Abstract
With nearly 42 million mild traumatic brain injuries (mTBIs) occurring worldwide every year, understanding the factors that may adversely influence recovery after mTBI is important for developing guidelines in mTBI management. Extensive clinical evidence exists documenting the detrimental effects of elevated temperature levels on recovery after moderate to severe TBI. However, whether elevated temperature alters recovery after mTBI or concussion is an active area of investigation. Individuals engaged in exercise and competitive sports regularly experience body and brain temperature increases to hyperthermic levels and these temperature increases are prolonged in hot and humid ambient environments. Thus, there is a strong potential for hyperthermia to alter recovery after mTBI in a subset of individuals at risk for mTBI. Preclinical mTBI studies have found that elevating brain temperature to 39°C before mTBI significantly increases neuronal death within the cortex and hippocampus and also worsens cognitive deficits. This review summarizes the pathology and behavioral problems of mTBI that are exacerbated by hyperthermia and discusses whether hyperthermia is a variable that should be considered after concussion and mTBI. Finally, underlying pathophysiological mechanisms responsible for hyperthermia-induced altered responses to mTBI and potential gender considerations are discussed.
Keywords: concussion, fluid-percussion brain injury, hyperthermia, hypothermia, temperature, traumatic brain injury
Introduction
Mild traumatic brain injuries (mTBIs) and concussions occur in about 100 to 300 people per 100,000 annually worldwide 1. This public health problem is underestimated because an estimated 50% of people with a concussion do not seek medical attention 2. This places the annual incidence of mTBI and concussion at about 42 million people worldwide 3. The current definition of concussion as recommended by the 4th International Conference on Concussion in Sport in 2012 is “concussion is a brain injury and is defined as a complex pathophysiological process affecting the brain, induced by biomechanical forces” 4. This dysfunction is mediated by a cascade of pathophysiological responses to the injury that ultimately alters cerebral function without necessarily causing concurrent macrostructural damage 4. For the purposes of this review, we will use concussion and mTBI synonymously, as a non-penetrating head injury that alters brain functioning with no or brief loss of consciousness 5. Other than management of symptoms, the patient with mTBI is typically recommended to rest until symptoms resolve, followed by a gradual increase in return to normal activity 6. Concussions and mTBIs are a significant clinical problem yet lack effective evidence-based therapeutics 2.
Concussions and mTBIs can result in significant cognitive, psychosocial, and physical issues, although many of these symptoms resolve rapidly. Clinical assessments of learning and memory ability have revealed that a single concussion can result in significant impairments in working memory, attention and concentration issues, and decreased processing and reaction time 7. However, several studies have demonstrated that mTBI may also result in chronic cognitive impairments lasting for weeks after injury 2, 8, 9. Additionally, many have more chronic physical symptoms such as headaches, vestibular issues, irritability, depression, and fatigue, collectively referred to as post-concussion syndrome 10– 12. Although there is some variability in the recovery trajectory, for 50%–60% of individuals with mTBI, symptoms will typically resolve in 7 to 10 days 8. However, imaging and more objective clinical assessments indicate that abnormalities in the brain may persist beyond 30 days after mTBI 13.
Prevalence of hyperthermia during mild traumatic brain injury
An aspect of mTBI that has been understudied and may affect recovery is the effect of temperature. It is well known in severe and moderate TBI that brain temperature has a profound effect on outcome 14, 15. If the patient is hypothermic, outcome is improved, but if the patient is hyperthermic, outcome is greatly worsened. However, hyperthermia has not been typically considered to be an important variable in mTBI studies despite the potential co-occurrence of hyperthermia with mTBI.
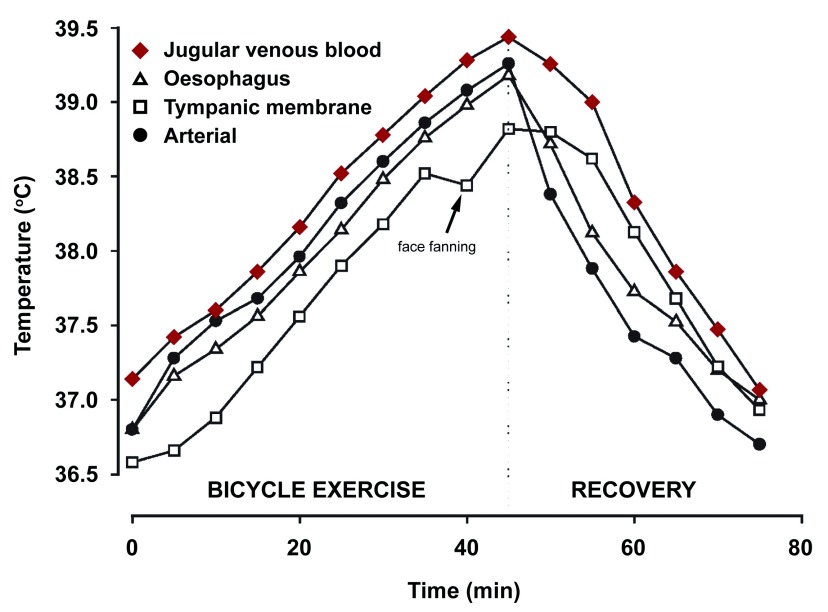
Many athletes experience mTBIs in the context of mild hyperthermia. Strenuous exercise can raise core temperature to 39–40°C, and this temperature increase is more long-lasting in hot environments 16– 18. Exercise-induced hyperthermia slowly recovers once an athlete has stopped exercising 19, 20. If core temperature has risen to 39–40°C, this temperature naturally returns to normothermic levels within 60 minutes to 3 hours after exercise has ceased 19, 21. The brain in particular is slow to cool, and published studies have found that after 1 hour of bicycle exercise, the jugular venous blood, a close approximation of brain temperature, is still significantly hyperthermic even 60 minutes after exercise has ceased ( Figure 1) 20.
Figure 1. Temperature responses during cycling.
Jugular venous blood temperature most closely reflects brain tissue temperature and is still elevated at 60 minutes during recovery (arrow). Figure reproduced with permission 20.
Another contribution to hyperthermia during mTBI is a hot ambient environment. Military personnel in Iraq and Afghanistan, countries with high environmental temperatures, are at risk to be hyperthermic when injured 22. The combination of high exertion and warm humid environments has the potential to raise the risk of experiencing mTBI while hyperthermic even further 16– 18. Thus, an mTBI experienced in the context of hyperthermia can be a common occurrence due to high environmental temperatures and, in particular, among people who are engaged in activities with a high relative risk for head injury.
Only a small proportion of energy produced by muscle metabolism is used by work, whereas over 75%–80% of the energy released by muscle metabolism is heat 23. Evaporation is effective in dissipating large amounts of heat and this limits core-temperature increases to 2–3°C in trained athletes 18, 24. Despite these natural cooling mechanisms, increases in core temperature to 39–40°C are commonly observed in people because of high-intensity activity 18, 25. Heat dissipation is also slowed by the environment temperature. Adaptation is well known to occur with exercise in warm environments and these physiological adaptations improve performance, but core temperature still rises to mildly hyperthermic levels with exercise in heat-adapted individuals 23, 24.
The brain is not protected against increases in core temperature and, in fact, stays warmer for a longer period of time after a rise in core temperature to hyperthermic levels 21. Exercise increases cerebral temperature because physical activity results in an increase in heat production of the muscles, which elevates arterial blood temperature. Heat removal from the brain is mediated by the heat capacity of the blood, cerebral blood blow, and the arterio-venous blood temperature difference. Increased arterial blood temperature elevates cerebral temperature at the same rate as the rise in core temperature 20. However, owing to the limited heat removal capacity of the brain, brain temperature returns more slowly than core temperature and is still hyperthermic for up to 60 minutes with passive rest after exercise 20.
Assessing brain temperature after an mTBI is complicated by the dissociation of brain temperature from oral, rectal, or tympanic membrane temperature 26, 27. Indeed, these temperature readings can be 1–3°C lower than brain temperature ( Figure 1). Temperature measurements from alternative locations such as the temporal artery more accurately reflect brain temperature but are not commonly used 26, 27. Thus, brain hyperthermia may be a likely variable at the time of mTBI, but studies assessing this variable in patients with mTBI are lacking.
Effects of hyperthermia
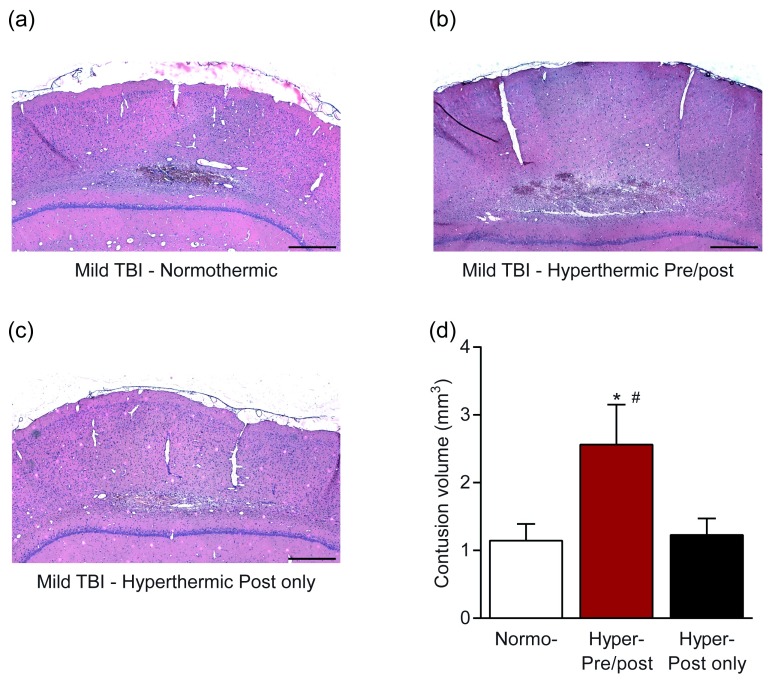
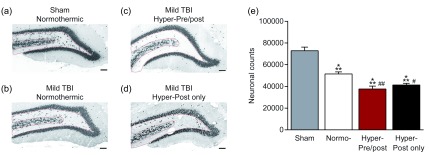
The detrimental consequences of post-traumatic hyperthermia following more moderate and severe TBI have been reported by multiple laboratories and previously summarized 14, 28. In various animal models of TBI, induced periods of mild hyperthermia at various post-traumatic time periods significantly aggravate histopathological and behavioral outcomes 29. In clinical investigations where severely brain-injured patients have been monitored, periods of fever are common and also result in worsening of functional outcomes 30. In contrast to these findings, the consequences of hyperthermia on mTBI and concussion symptoms and recovery are a fairly new and active area of investigation 31. To address this important question, we determined whether hyperthermia would also affect pathological and cognitive outcome after mTBI. We used a model well established to recapitulate several aspects of human mTBI, mild fluid-percussion brain injury, where saline is briefly pulsed on the dura of the parietal cortex. Using this preclinical model of mTBI in rats, mild fluid-percussion brain injury, we found that mTBI in the context of mild hyperthermia (39°C) both pre- and post-injury resulted in a significant exacerbation of pathology ( Figure 2) as assessed by cortical contusion volume 32. The mTBI pathology converted into pathology that was nearly equivalent to the pathology observed in moderate TBI. However, hyperthermia after, but not prior to or during, mTBI did not worsen cortical contusion volume. The exacerbation of pathology due to hyperthermia also occurred in the hippocampus, and increased neuronal loss occurred in mTBI animals that were hyperthermic both pre- and post-injury ( Figure 3). Furthermore, hyperthermia in only the post-injury period also increased hilar neuronal loss. Thus, preclinical studies demonstrate that mTBI experienced while hyperthermic significantly exacerbates neuronal loss and cortical damage.
Figure 2. Exacerbation of cortical contusion volume with hyperthermia after mild traumatic brain injury (TBI) in an experimental model of TBI in rats.
Animals received mild fluid-percussion brain injury while ( a) normothermic (37°C), ( b) hyperthermic (39°C) beginning 15 minutes prior to mild TBI and for 2 hours after injury, or ( c) hyperthermic (39°C) only for 2 hours after injury. ( d) Brains were sectioned and stained with hematoxylin and eosin to visualize and quantify cortical contusion volume. Scale bars = 300 µm. * P <0.05 versus normothermic, # P <0.05 versus hyperthermic post only. Adapted from 32.
Figure 3. Increased hippocampal cell loss after mild traumatic brain injury (TBI) and mild hyperthermia.
Animals received ( a) sham surgery, mild fluid-percussion brain injury while ( b) normothermic (37°C), ( c) hyperthermic (39°C) beginning 15 minutes prior to mild TBI and for 2 hours after injury, or ( d) hyperthermic (39°C) only for 2 hours after injury. ( e) Neuronal loss in the dentate hilus was quantified by stereology in NeuN-immunostained sections. Scale bars = 300 µm. *** P <0.001 versus Sham, # P <0.05, ## P <0.001 versus normothermic. Adapted from 32.
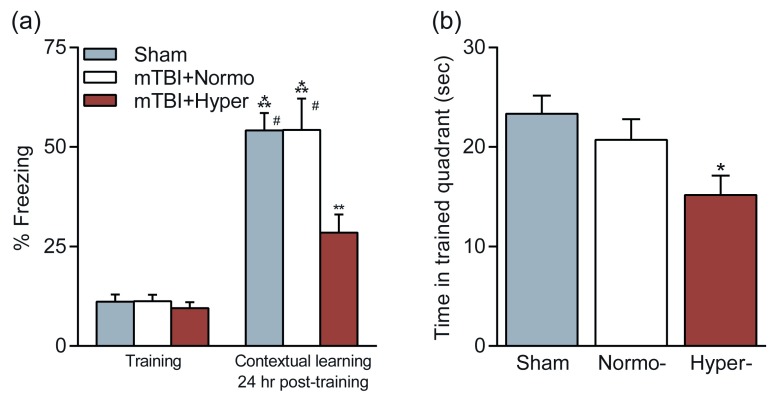
This conversion of pathology also resulted in an exacerbation of cognitive impairments 33. Animals that received mTBI when normothermic had no significant learning or retention deficits in contextual fear conditioning or the water maze ( Figure 4). However, animals that received mTBI while hyperthermic (39°C) had significant deficits in both hippocampal-dependent learning tasks as compared with animals that received mTBI during normothermic conditions or sham controls. Thus, a relatively mild elevation in brain temperature at the time of an mTBI causes hippocampal-dependent behavioral deficits. We tested whether reducing brain and body temperature to normal physiological temperatures 15 minutes after an mTBI would prevent the development of behavioral deficits. We found that animals that received an mTBI with mild hyperthermia but then were cooled to normothermic levels at 15 minutes post-TBI did not exhibit deficits in the water maze or with contextual fear conditioning and performed at sham, non-injured levels ( Figure 4). These results may have important implications in the treatment of mTBI for athletes in warm ambient environments. The simple treatment of cooling rapidly after mTBI has the potential to prevent the development of hippocampal-dependent learning deficits.
Figure 4. Effects of temperature manipulations on cognitive outcome after mild traumatic brain injury (mTBI).
Animals received mild fluid-percussion brain injury while normothermic (37°C, mTBI+Normo) or hyperthermic (39°C, mTBI+Hyper). Cognition was assessed at 2–3 weeks post-injury and compared with non-injured, sham animals. ( a) Contextual fear conditioning was unaffected by mTBI but impaired in hyperthermic mTBI animals. ( b) Water maze performance on the probe trial to assess retention of spatial learning. Time spent in the trained quadrant was significantly decreased in hyperthermic mTBI animals as compared with sham animals. Figure reproduced with permission 33.
Although the current discussion focused primarily of elevated brain temperature after brain injury, it is known that elevations in brain temperature under stressful conditions may have important biological effects independent of injury mechanisms 34, 35.
Temperature-sensitive biomarkers
The failure of multiple phase 3 clinical trials for TBI has directed National Institutes of Health and US Department of Defense working groups to conclude that non-invasive measures of therapeutic efficacy need to be developed to demonstrate that the therapy is engaging its proposed molecular target(s) and resulting in a biological effect 36. Determining whether a biomarker panel detects mTBI and is sensitive to temperature manipulations would greatly improve clinical guidance 37, 38. Efforts to develop biomarkers released from the injured brain and detected in the serum for mTBI have been hampered by the lack of sensitivity to concussion and the short half-life of most brain-derived biomarkers of mTBI 39, 40. Biomarkers sensitive to injury severity for TBI include glial fibrillary acidic protein (GFAP), which is released from injured astrocytes, and ubiquitin C-terminal hydrolase L1 (UCH-L1) 41, 42. However, both are limited in utility because of the relatively short half-life of the biomarkers. Other promising biomarkers that are being evaluated for their diagnostic utility for mTBI include auto-antibody to GFAP, auto-antibody to S100β, tau, select microRNAs (miRNAs), and plasma soluble cellular prion protein 43– 47. Preclinical and clinical studies have demonstrated that several of these biomarkers are sensitive to therapeutic hypothermia in moderate to severe TBI. In particular, GFAP and UCH-L1 are downregulated after therapeutic hypothermia in preclinical models of moderate TBI 48, 49. Therapeutic hypothermia decreases levels of GFAP in adult patients with severe TBI but not in pediatric patients with TBI 50, 51. However, both GFAP and UCH-L1 are limited in utility for mTBI because of the relatively short half-life of these biomarkers. Other potential biomarkers that are temperature-sensitive in moderate to severe TBI include neuron-specific enolase, S-100, brain-specific creatine kinase, and several miRNAs 52– 54. Whether they are regulated by temperature after concussion or mTBI remains to be established. Pro-inflammatory cytokines are highly sensitive to temperature manipulations after TBI, including hyperthermia 55– 59. However, cytokines are limited in utility for mTBI since mTBI often can be complicated by systemic injuries and these biomarkers do not differentiate well between brain-derived versus systemically derived sources. Biomarkers known to be sensitive to temperature in other studies include MMP-9 and HSP70 and merit study for mTBI 60– 63.
Interaction of gender and temperature as critical variables for traumatic brain injury outcome
The importance of gender on traumatic and ischemic outcome has been documented in various models of brain injury 64– 66. In one study, female rats after moderate fluid-percussion injury (FPI) demonstrated significantly smaller contusion volumes compared with male rats 66. In that study following ovariectomy, contusion volumes in females were not significantly different than those in males. These and other data emphasize the importance of estrogen and progesterone in the pathogenesis of TBI 64. Recent studies have also demonstrated that alterations in post-traumatic temperature have gender-specific effects on outcomes after moderate TBI. For example, Suzuki and colleagues 67 reported that while post-traumatic hypothermia following moderate FPI provided neuroprotection, no significant effect on contusion volume was seen in female rats. Thus, there appear to be gender-specific effects of temperature manipulations in preclinical models of brain injury.
In reference to the present discussion, more recent studies have evaluated the effects of post-traumatic hyperthermia in models of moderate TBI. In this regard, Suzuki and colleagues 66 reported that an induced period of mild hyperthermia after moderate TBI increased contusion volumes, cortical neuronal cell death, and axonal damage in intact female rats. Interestingly, in that study, the effects of post-traumatic hyperthermia were more pronounced in ovariectomized animals. These results therefore emphasize that hyperthermia worsens outcomes after moderate TBI in female rats and that neural hormones may protect against secondary hyperthermic insults. Future studies are required to determine whether gender is also an important factor in the pathogenesis of mTBI or concussion and the detrimental effects of hyperthermia on functional outcomes.
Underlying temperature-sensitive pathomechanisms and future research directions
The pathophysiology of TBI is complex and involves multiple injury cascades that have been reported to be temperature-sensitive 14, 28, 68. In regard to post-traumatic hypothermia, numerous reports have shown that lowering brain temperature after a moderate to severe TBI reduces excitotoxicity, free radical generation, apoptosis, and neuroinflammation 28, 69– 71. In the area of inflammation, post-traumatic hypothermia after moderate FPI reduces blood-brain barrier permeability to both large and small tracers and the infiltration of CD68-positive cells 72. Other studies have also shown that whereas hypothermia reduced the accumulation of polymorphonuclear leukocyte infiltration after TBI, post-traumatic hyperthermia increased inflammatory cell accumulation 73. These studies are also in agreement with post-traumatic temperature modifications (for example, that periods of hyperthermia significantly alter levels of several pro-inflammatory cytokines and inflammasome proteins) 74.
Most recently, the effects of post-traumatic hypothermia on microglial and macrophage phenotype polarization have been investigated after moderate TBI 75. In that study, temperature-sensitive effects on the various subsets of pro-inflammatory (M1) and anti-inflammatory (M2) microglia and macrophages were determined by using flow cytometry and reverse transcription–polymerase chain reaction. This study provided a link between temperature-sensitive alterations in macrophage/microglia activation and polarization toward an M2 phenotype with hypothermia that could be permissive for cell survival and repair. Studies to determine whether mild hyperthermic TBI also alters microglial and macrophage polarization are under way. Taken together, these studies emphasize the importance of post-traumatic temperature on pro-inflammatory signaling after brain injury.
Diffuse axonal injury (DAI) is a common consequence of mild and moderate TBI and is considered to be significantly related to the resulting functional deficit 76– 78. Previous studies have reported the importance of post-traumatic temperature in the frequency of damaged axons by using a variety of immunocytochemical techniques such as beta-amyloid precursor protein (β-APP). For example, after moderate TBI, hypothermia reduced the frequency of axonal damage at early post-traumatic time periods in several animal models 79. In reference to the effects of hyperthermia on DAI, Suzuki and colleagues 66 reported that post-traumatic hyperthermia after moderate TBI significantly increased axonal damage as indicated by β-APP in both intact and ovariectomized female rats. Following mTBI or concussion, evidence for white matter perturbations has been reported in animal models as well as clinical investigations 80– 82. Future studies are required to determine the effects of hyperthermia on DAI in models of mTBI and concussion.
An important cause of persistent behavioral problems after mTBI is pituitary dysfunction. Hypopituitarism has a prevalence rate of 25%–50% of patients with TBI overall and was found to occur in 37.5% of patients with mTBI 83, 84. Whether temperature at the time of injury alters this prevalence rate has yet to be studied. Interestingly, in an animal model of mTBI, core-temperature regulation was found to be disrupted in the post-injury recovery period during exercise 85. Understanding the interaction of pituitary dysfunction and temperature at the time of mTBI may facilitate clinical guidance in identifying which patients with mTBI need to be screened for potential pituitary dysfunction.
Conclusions
mTBI or concussion is a serious and fairly common medical problem that can affect all age groups and produce both short- and long-term consequences. Because of the high incidence of concussion in athletes and military personnel, a great appreciation of the detrimental effects of single and multiple concussions has led to more research on this particular type of brain injury. Recent studies have implicated gray and white matter pathologies that result in a spectrum of neurological symptoms. Complicated concussions that lead to long-term disturbances in memory function or other problems require patients to undergo extensive rehabilitation strategies to retrain the nervous system. In parallel, new treatment strategies that target selective neurotransmitter systems and secondary injury mechanisms are being tested in animal models and in some clinical studies.
It now appears from experimental and clinical work that small alterations in brain temperature at the time of an mTBI or concussion may be among several factors that can affect the brain’s response to injury through altering secondary injury mechanisms. Therefore, mild levels of hyperthermia that can occur in people during periods of strenuous activity or exercise may be considered a risk factor for more severe or long-term functional problems. In regard to potential treatment strategies, normalizing brain temperature after a hyperthermic brain injury appears to reduce the degree of structural damage and reduce behavioral outcomes. In this regard, new technologies are being used to develop cooling helmets to reduce brain temperature in acute injury settings such as brain trauma. Also, established targeted temperature management approaches currently being used in severely injured patients should be considered for individuals with milder brain injuries. In the future, new pharmacological strategies that target specific injury cascades that have been shown to be aggravated with elevated temperature need to be identified and tested in clinically relevant concussion models. In this regard, it might be reasonable to consider first testing US Food and Drug Administration–approved drugs that have shown some promise in clinical studies and trials for severe TBI. Only through continued research will new prevention and treatment strategies be discovered and tested to minimize the potentially devastating consequences of mTBI and concussion.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Peter Andrews, Department of Intensive Care / Department of Anaesthetics, University of Edinburgh, Edinburgh, UK
Jiyao Jiang, Department of Neurosurgery, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Funding Statement
This study was supported by National Institutes of Health grants R01 NS042133, NS056072, NS069721, and NS089443.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Cassidy JD, Carroll LJ, Peloso PM, et al. : Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004; (43 Suppl):28–60. 10.1080/16501960410023732 [DOI] [PubMed] [Google Scholar]
- 2. Harmon KG, Drezner JA, Gammons M, et al. : American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47(1):15–26. 10.1136/bjsports-2012-091941 [DOI] [PubMed] [Google Scholar]
- 3. Setnik L, Bazarian JJ: The characteristics of patients who do not seek medical treatment for traumatic brain injury. Brain Inj. 2007;21(1):1–9. 10.1080/02699050601111419 [DOI] [PubMed] [Google Scholar]
- 4. McCrory P, Meeuwisse W, Aubry M, et al. : Consensus statement on Concussion in Sport - The 4th International Conference on Concussion in Sport held in Zurich, November 2012. Phys Ther Sport. 2013;14(2):e1–e13. 10.1016/j.ptsp.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 5. Voss JD, Connolly J, Schwab KA, et al. : Update on the Epidemiology of Concussion/Mild Traumatic Brain Injury. Curr Pain Headache Rep. 2015;19(7):32. 10.1007/s11916-015-0506-z [DOI] [PubMed] [Google Scholar]
- 6. Levin HS, Diaz-Arrastia RR: Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015;14(5):506–17. 10.1016/S1474-4422(15)00002-2 [DOI] [PubMed] [Google Scholar]
- 7. Karlin AM: Concussion in the pediatric and adolescent population: "different population, different concerns". PM R. 2011;3(10 Suppl 2):S369–79. 10.1016/j.pmrj.2011.07.015 [DOI] [PubMed] [Google Scholar]
- 8. McCrea M, Guskiewicz KM, Marshall SW, et al. : Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2556–63. 10.1001/jama.290.19.2556 [DOI] [PubMed] [Google Scholar]
- 9. De Beaumont L, Henry LC, Gosselin N: Long-term functional alterations in sports concussion. Neurosurg Focus. 2012;33(6):E8: 1–7. 10.3171/2012.9.FOCUS12278 [DOI] [PubMed] [Google Scholar]
- 10. Valovich McLeod TC, Register-Mihalik JK: Clinical outcomes assessment for the management of sport-related concussion. J Sport Rehabil. 2011;20(1):46–60. 10.1123/jsr.20.1.46 [DOI] [PubMed] [Google Scholar]
- 11. Blume HK, Lucas S, Bell KR: Subacute concussion-related symptoms in youth. Phys Med Rehabil Clin N Am. 2011;22(4):665–81, viii-ix. 10.1016/j.pmr.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 12. Meares S, Shores EA, Taylor AJ, et al. : The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology. 2011;25(4):454–65. 10.1037/a0022580 [DOI] [PubMed] [Google Scholar]
- 13. Vagnozzi R, Signoretti S, Cristofori L, et al. : Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133(11):3232–42. 10.1093/brain/awq200 [DOI] [PubMed] [Google Scholar]
- 14. Dietrich WD, Bramlett HM: Hyperthermia and central nervous system injury. Prog Brain Res. 2007;162:201–17. 10.1016/S0079-6123(06)62011-6 [DOI] [PubMed] [Google Scholar]
- 15. Dietrich WD, Bramlett HM: The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7(1):43–50. 10.1016/j.nurt.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nybo L: Hyperthermia and fatigue. J Appl Physiol (1985). 2008;104(3):871–8. 10.1152/japplphysiol.00910.2007 [DOI] [PubMed] [Google Scholar]
- 17. Goosey-Tolfrey V, Swainson M, Boyd C, et al. : The effectiveness of hand cooling at reducing exercise-induced hyperthermia and improving distance-race performance in wheelchair and able-bodied athletes. J Appl Physiol (1985). 2008;105(1):37–43. 10.1152/japplphysiol.01084.2007 [DOI] [PubMed] [Google Scholar]
- 18. Ozgünen KT, Kurdak SS, Maughan RJ, et al. : Effect of hot environmental conditions on physical activity patterns and temperature response of football players. Scand J Med Sci Sports. 2010;20 Suppl 3:140–7. 10.1111/j.1600-0838.2010.01219.x [DOI] [PubMed] [Google Scholar]
- 19. DeMartini JK, Ranalli GF, Casa DJ, et al. : Comparison of body cooling methods on physiological and perceptual measures of mildly hyperthermic athletes. J Strength Cond Res. 2011;25(8):2065–74. 10.1519/JSC.0b013e3182259b1d [DOI] [PubMed] [Google Scholar]
- 20. Nybo L, Secher NH, Nielsen B: Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol. 2002;545(Pt 2):697–704. 10.1113/jphysiol.2002.030023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nybo L: Brain temperature and exercise performance. Exp Physiol. 2012;97(3):333–9. 10.1113/expphysiol.2011.062273 [DOI] [PubMed] [Google Scholar]
- 22. Wade CE, Salinas J, Eastridge BJ, et al. : Admission hypo- or hyperthermia and survival after trauma in civilian and military environments. Int J Emerg Med. 2011;4(1):35. 10.1186/1865-1380-4-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maughan RJ, Shirreffs SM, Ozgünen KT, et al. : Living, training and playing in the heat: challenges to the football player and strategies for coping with environmental extremes. Scand J Med Sci Sports. 2010;20 Suppl 3:117–24. 10.1111/j.1600-0838.2010.01221.x [DOI] [PubMed] [Google Scholar]
- 24. Yeargin SW, Casa DJ, Judelson DA, et al. : Thermoregulatory responses and hydration practices in heat-acclimatized adolescents during preseason high school football. J Athl Train. 2010;45(2):136–46. 10.4085/1062-6050-45.2.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Armstrong LE, Johnson EC, Casa DJ, et al. : The American football uniform: uncompensable heat stress and hyperthermic exhaustion. J Athl Train. 2010;45(2):117–27. 10.4085/1062-6050-45.2.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirk D, Rainey T, Vail A, et al. : Infra-red thermometry: the reliability of tympanic and temporal artery readings for predicting brain temperature after severe traumatic brain injury. Crit Care. 2009;13(3):R81. 10.1186/cc7898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuo JR, Lo CJ, Wang CC, et al. : Measuring brain temperature while maintaining brain normothermia in patients with severe traumatic brain injury. J Clin Neurosci. 2011;18(8):1059–63. 10.1016/j.jocn.2010.11.014 [DOI] [PubMed] [Google Scholar]
- 28. Dietrich WD, Atkins CM, Bramlett HM: Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J Neurotrauma. 2009;26(3):301–12. 10.1089/neu.2008.0806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dietrich WD, Alonso O, Halley M, et al. : Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery. 1996;38(3):533–41; discussion 541. 10.1097/00006123-199603000-00023 [DOI] [PubMed] [Google Scholar]
- 30. Kilpatrick MM, Lowry DW, Firlik AD, et al. : Hyperthermia in the neurosurgical intensive care unit. Neurosurgery. 2000;47(4):850–5; discussion 855-6. 10.1097/00006123-200010000-00011 [DOI] [PubMed] [Google Scholar]
- 31. Kochanek PM, Jackson TC: It might be time to let cooler heads prevail after mild traumatic brain injury or concussion. Exp Neurol. 2015;267:13–7. 10.1016/j.expneurol.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 32. Sakurai A, Atkins CM, Alonso OF, et al. : Mild hyperthermia worsens the neuropathological damage associated with mild traumatic brain injury in rats. J Neurotrauma. 2012;29(2):313–21. 10.1089/neu.2011.2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Titus DJ, Furones C, Atkins CM, et al. : Emergence of cognitive deficits after mild traumatic brain injury due to hyperthermia. Exp Neurol. 2015;263:254–62. 10.1016/j.expneurol.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang H, Wang B, Normoyle KP, et al. : Brain temperature and its fundamental properties: a review for clinical neuroscientists. Front Neurosci. 2014;8:307. 10.3389/fnins.2014.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gisolfi CV, Mora F: The Hot Brain.The MIT Press: Boston.2000. Reference Source [Google Scholar]
- 36. Diaz-Arrastia R, Kochanek PM, Bergold P, et al. : Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J Neurotrauma. 2014;31(2):135–58. 10.1089/neu.2013.3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adamczak S, Dale G, de Rivero Vaccari JP, et al. : Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg. 2012;117(6):1119–25. 10.3171/2012.9.JNS12815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DeFazio MV, Rammo RA, Robles JR, et al. : The potential utility of blood-derived biochemical markers as indicators of early clinical trends following severe traumatic brain injury. World Neurosurg. 2014;81(1):151–8. 10.1016/j.wneu.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Di Battista AP, Rhind SG, Baker AJ: Application of blood-based biomarkers in human mild traumatic brain injury. Front Neurol. 2013;4:44. 10.3389/fneur.2013.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zetterberg H, Smith DH, Blennow K: Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol. 2013;9(4):201–10. 10.1038/nrneurol.2013.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Okonkwo DO, Yue JK, Puccio AM, et al. : GFAP-BDP as an acute diagnostic marker in traumatic brain injury: results from the prospective transforming research and clinical knowledge in traumatic brain injury study. J Neurotrauma. 2013;30(17):1490–7. 10.1089/neu.2013.2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Papa L, Brophy GM, Welch RD, et al. : Time Course and Diagnostic Accuracy of Glial and Neuronal Blood Biomarkers GFAP and UCH-L1 in a Large Cohort of Trauma Patients With and Without Mild Traumatic Brain Injury. JAMA Neurol. 2016;73(5):551–60. 10.1001/jamaneurol.2016.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Pham N, Akonasu H, Shishkin R, et al. : Plasma soluble prion protein, a potential biomarker for sport-related concussions: a pilot study. PLoS One. 2015;10(2):e0117286. 10.1371/journal.pone.0117286 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Shahim P, Tegner Y, Wilson DH, et al. : Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 2014;71(6):684–92. 10.1001/jamaneurol.2014.367 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Bhomia M, Balakathiresan NS, Wang KK, et al. : A Panel of Serum MiRNA Biomarkers for the Diagnosis of Severe to Mild Traumatic Brain Injury in Humans. Sci Rep. 2016;6:28148. 10.1038/srep28148 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Zhang Z, Zoltewicz JS, Mondello S, et al. : Human traumatic brain injury induces autoantibody response against glial fibrillary acidic protein and its breakdown products. PLoS One. 2014;9(3):e92698. 10.1371/journal.pone.0092698 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Marchi N, Bazarian JJ, Puvenna V, et al. : Consequences of repeated blood-brain barrier disruption in football players. PLoS One. 2013;8(3):e56805. 10.1371/journal.pone.0056805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yokobori S, Gajavelli S, Mondello S, et al. : Neuroprotective effect of preoperatively induced mild hypothermia as determined by biomarkers and histopathological estimation in a rat subdural hematoma decompression model. J Neurosurg. 2013;118(2):370–80. 10.3171/2012.10.JNS12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao WY, Chen SB, Wang JJ, et al. : Establishment of an ideal time window model in hypothermic-targeted temperature management after traumatic brain injury in rats. Brain Res. 2017;1669:141–9. 10.1016/j.brainres.2017.06.006 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Lei J, Gao G, Feng J, et al. : Glial fibrillary acidic protein as a biomarker in severe traumatic brain injury patients: a prospective cohort study. Crit Care. 2015;19:362. 10.1186/s13054-015-1081-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Fraser DD, Close TE, Rose KL, et al. : Severe traumatic brain injury in children elevates glial fibrillary acidic protein in cerebrospinal fluid and serum. Pediatr Crit Care Med. 2011;12(3):319–24. 10.1097/PCC.0b013e3181e8b32d [DOI] [PubMed] [Google Scholar]
- 52. Li H, Lu G, Shi W, et al. : Protective effect of moderate hypothermia on severe traumatic brain injury in children. J Neurotrauma. 2009;26(11):1905–9. 10.1089/neu.2008.0828 [DOI] [PubMed] [Google Scholar]
- 53. Wang Y, Guo F, Pan C, et al. : Effects of low temperatures on proliferation-related signaling pathways in the hippocampus after traumatic brain injury. Exp Biol Med (Maywood). 2012;237(12):1424–32. 10.1258/ebm.2012.012123 [DOI] [PubMed] [Google Scholar]
- 54. Truettner JS, Alonso OF, Bramlett HM, et al. : Therapeutic hypothermia alters microRNA responses to traumatic brain injury in rats. J Cereb Blood Flow Metab. 2011;31(9):1897–907. 10.1038/jcbfm.2011.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang QW, Lu FL, Zhou Y, et al. : HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. J Cereb Blood Flow Metab. 2011;31(2):593–605. 10.1038/jcbfm.2010.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leira R, Rodríguez-Yáñez M, Castellanos M, et al. : Hyperthermia is a surrogate marker of inflammation-mediated cause of brain damage in acute ischaemic stroke. J Intern Med. 2006;260(4):343–9. 10.1111/j.1365-2796.2006.01694.x [DOI] [PubMed] [Google Scholar]
- 57. Kinoshita K, Chatzipanteli K, Vitarbo E, et al. : Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery. 2002;51(1):195–203; discussion 203. 10.1097/00006123-200207000-00027 [DOI] [PubMed] [Google Scholar]
- 58. Wang KY, Yu GF, Zhang ZY, et al. : Plasma high-mobility group box 1 levels and prediction of outcome in patients with traumatic brain injury. Clin Chim Acta. 2012;413(21–22):1737–41. 10.1016/j.cca.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 59. Liu T, Zhao DX, Cui H, et al. : Therapeutic hypothermia attenuates tissue damage and cytokine expression after traumatic brain injury by inhibiting necroptosis in the rat. Sci Rep. 2016;6:24547. 10.1038/srep24547 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Žurek J, Fedora M: The usefulness of S100B, NSE, GFAP, NF-H, secretagogin and Hsp70 as a predictive biomarker of outcome in children with traumatic brain injury. Acta Neurochir (Wien). 2012;154(1):93–103; discussion 103. 10.1007/s00701-011-1175-2 [DOI] [PubMed] [Google Scholar]
- 61. Campos F, Sobrino T, Vieites-Prado A, et al. : Hyperthermia in human ischemic and hemorrhagic stroke: similar outcome, different mechanisms. PLoS One. 2013;8(11):e78429. 10.1371/journal.pone.0078429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jia F, Pan YH, Mao Q, et al. : Matrix metalloproteinase-9 expression and protein levels after fluid percussion injury in rats: the effect of injury severity and brain temperature. J Neurotrauma. 2010;27(6):1059–68. 10.1089/neu.2009.1067 [DOI] [PubMed] [Google Scholar]
- 63. Meng Q, He C, Shuaib A, et al. : Hyperthermia worsens ischaemic brain injury through destruction of microvessels in an embolic model in rats. Int J Hyperthermia. 2012;28(1):24–32. 10.3109/02656736.2011.631963 [DOI] [PubMed] [Google Scholar]
- 64. Roof RL, Duvdevani R, Heyburn JW, et al. : Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp Neurol. 1996;138(2):246–51. 10.1006/exnr.1996.0063 [DOI] [PubMed] [Google Scholar]
- 65. Bramlett HM, Dietrich WD: Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 2001;18(9):891–900. 10.1089/089771501750451811 [DOI] [PubMed] [Google Scholar]
- 66. Suzuki T, Bramlett HM, Ruenes G, et al. : The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J Neurotrauma. 2004;21(7):842–53. 10.1089/0897715041526186 [DOI] [PubMed] [Google Scholar]
- 67. Suzuki T, Bramlett HM, Dietrich WD: The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp Neurol. 2003;184(2):1017–26. 10.1016/S0014-4886(03)00389-3 [DOI] [PubMed] [Google Scholar]
- 68. Jackson TC, Manole MD, Kotermanski SE, et al. : Cold stress protein RBM3 responds to temperature change in an ultra-sensitive manner in young neurons. Neuroscience. 2015;305:268–78. 10.1016/j.neuroscience.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Jin Y, Lin Y, Feng JF, et al. : Moderate Hypothermia Significantly Decreases Hippocampal Cell Death Involving Autophagy Pathway after Moderate Traumatic Brain Injury. J Neurotrauma. 2015;32(14):1090–100. 10.1089/neu.2014.3649 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Wang CF, Zhao CC, Jiang G, et al. : The Role of Posttraumatic Hypothermia in Preventing Dendrite Degeneration and Spine Loss after Severe Traumatic Brain Injury. Sci Rep. 2016;6:37063. 10.1038/srep37063 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Huang T, Solano J, He D, et al. : Traumatic injury activates MAP kinases in astrocytes: mechanisms of hypothermia and hyperthermia. J Neurotrauma. 2009;26(9):1535–45. 10.1089/neu.2008.0743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lotocki G, de Rivero Vaccari JP, Perez ER, et al. : Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of post-traumatic hypothermia. J Neurotrauma. 2009;26(7):1123–34. 10.1089/neu.2008.0802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chatzipanteli K, Alonso OF, Kraydieh S, et al. : Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immunocytochemical studies. J Cereb Blood Flow Metab. 2000;20(3):531–42. 10.1097/00004647-200003000-00012 [DOI] [PubMed] [Google Scholar]
- 74. Tomura S, de Rivero Vaccari JP, Keane RW, et al. : Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. J Cereb Blood Flow Metab. 2012;32(10):1939–47. 10.1038/jcbfm.2012.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Truettner JS, Bramlett HM, Dietrich WD: Posttraumatic therapeutic hypothermia alters microglial and macrophage polarization toward a beneficial phenotype. J Cereb Blood Flow Metab. 2017;37(8):2952–62. 10.1177/0271678X16680003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McGinn MJ, Povlishock JT: Pathophysiology of Traumatic Brain Injury. Neurosurg Clin N Am. 2016;27(4):397–407. 10.1016/j.nec.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 77. Hayes JP, Bigler ED, Verfaellie M: Traumatic Brain Injury as a Disorder of Brain Connectivity. J Int Neuropsychol Soc. 2016;22(2):120–37. 10.1017/S1355617715000740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Su E, Bell M: Diffuse Axonal Injury.In: Translational Research in Traumatic Brain Injury. Laskowitz D, Grant G. (eds): Boca Raton (FL).2016. 26583181 [Google Scholar]
- 79. Bramlett HM, Dietrich WD: The effects of posttraumatic hypothermia on diffuse axonal injury following parasaggital fluid percussion brain injury in rats. Ther Hypothermia Temp Manag. 2012;2(1):14–23. 10.1089/ther.2012.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shin SS, Pathak S, Presson N, et al. : Detection of white matter injury in concussion using high-definition fiber tractography. Prog Neurol Surg. 2014;28:86–93. 10.1159/000358767 [DOI] [PubMed] [Google Scholar]
- 81. Coughlin JM, Wang Y, Minn I, et al. : Imaging of Glial Cell Activation and White Matter Integrity in Brains of Active and Recently Retired National Football League Players. JAMA Neurol. 2017;74(1):67–74. 10.1001/jamaneurol.2016.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stemper BD, Shah AS, Pintar FA, et al. : Head rotational acceleration characteristics influence behavioral and diffusion tensor imaging outcomes following concussion. Ann Biomed Eng. 2015;43(5):1071–88. 10.1007/s10439-014-1171-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tanriverdi F, Unluhizarci K, Kelestimur F: Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary. 2010;13(2):146–53. 10.1007/s11102-009-0215-x [DOI] [PubMed] [Google Scholar]
- 84. Dubourg J, Messerer M: Sports-related chronic repetitive head trauma as a cause of pituitary dysfunction. Neurosurg Focus. 2011;31(5):E2. 10.3171/2011.8.FOCUS11182 [DOI] [PubMed] [Google Scholar]
- 85. Griesbach GS, Tio DL, Nair S, et al. : Temperature and heart rate responses to exercise following mild traumatic brain injury. J Neurotrauma. 2013;30(4):281–91. 10.1089/neu.2012.2616 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation