Abstract
Respiratory syncytial virus (RSV) is the leading cause of acute respiratory infections in children, yet no vaccine is available. The sole licensed preventive treatment against RSV is composed of a monoclonal neutralizing antibody (palivizumab), which targets a conformational epitope located on the fusion protein (F). Palivizumab reduces the burden of bronchiolitis but does not prevent infection. Thus, the development of RSV vaccines remains a priority. We previously evaluated nanorings formed by RSV nucleoprotein (N) as an RSV vaccine, as well as an immunostimulatory carrier for heterologous antigens. Here, we linked the palivizumab-targeted epitope (called FsII) to N, to generate N-FsII-nanorings. Intranasal N-FsII immunization elicited anti-F antibodies in mice that were non-neutralizing in vitro. Nevertheless, RSV-challenged animals were better protected against virus replication than mice immunized with N-nanorings, especially in the upper airways. In conclusion, an N-FsII–focused vaccine is an attractive candidate combining N-specific cellular immunity and F-specific antibodies for protection.
Introduction
Respiratory syncytial virus (RSV) is the major cause of acute respiratory infections in the pediatric population.1 Bronchiolitis and pneumonia, the most severe clinical manifestations of RSV, affect 25 to 40% of infected children, leading to hospitalization in 0.5 to 2% of cases.2
There is still no marketed vaccine against RSV although many candidates are under clinical evaluation. In fact, the first vaccine trial in the 1960s using formalin-inactivated RSV had dramatic consequences. Indeed, this vaccinal formulation exacerbated clinical symptoms after infection, leading to the hospitalization of almost 80% of the participants.3
Currently, the sole licensed RSV preventive treatment is composed of a humanized mouse monoclonal antibody (mAb 1129) named palivizumab (Synagis®, Medimmune).4 This antibody can reduce the burden of severe bronchiolitis in at-risk populations,5 but requires several injections throughout the RSV season, is expensive and does not prevent viral replication in the upper airways.6 Thus, the development of an efficient RSV vaccine remains a priority for public health.
Palivizumab binds to a neutralizing epitope located in the antigenic site II of the RSV fusion glycoprotein (F).4,7–9 This epitope will be named FsII throughout this manuscript. FsII folds as a helix-loop-helix secondary structure that is conserved in the two F conformations found on the virion surface (metastable prefusion and stable postfusion states).10–13 Attempts to immunize mice against this epitope using a free peptide were unsuccessful, suggesting that the conservation of FsII antigenicity is dependent upon the preservation of its native conformation.14 However, Correia et al recently provided the evidence of the feasibility of FsII epitope-focused vaccines by using computationally designed protein scaffolds to stabilize FsII in its correct folding.15,16
RSV vaccines currently in clinical trials are designed to generate neutralizing antibodies against epitopes of the F protein that would be able to block infection at an early state.17 Additionally, a good correlation has been observed in human between the presence of naturally-acquired RSV-neutralizing antibodies and protection.18 However, in the context of a large scale vaccination campaign resulting in a high immune selection pressure, there would be a risk of virus escape from a vaccine targeting the sole F protein.
By contrast to F, nucleoprotein (N) is a major target of CD8 T-cell response and is the most conserved of RSV proteins with high structure constraints. Thus, we believe that a vaccine formulation combining N protein and F neutralizing epitopes would induce a broad spectrum and cross-protective immunity against divergent RSV strains and would limit the emergence of escape mutants. The importance of CD8 T-cell-mediated immunity is demonstrated in calves, a natural host for bovine RSV, in which in vivo depletion of CD8 T-cells lead to prolonged virus shedding and increased pathology.19 Moreover, appearance of RSV-specific CD8 T-cells correlates in calves as well as in infants with virus clearance and not with pneumonic lesions.20
We have documented the immunogenicity of a recombinant N which assembles as soluble nanorings of 15 nm diameter, composed of 10 to 11 N protomers bound to a bacterial RNA of 70 to 77 bases.21,22 In mice, intranasal immunization with N-nanorings induces antigen-specific CD4 and CD8 T-cell responses and confers protection against RSV challenge.23 In calves, N-nanorings alone or co-administered with RSV P and M2-1 proteins, provide strong cellular immunity and significant but partial protection against virus replication and clinical symptoms upon bovine RSV challenge.24,25 Importantly, we did not record signs of pulmonary disease exacerbation upon challenge of vaccinated mice or calves.23,24
Recently, we made the proof of concept that N-nanorings could be used as a vaccinal carrier for heterologous antigens such as the ectodomain of influenza M2 (M2e). Indeed, by contrast to M2e peptide alone, M2e-fused N-nanorings induce anti-M2e antibody responses and protect against influenza replication.26
Using N-nanorings to present FsII in an RSV vaccine strategy would have the advantage to combine a T-cell immunity (afforded by N) and a neutralizing antibody response (afforded by FsII), in the context of an immunostimulatory carrier (afforded by the repeated nanostructure organization).
Here, we designed FsII-fused chimeric N-nanorings (N-FsII), in which the sequence of the human RSV FsII epitope was used. The length of the FsII sequence has been slightly extended to place the epitope in a more favorable structural environment, while avoiding the presence of hydrophobic residues at the extremities that could impair solubility. Additionally, two sites of insertion in N protein have been selected: (i) N-terminus or (ii) a well-exposed loop visible on the 3D structure of nanorings.22 These two N-FsII constructs were able to form soluble nanorings that were efficiently recognized by anti-F antibodies and palivizumab. In mice, N-FsII nanorings induced anti-F antibody responses and protected animals against RSV replication in lungs. Importantly, the protection afforded by N-FsII was improved in upper airways (nasal turbinates) compared to N-nanorings alone, although neutralizing antibodies were not detected.
Methods
Plasmid construction, protein expression and purification
N-FsII (Nter) and N-FsII (G106) fusion proteins were engineered by inserting the FsII epitope sequence (STYMLTNSELLSLINDMPITNDQKKLMSNNVQIVRQQS) either at the N-terminus or between residues G106 and K107 of N protein, respectively. For N-FsII (Nter), the cDNA coding for FsII epitope (Long strain) was amplified by PCR using DreamTaq™ polymerase (Thermo Scientific) and cloned at NheI and BamH1 restriction sites in pET-N plasmid.27 For N-FsII (G106), a KpnIrestriction site was created by site-directed mutagenesis at nucleotides 316–321 (T107 K substitution) using the QuickChange® kit (Agilent), in which the amplified cDNA coding for FsII was inserted after the sequence coding for glycine 106 (G106). All sequences were validated by DNA sequencing.
Expression and purification of recombinant N-FsII (Nter) and N-FsII (G106) were performed as previously described.21 Briefly, the purification of N is permitted by its interaction with the C-terminus of RSV P protein (PCT) fused to GST. E.coli BL21 (DE3) bacteria were co-transformed with pGEX-PCT and pET-N-FsII (Nter or G106) and grown at 37 °C for 8 h in 1 L of LB + 100 μg/mL ampicillin and 50 μg/mL kanamycin. The same volume of LB was then added, and protein expression was induced with 80 μg/mL isopropyl-β-D-thiogalactopyranoside. Bacteria were incubated for a further 15 h at 28 °C and harvested. Complexes were purified from pellets on glutathione-Sepharose 4B beads (GE Healthcare) as previously described.27 To isolate N-nanorings, beads were incubated 16 h at 20 °C with biotinylated thrombin that was further removed using the cleavage capture kit (Millipore). Purified N-nanorings were concentrated on Vivaspin® (Sartorius) and sterilely filtered. A small amount of PCT was co-purified with N-nanorings, but we previously demonstrated that PCT is poorly immunogenic compared to the entire P protein.26 All protein sequences were controlled by mass spectrometry.
SDS-PAGE and native gel electrophoresis (NGE)
For SDS-PAGE, samples were prepared in Laemmli buffer, denatured 5 min at 95 °C, separated on 12.5% polyacrylamide gel (Criterion™ TGX™ any kD™, BioRad) and detected by Coomassie brilliant blue. ProSieve™ protein ladder (Lonza) was used. For NGE, samples were prepared in 50% saccharose, separated on 4% polyacrylamide gel in 0.2× TBE (pH 8.0) and stained with Coomassie brilliant blue.
Western blot assay
Proteins were run on a 12.5% SDS-PAGE and transferred to PVDF transfer Turbo® pack membrane using Trans-Blot® Turbo™ (BioRad). Membranes were blocked 1 h with PBS-0.2% Tween-5% skim milk, then incubated overnight at 4 °C with anti-N polyclonal serum,27 [1:1000] or each of two monoclonal antibodies against FsII (mAb11 [1:1000] or AbD Serotec, clone RSV3216 [1:200]) in PBS-0.2% Tween-5% skim milk. Membranes were washed with PBS-0.25% Tween and incubated 1 h at room temperature with peroxidase conjugated secondary antibody (p.a.r.i.s biotech). Proteins were visualized by chemiluminescence using SuperSignal™ West Pico substrate (Thermo).
Size measurements by dynamic light scattering (DLS)
The size measurements with the Zetasizer Nano (Malvern), based on the principle of dynamic light scattering, was performed at 20 °C using a helium-neon laser wavelength of 633 nm and detection angle of 173°. The results were presented as size distribution.
Mice: Immunization, challenge and sample collection
Female BALB/c mice aged 6–8 weeks were purchased from Janvier (France) and housed under Bio-Safety Level (BSL)-2 (IERP, INRA, Jouy-en-Josas, France). All experiments were approved by the local ethics committee COMETHEA (authorizations 12–125 and 12–126).
Mice were anesthetized with ketamine and xylazine (50 and 10 mg/kg, respectively) and immunized intranasally, twice at 2 weeks interval, with 50 μL of 0.9% NaCl containing 2 μg of N-nanorings, stabilized postfusion F (F) [provided by Jason S McLellan and produced as previously described],11 N-FsII (Nter) or N-FsII (G106). All immunogens were adjuvanted with 5% Montanide™ Gel 01 (Seppic).26,28 Two weeks after the boost, mice were either autopsied or anesthetized and inoculated intranasally with 8.8 × 104 PFU of RSV-Luciferase.29 Body weight and clinical score were monitored daily. We implemented a clinical score scale (0: no symptoms; 1: slightly ruffled fur; 2: ruffled fur but active; 3: inactive).
Blood samples were collected 14 days after the prime or the boost immunizations. Broncho-alveolar lavages were performed by flushing lungs with PBS-1 mM EDTA.
Evaluation of antibody responses by ELISA
Sera and BAL were assayed for N- or F-specific antibodies by indirect ELISA using N-nanorings or postfusion F as coating (200 ng per well on microplates), as previously described.23 End-point antibody titers were calculated by regression analysis, plotting serum dilution versus absorbance at 450 nm using Microcal Origin (OriginLab) (regression curve y = (b + cx)/(1 + ax)). Titers were defined as the highest dilution resulting in an absorbance value twice that of blank points.
Binding of mice anti-N and anti-F antibodies to infected cells
Subconfluent monolayer of HEp-2 cells was infected with RSV strain Long at a multiplicity of infection of 0.1. Cells were collected at 12 h post-infection in Laemmli buffer. Then, lysates were analyzed by Western blotting using sera collected from mice immunized twice with N, N-FsII (G106) or postfusion F at a dilution of 1:100.
RSV microneutralization assay
Serial dilutions of heat-inactivated (56 °C, 30 min) serum samples were mixed with 600 PFU’s of RSV-Cherry,29 in 100 μL of Eagle’s Minimum Essential with 2 mM L-glutamine, 100 IU/mL Penicillin and 100 μg/mL Streptomycin. Mixtures were incubated 1 h at 37 °C and added to a subconfluent monolayer of HEp-2 cells on microplates. Cherry fluorescence was measured at 620 nm, 60 h post-infection using an Infinite® 200 pro Microplates reader (Tecan).
Determination of RSV viral load in mice
Viral loads were measured in living mice via photon emission that was representative of RSV-Luciferase replication, as previously described.29 Briefly, mice were anesthetized with ketamine and xylazine and 0.75 mg/kg of D-luciferine (Sigma-Aldrich) was instilled intranasally. Replication was estimated using an “In Vivo Imaging System” (IVIS-200) and Live Imaging software (Xenogen), by measuring the photon emission from regions of interest (Figure S1).
Statistical data analysis
Data were expressed as arithmetic mean ± SEM. Non-parametric unpaired t test (two groups) or one-way ANOVA Tukey’s multiple comparison test (>2 groups) were used to compare unpaired values (GraphPad Prism®). The level of significance is indicated on graphs: *P < 0.05; **P < 0.01; ***P < 0.001.
Results
Design and characterization of F epitope-fused N-nanorings (N-FsII)
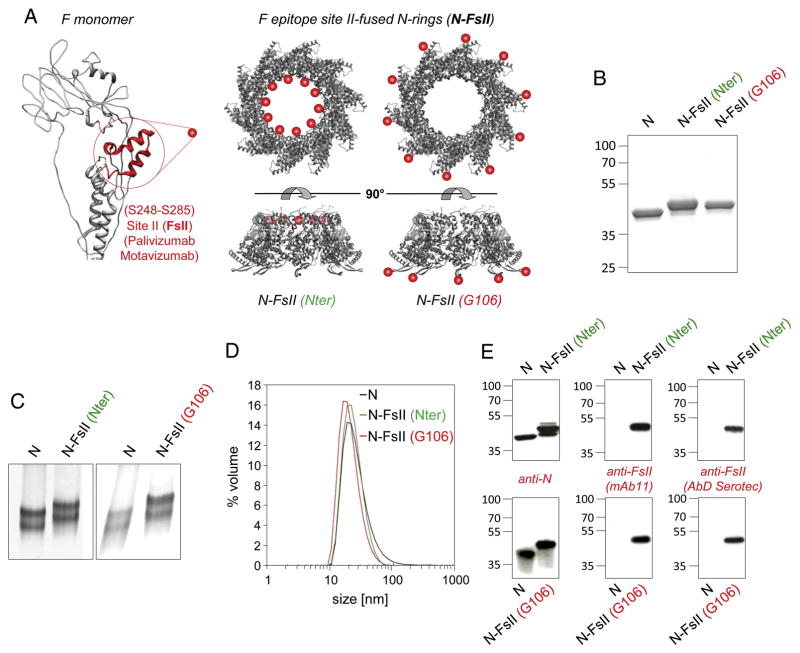
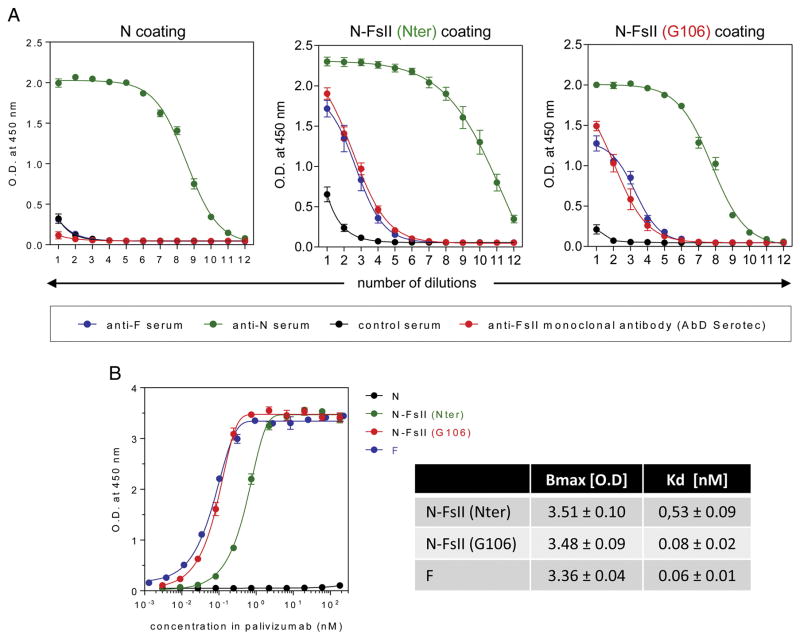
FsII sequence (amino-acids S248 to S285) was fused to RSV N. Two anchoring sites were investigated: the N-terminus (Nter) and an exposed loop (position G106-K107) (Figure 1, A). The insertion between G106 and K107, which form the loop at the top of the β-hairpin projecting away from the N-terminal domain of N, was designed to stabilize FsII and to maintain its antigenic conformation. The two N-FsII proteins [(Nter) and (G106)] were analyzed by SDS-PAGE. A single band migrating at the expected size was obtained for both constructs (Figure 1, B). The capacity of N-FsII (Nter) and N-FsII (G106) to assemble as nanorings was assessed by Native Gel Electrophoresis (NGE) and Dynamic Light Scattering (DLS). NGE revealed two bands corresponding to nanorings made of 10 and 11 protomers as previously shown for epitope-free N-nanorings (Figure 1, C).21,22 DLS plot showed one major peak for both N-FsII-nanorings with a mean hydrodynamic diameter of 15 nm, in agreement with the diameter of N-nanorings (Figure 1, D). Analysis of the protein content of N-FsII-nanorings was carried out by Western blot using anti-N polyclonal antibody or FsII specific monoclonal antibodies (Figure 1, E), validating the presence of FsII epitopes. Finally, the presence of FsII epitopes on chimeric N-nanorings was confirmed by indirect ELISA using N, N-FsII (Nter) or N-FsII (G106) as coating and either pooled sera collected from mice immunized with postfusion F (F), FsII-specific monoclonal antibody (Figure 2, A) or palivizumab (Figure 2, B). Interestingly, palivizumab presented an increased apparent affinity for N-FsII (G106) as compared to N-FsII (Nter) (Kd of 0.08 ± 0.02 nM and 0.53 ± 0.09 nM, respectively). Moreover, this affinity was similar to that measured for postfusion F (Kd of 0.06 ± 0.02 nM) (Figure 2, B). Overall, these results indicate that the fusion of FsII to N protein at Nter or G106 positions allowed the formation of N-FsII-nanorings that are well recognized by FsII-specific antibodies.
Figure 1. Design and purification of F epitope-fused N-nanorings (N-FsII).
F antigenic site II (FsII) was fused to the N-terminus (Nter) or at the top of a β-hairpin between G106 and K107 residues (G106) of N. (A) Schematic representation of the two N-FsII constructs, based on the 3D structure of N-nanorings. FsII is highlighted in red on the structure of a postfusion RSV F monomer (adapted from Swanson et al)10 and is represented by a red sphere on N-nanorings. N-FsII proteins were analyzed by SDS-PAGE (B) or Native Gel Electrophoresis (NGE) (C), in comparison with FsII-free N-nanorings (N). (D) The hydrodynamic radius of the two purified N-FsII was estimated by Dynamic Light Scattering (DLS). DLS plot is representative of 2 independent measures. (E) Western blot assay using anti-N polyclonal antibodies or FsII-specific monoclonal antibodies (mAb11 and AbD Serotec). The relative molecular mass (kDa) is indicated on the left.
Figure 2. Reactivity of N-FsII-nanorings with specific antibodies.
(A) The reactivity of N-FsII-nanorings or N-nanorings (N) with specific antibodies was evaluated by ELISA using sera collected from postfusion F (F) immunized mice (anti-F serum), N-nanorings immunized mice (anti-N serum) or naive mice (control serum), or FsII-specific monoclonal antibody (AbD Serotec). Data are mean ± SEM of triplicate to septuplicate values (two experiments). Nonlinear regression was done using Boltzmann sigmoidal equation. (B) Reactivity of N-FsII-nanorings or F with palivizumab was evaluated by ELISA. Data are mean ± SEM of triplicate to quadruplicate values. Non-linear regression was done using Boltzmann sigmoidal equation. Dissociation constants (Kd) were calculated from saturation curves obtained after baseline correction (N curve) and non-linear regression.
N-FsII-nanorings induced anti-N and anti-F antibodies in mice
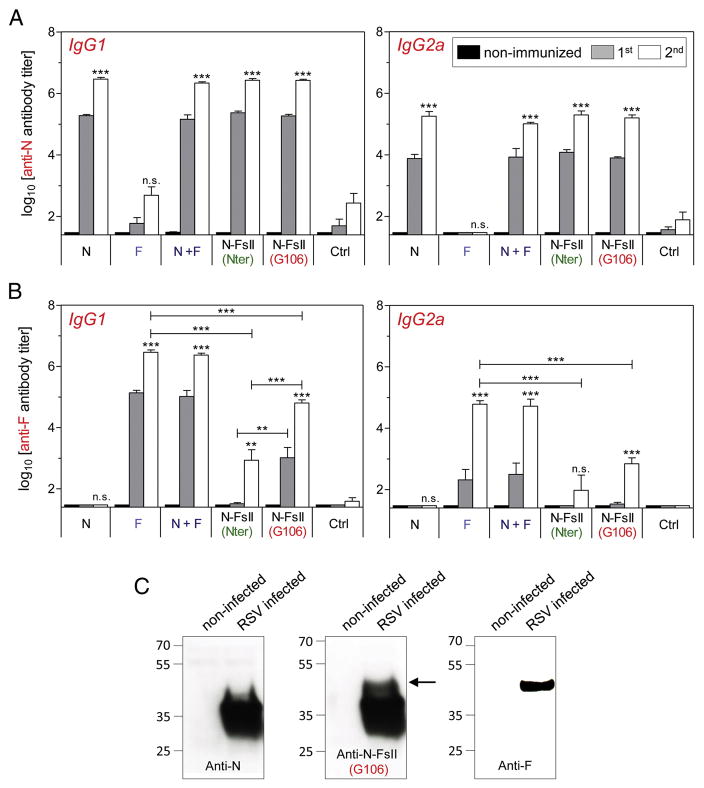
Mice were immunized intranasally with postfusion F (F), N-nanorings associated with postfusion F (N + F), N-FsII-nanorings or N-nanorings alone (N). Mice immunized with F elicited high anti-F antibody titers after two immunizations (6.5 ± 0.2 log10 for IgG1 and 4.8 ± 0.3 log10 for IgG2a) (Figure 3, A and B). Additionally, mice immunized with N + F elicited high anti-N and anti-F antibody titers (6.3 ± 0.1 log10 for anti-N IgG1, 5.0 ± 0.1 log10 for anti-N IgG2a, 6.4 ± 0.1 log10 for anti-F IgG1 and 4.7 ± 0.5 log10 for anti-F IgG2a). On the other hand, N-FsII induced high level of anti-N antibody titers after two immunizations (N-FsII (Nter): 6.4 ± 0.1 log10 for IgG1 and 5.3 ± 0.3 log10 for IgG2a; N-FsII (G106): 6.4 ± 0.1 log10 for IgG1 and 5.2 ± 0.2 log10 for IgG2a) and significant levels of anti-F antibody titers albeit at lower levels than after immunizations with F (P < 0.001). Interestingly, the anti-F antibody response was significantly stronger in N-FsII (G106)-immunized mice than in N-FsII (Nter)-immunized mice (4.8 ± 0.2 log10 for IgG1 versus 2.9 ± 0.8 log10 for IgG1 after two immunizations, respectively, P < 0.001). Again, this suggests that the insertion of FsII between residues G106 and K107 of N provided a better maintenance of its antigenic conformation (Figure 3, B). Analysis of RSV-infected cell lysates by Western blotting, using pooled sera collected from mice immunized with N-FsII (G106), showed a band migrating with an apparent molecular mass of 48 kDa corresponding to the F1 subunit of the F protein (Figure 3, C). This indicates that antibodies generated in mice against N-FsII (G106) recognize the F protein expressed in RSV-infected cells.
Figure 3. Measure of serum antibody responses.
BALB/c mice were immunized intranasally twice at two weeks interval with 2 μg of FsII-free N-nanorings (N) or postfusion F (F), 2 μg of N associated to 2 μg of F (N + F) or 2 μg of N-FsII (Nter or G106), adjuvanted with 5% Montanide™ Gel. As a control, mice received adjuvant alone (Ctrl). Sera were collected before (non-immunized) and two weeks after the prime (1st) or the boost (2nd) immunizations. N (A) and F (B) specific antibody titers were determined by ELISA. Data are mean + SEM of individual titers (n = 5–6). P values were determined according to the Student’s t test (**P < 0.01; ***P < 0.001; n.s., non-significant). The significance measured between vaccinated groups was indicated above horizontal lines. The significance measured between each vaccinated groups and the negative control (Ctrl) group after the boost immunization was indicated above each histogram bar. (C) HEp-2 cells were infected with RSV strain Long. Cell lysates were analyzed by Western blotting using pooled sera collected from N-, N-FsII- or postfusion F-immunized mice. The relative molecular mass (kDa) is indicated on the left. The band corresponding to F1 is indicated by an arrow.
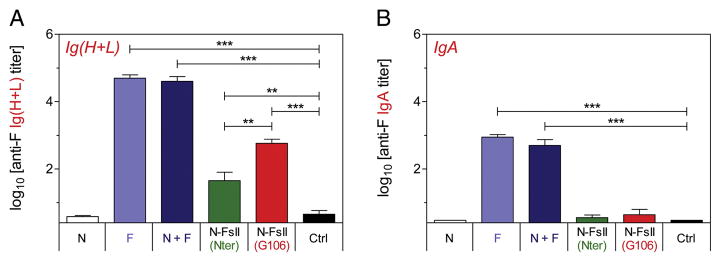
In broncho-alveolar lavages, the highest titers of anti-F Ig(H + L) and IgA were found in mice immunized intranasally with F (4.7 ± 0.2 log10 and 4.6 ± 0.3 log10, respectively) and N + F (2.9 ± 0.2 log10 and 2.7 ± 0.4 log10, respectively) (Figure 4, A and B). Immunization with N-FsII-nanorings gave rise to significant levels of anti-F Ig (H + L) (N-FsII (Nter): 1.7 ± 0.6 log10; N-FsII (G106): 2.8 ± 0.6 log10) but to low levels of IgA (N-FsII (Nter): 0.6 ± 0.2 log10; N-FsII (G106): 0.6 ± 0.4 log10). Interestingly, in accordance with antibody responses measured in serum, anti-F antibody titers were significantly higher in N-FsII (G106) group as compared to N-FsII (Nter) group (P < 0.01 for Ig(H + L)). Overall, these results indicate that N-FsII was immunogenic in mice and induced both systemic and local anti-N and anti-F antibody responses. Moreover, N-FsII (G106) presented a significant advantage for the induction of anti-F antibody response as compared to N-FsII (Nter).
Figure 4. Measure of mucosal antibody responses.
Mice were immunized as described in Figure 3. Broncho-alveolar lavages were collected two weeks after the boost immunization. F-specific Ig (H + L) (A) and IgA (B) titers were determined by ELISA. Data are mean + SEM of individual titers (n = 5–6). P values were determined according to the Student’s t test (**P < 0.01; ***P < 0.001).
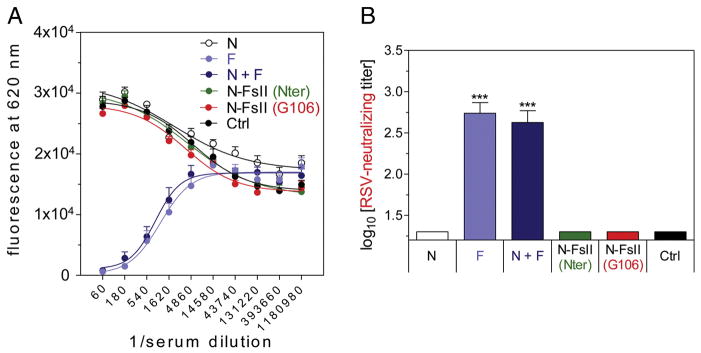
For RSV-neutralization assay, we used a recombinant RSV expressing the fluorescent protein mCherry (RSV-Cherry) (Figure 5, A and B).29 We validated in the present study that our miniaturized RSV neutralization assay was robust and reproducible. As expected, F and N + F immunizations induced strong neutralization titers (2.7 ± 0.3 and 2.6 ± 0.3 log10, respectively). However, N-FsII immunization did not induce detectable neutralization titers (<1.3 log10).
Figure 5. Measure of RSV-neutralizing responses.
Mice were immunized as described in Figure 3. RSV-neutralizing antibody titers were determined from sera using RSV virus expressing Cherry fluorescent protein. (A) Fluorescence curves at 620 nm. Data are mean + SEM of individual values (n = 5–6). Non-linear regression was done using Boltzmann sigmoidal equation. (B) Neutralizing titer was defined as the reverse dilution of serum inhibiting 50% of fluorescence at 620 nm. Data are mean + SEM of individual titers (n = 5–6). P values were determined according to the Student’s t test (***P < 0.001).
Immunization with N-FsII-nanorings protected mice against RSV challenge
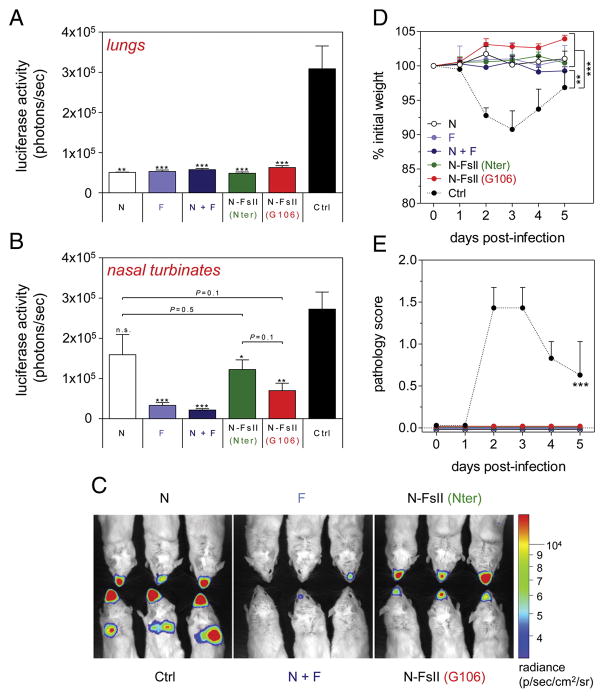
Mice were challenged two weeks after the boost immunization with a luciferase-expressing recombinant RSV (RSV-Luciferase).29 This recombinant virus allowed us to monitor viral replication independently and simultaneously in lower and upper airways of living mice (Figure S1). Viral replication was monitored at the peak of replication (day 4 post-infection). All vaccinated mice were totally protected against viral replication in lungs (Figure 6, A and C). However, in nasal turbinates, the best protection was afforded by F and N + F immunizations whereas N immunization failed to significantly reduce viral replication (P = 0.1) (Figure 6, B and C). Interestingly, N-FsII (Nter) and especially N-FsII (G106) immunizations significantly reduced viral replication in upper airways as compared to control group (P = 0.01 and P = 0.001, respectively) (Figure 6, B and C). Of note, despite a marked trend in the favor of N-FsII (G106) for protection, no significant differences were observed between the N group and the two N-FsII groups. In agreement with the absence of RSV replication in lungs, all vaccinated mice were protected against weight loss and clinical symptoms, as opposed to the significant weight loss (9% of initial weight at day 3 post-infection, P < 0.01) and clinical symptoms (P < 0.001) recorded in the control group (Figure 6, D and E).
Figure 6. Evaluation of the protective efficacy of N-FsII-nanorings.
Mice were immunized as described in Figure 3. Two weeks after the boost immunization, mice were challenged with RSV-Luciferase. Viral replication in lungs (A) and nasal turbinates (B) was evaluated at day 4 post-infection by measuring luminescence as described in Figure S1. Data are mean + SEM of individual values (n = 5–6). The significance observed between the control group (Ctrl) and each other groups was evaluated according to the Student’s t test (*P < 0.05; **P < 0.01; ***P < 0.001; n.s., non-significant). (C) Snapshot of 3 representative mice of each group. (D) Body weight and (E) clinical symptoms. Data are mean + SEM of individual values (n = 5–6). The significance observed between the control group (Ctrl) and each other groups was evaluated according to the one-way ANOVA Tukey’s multiple comparison test (**P < 0.01; ***P < 0.001).
Overall, these results indicate that N-FsII immunization gave rise to a significant protection against RSV challenge in mice.
Discussion
The main objective of this work was to evaluate the potency of nanoparticles generated by RSV N (N-nanorings) as a vaccinal carrier to present a conformational neutralizing epitope of RSV F.
Our first goal was to display an epitope of the human RSV F antigenic site II (FsII, target of palivizumab) on N-nanorings, in a way to maintain its antigenic tertiary structure. In a previous attempt to design chimeric N-nanorings fused to bovine FsII, we failed to generate anti-F antibodies in calves, strongly suggesting that bovine FsII was not correctly exposed.25 In the present study, we fused an extended human FsII either at the N-terminus (Nter) or on the top of the β-hairpin located between G106 and K107 residues (G106) of N. Indeed, the two FsII-fused N protein constructs (N-FsII) were able to form soluble nanorings. These N-FsII-nanorings were efficiently recognized by either monoclonal antibodies specific for FsII, sera of F-immunized mice or palivizumab. Notably, the apparent affinity of palivizumab for N-FsII (G106) was significantly increased in comparison to N-FsII (Nter) and as good as for postfusion F. This suggests that FsII antigenic structure would be better preserved when it is inserted in the G106-K107 β-hairpin.
Our second goal was to evaluate the capacity of N-FsII-nanorings to induce anti-F antibody responses in mice, and investigate if they provide protection upon RSV challenge. For all of these aspects, we systematically compared N-FsII to postfusion F (F) and to epitope-free N-nanorings (N) (separately or in combination). We chose the intranasal route for immunizing mice with N-FsII. Indeed, it was the best method for N-FsII vaccination, since neither of the other immunization routes that we have tested induced significant anti-F antibodies (data not shown). This result is consistent with our previous studies using N-nanorings as a vaccine or as a vaccinal carrier for influenza M2e which show that intranasal is the best route of immunization for conferring protection against respiratory virus challenge (comparison made with subcutaneous or intradermal routes).26 This choice was further supported by a recent study published by Pierantoni et al, showing that, when administered intranasally, a vaccinal adenovirus encoding F, N and M2-1 is more efficient in mice at inducing F-specific antibodies and at protecting animals upon challenge.30
Remarkably, using RSV-Luciferase for challenge, we noticed significant differences for viral replication in the upper airways between the two groups of F- or N-immunized mice. Replication remained particularly low in nasal turbinates of F-immunized mice, suggesting that intranasal immunization with F induced local neutralizing effectors capable of blocking viral entry at the delivery site. By contrast, N-induced immunity is permissive with viral replication occurring in upper airways. Indeed, N essentially induces cellular effectors located in lungs.23,24 Nevertheless, these two groups were totally protected from clinical symptoms, suggesting that the inhibition of RSV replication in lungs is sufficient to protect animals from pathology. Our results also suggest that N-induced immunity would not prevent viral transmission between individuals since infectious virus is still produced in the nose. Of note, the mix of N-nanorings and postfusion F proteins in the same formulation did not provide any additional benefits for protection. This was consistent with our first observation that postfusion F protein alone induced a sterilizing immunity. Yet, the fusion of FsII neutralizing epitope on N-nanorings would remain a competitive strategy in terms of cost and manufacturability, since soluble F protein that has to be produced in mammalian cells is more difficult and more expensive to obtain than N-nanorings carrying epitopes produced in bacteria. However, in order to reinforce the competitiveness of N-FsII, optimizations are needed to obtain a better viral protection in upper airways that would be, at least, as good as with entire F.
N-FsII-immunized mice developed significant anti-F antibody responses. Interestingly, N-FsII (G106) was significantly better than N-FsII (Nter) at inducing F-specific antibodies. This observation may be related to the results showing a better recognition of N-FsII (G106) by palivizumab and suggests a better 3D conformation of FsII when inserted within the G106 loop and therefore anchored by its two extremities. Of note, FsII epitope should also be more exposed in the G106 construct (Figure 1, A), resulting in an increased accessibility for antibodies. To go further, we are currently investigating the possibility to determine the 3D structure of N-FsII (G106). This result could potentially extend the application of N-nanorings as a platform for the presentation of various conformational epitopes requiring a specific set up to maintain their antigenicity.
N-FsII-immunized mice were efficiently protected against RSV replication, both in lungs and in nasal turbinates, without any signs of disease. Of note, the viral protection observed in lung for N-FsII-vaccinated groups may be largely mediated by N-specific immunity, since N-vaccinated group was totally protected from viral infection at this site of replication. However, in contrast to what was observed with N alone, immunization with N-FsII provided significant viral protection in the upper airways, suggesting that FsII-specific immune effectors were involved in protection. A potential role of FsII-targeting neutralizing antibodies is unlikely since RSV-neutralizing antibodies were undetectable in the sera of N-FsII vaccinated mice. In agreement with this result, other studies highlighted the difficulty to induce RSV-neutralizing antibodies in rodents with FsII-focused vaccination strategies. In one of them, stable protein scaffolds were designed to mimic the 3D structure of RSV FsII.16 This scaffold immunogen induced poor anti-F antibody response and failed to induce RSV-neutralizing antibodies in mice. However, RSV-neutralizing antibodies were found in macaque but only in 7 out of 16 animals after 3 immunizations and in 12 out of 16 animals after 5 immunizations.16 More recently, Schickli et al developed woodchuck hepadnavirus core-based VLPs containing 240 copies of F254-277 epitope.31 Some of these hybrid VLPs were recognized by palivizumab and elicited neutralizing antibodies in mice. However, authors pointed to individual variability for RSV-neutralizing responses, suggesting the presence of functional “holes” in the mouse B cell repertoire. This hypothesis is further supported by our recent results showing that N-FsII (G106) is able to induce low but significant RSV-neutralizing antibodies in piglets upon intramuscular immunization (Hervé et al, unpublished data). Alternatively, anti-F antibodies induced by N-FsII vaccination could contribute to reduce viral replication in vivo by other mechanisms than neutralization. Indeed, antibodies targeting viral proteins expressed at the surface of infected cells can hamper viral spread in vivo by complement activation (CDC) and/or antibody-dependent cellular cytotoxicity (ADCC). This was demonstrated for gp120-targeting anti-HIV non-neutralizing antibodies,32,33 and for M2e-targeting anti-influenza antibodies.34,35 Our results showing a binding of anti-F antibodies generated in mice after N-FsII (G106) immunization to RSV infected cells would support this hypothesis. Very recently, we obtained preliminary data showing that the passive transfer of immune sera issued from N-FsII (G106)-immunized mice gave a modest but significant virological protection in the nasal turbinates of recipient mice (Figure S2). Since we had demonstrated before that passive transfer of sera collected from mice immunized with N-nanorings alone is not protective,23 these results suggest that non-neutralizing anti-FsII antibodies could be involved in upper airways protection. Nevertheless, these results need to be completed to better understand the role of non-neutralizing anti-FsII antibodies in protection.
We are currently considering several approaches to optimize the induction of RSV-neutralizing antibodies after N-FsII (G106) immunization. We are also investigating the possibility to fuse F epitope Ø to N-nanorings.12,36 This epitope specific to prefusion F is the main target of RSV-neutralizing antibodies in human. Moreover, antibodies targeting epitope Ø were significantly more potent than palivizumab to bind to prefusion F.36 However it may be technically more challenging since this epitope is non-linear (spread over distal segments of the F protein).12
To conclude, we demonstrated the feasibility and interest of F epitope-focused vaccination using N-FsII-nanorings. Interestingly, we highlighted the potency of RSV-Luciferase challenge model to discriminate between viral replication in lungs and nasal turbinates. The different ability of N- and F-based vaccine candidates to provide protection in the upper airways points to the importance of assessing viral load at this site of replication, especially if the vaccine aims at reducing RSV transmission. Moreover, our study points to the importance of elucidating more in depths the mechanisms of protection mediated by anti-F antibodies in upper airways.
Supplementary Material
Acknowledgments
We thank Jérôme Pottier, Mathilde Beauducel, Marlène Héry and Charline Pontlevoy from the Fish and Rodent Experimental Infectiology unit (IERP, INRA, Jouy-en-Josas, France), for their support in the animal facilities. We also thank Marie Galloux and Christophe Chevalier for their important support and for providing pET-N and pGEX-PCT plasmids. We thank the MIMA2 platform for access to the IVIS-200, which was financed by the Ile de France region (SESAME). We thank Geraldine Taylor from the Pirbright Institute for providing mAb11 antibody. We thank Juliette Ben Arous from Air Liquide for providing Montanide™ adjuvant. We also thank Isabelle Schwartz-Cornil for the final read-through of the manuscript and for helpful discussions.
References
- 1.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNamara PS, Smyth RL. The pathogenesis of respiratory syncytial virus disease in childhood. Br Med Bull. 2002;61:13–28. doi: 10.1093/bmb/61.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Zea Hernandez J, Batalle JP, et al. Lack of antibody affinity maturation due to poor toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beeler JA, van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol. 1989;63:2941–50. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wegzyn C, Toh LK, Notario G, Biguenet S, Unnebrink K, Park C, et al. Safety and effectiveness of palivizumab in children at high risk of serious disease due to respiratory syncytial virus infection: a systematic review. Infect Dis Ther. 2014:133–58. doi: 10.1007/s40121-014-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–7. [PubMed] [Google Scholar]
- 7.Taylor G, Stott EJ, Furze J, Ford J, Sopp P. Protective epitopes on the fusion protein of respiratory syncytial virus recognized by murine and bovine monoclonal antibodies. J Gen Virol. 1992;73(Pt 9):2217–23. doi: 10.1099/0022-1317-73-9-2217. [DOI] [PubMed] [Google Scholar]
- 8.Arbiza J, Taylor G, Lopez JA, Furze J, Wyld S, Whyte P, et al. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial. J Gen Virol. 1992;73:2225–34. doi: 10.1099/0022-1317-73-9-2225. [DOI] [PubMed] [Google Scholar]
- 9.Magro M, Andreu D, Gómez-Puertas P, Melero JA, Palomo C. Neutralization of human respiratory syncytial virus infectivity by antibodies and low-molecular-weight compounds targeted against the fusion glycoprotein. J Virol. 2010;84:7970–82. doi: 10.1128/JVI.00447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, et al. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A. 2011;108:9619–24. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLellan JS, Yang Y, Graham BS, Kwong PD. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol. 2011;85:7788–96. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLellan JS. Neutralizing epitopes on the respiratory syncytial virus fusion glycoprotein. Curr Opin Virol. 2015;11:70–5. doi: 10.1016/j.coviro.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLellan JS, Chen M, Kim A, Yang Y, Graham BS, Kwong PD. Structural basis of respiratory syncytial virus neutralization by motavizumab. Nat Struct Mol Biol. 2010;17:248–50. doi: 10.1038/nsmb.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez JA, Andreu LD, Carreno C, Whyte P, Taylor G, Melero JA. Conformational constraints of conserved neutralizing epitopes from a major antigenic area of human respiratory syncytial virus fusion glycoprotein. J Gen Virol. 1993;74:2567–77. doi: 10.1099/0022-1317-74-12-2567. [DOI] [PubMed] [Google Scholar]
- 15.McLellan JS, Correia BE, Chen M, Yang Y, Graham BS, Schief WR, et al. Design and characterization of epitope-scaffold immunogens that present the motavizumab epitope from respiratory syncytial virus. J Mol Biol. 2011;409:853–66. doi: 10.1016/j.jmb.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, et al. Proof of principle for epitope-focused vaccine design. Nature. 2014;507:201–6. doi: 10.1038/nature12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins D, Trujillo C, Keech C. Advances in RSV vaccine research and development – a global agenda. Vaccine. 2016;34:2870–5. doi: 10.1016/j.vaccine.2016.03.109. [DOI] [PubMed] [Google Scholar]
- 18.Piedra P, Jewell A, Cron S, Atmar R, Glezen W. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine. 2003;21:3479–82. doi: 10.1016/s0264-410x(03)00355-4. [DOI] [PubMed] [Google Scholar]
- 19.Taylor G, Thomas LH, Wyld SG, Furze J, Sopp P, Howard CJ. Role of T-lymphocyte subsets in recovery from respiratory syncytial virus infection in calves. J Virol. 1995;69:6658–64. doi: 10.1128/jvi.69.11.6658-6664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzman E, Taylor G. Immunology of bovine respiratory syncytial virus in calves. Mol Immunol. 2015;66:48–56. doi: 10.1016/j.molimm.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Tran T-L, Castagné N, Bhella D, Varela PF, Bernard J, Chilmonczyk S, et al. The nine C-terminal amino acids of the respiratory syncytial virus protein P are necessary and sufficient for binding to ribonucleoprotein complexes in which six ribonucleotides are contacted per N protein protomer. J Gen Virol. 2007;88:196–206. doi: 10.1099/vir.0.82282-0. [DOI] [PubMed] [Google Scholar]
- 22.Tawar RG, Duquerroy S, Vonrhein C, Varela PF, Damier-Piolle L, Castagné N, et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science. 2009;326:1279–83. doi: 10.1126/science.1177634. [DOI] [PubMed] [Google Scholar]
- 23.Roux X, Dubuquoy C, Durand G, Tran-Tolla T-L, Castagné N, Bernard J, et al. Sub-nucleocapsid nanoparticles: a nasal vaccine against respiratory syncytial virus. PLoS One. 2008;3:e1766. doi: 10.1371/journal.pone.0001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riffault S, Meyer G, Deplanche M, Dubuquoy C, Durand G, Soulestin M, et al. A new subunit vaccine based on nucleoprotein nanoparticles confers partial clinical and virological protection in calves against bovine respiratory syncytial virus. Vaccine. 2010;28:3722–34. doi: 10.1016/j.vaccine.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blodörn K, Hägglund S, Fix J, Dubuquoy C, Makabi-Panzu B, Thom M, et al. Vaccine safety and efficacy evaluation of a recombinant bovine respiratory syncytial virus (BRSV) with deletion of the SH gene and subunit vaccines based on recombinant human RSV proteins: N-nanorings, P and M2-1, in calves with maternal antibodies. PLoS One. 2014;9:e100392. doi: 10.1371/journal.pone.0100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hervé P-L, Raliou M, Bourdieu C, Dubuquoy C, Petit-Camurdan A, Bertho N, et al. A novel subnucleocapsid nanoplatform for mucosal vaccination against influenza virus that targets the ectodomain of matrix protein 2. J Virol. 2014;88:325–38. doi: 10.1128/JVI.01141-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castagne N, Barbier A, Bernard J, Rezaei H, Huet J, Henry C, et al. Biochemical characterization of the respiratory syncytial virus P–P and P–N protein complexes and localization of the P protein oligomerization domain. J Gen Virol. 2004;85:1643–53. doi: 10.1099/vir.0.79830-0. [DOI] [PubMed] [Google Scholar]
- 28.Parker R, Deville S, Dupuis L, Bertrand F, Aucouturier J. Adjuvant formulation for veterinary vaccines: Montanide™ gel safety profile. Procedia Vaccinol. 2009;1:140–7. [Google Scholar]
- 29.Rameix-Welti M-A, Le Goffic R, Hervé P-L, Sourimant J, Rémot A, Riffault S, et al. Visualizing the replication of respiratory syncytial virus in cells and in living mice. Nat Commun. 2014;5:5104. doi: 10.1038/ncomms6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierantoni A, Esposito ML, Ammendola V, Napolitano F, Grazioli F, Abbate A, et al. Mucosal delivery of a vectored RSV vaccine is safe and elicits protective immunity in rodents and nonhuman primates. Mol Ther - Methods Clin Dev. 2015;2:15018. doi: 10.1038/mtm.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schickli JH, Whitacre DC, Tang RS, Kaur J, Lawlor H, Peters CJ, et al. Palivizumab epitope–displaying virus-like particles protect rodents from RSV challenge. J Clin Invest. 2015;125:1637–47. doi: 10.1172/JCI78450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol. 2009;182:3718–27. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hessell AJ, Hangartner L, Hunter M, Havenith CEG, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–4. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 34.Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. 2004;172:5598–605. doi: 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
- 35.El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, et al. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: fc receptors and alveolar macrophages mediate protection. J Immunol. 2011;186:1022–31. doi: 10.4049/jimmunol.0902147. [DOI] [PubMed] [Google Scholar]
- 36.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–7. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.