the beta adrenoceptor antagonist propranolol (2 mg/kg, s.c.), the adrenergic blocker guanethidine (16 mg/kg, s.c.), the muscarinic acetylcholine receptor antagonist atropine (1 mg/kg, s.c.) was examined on antinociception caused by spironolactone (10 mg/kg, s.c.). Drugs were administered 30 min prior to acetic acid challenge. Furthermore, the effect of co-administered spironolactone (40 or 80 mg/kg, s.c.) and melatonin (4 mg/kg, i.p.) was examined. Drugs were administered 30 min prior to the abdominal constriction assay.
Capsaicin-induced hind paw licking
Spironolactone (20, 40, 80 or 160 mg/kg, s.c.) or saline was administered 30 min before injection of capsaicin (1.6 μg/paw; 25 μl) under the skin of the dorsal surface of the right hind paw. Observation started after capsaicin injection and lasted for 5 min. The time the animals spent licking the injected paw was determined using a stopwatch (Sakurada et al., 1992[36]).
Rotarod testing
Motor performance was measured as the latency to fall from an accelerating rotarod located over plates connected to an automatic counter (Ugo Basile, Varese, Italy). Mice were trained to remain on a rotating rod for 2 min as the rod rotated toward the animal. After the 2-min training period, the mice were administered spironolactone (10, 20, 40 or 80 mg/kg, i.p.) or saline (control) and 30 min later placed on the rotating rod as it accelerated from 4 to 40 rpm over 5 min and the time that they could remain on the accelerating rod was noted (Millan et al., 1994[31]). The cutoff time was 600 sec. The time was measured from the start of the acceleration period.
Haloperidol-induced catalepsy
Catalepsy defined as a reduced ability to initiate movement and a failure to correct posture, was measured by the bar test. Mice were positioned so that their hindquarters were on the bench and their forelimbs rested on a 1 cm diameter horizontal bar, 4 cm above the bench. The length of time the mouse maintained this position was recorded by a stopwatch to a maximum of 180 s. This procedure was performed 30 min after haloperidol (2 mg/kg, i.p.) administration. Spironolactone (10, 20, 40 or 80 mg/kg, i.p.) was co-administered with haloperidol. Mice were judged to be cataleptic if they maintained this position for 30 s or more.
Haloperidol-induced locomotor impairment
Mice were administered saline, haloperidol (2 mg/kg, i.p.) or haloperidol + spironolactone (10, 20, 40 or 80 mg/kg, i.p.). Thirty min after treatment with drugs or vehicle, mice were individually placed into a 40-cm3 activity monitor equipped with photoelectric detectors and the total number of horizontal beam interruptions (spontaneous locomotor activity) was counted over a 6-min period for each animal.
Statistical analysis
Data are expressed as mean ± SE. Data were analyzed by one-way analysis of variance, followed by a Tukey's multiple range test for post hoc comparison of group means. When there were only two groups a two-tailed Student's t test was used. For all tests, effects with a probability of p<.05 were considered to be significant.
Results
Effect of spironolactone on thermal nociception
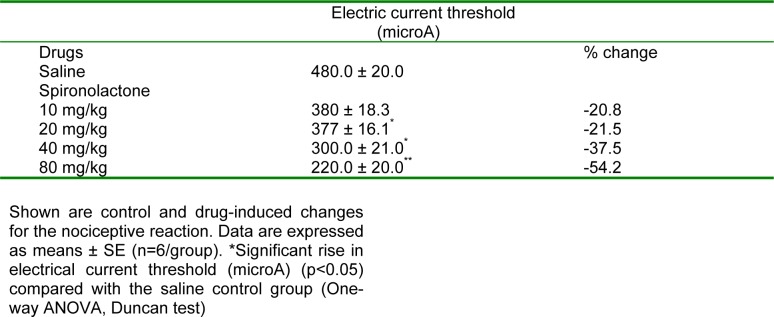
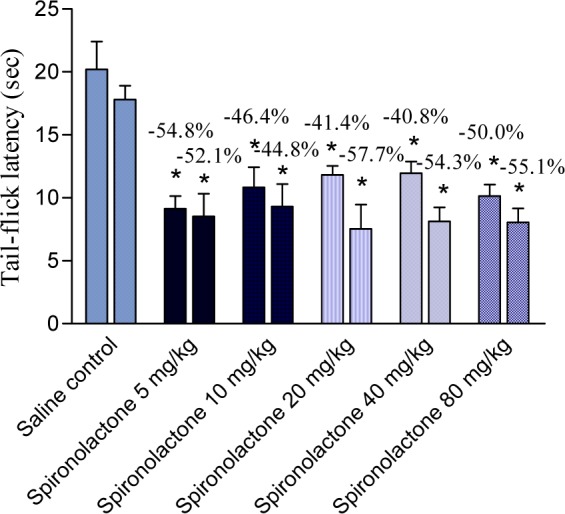
Spironolactone significantly and markedly shortened response latency in the mouse tail-flick test at all doses examined (5, 10, 20, 40 or 80 mg/kg) by 41.4-54.8 % (Figure 1(Fig. 1)). Meanwhile, spironolactone at 10 mg/kg did not affect response latency in the mouse hot plate test, but the drug at 20, 40 or 80 mg/kg decreased hot plate latencies 1h after drug administration by 26.5, 31.9 and 16.6 %, respectively (Figure 2(Fig. 2)).
Figure 1. 1 h and 2 h values (first and second column, respectively) of tail flick latency (seconds) of saline (control) and spironolactone-treated mice. Each column represents mean ± SE of 6 mice/group.
*p<0.05 compared to saline group at the corresponding time
Figure 2. Basal (pre-drug), 30 min and 1 h values (first, second and third column, respectively) of hot-plate latency (seconds) of saline (control) and spironolactone-treated mice. Each column represents mean ± SE of 6 mice/group. *p<0.05, **p<0.01 compared to its basal value.
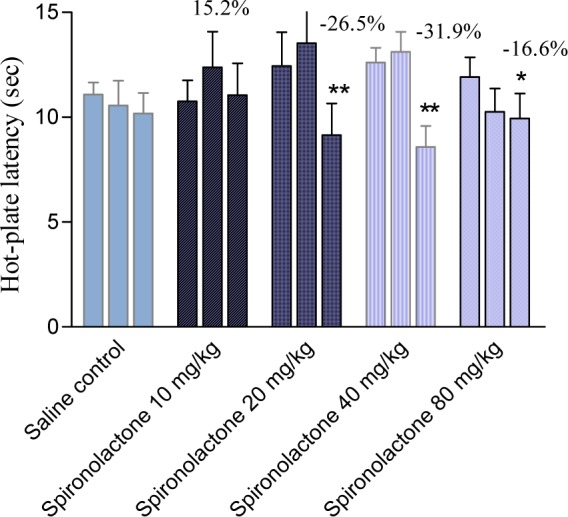
Tramadol administered at 10 mg/kg, i.p., increased hot-plate latencies by 62.5, 38.4 and 19.3 %, at 30 min, 1 h and 2 h after drug administration, respectively. Tramadol (10 mg/kg) co-administered with spironolactone at 40 or 80 mg/kg increased hot plate response latencies by 5.4, 30.2, and 29.7 % and by 4.3, 12.7, and 22.2 % at 30 min, 1 h and 2 h post-drug, respectively. Tramadol administered at 20 mg/kg, i.p., increased hot-plate latencies by 68.4, 43.6 and 23.5 %, 30 min, 1 h and 2 h after drug administration, respectively. Tramadol (20 mg/kg) co-administered with spironolactone at 40 mg/kg resulted in 55 and 57 % increase in hot-plate latency, 30 min and 1 h post-drug, respectively. Tramadol (20 mg/kg) co-administered with spironolactone at 80 mg/kg caused 13.4, 31.9 and 24.3 % increase in response latency in the hot-plate test, 30 min, 1 h and 2 h post-drug (Figure 3(Fig. 3)).
Figure 3. Basal (pre-drug), 30 min, 1 h and 2 h values (first, second, third and fourth column, respectively) of hot-plate latency (seconds) of tramadol and tramadol + spironolactone-treated mice. Each column represents mean ± SE of 6 mice/group.
*p<0.05, **p<0.01 compared to its basal value
Effect of spironolactone on tail electric stimulation
Spironolactone (10, 20, 40 and 80 mg/kg, i.p.) produced a dose-related decrease in electrical current threshold in the tail stimulation test in mice by 20.8, 21.5, 37.5 and 54.2 % vs control values 1 h post-drug, respectively (Table 1(Tab. 1)).
Table 1. Antinociceptive activity of spironolactone in the tail electric stimulation test in mice.
Effect of spironolactone on visceral nociception
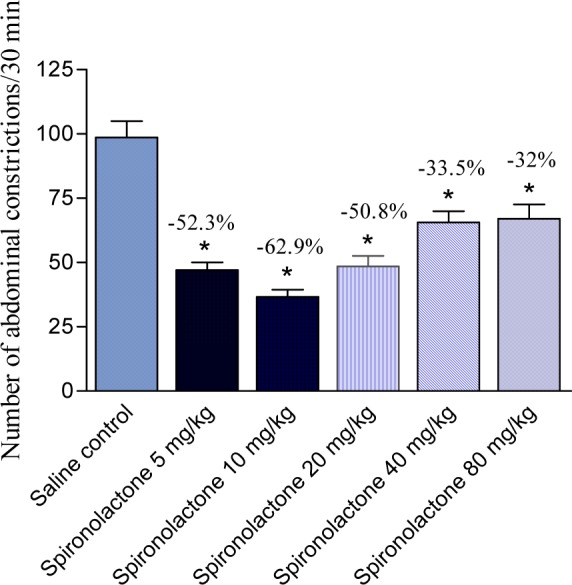
Spironolactone administered via oral route produced a decrease in acetic acid-induced writhing in mice. It was noted that lower doses were more effective in reducing pain response. Spironolactone given at 5, 10, 20, 40 or 80 mg/kg produced 52.3, 62.9, 50.8, 33.5 and 32 % of the number of contractions induced by acetic acid (Figure 4(Fig. 4)).
Figure 4. Effect of orally administered spironolactone on the number of abdominal constrictions in the acetic acid-induced writhing assay in mice. Data represent mean values of 6 mice per group (± SE) and percent inhibition (%) compared to the control animals. Statistical differences vs. control group are indicated by asterisks.
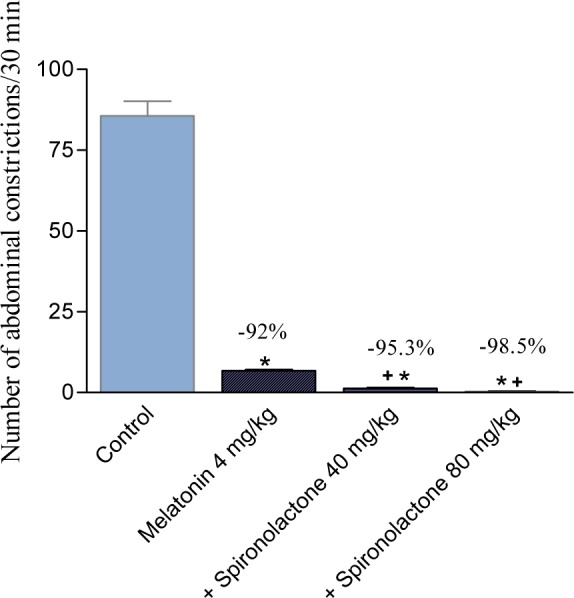
The effect of spironolactone (10 mg/kg, s.c.) was unaffected by co-administration of atropine, propranolol or guanethidine, but yohimbine reduced the antinociceptive effect of spironolactone on the writhing response (Figure 5(Fig. 5)). The effect of spironolactone and melatonin was additive (Figure 6(Fig. 6)).
Figure 5. Effect of yohimbine, propranolol, atropine and guanethidine on the antinociception caused by the administration of spironolactone (10 mg/kg. s.c.) in the acetic acid-induced writhing assay in mice. Data represent mean values of 6 mice per group (± SE) and percent inhibition (%) compared to the control animals. Statistical differences vs. control group are indicated by asterisks. The plus sign indicates significant change from yohimbine + spironolactone-treated group.
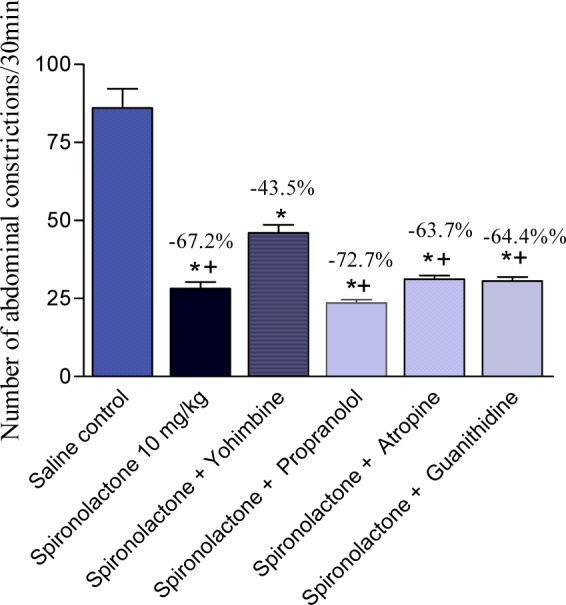
Figure 6. The effect of co-administered spironolactone (40 or 80 mg/kg, s.c.) and melatonin (4 mg/kg, i.p.) in the acetic acid-induced writhing assay in mice. Drugs were administered 30 min prior to the abdominal constriction assay. Data represent mean values of 6 mice per group (± SE) and percent inhibition (%) compared to the control animals. Statistical differences vs. control group are indicated by asterisks. The plus sign indicates significant change from melatonin only-treated group.
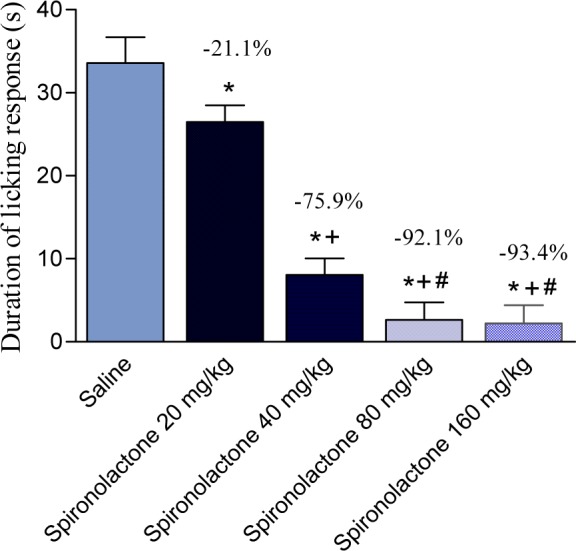
Effect of spironolactone on capsaicin-induced paw licking
The duration of paw licking following intraplantar capsaicin injection was decreased by 21.2, 75.9, 92.1 and 93.4 % following administration of 20, 40, 80 and 160 mg/kg spironolactone, respectively (Figure 7(Fig. 7)).
Figure 7. Effect of spironolactone on the duration of licking response to intraplantar capsaicin injection in mice. Data represent mean values (± SE) and percent inhibition (%) (Percent analgesic effect) compared to the control animals. Statistical differences vs. control group are indicated by asterisks. The plus sign indicates a significant change from the spironolactone 20 mg/kg-treated group. The # sign indicates a significant change from the spironolactone 40 mg/kg-treated group.
Rotarod testing
Spironolactone administered at 10 or 20 mg/kg did not affect rotarod performances compared to saline-treated mice (347 ± 22.6 vs. 342 ± 19.6 and 338 ± 26.0). Spironolactone administered at 40 or 80 mg/kg significantly impaired rotarod performances of the mice. Time spent on rotarod decreased from 347 ± 22.6 to 271.5 ± 18 and 206 ± 12.2, respectively.
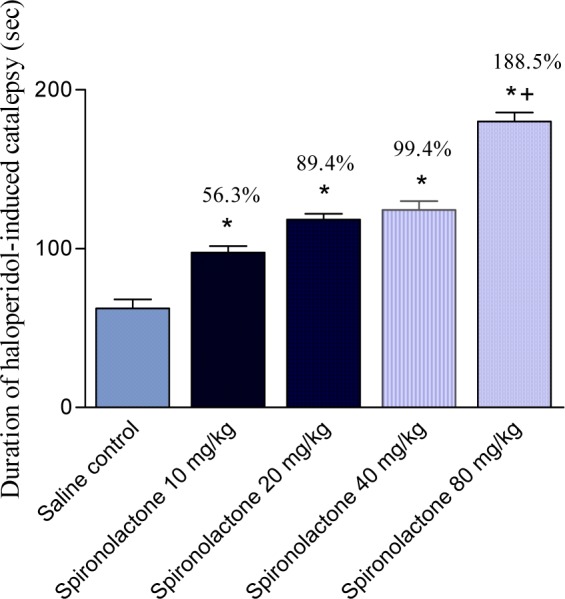
Effect of spironolactone on the duration of haloperidol-induced catalepsy
Haloperidol administered i.p. at a dose of 2 mg/kg produced a significant cataleptic response. The duration of haloperidol-induced catalepsy was significantly increased by 56.3, 89.4, 99.4 and 188.5 % by 10, 20, 40 and 80 mg/kg of spironolactone, respectively (Figure 8(Fig. 8)).
Figure 8. Effect of spironolactone on haloperidol-induced catalepsy in mice. Data represent mean values (± SE) of 6 mice per group and percent increase (%) compared to the control animals. Statistical differences vs. control group are indicated by asterisks.
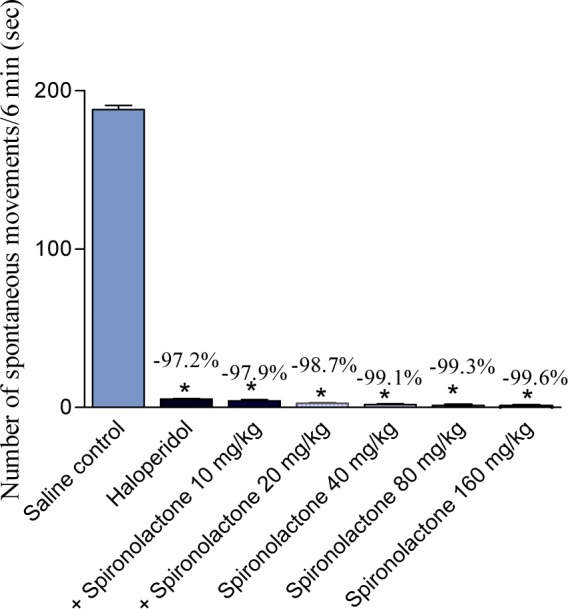
Effect of spironolactone on haloperidol-induced locomotor impairment
Spontaneous motor activity was markedly and significantly reduced in haloperidol-treated mice. Treatment with spironolactone resulted in further reduction in motor activity (Figure 9(Fig. 9)).
Figure 9. Effect of spironolactone on haloperidol-induced motor impairment in mice. Data represent mean values (± SE) of 6 mice per group and percent inhibition (%) compared to the control animals. Statistical differences vs. control group are indicated by asterisks.
Discussion
Results of the present study provide the evidence that the administration of spironolactone, a non-selective aldosterone antagonist, have differing effects on acute nociceptive pain. Thus in models of tail flick and hot plate in mice, which are measures of thermally-induced pain, the drug decreased nociceptive pain threshold. Spironolactone in addition decreased the antinociceptive effect of tramadol in the hot-plate test. Spironolactone also displayed pronociceptive effect on electrically-induced pain. In contrast, spironolactone displayed an antinociceptive effect upon the capsaicin-induced chemogenic pain. Spironolactone also inhibited the behavioral nociceptive effects evoked by injection of acetic acid into the peritoneal cavity of mice, a widely used model for visceral pain. The effect of the oral administration of the drug on visceral pain responses, however, was not dose-dependant.
The tail-flick response is considered to be a spinal reflex whereas the hotplate test involves supraspinal processing (Sinclair et al., 1988[38]). For the tail electric stimulation, the motor and simple vocalization responses have been suggested to be organized at the spinal cord and caudal brainstem levels, respectively (Hoffmeister, 1968[24]). Spironolactone thus is likely to act at spinal and supraspinal sites to modulate pain responses. The presence of mineralocorticoid receptors in some areas within the brain such as the hippocampus and within the nucleus of the solitary tract (Krozowski and Funder, 1983[27]; Geerling et al., 2006[16]) have been demonstrated. Mineralocorticoid effects in the brain include the control of cardiac vagal activity (Fletcher et al., 2004[13]), induction of salt appetite (Zhang et al., 1993[46]), sympathetic excitation and blood pressure elevation (Gomez-Sanchez, 1997[17]; Wang et al., 2003[45]; Zhang et al., 2006[47]). Studies suggest that aldosterone, originating in the periphery and acting on brain MR, activates brain MAPK pathways to produce sympatho-excitatory responses (Geerling et al., 2006[16]). In contrast, central MR blockade has been shown to reduce sympathetic drive (Francis et al., 2001[15]). Significant changes in adrenal and blood aldosterone levels were seen 2.5 min after acute nociception (Belyakova and Mendzheritskii, 2006[3]). Furthermore, in intact rats, the brain concentration of aldosterone reflected that in the plasma and aldosterone was consistently found in the brains of adrenalectomized rats, suggesting that the small amounts of aldosterone synthesized in the brain could provide a local ligand for autocrine or paracrine activation of the MR (Gomez-Sanchez et al., 2005[18]).
The effect of spironolactone on pain responses are not clear, but it has been shown that, aldosterone micropellets implanted in amygdala led to increased visceromotor behavioral responses to colorectal distention. More spinal neurons had spontaneous activity and exhibited low thresholds for excitability responses to colorectal distention and these responses were higher magnitude and longer duration. Thus MR-mediated mechanisms are likely to be involved in descending pathways into lumbosacral spinal neurons that induce colorectal hypersensitivity to luminal distention (Qin et al., 2003[35]). Aldosterone implants into the amygdala increased the number of abdominal muscle contractions in response to all levels of colorectal distension. MR mediated-mechanisms thus induce visceral hypersensitivity via processes originating in the amygdala (Myers and Greenwood-Van Meerveld, 2010[32]). Blockade of central MRs by spironolactone is therefore expected to reduce visceral pain responses. Spironolactone also possesses potent anti-inflammatory and immune modifying properties (Francis et al., 2003[14]; Hansen et al., 2004[20]; Sonder et al., 2006[39]) which could be relevant to its visceral pain inhibiting properties shown in the present study. The model of inflammatory pain evoked by acetic acid injection into the peritoneal cavity involves the local release of prostaglandins in the peritoneal cavity (Berkenkopf and Weichman, 1988[4]). It can be inhibited by the administration of cyclo-oxygenase inhibitors (Santos et al., 1998[37]), as well as by drugs acting on central monoaminergic neurotransmission, e. g., fluoxetine (Abdel-Salam, 2005[1]), or by morphine (Baamonde et al., 1989[2]). We further attempted to elucidate mechanism by which spironolactone alleviate visceral pain. The analgesic response to spironolactone was partially diminished by administration the alpha-2 receptor antagonist yohimbine, suggesting that alpha (2)-adrenoceptor-mediated mechanism could be involved. Spinal noradrenergic systems participate in pain-relieving mechanisms, and the alpha-2 receptor agonists clonidine and dexmetomidine are effective analgesics and potentiate the analgesic effects of morphine (Chen et al., 2009[7]; Gulati et al., 2009[19]). Spironolactone at doses, which caused effective visceral anti-nociception, did not impair mouse performance evaluated by the rotarod test, thus ruling out the confounding influence of a possible sedative effect. Significantly impaired rotarod performances occurred only with in contrast, higher doses of 40 and 80 mg/kg of the drug.
Catalepsy occurs following high dopamine D2 receptor blockade by the typical antipsychotic drug haloperidol. The haloperidol-induced catalepsy is a behavioral predictor of extrapyramidal symptoms liability (Farde et al., 1992[11]; Hoffman and Donovan, 1995[23]). In the present study, spironolactone increased the duration of haloperidol-induced catalepsy in a dose-related manner. Symptoms of Parkinson's disease are mainly related to a progressive degeneration of dopamine producing neurons in the substantia nigra pars compacta (SNc) and to a lesser extent in the ventral tegmental area (VTA), the most prominent dopamine nuclei in the brain (Torack and Morris, 1992[42]; Hirsch et al., 2003[22]). There is no evidence yet that spironolactone affects dopamine release. But, the nucleus of the solitary tract contains a subpopulation of neurons which are uniquely sensitive to aldosterone and send minor axonal projections to the midbrain VTA (Geerling et al., 2006[16]). The latter is a cluster of neurons in the midbrain tegmentum located just medial to the SNc, implicated mainly in attention, memory, reward and motivation (Nowak et al., 2000[33]; Howard et al., 2007; de Oliveira et al., 2009[9]), but is involved in some motor behaviors as well (Stinus et al., 1980[40]; Hull et al., 1991[25]; Trojniar and Klejbor, 1999[43]). This raises an intriguing question whether central aldosterone might influence the activity of dopaminergic neurons of this structure and thus is involved in the control of movements. Studies suggested that spironolactone act centrally to affect mood and behavior (Hendler, 1978[21]). Balanced activation of MRs and GRs is also necessary for optimal memory function in humans (Tytherleigh et al., 2004[44]) and spironolactone impairs cognitive function by significantly impairing selective attention and delayed recall of visuospatial memory (Otte et al., 2007[34]). Studies also indicated that increased neuronal MR has neuroprotective effects. Transgenic mice over expressing MR in forebrain had significantly reduced neuronal death, improved spatial memory retention, and reduced anxiety following transient cerebral global ischemia compared to wild-type littermates. This effect was independent on alterations in basal or post stress HPA axis function or in arterial blood pressure (Lai et al., 2007[28]). Consequently it might be hypothesized that blockade of central MR receptors could have adverse effects on neuronal integrity in vulnerable regions.
In conclusion, systemic injections of spironolactone increased pain behavior in models of thermal, chemogenic and electrically-induced pain, whereas visceral pain was reduced by the drug. The effect of spironolactone on motor symptoms in patients with Parkinson's disease or on antipsychotic drug therapy needs further investigation.
References
- 1.Abdel-Salam OME. anti-inflammatory, anti-nociceptive, and gastric effects of Hypericum Perforatum in rats. Scient World J. 2005;5:585–96. doi: 10.1100/tsw.2005.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baamonde A, Hidalgo A, Andres-Trelles F. Sex-related differences in the effects of morphine and stress on visceral pain. Neuropharmacology. 1989;28:967–70. doi: 10.1016/0028-3908(89)90197-4. [DOI] [PubMed] [Google Scholar]
- 3.Belyakova EI, Mendzheritskii AM. Adrenocortical and thyroid systems of rats during the initial period of nociceptive influences. Neurosci Behav Physiol. 2006;36:561–564. doi: 10.1007/s11055-006-0056-2. [DOI] [PubMed] [Google Scholar]
- 4.Berkenkopf JW, Weichman BM. Production of prostacyclin in mice following intraperitoneal injection of acetic acid, phenylbenzoquinone and zymosan: its role in the writhing response. Prostaglandins. 1988;36:693–709. doi: 10.1016/0090-6980(88)90014-7. [DOI] [PubMed] [Google Scholar]
- 5.Calvo N, Volosin M. Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology. 2001;73:261–271. doi: 10.1159/000054643. [DOI] [PubMed] [Google Scholar]
- 6.Carmina E. Anti-androgens for the treatment of hirsutism. Expert Opin Investig Drugs. 2002;11:357–363. doi: 10.1517/13543784.11.3.357. [DOI] [PubMed] [Google Scholar]
- 7.Chen BS, Peng H, Wu SN. Dexmedetomidine, an alpha2-adrenergic agonist, inhibits neuronal delayed-rectifier potassium current and sodium current. Br J Anaesth. 2009;103:244–254. doi: 10.1093/bja/aep107. [DOI] [PubMed] [Google Scholar]
- 8.Conn JW, Hinerman DL. Spironolactone-induced inhibition of aldosterone biosynthesis in primary aldosteronism: morphological and functional studies. Metabolism. 1977;26:1293–1307. doi: 10.1016/0026-0495(77)90026-9. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira AR, Reimer AE, Brandão ML. Role of dopamine receptors in the ventral tegmental area in conditioned fear. Behav Brain Res. 2009;199:271–277. doi: 10.1016/j.bbr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Dib N, Oberti F, Cales P. Current management of the complications of portal hypertension: variceal bleeding and ascites. Can Med Assoc J. 2006;174:1433–1443. doi: 10.1503/cmaj.051700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- 12.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher J, Buch AN, Routledge HC, Chowdhary S, Coote JH, Townend JN. Acute aldosterone antagonism improves cardiac vagal control in humans. J Am Coll Cardiol. 2004;43:1270–1275. doi: 10.1016/j.jacc.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 14.Francis J, Beltz T, Johnson AK, Felder RB. Mineralocorticoids act centrally to regulate blood-borne tumor necrosis factor-alpha in normal rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1402–R1409. doi: 10.1152/ajpregu.00027.2003. [DOI] [PubMed] [Google Scholar]
- 15.Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H2241–H2251. doi: 10.1152/ajpheart.2001.281.5.H2241. [DOI] [PubMed] [Google Scholar]
- 16.Geerling JC, Kawata M, Loewy AD. Aldosterone-sensitive neurons in the rat central nervous system. J Comp Neurol. 2006;494:515–527. doi: 10.1002/cne.20808. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Sanchez EP. Central hypertensive effects of aldosterone. Front Neuroendocrinol. 1997;18:440–62. doi: 10.1006/frne.1997.0157. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab. 2005;288:E342–E346. doi: 10.1152/ajpendo.00355.2004. [DOI] [PubMed] [Google Scholar]
- 19.Gulati A, Bhalla S, Matwyshyn G, Zhang Z, Andurkar SV. Determination of adrenergic and imidazoline receptor involvement in augmentation of morphine and oxycodone analgesia by clonidine and BMS182874. Pharmacology. 2009;83:45–58. doi: 10.1159/000178812. [DOI] [PubMed] [Google Scholar]
- 20.Hansen PR, Rieneck K, Bendtzen K. Spironolactone inhibits production of proinflammatory cytokines by human mononuclear cells. Immunol Lett. 2004;91:87–91. doi: 10.1016/j.imlet.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Hendler NH. Spironolactone prophylaxis in manic depressive disease. J Nerv Ment Dis. 1978;166:517–20. doi: 10.1097/00005053-197807000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch EC, Orieux G, Muriel MP, Francois C, Feger J. Nondopaminergic neurons in Parkinson's disease. Adv Neurol. 2003;91:29–37. [PubMed] [Google Scholar]
- 23.Hoffman DC, Donovan H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal side effect liability. Psychopharmacology. 1995;120:128–33. doi: 10.1007/BF02246184. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmeister F. Effects of psychotropic drugs on pain. In: Soulairac A, Cohn J, Charpentier J, editors. Pain. New York: Academic Press; 1968. p. 309–335. [Google Scholar]
- 25.Hull EM, Weber MS, Eaton RC, Dua R, Markowski VP, Lumley L, Moses J. Dopamine receptors in the ventral tegmental area affect motor, but not motivational or reflexive, components of copulation in male rats. Brain Res. 1991;554:72–76. doi: 10.1016/0006-8993(91)90173-s. [DOI] [PubMed] [Google Scholar]
- 26.Koster R, Anderson M, De Beer EJ. Acetic acid for analgesic screening. Fed Proc. 1959;18:412. [Google Scholar]
- 27.Krozowski ZS, Funder JW. Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc Natl Acad Sci U S A. 1983;80:6056–6060. doi: 10.1073/pnas.80.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai M, Horsburgh K, Bae SE, Carter RN, Stenvers DJ, Fowler JH, Yau JL, Gomez-Sanchez CE, Holmes MC, Kenyon CJ, Seckl JR, Macleod MR. Forebrain mineralocorticoid receptor overexpression enhances memory, reduces anxiety and attenuates neuronal loss in cerebral ischaemia. Eur J Neurosci. 2007;25:1832–1842. doi: 10.1111/j.1460-9568.2007.05427.x. [DOI] [PubMed] [Google Scholar]
- 29.Mantero F, Lucarelli G. Aldosterone antagonists in hypertension and heart failure. Ann Endocrinol (Paris) 2000;61:52–60. [PubMed] [Google Scholar]
- 30.Michael-Titus A, Costentin J. Analgesic effects of metapramine and evidence against the involvement of endogenous enkephalins in the analgesia induced by tricyclic antidepressants. Pain. 1987;31:391–400. doi: 10.1016/0304-3959(87)90167-9. [DOI] [PubMed] [Google Scholar]
- 31.Millan MJ, Bervoets K, Rivet JM, Widdowson P, Renouard A, Le Marouille-Girardon S, Gobert A. Multiple alpha2-adrenergic receptor subtypes: II. Evidence for a role of rat R and 2A-ARs in the control of nociception, motor behaviour and hippocampal synthesis of noradrenaline. J Pharmacol Exp Ther. 1994;270:958–972. [PubMed] [Google Scholar]
- 32.Myers B, Greenwood-Van Meerveld B. Divergent effects of amygdala glucocorticoid and mineralocorticoid receptors in the regulation of visceral and somatic pain. Am J Physiol Gastrointest Liver Physiol. 2010;298:G295–G303. doi: 10.1152/ajpgi.00298.2009. [DOI] [PubMed] [Google Scholar]
- 33.Nowak KL, McBride WJ, Lumeng L, Li TK, Murphy JM. Involvement of dopamine D2 autoreceptors in the ventral tegmental area on alcohol and saccharin intake of the alcohol-preferring P rat. Alcohol Clin Exp Res. 2000;24:476–483. [PubMed] [Google Scholar]
- 34.Otte C, Moritz S, Yassouridis A, Koop M, Madrischewski AM, Wiedemann K, Kellner M. Blockade of the mineralocorticoid receptor in healthy men: effects on experimentally induced panic symptoms, stress hormones, and cognition. Neuropsychopharmacology. 2007;32:232–238. doi: 10.1038/sj.npp.1301217. [DOI] [PubMed] [Google Scholar]
- 35.Qin C, Greenwood-Van Meerveld B, Foreman RD. Visceromotor and spinal neuronal responses to colorectal distension in rats with aldosterone onto the amygdala. J Neurophysiol. 2003;90:2–11. doi: 10.1152/jn.00023.2003. [DOI] [PubMed] [Google Scholar]
- 36.Sakurada T, Katsumata K, Tan-No K, Sakurada S, Kisara K. The capsaicin test in mice for evaluating tachykinin antagonists in the spinal cord. Neuropharmacology. 1992;31:1279–1285. doi: 10.1016/0028-3908(92)90057-v. [DOI] [PubMed] [Google Scholar]
- 37.Santos AR, Vedana EM, De-Freitas GA. Antinociceptive effect of meloxicam, in neurogenic and inflammatory nociceptive models in mice. Inflamm Res. 1998;47:302–307. doi: 10.1007/s000110050333. [DOI] [PubMed] [Google Scholar]
- 38.Sinclair JG, Main CD, Lo GF. Spinal vs. supraspinal actions of morphine on the rat tail-flick reflex. Pain. 1988;33:357–362. doi: 10.1016/0304-3959(88)90296-5. [DOI] [PubMed] [Google Scholar]
- 39.Sonder SU, Mikkelsen M, Rieneck K, Hedegaard CJ, Bendtzen K. Effects of spironolactone on human blood mononuclear cells: mineralocorticoid receptor independent effects on gene expression and late apoptosis induction. Br J Pharmacol. 2006;148:46–53. doi: 10.1038/sj.bjp.0706700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stinus L, Koob GF, Ling N, Bloom FE, Le Moal M. Locomotor activation induced by infusion of endorphins into the ventral tegmental area: evidence for opiate-dopamine interactions. Proc Natl Acad Sci U S A. 1980;77:2323–2327. doi: 10.1073/pnas.77.4.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang WH, Parameswaran AC, Maroo AP, Francis GS. Aldosterone receptor antagonists in the medical management of chronic heart failure. Mayo Clin Proc. 2005;80:1623–1630. doi: 10.4065/80.12.1623. [DOI] [PubMed] [Google Scholar]
- 42.Torack RMI, Morris JC. Tyrosine hydroxylase-like (TH) immunoreactivity in Parkinson's disease and Alzheimer's disease. J Neural Transm [P-D Sect] 1992;4:165–171. doi: 10.1007/BF02251479. [DOI] [PubMed] [Google Scholar]
- 43.Trojniar W, Klejbor I. Facilitatory effect of unilateral lesion of the ventral tegmental area on locomotor response to stimulation of the contralateral ventral tegmental area: involvement of GABAergic transmission. Brain Res. 1999;842:419–430. doi: 10.1016/s0006-8993(99)01865-x. [DOI] [PubMed] [Google Scholar]
- 44.Tytherleigh MY, Vedhara K, Lightman SL. Mineralocorticoid and glucocorticoid receptors and their differential effects on memory performance in people with Addison's disease. Psychoneuroendocrinology. 2004;29:712–723. doi: 10.1016/S0306-4530(03)00103-3. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Huang BS, Leenen FH. Brain sodium channels and ouabainlike compounds mediate central aldosterone-induced hypertension. Am J Physiol Heart Circ Physiol. 2003;285:H2516–H2523. doi: 10.1152/ajpheart.00299.2003. [DOI] [PubMed] [Google Scholar]
- 46.Zhang DM, Epstein AN, Schulkin J. Medial region of the amygdala: involvement in adrenal-steroid-induced salt appetite. Brain Res. 1993;600:20–6. doi: 10.1016/0006-8993(93)90396-5. [DOI] [PubMed] [Google Scholar]
- 47.Zhang ZH, Kang YM, Yu Y, Wei SG, Schmidt TJ, Johnson AK, Felder RB. 11beta-hydroxysteroid dehydrogenase type 2 activity in hypothalamic paraventricular nucleus modulates sympathetic excitation. Hypertension. 2006;48:127–133. doi: 10.1161/01.HYP.0000224296.96235.dd. [DOI] [PubMed] [Google Scholar]