Abstract
Risky decision making is prominent during adolescence, perhaps contributed to by heightened sensation seeking and ongoing maturation of reward and dopamine systems in the brain, which are, in part, modulated by sex hormones. In this study, we examined sex differences in the neural substrates of reward sensitivity during a risky decision-making task and hypothesized that compared with girls, boys would show heightened brain activation in reward-relevant regions, particularly the nucleus accumbens, during reward receipt. Further, we hypothesized that testosterone and estradiol levels would mediate this sex difference. Moreover, we predicted boys would make more risky choices on the task. While boys showed increased nucleus accumbens blood oxygen level-dependent (BOLD) response relative to girls, sex hormones did not mediate this effect. As predicted, boys made a higher percentage of risky decisions during the task. Interestingly, boys also self-reported more motivation to perform well and earn money on the task, while girls self-reported higher state anxiety prior to the scan session. Motivation to earn money partially mediated the effect of sex on nucleus accumbens activity during reward. Previous research shows that increased motivation and salience of reinforcers is linked with more robust striatal BOLD response, therefore psychosocial factors, in addition to sex, may play an important role in reward sensitivity. Elucidating neurobiological mechanisms that support adolescent sex differences in risky decision making has important implications for understanding individual differences that lead to advantageous and adverse behaviors that affect health outcomes.
Keywords: Reward, Risk taking, Adolescence, Sex hormones, Sex differences, Motivation
1. Introduction
Following perinatal neural organization, adolescence marks a second wave of plasticity, during which numerous behavioral, social, and physiological changes occur that act to re-organize and activate the brain (Spear, 2013). This extended brain plasticity can be viewed as a double-edged sword, serving to augment vulnerability to biological and psychological insult, as well as support healthy neurodevelopment (Telzer, 2016). Processing of rewarding stimuli is particularly relevant during the adolescent period, given the rise in sensation seeking, which may contribute to increased reward sensitivity and risk taking in some youth (Romer & Hennessy, 2007). Dysregulated reward processing has been linked with affective and substance use disorders, the incidence of which increase substantially during adolescence (Davey, Yucel, & Allen, 2008; Ernst, Pine, & Hardin, 2006; Fairchild, 2011; MacPherson, Magidson, Reynolds, Kahler, & Lejuez, 2010). As such, elucidating the neural mechanisms underlying adolescent reward sensitivity may help in promoting beneficial, rather than adverse, neuroplastic change.
Psychobiological models of adolescent risk taking posit an imbalance between reward processing and self-control, mirrored by enhanced functional activation of reward-sensitive regions (i.e. striatum, including nucleus accumbens) and diminished activation of self-regulatory brain regions (i.e. medial prefrontal cortex), which drives risk taking via inefficient regulation of reward-sensitive brain regions by self-regulatory regions (Casey, 2015; Ernst, 2014; Smith, Chein, & Steinberg, 2013; Somerville, Jones, & Casey, 2010). However, there is a paucity of data showing a direct relationship between reward sensitivity and risk taking (Braams, Peper, van der Heide, Peters, & Crone, 2016; Braams, van Duijvenvoorde, Peper, & Crone, 2015; Galvan et al., 2006; van Duijvenvoorde et al., 2014, 2015; Vorobyev, Kwon, Moe, Parkkola, & Hamalainen, 2015), likely because there is substantial individual variability in reward sensitivity (Bjork & Pardini, 2015; Braams et al., 2015; Chick, 2015; Cservenka, Herting, Seghete, Hudson, & Nagel, 2012). Some of this variability may be due to individual differences in personality traits, such as sensation seeking (Cservenka et al., 2012; van Duijvenvoorde et al., 2014) and impulsivity (Forbes et al., 2009; Piray, den Ouden, van der Schaaf, Toni, & Cools, 2015). Moreover, the link between reward sensitivity and risk taking may be partly explained by pubertal influences (Forbes et al., 2010; Urosevic, Collins, Muetzel, Lim, & Luciana, 2014), given that puberty has been shown to correlate with sensation seeking (Forbes & Dahl, 2010; Martin et al., 2002, 2006; Steinberg, 2004; Steinberg et al., 2008), reward sensitivity (Urosevic et al., 2014) and nucleus accumbens activity in response to rewards (Braams et al., 2015). Indeed, there is evidence that pubertal increases in sensation seeking predict real-world risky behavior, such as substance use (Kirillova, Vanyukov, Gavaler, Pajer, & Tarter, 2001; Martin et al., 2002).
Gonadal hormones, which are re-activated at the onset of puberty, have also been linked to reward processing. Previous work in adolescents showed a positive association between striatal activity in response to reward and endogenous levels of testosterone (Braams et al., 2015; Op de Macks et al., 2011) and estradiol (Op de Macks et al., 2011) in both males and females. Moreover, sex hormone levels have been positively associated with risk-taking behavior in adolescence (de Water, Braams, Crone, & Peper, 2013; Martin, Mainous, Curry, & Martin, 1999; Peper, Mandl, et al., 2013; Peters, Jolles, Van Duijvenvoorde, Crone, & Peper, 2015; Vermeersch, T’Sjoen, Kaufman, & Vincke, 2008a, 2008b). In studies that compared boys and girls directly, there is more evidence of a positive relationship between sex hormones and risky behavior in boys relative to girls (de Water et al., 2013; Peper, de Reus, van den Heuvel, & Schutter, 2015; Peters et al., 2015), or compared to evidence indicating no sex difference (Peper, Koolschijn, & Crone, 2013). In young adults, sex hormone levels have been shown to predict risky behavior in both sexes to the same degree (Braams et al., 2016; Mehta, Welker, Zilioli, & Carre, 2015; Nguyen et al., 2016; Stanton, Liening, & Schultheiss, 2011). The majority of this research supports a link between testosterone and risk taking (Braams et al., 2016; de Water et al., 2013; Martin et al., 1999; Mehta et al., 2015; Nguyen et al., 2016; Peper et al., 2015; Peper, Koolschijn, et al., 2013; Peters et al., 2015; Stanton et al., 2011; Vermeersch et al., 2008b), while a subset of studies also support a positive association between estradiol and risk taking (de Water et al., 2013; Martin et al., 1999; Peper et al., 2015; Vermeersch et al., 2008a). Only two studies have examined the relationship between reward sensitivity, as indexed by nucleus accumbens activity, sex hormones and risk taking (Braams et al., 2015, 2016). One of these studies reported that puberty, testosterone and risk taking explained nucleus accumbens activation during a gambling game in both males and females (Braams et al., 2015). The second study indicated that testosterone levels, but not nucleus accumbens activation during the same gambling task, predicted risky behavior, as indexed by self-reported alcohol use, two years later in males and females (Braams et al., 2016). The mechanism linking sex hormones, reward sensitivity and risk taking remains to be fully elucidated; however, the extant literature suggests that both testosterone and estradiol may be important in explaining risk-taking behavior during adolescence, particularly in boys.
Intriguingly, sex differences in striatal reactivity during reward processing have not been reported or examined in previous studies of adolescents (Braams et al., 2015, 2016; Forbes et al., 2010; Op de Macks et al., 2011). This is somewhat surprising, given the presence of sex differences in pubertal maturation, sex hormone levels (Tanner & Whitehouse, 1976), prefrontal cortical maturation (on average, girls mature approximately two years earlier than boys) (Lenroot et al., 2007) and sensation seeking (on average, boys report more sensation seeking than girls) (Romer & Hennessy, 2007; Steinberg et al., 2008; Zuckerman & Kuhlman, 2000). Thus, sex may be an important variable to consider for understanding individual differences in reward sensitivity and risk taking during adolescence. Indeed, one of the primary neurotransmitters involved in reward processing - dopamine (Berridge & Kringelbach, 2008) - develops in a sexually dimorphic manner during adolescence. Studies in rodents demonstrate enhanced dopamine release in females compared to males due to elevations in estradiol levels during puberty (Di Paolo, Rouillard, & Bedard, 1985; Sarvari et al., 2014; Xiao & Becker, 1994). In contrast, testosterone metabolites have been shown to mediate reward response following direct administration into the nucleus accumbens, which may be mediated by binding at γ-Aminobutyric acid (GABA) (Frye, Park, Tanaka, Rosellini, & Svare, 2001) and dopamine (Mhillaj et al., 2015) receptors. Additionally, both sex hormones have been shown to influence sensation seeking in adolescence (Kerschbaum, Ruemer, Weisshuhn, & Klimesch, 2006; Vermeersch, T’Sjoen, Kaufman, & Vincke, 2009), indicating a role for sex hormones in dopamine activity and sensation seeking. Thus, examining the influence of sensation seeking and sex hormones on potential sex differences in reward sensitivity may inform psychobiological models of risk taking in adolescence.
The current study adds to this literature by examining sex differences in reward processing in a large sample of healthy adolescents, as well as the potentially mediating influence of sex hormones on observed sex differences. We hypothesized boys would show increased blood oxygen level-dependent (BOLD) response in the striatum, including nucleus accumbens, during reward receipt feedback, as well as heightened risky behavior during a risky decision-making task, compared to girls. These hypotheses were based on research showing higher sensation seeking in adolescent boys (Romer & Hennessy, 2007; Steinberg et al., 2008) and delayed prefrontal gray matter maturation in boys, compared to age-matched girls (Lenroot et al., 2007). We also predicted testosterone and estradiol would mediate sex differences in nucleus accumbens BOLD response, given their important role in pubertal development, sensation seeking and in modulating reward-relevant brain regions (Braams et al., 2015; Di Paolo et al., 1985; Frye et al., 2001; Op de Macks et al., 2011; Sarvari et al., 2014; Xiao & Becker, 1994).
2. Material and methods
2.1. Participant screening and exclusionary criteria
Participants underwent comprehensive structured interviews by trained research assistants to determine eligibility. Youth and parents completed separate structured telephone interviews that included the Diagnostic Interview Schedule for Children Predictive Scales (Lucas et al., 2001), the Family History Assessment Module (Rice et al., 1995), and the Brief Lifetime version of the Customary Drinking and Drug Use Record (Brown et al., 1998). Exclusionary criteria included current diagnosis of DSM-IV disorders (lifetime history of DSM-IV disorders was not assessed), significant substance use (>10 lifetime alcoholic drinks or >2 drinks/occasion, >5 uses of marijuana, any other drug use, or >4 cigarettes per day), neurological illness/head trauma, serious medical problems, prenatal exposure to drugs or alcohol, reported history of psychotic disorders in biological parents, current medication that may affect neural (e.g. psychoactive medication) or endocrine (e.g. birth control) function, the inability of a parent to provide family history information, left-handedness (Edinburgh Handedness Inventory, Oldfield, 1971), pregnancy, and MRI contraindications. This study was reviewed and approved by the Oregon Health & Science University’s (OHSU) Institutional Review Board. Written assent and consent was obtained from all children and their parents, respectively.
Two-hundred one participants from an ongoing longitudinal study of adolescent neurodevelopment completed a reward processing task (see Section 2.4). From this sample, 21 were excluded due to missing sex hormone data or values that exceeded normal ranges based on sex, age, and pubertal status (see Section 2.3). An additional 5 participants were excluded for excessive head motion during scanning (see Section 2.6) and 8 participants were excluded for taking birth control or other endocrine-disrupting medication. The data for the remaining 167 participants were used in subsequent data analyses.
2.2. Participant characteristics and questionnaires
Eligible youth were administered the following: a 2-subtest (Vocabulary and Matrix Reasoning) version of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), the Pubertal Development Scale (PDS) (Petersen, Crockett, Richards, & Boxer, 1988), the Children’s Sleep Habits Questionnaire (CSHQ) (Owens, Spirito, & McGuinn, 2000) and the Impulsive Sensation Seeking scale from the Zuckerman-Kuhlman Personality Questionnaire (Zuckerman, Kuhlman, Joireman, Teta, & Kraft, 1993). In addition, parents completed the Hollingshead Index of Social Position to determine family socioeconomic status (SES) (Hollingshead, 1975). Prior to the scan session, participants filled out the state anxiety sub-scale from the State-Trait Anxiety Inventory (STAI) (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). Measures on sleep habits, SES and state anxiety were collected because they have been shown to impact reward processing (Forbes et al., 2012; Gianaros et al., 2011; Hasler et al., 2012; Hasler, Sitnick, Shaw, & Forbes, 2013; Holm et al., 2009; Kumar et al., 2014; Mullin et al., 2013; Telzer, Fuligni, Lieberman, & Galvan, 2013). At the conclusion of the scan, participants completed an Exit Questionnaire assessing their motivation to perform well on the task (i.e. “How important was it for you to do well?” rated on a scale from 1 to 5, or “Not important at all” to “Very important”) and earn money (i.e. “How much did earning money motivate you?” rated on a scale from 1 to 4, or “Not at all” to “Very much”), as well as general feelings about winning (e.g. “On average, how did you feel when you won $7 on this wheel?” rated on a scale from 1 to 10 or “Very Sad” to “Very Happy”) and not winning (e.g. “On average, how did you feel when you did not win $1 on this wheel?” rated on a scale from 1 to 10 or “Very Sad” to “Very Happy”) during low- and high-risk trials (see Section 2.4).
2.3. Sex hormone assays
Serum sex hormone levels were measured within seven days of the scan procedure. Four mL of blood was collected via venipuncture from all subjects at the Oregon Clinical and Translational Research Institute. To reduce diurnal heterogeneity of hormone levels, blood was collected between 7:00 and 10:00 a.m. In addition, samples from post-menarche girls were drawn during the follicular phase of the menstrual cycle (days 1–10) to further minimize variability, as well as interactions with progesterone (Gillies & McArthur, 2010; Wallach, 2000). Menstrual cycle phase was determined by self-report. Testosterone levels were determined by Coat-A-Count radioimmunoassay (Diagnostic Product Corp., Los Angeles, CA). The intra-assay and inter-assay CVs were 7.0% and 7.4%, respectively, with a lower level of detection of 10 ng/dL. Normal levels of testosterone range from <7 to 75 and <7 to 1200 ng/dL, in pubertal girls and boys, respectively (Wallach, 2000). Estradiol levels were determined using the DSL-4800 Ultra-sensitive Estradiol Radioimmunoassay Kit (Beckman Coulter, Fullerton, CA). The intraassay and interassay CVs were 7.4% and 12.6%, respectively, with a lower level of detection of >2.2 pg/mL. Normal levels of estradiol range from <2 to 350 pg/mL and <2 to 40 pg/mL in pubertal girls and boys, respectively (Wallach, 2000). Hormone levels were examined to ensure none exceeded expected levels, as determined by norms for age, sex, and pubertal status.
2.4. Reward processing task
A modified version of the Wheel of Fortune (WOF) Task (Cservenka & Nagel, 2012), adapted from the original WOF paradigm (Ernst et al., 2004), was used to assess neural response during reward processing. Details of the task have been described in depth previously (Cservenka & Nagel, 2012). Briefly, the WOF is a computerized decision-making task in which participants chose between two options associated with distinct probabilities of winning various monetary amounts, represented as portions of a wheel adding up to 100% (10/90, 30/70, or 50/50%). Selection of the low probability/high magnitude option of the wheel (10% chance of winning $7 or 30% chance of winning $2) was considered a risky choice, while selection of the high probability/low magnitude option (90% or 70% chance of winning $1) represented a safe choice. Lastly, selection of a wheel with equal probabilities and magnitudes (50% of winning $2) was considered a chance or neutral choice. Seventy-two trials were presented over two 10-min runs, with each run including 12 10/90, 14 30/70, and 10 50/50 probability wheels. In order to “Win” a trial, a participant’s selection had to match the computer’s choice, based on predefined probabilities, while a choice that did not match, resulted in a “No Win” trial. Participants were instructed to select the portion of the wheel they thought would win them money and to try to win as much money as possible because they would receive “a portion” of their total earnings at the end of the scan session. Each trial was 10.5 s and included a selection (3 s), anticipation (3.5 s) and feedback (4 s) phase, with intertrial fixation intervals jittered between 1 and 11 s. Trial numbers included in the decision making and anticipation phases of the task were determined by participant selections (safe, risky or neutral), while the number of trials for the reward receipt (feedback) phase included wins from all trial types. Only the feedback phase (combining wins across all types of trials) was analyzed in the present study, because it offers the most power for statistical analysis (i.e. number of trials is not limited by participant selections) (Jones, Cservenka, & Nagel, 2016; Steele et al., 2016). During this phase, the screen indicated whether the participant won or did not win money during that trial, as well as the cumulative dollar amount won up to that point. To confirm participant attention during this phase, youth were asked to indicate whether or not they won money during each trial. The task was displayed with E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA). Average accuracy and reaction times (RT) for all phases across different trial probabilities/magnitudes were recorded for both runs of the task.
2.5. MRI data acquisition
Youth were scanned on a 3 Tesla Siemens Magnetom Tim Trio system (Siemens Medical Solutions, Erlangen, Germany) using a twelve-channel head coil at the Advanced Imaging Research Center at OHSU. Functional images were collected in the axial plane oblique to the anterior – posterior commissure, using a high-angular resolution T2*-weighted echo-planar BOLD sequence (TR = 2000 ms, TE = 30 ms, matrix = 240 × 176, FOV = 256 mm, flip angle = 90°, 33 slices, no gap, slice thickness = 3.8 mm, 300 repetitions/run). A whole-brain, high-resolution structural image series was collected in the sagittal plane using a T1-weighted MPRAGE scanning sequence (TI = 900 ms, flip angle = 10°, TE = 3.58 ms, TR = 2300 ms, matrix = 256 × 240, FOV = 240 mm, slice thickness = 1 mm, 33 slices) for co-registration to functional data.
2.6. Image processing
Imaging data were processed and analyzed using Analysis of Functional NeuroImages (AFNI) (Cox, 1996) using the following steps: slice time correction, correction for head movement, spatial smoothing with a 6.0 mm full-width half-maximum Gaussian kernel, within-run intensity normalization to a whole-brain signal, and co-registration of functional images to the anatomical image. TRs exceeding movement of 2.5 mm or 2.5° in any of the three displacement or three rotational parameters, respectively, were censored. To assess within-run motion, an average root mean square (RMS) value was calculated using these six motion parameters and compared across groups. Any subject with a two-run average RMS value exceeding 1.5 mm was excluded from analyses.
Regressors representing selection, anticipation and feedback trials of the task were modeled. Stimulus times corresponding to the onset time of each phase and duration of the event coded as the length of each phase were convolved with a gamma-variate hemodynamic response function (Cohen, 1997). The estimated implicit baseline model corresponded to mean BOLD signal from the entire time course of the task, linear drift, periods of fixation, and nuisance regressors (Cox, 1996). Functional data were then transformed into Talairach space (Talairach & Tournoux, 1988) and resampled into 3 mm3 voxels. Contrast images Win - No Win, No Win - baseline, and Win - baseline were used for fMRI statistical analysis.
2.7. Statistical analyses
2.7.1. Demographics and behavior
Demographic and task performance data were examined for normality and occurrence of outliers using SPSS Statistics 20 (Armonk, NY: IBM Corp.). Non-normal variables with absolute skew/kurtosis values exceeding 2.0 were log transformed. Sex differences for self-reported race were assessed with chi-square analysis, while differences in PDS and responses on the post-scan Exit Questionnaire were determined with a Mann-Whitney U analysis. All other variables were examined with independent samples t-tests.
2.7.2. Task activation-masked fMRI analysis
To best represent task-related activity for both boys and girls, task activation maps were created for each sex. One-sample t-tests of task activation were thresholded at a voxel level of p < 0.001, uncorrected, summed and binarized to create a task-relevant mask (33,903 total voxels). By using this approach, we limit the detection of findings to those pertinent to the task conditions, as previously published (Cservenka et al., 2012; Cservenka, Jones, & Nagel, 2015; Jones et al., 2016). Sex differences were then examined within this reward-related activity mask. Sex differences in Win - No Win activation were examined with an analysis of covariance (ANCOVA) that included age as a covariate because previous studies have demonstrated changes in reward processing across this period of development (Braams et al., 2015; Forbes et al., 2010; Op de Macks et al., 2011). Although pubertal status was significantly different between boys and girls (Table 1), it was not included as a covariate due to its strong collinearity with age. Contributing effects of puberty were examined post hoc in SPSS. AFNI’s AlphaSim (Cox, 1996) was used to correct for between-group multiple comparisons at a voxel- and cluster-level (threshold of p < 0.001 and α < 0.01, respectively; minimum cluster size = 19 voxels).
Table 1.
Demographic and behavioral measures.
| Boys (n = 96) | Girls (n = 71) | Statistic | |
|---|---|---|---|
| Age (years) | 14.2 (1.3) | 14.4 (1.3) | t165 = 0.63 |
| Range | 12.3–17.0 | 12.0–16.9 | |
| Pubertya | 3.1 (0.9) | 4.0 (0.6) | Z = 6.45** |
| Socioeconomic statusb | 29.9 (13.5) | 30.5 (14.6) | t165 = 0.28 |
| IQc | 110.3 (12.4) | 110.8 (10.0) | t165 = 0.31 |
| Log Testosterone (ng/dL) | 2.3 (0.5) | 1.2 (0.3) | t153.37 = 20.68** |
| Range | 7.1–809.5 | 2.4–53.3 | |
| Log Estradiol (pg/dL) | 1.2 (0.2) | 1.5 (0.2) | t162.76 = 11.12** |
| Range | 6.1–38.0 | 11.9–85.0 | |
| Sensation Seekingd | 42.5 (21.0) | 42.8 (22.0) | t165 = 0.09 |
| CHSQ Sleepinesse | 5.8 (1.7) | 6.2 (1.9) | t165 = 1.24 |
| RMS head movementf | 0.31 | 0.27 | t165 = 1.19 |
Crockett Pubertal Development Scale; values range from 1 to 5, with larger values referring to more advanced pubertal development.
Hollingshead Index of Social Position; larger values indicate lower socioeconomic status (middle class corresponds to 32–47 range); parent-rated.
Wechsler Abbreviated Scale of Intelligence (2-subtest version).
Impulsive Sensation Seeking scale from Zuckerman-Kuhlman Personality Questionnaire.
Children’s Sleep Habits Questionnaire.
Root mean square; index of averaged within-run motion.
Statistical significance at p < 0.001.
2.7.3. Region of interest and mediation analyses
To test if sex hormones mediated the relationship between sex and striatal BOLD response, a region of interest (ROI) analysis of bilateral nucleus accumbens was conducted. A mask of the nucleus accumbens was created with the following Talairach coordinates: 12, −8, −8 (right) and −12, −8, −8 (left) corresponding to this region. Surrounding the peak coordinates, two spheres with 4 mm radii where created (20 total voxels; Fig. 3A). Next, an ANCOVA comparing sex differences in reward processing (Win - No Win), while controlling for age, was conducted. AFNI’s AlphaSim was used again to account for multiple comparisons at a voxel- and cluster-level threshold of p < 0.05 and α < 0.05, respectively, which yielded a minimum cluster size of 5 voxels. A more liberal voxel- and cluster-wise threshold was implemented relative to the task activation-masked ANCOVA to account for the small ROI. Values of percent BOLD signal change from significant clusters were included in a nonparametric bootstrapping procedure (5000 re-samples) to assess mediation by sex hormones (Hayes, 2013). The model included sex (independent variable), sex hormone (mediator variable), percent BOLD signal change from significant clusters (dependent variables), and age as a covariate.
Fig. 3.
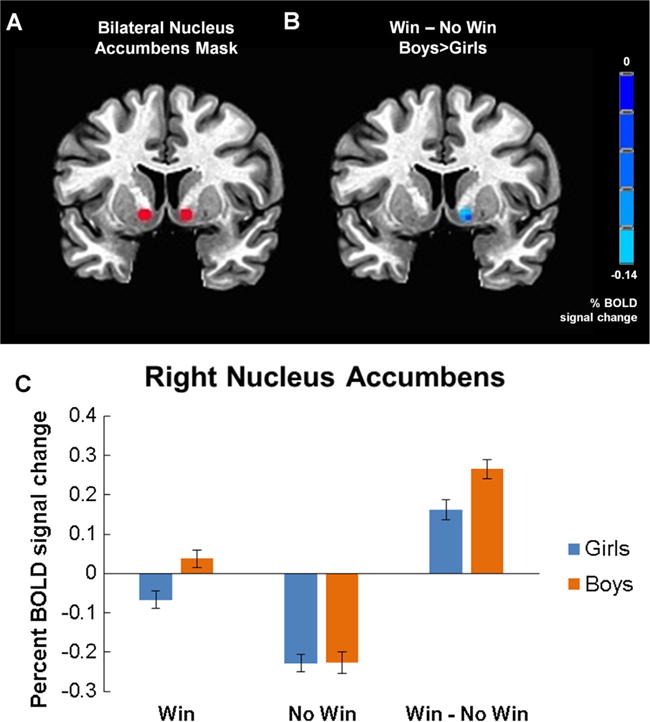
Boys showed greater BOLD response in nucleus accumbens during reward processing. (A) Bilateral nucleus accumbens mask (peak coordinates: 12, −8, −8 and −12, −8, −8) overlaid on standard Talairach atlas. (B) Statistical map of sex differences in nucleus accumbens region of interest analysis overlaid on a standard Talairach template. Percent BOLD signal change in the Win – No Win contrast is displayed for one significant cluster in right nucleus accumbens (p < 0.05 voxel and α < 0.05 cluster correction) in which boys had significantly more activation than girls. (C) Mean ± SEM percent BOLD signal change in right nucleus accumbens is plotted by trial type.
2.7.4. Sex hormone linear regressions
To examine the relationships between sex hormones and reward-relevant brain activation, multiple regressions with either log testosterone or log estradiol (controlling for age) were conducted separately for boys and girls. Like the previous analysis, multiple regressions were restricted by the task-activation masks created for each sex. Results of the linear regressions were corrected for multiple comparisons with a voxel-wise correction of p < 0.001 and cluster-wise correction of α < 0.01. Because task activation maps differed slightly by sex, the minimum cluster sizes were 15 voxels for girls (of 11,039 total voxels) and 16 voxels for boys (of 32,885 total voxels).
3. Results
3.1. Participant characteristics and task behavior
Details on participant characteristics can be found in Table 1. Boys and girls did not differ in age, SES, IQ, sensation seeking, or general quality of sleep. Two-run averaged RMS head movement values exceeded 1.5 mm for five participants that were subsequently excluded from further analyses; RMS was not statistically different between boys and girls of the remaining sample. Sex hormone levels were not normally distributed and underwent log transformation. Boys had statistically greater serum levels of log testosterone, while girls had statistically higher log estradiol levels. Girls also reported more advanced pubertal maturation. Sensation seeking was not statistically different by sex, nor was it correlated with pubertal status or age across the whole sample. However, examined separately by sex, sensation seeking was correlated to puberty in boys, but not girls, while sensation seeking was not correlated to age in either boys or girls. Self-reported state anxiety prior to the scan session was significantly higher in girls (T-score = 42.7 ± 5.7), compared to boys (T-score = 39.6 ± 7.5; t164 = 2.95, p = 0.004). State anxiety was positively correlated with log estradiol across the whole sample, but not within sex. Percent of risky selections was statistically higher in boys (64.5%), compared to girls (56.2%; t164 = 2.00, p < 0.05), but was not correlated with sensation seeking in the whole sample or by sex. Log testosterone and estradiol were not significantly related to percent of risky selections across the whole sample or by sex. Correlation tables can be found in Supplementary Material.
Responses on the post-scan Exit Questionnaire revealed that compared to girls, boys believed it was more important to perform well on the task (Z = 2.08, p = 0.04) and were more motivated by money (Z = 2.01, p < 0.05). All participants understood the task, and boys and girls did not feel differently after winning or losing (all Z ≤ 1.62, p ≥ 0.11). Additionally, log estradiol was negatively correlated with ‘Importance of Performance’ and ‘Motivation to Earn Money’ across the whole sample, but not within sex. Log testosterone was not related to either measure (Supplementary Material).
3.2. Sex differences in BOLD response during reward processing
3.2.1. Task activation-masked fMRI analysis
Sex differences in reward processing were assessed with an ANCOVA (controlling for age) masked with a task activation-related mask. Sex differences in percent BOLD signal change during Win - No Win were such that boys showed an increase in response, as compared to girls, in the following brain regions: left lentiform nucleus (extending to thalamus, putamen and caudate nucleus), right lentiform nucleus (extending to putamen, caudate nucleus and nucleus accumbens), right thalamus, left paracentral lobule (extending to cingulate cortex and precuneus), right superior parietal lobule (extending to precuneus and inferior parietal lobule) and right fusiform gyrus (extending to lingual gyrus) (Table 2; Figs. 1 and 2). Effect sizes for these results were in the medium range (Partial η2 ~ 0.10) (Table 2).
Table 2.
Sex differences in BOLD response during reward processing.
| Voxels | x | y | z | Partial η2a | |
|---|---|---|---|---|---|
| Peak Coordinate Brain Region | |||||
| Boys > Girls | |||||
| L Lentiform/Caudate nucleus/Thalamus/Putamen | 163 | −19 | −13 | −7 | 0.10 |
| L Paracentral gyrus/Cingulate cortex/Precuneus | 124 | −1 | −28 | 44 | 0.10 |
| R Superior/Inferior parietal lobule/Precuneus | 87 | 32 | −52 | 56 | 0.12 |
| R Lentiform nucleus/Putamen | 43 | 26 | −10 | 5 | 0.09 |
| R Thalamus | 40 | 5 | −7 | 11 | 0.09 |
| R Lentiform/Caudate nucleus/Nucleus accumbens | 34 | 14 | 5 | −10 | 0.11 |
| R Fusiform/Lingual gyrus | 33 | 23 | −67 | −10 | 0.10 |
| R Lentiform nucleus/Thalamus | 19 | 23 | −16 | −7 | 0.10 |
| Region of Interest Analysis | |||||
| Boys > Girls | |||||
| R Nucleus accumbens | 9 | 14 | 8 | −4 | 0.05 |
Partial η2 effect sizes range from small (0.01) to medium (0.09) to large (0.25). R = right; L = left.
Fig. 1.
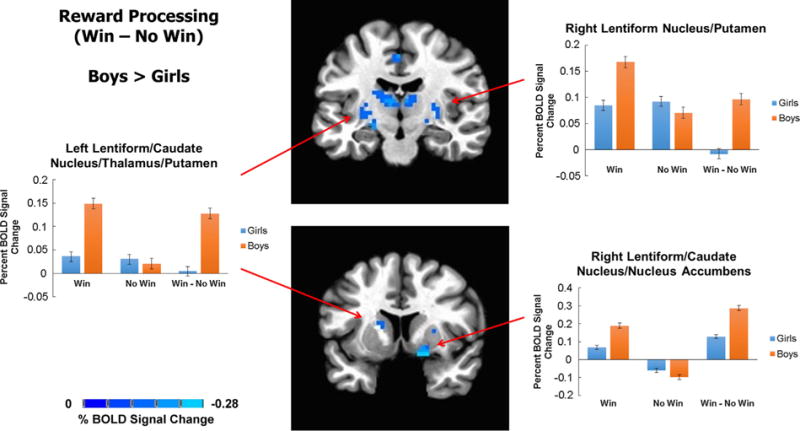
Boys showed greater striatal BOLD response during reward processing. Statistical maps of sex differences in percent BOLD activation in Win – No Win contrast (controlling for age) overlaid on a standard Talairach template are depicted here. Boys showed more activation (orange) than girls (blue) in bilateral lentiform nucleus (extending to caudate nucleus, putamen, nucleus accumbens and thalamus). Percent BOLD signal change in these regions is also depicted by trial type (No Win and Win) and their contrast (Win – No Win). In all cases, boys showed higher percent BOLD signal change during Win trials, compared to girls. In contrast, there were no sex differences in No Win BOLD activation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
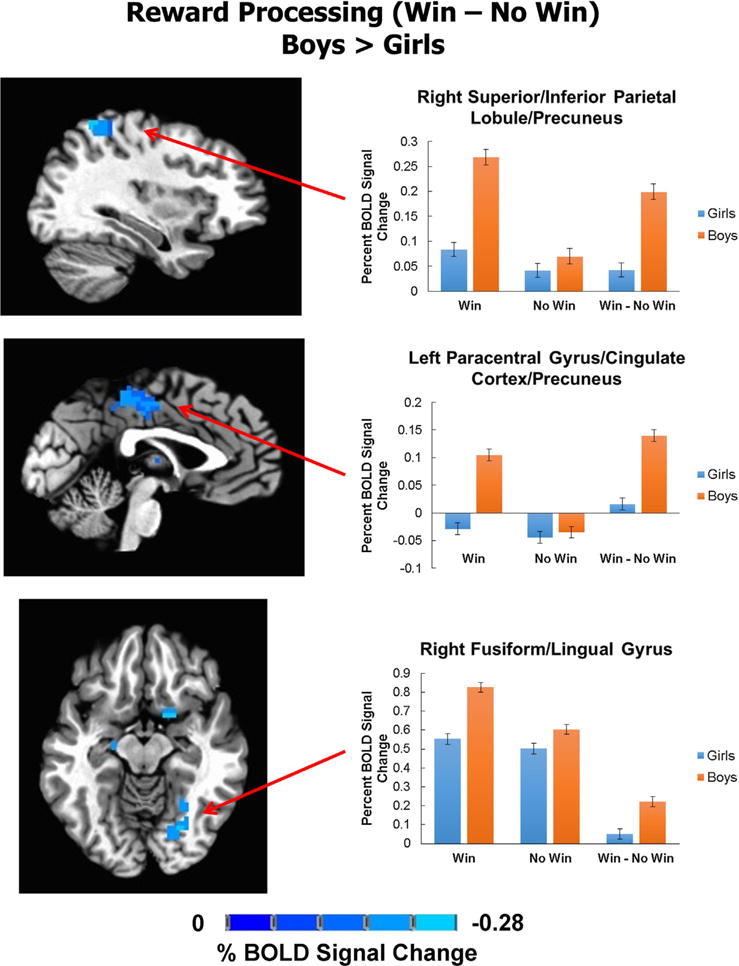
Boys showed greater cortical BOLD response during reward processing. Statistical maps of sex differences in percent BOLD activation in Win – No Win contrast (controlling for age) overlaid on a standard Talairach template are depicted here. Boys showed more activation (orange) than girls (blue) in paracentral gyrus (extending to cingulate cortex and precuneus), superior/inferior parietal lobule (extending to precuneus) and fusiform gyrus (extending to lingual gyrus). Percent BOLD signal change in these regions is also depicted by trial type (No Win and Win) and their contrast (Win – No Win). In all cases, boys showed higher percent BOLD signal change during Win trials, compared to girls. In contrast, there were no sex differences in No Win BOLD activation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Percent BOLD signal change values from significant clusters of group differences were plotted for Win - baseline and No Win -baseline contrasts to examine whether group differences were driven by changes in BOLD response during Win trials, No Win trials, or both. Boys and girls showed comparable BOLD response during No Win trials; however, they differed significantly in their BOLD response during Win trials. In all clusters, boys showed greater BOLD signal during Win trials, relative to girls (Figs. 1 and 2).
To confirm that sex differences in reward processing were not attributed to differences in task performance, percent of risky selections, which was significantly higher in boys compared to girls (Table 1), was examined post hoc. Addition of a percent risky selections covariate did not change the significant effects of sex on percent BOLD signal change at any cluster (all F(1,162) ≥ 12.99, p < 0.001). However, percent of risky selections also explained some variance of BOLD activation during Win – No Win in the following clusters: left lentiform nucleus/caudate nucleus/thalamus/putamen (F(1,162) = 7.61, p = 0.006, partial η2 = 0.05), right lentiform nucleus/putamen (F(1,162) = 4.76, p = 0.03, partial η2 = 0.03), right lentiform nucleus/caudate nucleus/nucleus accumbens (F(1,162) = 6.31, p = 0.01, partial η2 = 0.04), right fusiform/lingual gyrus (F(1,162) = 7.04, p = 0.009, partial η2 = 0.04) and right lentiform nucleus/thalamus (F(1,162) = 11.09, p = 0.001, partial η2 = 0.06). Percent of risky selections did not explain variance in Win – No Win BOLD response in left paracentral gyrus/cingulate cortex/precuneus (F(1,162) = 2.77, p = 0.10), right superior/inferior parietal lobule/precuneus (F(1,162) = 2.70, p = 0.10) or right thalamus (F(1,162) = 3.71, p = 0.06).
Further, given the relevance of pubertal status in the neuromaturation of reward processing (Braams et al., 2015; Forbes et al., 2010), its effect on reward response was also analyzed post hoc. With the addition of a PDS covariate, the results of the ANCOVA replicated; pubertal status did not change the significant effects of sex on percent BOLD signal change at any cluster (all F (1,163) ≥ 11.43, p ≤ 0.001). However, PDS explained additional variance of Win – No Win BOLD response in right fusiform/lingual gyrus (F(1,163) = 3.92, p < 0.05, partial η2 = 0.02), such that PDS was associated with an increase in fusiform gyrus activation independent of age and sex. PDS did not explain a significant amount of variance in the remaining clusters (all F(1,163) ≤ 2.88, p ≥ 0.09).
3.3. ROI and mediation analyses
Sex differences in reward processing were assessed with an ANCOVA (controlling for age) masked with a nucleus accumbens mask (Fig. 3A). Boys showed more BOLD activation in Win - No Win contrasts compared to girls in right, but not left nucleus accumbens (Table 2, Fig. 3B and C). Log testosterone was positively correlated with right nucleus accumbens percent BOLD signal change, controlling for age, across the whole sample (partial 2 = 0.22, p = 0.005; when girls and boys where examined separately, this correlation did not stand (all partial r2 ≤ −0.10, p ≥ 0.41)); therefore, a nonparametric mediation analysis was pursued (Hayes, 2013). Log testosterone did not statistically mediate the effect of sex on nucleus accumbens percent BOLD signal change (Bootstrapped CI95: −0.0834, 0.0859). A mediation analysis with estradiol was not pursued because estradiol did not relate to nucleus accumbens BOLD response, controlling for age (partial r2 = −0.10, p = 0.22).
Since we observed sex differences in ‘Importance of Performance’, ‘Motivation to Earn Money’ and state anxiety - variables known to impact striatal response to rewards (Kumar et al., 2014; Lighthall et al., 2012; Zink, Pagnoni, Martin-Skurski, Chappelow, & Berns, 2004) - we examined correlations between right nucleus accumbens BOLD response and these variables. Neither ‘Importance of Performance’ (ρ = 0.10, p = 0.21) nor state anxiety (r2 = −0.03, p = 0.72) were related to nucleus accumbens BOLD response. ‘Motivation to Earn Money’ was significantly related to nucleus accumbens BOLD activity (ρ = 0.17, p = 0.04), thus, a mediation analysis was pursued. ‘Motivation to Earn Money’ partially mediated the effect of sex on nucleus accumbens activity (Bootstrapped CI95: 0.0005, 0.0303); the direct effect of sex on nucleus accumbens activity remained statistically significant (CI95 = 0.0301, 0.1556).
3.4. Task-activation masked sex hormone linear regressions
To determine any associations between sex hormones and BOLD response during reward processing, multiple regressions (controlling for age) with testosterone or estradiol as regressors were conducted in girls and boys separately. These analyses did not yield significant effects of testosterone or estradiol in either boys or girls.
4. Discussion
In this study, sex differences in brain response to reward were observed in a large and carefully matched sample of adolescent boys and girls. Specifically, BOLD response following notification of receipt of monetary rewards was higher in males, relative to females, in several reward-relevant brain regions (Liu, Hairston, Schrier, & Fan, 2011; Mohr, Biele, & Heekeren, 2010), including the nucleus accumbens. Although testosterone was positively related with nucleus accumbens BOLD response, it did not mediate the relationship between sex and nucleus accumbens activation during reward processing. Estradiol was not related to nucleus accumbens response. Notably, although self-reported sensation seeking was not different by sex, boys made a higher percentage of risky selections on the task. In addition, boys reported higher motivation to perform well and earn money on the task, while girls reported more state anxiety, both of which have been shown to impact reward processing (Kumar et al., 2014; Lighthall et al., 2012; Zink et al., 2004). Notably, motivation to earn money on the task partially mediated the effect of sex on nucleus accumbens BOLD signal during reward processing, indicating that both sex and task motivation play an important role in determining striatal reactivity to rewards.
4.1. Sex differences mechanisms: sensation seeking
While our primary hypothesis was supported (i.e. increased nucleus accumbens BOLD response to reward in males), the mechanisms underlying this difference were not as predicted. First, it was hypothesized that males would show increased activation of nucleus accumbens during receipt of rewards, due in part to previous evidence showing heightened sensation seeking in age-matched males versus females (Romer & Hennessy, 2007; Steinberg et al., 2008). Interestingly, no sex differences in sensation seeking were observed in this study; however, sensation seeking and percent of risky selections were positively correlated only in males, who made a statistically greater number of risky selections compared to girls. The higher rates of risky selections in males may still be partly due to sensation seeking, but not impulsive sensation seeking per se. The Impulsive Sensation Seeking scale (Zuckerman et al., 1993) employed in the present study measures a specific type of sensation seeking that hinges on impulsivity; however, not all sensation seeking is done impulsively; a different assessment tool may have detected sex differences in sensation seeking that may better explain group differences in BOLD response during reward.
4.2. Sex differences mechanisms: testosterone
Second, a link between testosterone and ventral striatal brain response during reward processing has been shown in early adolescent samples (Braams et al., 2015; Op de Macks et al., 2011) and animal models (Frye et al., 2001); therefore, testosterone was a relevant mechanistic target explaining group differences in reward processing. Although testosterone was related to nucleus accumbens BOLD response in the entire sample, replicating previous work (Braams et al., 2015; Op de Macks et al., 2011), it did not explain sex differences in reward processing BOLD activation. Moreover, testosterone did not relate to reward processing in either sex when regressed directly onto reward feedback BOLD signal, indicating that testosterone alone may not predict a neural response to reward, but might be correlated to this process insofar as it relates to sex and the biological processes that differentiate males and females during adolescence. However, it is possible that the reduction in sample size when conducting these analyses separately in males and females may have reduced our power to detect an effect. Future work should address these questions with much larger samples in order to account for the broad range of individual variability in sex hormones levels during adolescence.
4.3. Sex differences mechanisms: estradiol
Estradiol is another neurophysiological mechanism that may contribute to sex differences in reward-related BOLD response, as it has been shown to modulate dopaminergic systems (Di Paolo et al., 1985; Sarvari et al., 2014; Xiao & Becker, 1994) and relate to impulsivity (Smith, Sierra, Oppler, & Boettiger, 2014) and risk taking (de Water et al., 2013). Indeed, estradiol levels in adolescent girls have been associated with dorsal striatal BOLD response during reward processing (Op de Macks et al., 2011), and brain response during reward anticipation and receipt has been shown to fluctuate across the menstrual cycle, at least in adults (Bayer, Bandurski, & Sommer, 2013; Dreher et al., 2007; Ossewaarde et al., 2011). An effect of estradiol was explored in the present study by relating estradiol levels to BOLD response in reward-relevant brain regions that differentiated boys and girls, including the nucleus accumbens. However, estradiol was not significantly related to reward-related brain response. Moreover, regression of estradiol with reward-related BOLD response did not yield any significant effects in either boys or girls. It is possible that our hormone data collection approach obfuscated the relationship between estradiol and BOLD activation to reward. In the case of girls, the sampling of sex hormones levels was truncated to the first 10 days of the follicular phase; thus, establishing a physiological context through which individual differences in estradiol and testosterone could be interpreted. Given that sex hormones, particularly estradiol, fluctuate across the menstrual cycle, sampling these hormones during the same phase provides a reference point and added meaning to the values. However, one limitation of sampling from only one phase of the menstrual cycle, particularly the early follicular phase when estradiol levels are at their lowest, is that our hormone values have restricted variance. Reductions in estradiol, like those observed in premenstrual and early follicular phases of the menstrual cycle, acutely down-regulate endogenous dopamine activity (Di Paolo et al., 1985; Thompson & Moss, 1994), which in turn, diminishes reward signaling from the ventral striatum (Tzschentke & Schmidt, 2000). Therefore, the relatively low levels of estradiol, reflective of the follicular phase, observed in our female sample may have precipitated reductions in striatal BOLD response via blunted dopamine signaling during reward outcome. This interpretation may explain the weaker nucleus accumbens BOLD response observed in girls, relative to boys, during reward processing. Research comparing hormone states across the menstrual cycle is needed to confirm an independent role for estradiol in reward-related brain response during adolescence.
4.4. Sex differences mechanisms: motivation
Psychosocial factors can also interact with physiological and reward processes. Previous research has shown that degree of striatal BOLD response depends, in part, on the saliency of a reinforcer (Zink et al., 2004). Based on post-scanning questionnaire responses, in the current study, boys self-reported higher motivation to obtain monetary rewards and perform well on the task, which may have made the task more salient for males than females. This is supported by the finding that males made more risky selections during the WOF task. Further, percent of risky selections explained a significant amount of variance (in a model controlling for sex and age) in the activation of several reward-relevant brain regions that differentiated boys and girls, particularly in ventral and dorsal striatum, which may indicate that sex differences in activation of these regions underlies sex differences in percent of risky selections made during the task. Interestingly, ‘Motivation to Earn Money’ partially mediated the effect of sex on nucleus accumbens activation, suggesting that both sex and motivation are critical components of salience and sensitivity to rewards. Importantly, girls reported more state anxiety prior to their scan sessions, which can blunt striatal BOLD response (Kumar et al., 2014; Lighthall et al., 2012); thus, we cannot rule out the possibility that anxiety during the scan, which was not measured, may have impacted BOLD activity. The combination of heightened motivation in males and enhanced state anxiety in females may contribute to the sex differences in nucleus accumbens BOLD response observed in this adolescent sample; however, this remains to be tested. In fact, individual differences in such variables may explain why previous studies have not found sex differences in reward sensitivity of the striatum (Braams et al., 2015; Forbes et al., 2010; Op de Macks et al., 2011); motivation, and not sex per se, may be a better predictor of nucleus accumbens reward activation. Future studies must parametrically modulate motivation and examine reward-relevant brain regions outside the ventral striatum. Regardless, we can conclude that task motivation is an important factor when assessing adolescent sex differences in reward sensitivity, as a function of striatal BOLD response.
4.5. Limitations
The primary limitation of this study was the method for analyzing sex hormone levels. Blood samples were collected the week of MRI scanning, thus providing a measure of trait, rather than state sex hormone levels. In the case of the females, this trait was specific to the early follicular phase of the menstrual cycle. However, a single measurement does not reliably describe a trait that can vary as much as sex hormone levels. In an attempt to account for diurnal heterogeneity, blood samples were collected before 10 a.m. for all participants; however, we cannot ascertain the reliability of this measure across days. Measurement of sex hormone levels immediately prior to scanning or administration of exogenous sex hormones would provide some confidence that acute mechanisms of action could be at play. Consideration of other variables that influence sex hormone levels would also be beneficial. Indeed, both genetic and environmental factors influence testosterone levels in adolescence (Harden, Kretsch, Tackett, & Tucker-Drob, 2014). For instance, maternal social instability during pregnancy or lactation leads to a delay in increase of testosterone in male adolescents (Siegeler et al., 2013), while anticipation of a challenge or competition, as well as winning lead to more acute increases in testosterone concentration in young men (Booth, Shelley, Mazur, Tharp, & Kittok, 1989; van der Meij, Buunk, Almela, & Salvador, 2010; Zilioli & Watson, 2014). Understanding variability in the factors linked to reward sensitivity and risk taking during adolescence will offer the best opportunity to uncover the mechanism(s) underlying problem risky behavior.
5. Conclusions
In sum, sex differences in BOLD activity during reward processing were observed in a large sample of healthy adolescents. Regions related to reward processing, including nucleus accumbens (Liu et al., 2011; Mohr et al., 2010), were recruited more robustly in males during reward trials. Sex hormones did not mediate the effect of sex on nucleus accumbens activation even though testosterone was positively correlated with activation of this region, indicating that sex may be a stronger predictor of reward sensitivity than sex hormones. Task motivation partially mediated the effect of sex on nucleus accumbens BOLD response, suggesting that motivation may serve to explain sex differences in reward sensitivity. Importantly, our findings of sex differences in reward processing BOLD activity had medium effect sizes, which emphasize the notion that neurobiological sex differences are nuanced. However, studying subtle differences in male and female brains, particularly during adolescence, can help elucidate healthy developmental trajectories and individual differences in psychosocial and neurophysiological factors that affect relevant processes, such as risk taking and reward sensitivity, which can promote both beneficial and adverse neuroplastic events with long-term consequences.
Supplementary Material
Acknowledgments
Members of the Developmental Brain Imaging Lab at OHSU are thanked for their efforts in data collection.
Funding
This work was supported by the National Institutes of Health [AA017664 to B.J.N., AA2368801 to G.A.]; and the American Psychological Association [APA 110415 to G.A.]. Funding sources had no involvement in study design, collection, analysis or interpretation of the data and no involvement in writing or deciding to publish this manuscript.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bandc.2016.10.003.
References
- Bayer J, Bandurski P, Sommer T. Differential modulation of activity related to the anticipation of monetary gains and losses across the menstrual cycle. European Journal of Neuroscience. 2013;38(10):3519–3526. doi: 10.1111/ejn.12347. http://dx.doi.org/10.1111/ejn.12347. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology (Berl) 2008;199(3):457–480. doi: 10.1007/s00213-008-1099-6. http://dx.doi.org/10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Pardini DA. Who are those “risk-taking adolescents”? Individual differences in developmental neuroimaging research. Developmental Cognitive Neuroscience. 2015;11:56–64. doi: 10.1016/j.dcn.2014.07.008. http://dx.doi.org/10.1016/j.dcn.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A, Shelley G, Mazur A, Tharp G, Kittok R. Testosterone, and winning and losing in human competition. Hormones and Behavior. 1989;23(4):556–571. doi: 10.1016/0018-506x(89)90042-1. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/2606468>. [DOI] [PubMed] [Google Scholar]
- Braams BR, Peper JS, van der Heide D, Peters S, Crone EA. Nucleus accumbens response to rewards and testosterone levels are related to alcohol use in adolescents and young adults. Developmental Cognitive Neuroscience. 2016;17:83–93. doi: 10.1016/j.dcn.2015.12.014. http://dx.doi.org/10.1016/j.dcn.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde AC, Peper JS, Crone EA. Longitudinal changes in adolescent risk-taking: A comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. The Journal of Neuroscience. 2015;35(18):7226–7238. doi: 10.1523/JNEUROSCI.4764-14.2015. http://dx.doi.org/10.1523/JNEUROSCI.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol and Drugs. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. http://dx.doi.org/10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. http://dx.doi.org/10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Chick CF. Reward processing in the adolescent brain: Individual differences and relation to risk taking. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2015;35(40):13539–13541. doi: 10.1523/JNEUROSCI.2571-15.2015. http://dx.doi.org/10.1523/JNEUROSCI.2571-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. http://dx.doi.org/10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. http://dx.doi.org/10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Herting MM, Seghete KL, Hudson KA, Nagel BJ. High and low sensation seeking adolescents show distinct patterns of brain activity during reward processing. NeuroImage. 2012;66C:184–193. doi: 10.1016/j.neuroimage.2012.11.003. http://dx.doi.org/10.1016/j.neuroimage.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Jones SA, Nagel BJ. Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. Developmental Cognitive Neuroscience. 2015;16:110–120. doi: 10.1016/j.dcn.2015.06.004. http://dx.doi.org/10.1016/j.dcn.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Nagel BJ. Risky decision-making: An FMRI study of youth at high risk for alcoholism. Alcoholism: Clinical and Experimental Research. 2012;36(4):604–615. doi: 10.1111/j.1530-0277.2011.01650.x. http://dx.doi.org/10.1111/j.1530-0277.2011.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neuroscience & Biobehavioral Reviews. 2008;32(1):1–19. doi: 10.1016/j.neubiorev.2007.04.016. http://dx.doi.org/10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- de Water E, Braams BR, Crone EA, Peper JS. Pubertal maturation and sex steroids are related to alcohol use in adolescents. Hormones and Behavior. 2013;63(2):392–397. doi: 10.1016/j.yhbeh.2012.11.018. http://dx.doi.org/10.1016/j.yhbeh.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Rouillard C, Bedard P. 17 Beta-estradiol at a physiological dose acutely increases dopamine turnover in rat brain. European Journal of Pharmacology. 1985;117(2):197–203. doi: 10.1016/0014-2999(85)90604-1. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/4076343. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proceedings of the National Academy of Science of the United States of America. 2007;104(7):2465–2470. doi: 10.1073/pnas.0605569104. http://dx.doi.org/10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain and Cognition. 2014;89:104–111. doi: 10.1016/j.bandc.2014.01.006. http://dx.doi.org/10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Pine DS. Choice selection and reward anticipation: An fMRI study. Neuropsychologia. 2004;42(12):1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. http://dx.doi.org/10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. http://dx.doi.org/10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G. The developmental psychopathology of motivation in adolescence. Developmental Cognitive Neuroscience. 2011;1(4):414–429. doi: 10.1016/j.dcn.2011.07.009. http://dx.doi.org/10.1016/j.dcn.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2009;14(1):60–70. doi: 10.1038/sj.mp.4002086. http://dx.doi.org/10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain and Cognition. 2010;72(1):66–72. doi: 10.1016/j.bandc.2009.10.007. http://dx.doi.org/10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE, Almeida JR, Ferrell RE, Nimgaonkar VL, Mansour H, Phillips ML. PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biological Psychiatry. 2012;71(5):451–457. doi: 10.1016/j.biopsych.2011.10.012. http://dx.doi.org/10.1016/j.biopsych.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Dahl RE. Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(2):162–172. e161–165. doi: 10.1097/00004583-201002000-00010. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/20215938>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Park D, Tanaka M, Rosellini R, Svare B. The testosterone metabolite and neurosteroid 3alpha-androstanediol may mediate the effects of testosterone on conditioned place preference. Psychoneuroendocrinology. 2001;26(7):731–750. doi: 10.1016/s0306-4530(01)00027-0. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/11500254>. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. http://dx.doi.org/10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Manuck SB, Sheu LK, Kuan DC, Votruba-Drzal E, Craig AE, Hariri AR. Parental education predicts corticostriatal functionality in adulthood. Cerebral Cortex. 2011;21(4):896–910. doi: 10.1093/cercor/bhq160. http://dx.doi.org/10.1093/cercor/bhq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacological Reviews. 2010;62(2):155–198. doi: 10.1124/pr.109.002071. http://dx.doi.org/10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Kretsch N, Tackett JL, Tucker-Drob EM. Genetic and environmental influences on testosterone in adolescents: Evidence for sex differences. Developmental Psychobiology. 2014;56(6):1278–1289. doi: 10.1002/dev.21207. http://dx.doi.org/10.1002/dev.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Dahl RE, Holm SM, Jakubcak JL, Ryan ND, Silk JS, Forbes EE. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biological Psychology. 2012;91(3):334–341. doi: 10.1016/j.biopsycho.2012.08.008. http://dx.doi.org/10.1016/j.biopsycho.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Sitnick SL, Shaw DS, Forbes EE. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Research. 2013;214(3):357–364. doi: 10.1016/j.pscychresns.2013.08.005. http://dx.doi.org/10.1016/j.pscychresns.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. 1st. New York: The Guilford Press; 2013. [Google Scholar]
- Hollingshead AB. Four-factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. Journal of Adolescent Health. 2009;45(4):326–334. doi: 10.1016/j.jadohealth.2009.04.001. http://dx.doi.org/10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Cservenka A, Nagel BJ. Binge drinking impacts dorsal striatal response during decision making in adolescents. NeuroImage. 2016;129:378–388. doi: 10.1016/j.neuroimage.2016.01.044. http://dx.doi.org/10.1016/j.neuroimage.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschbaum HH, Ruemer M, Weisshuhn S, Klimesch W. Gender-dependent differences in sensation seeking and social interaction are correlated with saliva testosterone titre in adolescents. Neuroendocrinology Letters. 2006;27(3):315–320. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/16807524>. [PubMed] [Google Scholar]
- Kirillova GP, Vanyukov MM, Gavaler JS, Pajer K, Tarter RE. Substance abuse in parents and their adolescent offspring: The role of sexual maturation and sensation seeking. Journal of Child & Adolescent Substance Abuse. 2001;10:77–89. [Google Scholar]
- Kumar P, Berghorst LH, Nickerson LD, Dutra SJ, Goer FK, Greve DN, Pizzagalli DA. Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience. 2014;266:1–12. doi: 10.1016/j.neuroscience.2014.01.058. http://dx.doi.org/10.1016/j.neuroscience.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. http://dx.doi.org/10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall NR, Sakaki M, Vasunilashorn S, Nga L, Somayajula S, Chen EY, Mather M. Gender differences in reward-related decision processing under stress. Social Cognitive & Affective Neuroscience. 2012;7(4):476–484. doi: 10.1093/scan/nsr026. http://dx.doi.org/10.1093/scan/nsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2011;35(5):1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. http://dx.doi.org/10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Friman P. The DISC Predictive Scales (DPS): Efficiently screening for diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(4):443–449. doi: 10.1097/00004583-200104000-00013. http://dx.doi.org/10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- MacPherson L, Magidson JF, Reynolds EK, Kahler CW, Lejuez CW. Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcoholism, Clinical and Experimental Research. 2010;34(8):1400–1408. doi: 10.1111/j.1530-0277.2010.01223.x. http://dx.doi.org/10.1111/j.1530-0277.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CA, Guenthner G, Bingcang C, Smith WJ, Curry T, Omar HA, Kelly TH. A pilot study: Attention deficit hyperactivity disorder, sensation seeking, and pubertal changes. The Scientific World Journal. 2006;6:637–642. doi: 10.1100/tsw.2006.129. http://dx.doi.org/10.1100/tsw.2006.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(12):1495–1502. doi: 10.1097/00004583-200212000-00022. http://dx.doi.org/10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Martin CA, Mainous AG, 3rd, Curry T, Martin D. Alcohol use in adolescent females: Correlates with estradiol and testosterone. The American Journal on Addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 1999;8(1):9–14. doi: 10.1080/105504999306036. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/10189510>. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Welker KM, Zilioli S, Carre JM. Testosterone and cortisol jointly modulate risk-taking. Psychoneuroendocrinology. 2015;56:88–99. doi: 10.1016/j.psyneuen.2015.02.023. http://dx.doi.org/10.1016/j.psyneuen.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Mhillaj E, Morgese MG, Tucci P, Bove M, Schiavone S, Trabace L. Effects of anabolic-androgens on brain reward function. Frontiers in Neuroscience. 2015;9:295. doi: 10.3389/fnins.2015.00295. http://dx.doi.org/10.3389/fnins.2015.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr PN, Biele G, Heekeren HR. Neural processing of risk. The Journal of Neuroscience. 2010;30(19):6613–6619. doi: 10.1523/JNEUROSCI.0003-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin BC, Phillips ML, Siegle GJ, Buysse DJ, Forbes EE, Franzen PL. Sleep deprivation amplifies striatal activation to monetary reward. Psychological Medicine. 2013;43(10):2215–2225. doi: 10.1017/S0033291712002875. http://dx.doi.org/10.1017/S0033291712002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken JT, Albaugh MD, Botteron KN, Hudziak JJ, Ducharme S. A testosterone-related structural brain phenotype predicts aggressive behavior from childhood to adulthood. Psychoneuroendocrinology. 2016;63:109–118. doi: 10.1016/j.psyneuen.2015.09.021. http://dx.doi.org/10.1016/j.psyneuen.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. http://dx.doi.org/10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Op de Macks ZA, Gunther Moor B, Overgaauw S, Guroglu B, Dahl RE, Crone EA. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Developmental Cognitive Neuroscience. 2011;1(4):506–516. doi: 10.1016/j.dcn.2011.06.003. http://dx.doi.org/10.1016/j.dcn.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L, van Wingen GA, Kooijman SC, Backstrom T, Fernandez G, Hermans EJ. Changes in functioning of mesolimbic incentive processing circuits during the premenstrual phase. Social Cognitive & Affective Neuroscience. 2011;6(5):612–620. doi: 10.1093/scan/nsq071. http://dx.doi.org/10.1093/scan/nsq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/11145319>. [PubMed] [Google Scholar]
- Peper JS, de Reus MA, van den Heuvel MP, Schutter DJ. Short fused? Associations between white matter connections, sex steroids, and aggression across adolescence. Human Brain Mapping. 2015;36(3):1043–1052. doi: 10.1002/hbm.22684. http://dx.doi.org/10.1002/hbm.22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Koolschijn PC, Crone EA. Development of risk taking: Contributions from adolescent testosterone and the orbito-frontal cortex. Journal of Cognitive Neuroscience. 2013;25(12):2141–2150. doi: 10.1162/jocn_a_00445. http://dx.doi.org/10.1162/jocn_a_00445. [DOI] [PubMed] [Google Scholar]
- Peper JS, Mandl RC, Braams BR, de Water E, Heijboer AC, Koolschijn PC, Crone EA. Delay discounting and frontostriatal fiber tracts: A combined DTI and MTR study on impulsive choices in healthy young adults. Cerebral Cortex. 2013;23(7):1695–1702. doi: 10.1093/cercor/bhs163. http://dx.doi.org/10.1093/cercor/bhs163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Jolles DJ, Van Duijvenvoorde AC, Crone EA, Peper JS. The link between testosterone and amygdala-orbitofrontal cortex connectivity in adolescent alcohol use. Psychoneuroendocrinology. 2015;53:117–126. doi: 10.1016/j.psyneuen.2015.01.004. http://dx.doi.org/10.1016/j.psyneuen.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. http://dx.doi.org/10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Piray P, den Ouden HE, van der Schaaf ME, Toni I, Cools R. Dopaminergic modulation of the functional ventrodorsal architecture of the human striatum. Cerebral Cortex. 2015 doi: 10.1093/cercor/bhv243. http://dx.doi.org/10.1093/cercor/bhv243. [DOI] [PubMed]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19(4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/7485811>. [DOI] [PubMed] [Google Scholar]
- Romer D, Hennessy M. A biosocial-affect model of adolescent sensation seeking: The role of affect evaluation and peer-group influence in adolescent drug use. Prevention Science. 2007;8(2):89–101. doi: 10.1007/s11121-007-0064-7. http://dx.doi.org/10.1007/s11121-007-0064-7. [DOI] [PubMed] [Google Scholar]
- Sarvari M, Deli L, Kocsis P, Mark L, Maasz G, Hrabovszky E, Liposits Z. Estradiol and isotype-selective estrogen receptor agonists modulate the mesocortical dopaminergic system in gonadectomized female rats. Brain Research. 2014;1583:1–11. doi: 10.1016/j.brainres.2014.06.020. http://dx.doi.org/10.1016/j.brainres.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Siegeler K, Wistuba J, Damm OS, von Engelhardt N, Sachser N, Kaiser S. Early social instability affects plasma testosterone during adolescence but does not alter reproductive capacity or measures of stress later in life. Physiology & Behavior. 2013;120:143–149. doi: 10.1016/j.physbeh.2013.08.008. http://dx.doi.org/10.1016/j.physbeh.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Smith Chein CJ, Steinberg L. Impact of socio-emotional context, brain development, and pubertal maturation on adolescent risk-taking. Hormones and Behavior. 2013;64(2):323–332. doi: 10.1016/j.yhbeh.2013.03.006. http://dx.doi.org/10.1016/j.yhbeh.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, Sierra Y, Oppler SH, Boettiger CA. Ovarian cycle effects on immediate reward selection bias in humans: A role for estradiol. The Journal of Neuroscience. 2014;34(16):5468–5476. doi: 10.1523/JNEUROSCI.0014-14.2014. http://dx.doi.org/10.1523/JNEUROSCI.0014-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72(1):124–133. doi: 10.1016/j.bandc.2009.07.003. http://dx.doi.org/10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurodevelopment. The Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine. 2013;52(Suppl 2):S7–13. doi: 10.1016/j.jadohealth.2012.05.006. http://dx.doi.org/10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stanton SJ, Liening SH, Schultheiss OC. Testosterone is positively associated with risk taking in the Iowa Gambling Task. Hormones and Behavior. 2011;59(2):252–256. doi: 10.1016/j.yhbeh.2010.12.003. http://dx.doi.org/10.1016/j.yhbeh.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Steele VR, Anderson NE, Claus ED, Bernat EM, Rao V, Assaf M, Kiehl KA. Neuroimaging measures of error-processing: Extracting reliable signals from event-related potentials and functional magnetic resonance imaging. NeuroImage. 2016;132:247–260. doi: 10.1016/j.neuroimage.2016.02.046. http://dx.doi.org/10.1016/j.neuroimage.2016.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: What changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. http://dx.doi.org/10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764–1778. doi: 10.1037/a0012955. http://dx.doi.org/10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-Dimensional proportional system: An approach to cerebral imaging. Stuttgart, New York: Georg Thieme; 1988. [Google Scholar]
- Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Archives of Disease in Childhood. 1976;51(3):170–179. doi: 10.1136/adc.51.3.170. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/952550>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH. Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience. 2016;17:57–67. doi: 10.1016/j.dcn.2015.10.010. http://dx.doi.org/10.1016/j.dcn.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galvan A. The effects of poor quality sleep on brain function and risk taking in adolescence. NeuroImage. 2013;71:275–283. doi: 10.1016/j.neuroimage.2013.01.025. http://dx.doi.org/10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: Genomic- and nongenomic-mediated effects. Journal of Neurochemistry. 1994;62(5):1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/8158125>. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Critical Reviews in Neurobiology. 2000;14(2):131–142. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/11513242>. [PubMed] [Google Scholar]
- Urosevic S, Collins P, Muetzel R, Lim KO, Luciana M. Pubertal status associations with reward and threat sensitivities and subcortical brain volumes during adolescence. Brain and Cognition. 2014;89:15–26. doi: 10.1016/j.bandc.2014.01.007. http://dx.doi.org/10.1016/j.bandc.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meij L, Buunk AP, Almela M, Salvador A. Testosterone responses to competition: The opponent’s psychological state makes it challenging. Biological Psychology. 2010;84(2):330–335. doi: 10.1016/j.biopsycho.2010.03.017. http://dx.doi.org/10.1016/j.biopsycho.2010.03.017. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde AC, Huizenga HM, Somerville LH, Delgado MR, Powers A, Weeda WD, Figner B. Neural correlates of expected risks and returns in risky choice across development. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2015;35(4):1549–1560. doi: 10.1523/JNEUROSCI.1924-14.2015. http://dx.doi.org/10.1523/JNEUROSCI.1924-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde AC, Op de Macks ZA, Overgaauw S, Gunther Moor B, Dahl RE, Crone EA. A cross-sectional and longitudinal analysis of reward-related brain activation: Effects of age, pubertal stage, and reward sensitivity. Brain and Cognition. 2014;89:3–14. doi: 10.1016/j.bandc.2013.10.005. http://dx.doi.org/10.1016/j.bandc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Vermeersch H, T’Sjoen G, Kaufman JM, Vincke J. Estradiol, testosterone, differential association and aggressive and non-aggressive risk-taking in adolescent girls. Psychoneuroendocrinology. 2008a;33(7):897–908. doi: 10.1016/j.psyneuen.2008.03.016. http://dx.doi.org/10.1016/j.psyneuen.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Vermeersch H, T’Sjoen G, Kaufman JM, Vincke J. The role of testosterone in aggressive and non-aggressive risk-taking in adolescent boys. Hormones and Behavior. 2008b;53(3):463–471. doi: 10.1016/j.yhbeh.2007.11.021. http://dx.doi.org/10.1016/j.yhbeh.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Vermeersch H, T’Sjoen G, Kaufman JM, Vincke J. The relationship between sex steroid hormones and behavioural inhibition, (BIS) and behavioural activation (BAS) in adolescent boys and girls. Personality and Individual Differences. 2009;47:3–7. [Google Scholar]
- Vorobyev V, Kwon MS, Moe D, Parkkola R, Hamalainen H. Risk-taking behavior in a computerized driving task: Brain activation correlates of decision-making, outcome, and peer influence in male adolescents. PLoS ONE. 2015;10(6):e0129516. doi: 10.1371/journal.pone.0129516. http://dx.doi.org/10.1371/journal.pone.0129516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach JB. Interpretation of diagnostic tests. 7th. Philadelphia, PA: Lippincott Williams and Wilkins; 2000. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: Effects of estrous cycle and gonadectomy. Neuroscience Letters. 1994;180(2):155–158. doi: 10.1016/0304-3940(94)90510-x. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/7700570>. [DOI] [PubMed] [Google Scholar]
- Zilioli S, Watson NV. Testosterone across successive competitions: Evidence for a ’winner effect’ in humans? Psychoneuroendocrinology. 2014;47:1–9. doi: 10.1016/j.psyneuen.2014.05.001. http://dx.doi.org/10.1016/j.psyneuen.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42(3):509–517. doi: 10.1016/s0896-6273(04)00183-7. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/15134646>. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM. Personality and risk-taking: Common biosocial factors. Journal of Personality. 2000;68(6):999–1029. doi: 10.1111/1467-6494.00124. Retrieved from < http://www.ncbi.nlm.nih.gov/pubmed/11130742>. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM, Joireman J, Teta P, Kraft M. A comparison of three structural models for personality: The big three, the big five, and the alternative five. Journal of Personality and Social Psychology. 1993;65:757–768. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


