An editorial describing “minimal experimental requirements for definition of extracellular vesicles (EVs)”, or more simply “minimal information for studies of EVs (MISEV)” was published in the Journal of Extracellular Vesicles in late 2014 [1]. Similar to guidelines in other scientific fields [2–4], “MISEV2014”, as we will call it here, provided recommendations on experimental methods and minimal information in reporting. Specifically, three key areas were addressed: EV isolation/purification, EV characterization and EV functional studies (see Text Box 1).
Text Box 1.
Major recommendations of MISEV2014.
| 1. EV separation/isolation a) There is no single optimal isolation method, so choose based on the downstream applications and scientific question b) Report all details of the method(s) for reproducibility 2. EV characterization a) General characterization. Show: i. At least three positive protein markers of EVs, including at least one -transmembrane/lipid-bound protein -cytosolic protein ii. At least one negative protein marker b) Characterization of single vesicles: use two different but complementary techniques, for example: i. electron or atomic force microscopy (and show both close-up and wide-field) ii. single particle tracking 3. Functional studies should include: a) Dose–response studies b) Process controls to rule out influence of serum components/other possible contaminants c-i.) Density gradients to show activity is intrinsic to EVs, not just associated or c-ii.) EV depletion to remove activity or c-iii.) EV/cell labelling (e.g., fluorescent labelling, with careful interpretation) |
Brief review of MISEV2014
On separation of EVs (also referred to as isolation, purification or concentration), MISEV2014 acknowledged that there is no consensus on an “optimal” method, such as ultracentrifugation, size exclusion or immunoaffinity. Instead, selection of a method should be guided by the relevant scientific question and downstream applications, and all details of the method should be provided to ensure reproducibility.
MISEV2014 recommended that EV characterization be performed at both the population and single vesicle levels. For the former, both positive and negative protein marker characterization was endorsed, following four categories of proteins: (1) transmembrane or lipid-bound EV protein, (2) EV cytosolic protein, (3) intracellular but not associated with plasma membrane or endosomes (i.e. relatively less abundant in exosomal EVs than in cells) and (4) extracellular but not typically EV-associated proteins. Several examples of proteins in each category were provided.
For characterization of single vesicles, as a means to assess population heterogeneity, MISEV2014 called for the use of at least two different but complementary technologies. For example, electron or atomic force microscopy could be paired with one of the single particle tracking methods. Furthermore, close-up images should be accompanied by wide-field views to allow assessment of heterogeneity.
For EV functional studies, MISEV2014 stated that quantitation of EVs and their activity should include dose–response studies. Process controls should be included, since contaminants might contribute activity. Use of density gradients was encouraged to show that any observed activity is intrinsic to EVs and not to closely associated, commonly co-purifying particles; alternatively, this activity should be depletable, e.g. by immunodepletion of EVs. Labelling studies were also recommended.
Rigour vs flexibility
MISEV2014 reflected a field in flux, with a need for standard development tempered by remaining uncertainties. Indeed, this is still the case. For this reason, the protein markers for general characterization that were listed in a table in the manuscript were presented as non-exclusive examples only, and no specific antibodies were recommended. Our understanding of categories such as “EV proteins” and “non-EV proteins” is still evolving, and these categories might better be labelled “EV-enriched” and “EV-depleted”. Some described non-EV proteins have been found in vesicles and have biological effects. Instead of an inflexible approach, MISEV2014 urged that markers from different categories be used, and that all antibody details and sample manipulations be reported to ensure interpretability and reproducibility. What if scientists could not follow all guidelines? In this case, they were encouraged to explain why certain experiments could not be performed. The example of low-abundance, precious samples was given explicitly. That is, guidance was accompanied by acknowledgement of the realities of the field.
Utility of MISEV2014
The value of MISEV2014 is supported by a key finding of van Deun and Hendrix [5] reporting on the release of the EV-TRACK knowledgebase [6]. The EV-TRACK project includes an “EV-METRIC” that can be calculated to gauge the quality of reporting of each EV-related paper. van Deun et al. found that, in the year after the release of MISEV2014, the EV-METRICs of publications overall did not improve significantly. However, publications that cited MISEV2014 had significantly higher EV-METRICs. Those who engaged with MISEV2014, then, seemed to be better equipped to report important experimental parameters. Of course, it takes time for laboratories to incorporate new methodologies and then go on to publish a paper based on them. Laboratories that were unaware of the MISEV effort before its publication may simply not have had time to incorporate the guidelines by the time our survey took place. How far has MISEV reached? Citations of MISEV2014 reached 400 in September 2017, according to Google Scholar. However, this number represents only a minority of EV-related publications. While MISEV is likely followed in many publications that do not cite it, and citations per se are certainly not the goal of standardization efforts, the MISEV2014 citation rate nonetheless suggests that broader dissemination and researcher involvement could be achieved.
Opportunities and pulse-checking
The ISEV community now faces choices to extend the value and reach of MISEV2014. Whereas MISEV2014 was produced by the ISEV Board of Directors as the elected representatives of ISEV – at that time including the three editors-in-chief of the Journal of Extracellular Vesicles – future developments will benefit from the input of a much broader authorship. Drawing on more expertise will allow a future iteration or offshoot of MISEV2014 to offer more detailed guidance in more areas, and/or to reflect greater consensus. A first step in this direction was to take the pulse of the community on where we came from (MISEV2014) and where we are going with standardization.
2016 MISEV survey: obtaining guidance from the experts
A 14-question MISEV survey was released to the ISEV membership on 24 October 2016. See Text Box 2 for an outline of the survey. A total of 187 responses were submitted: 81% (152) within a 24-hour period, and almost 95% within 5 days. The survey was closed in early 2017.
Text Box 2.
ISEV 2016 MISEV survey.
| 1. I have read the MISEV minimal requirements paper (Lötvall et al., JEV, 2014) (yes/no) 2. I have published on extracellular vesicles in 2015 and/or 2016 (yes/no) 3. In your recent EV publication(s), did you (follow/cite/not follow or cite) MISEV? 4. Do you think that minimal reporting and/or methodologic requirements are important for the EV field? (yes/no) 5. Which of the following most closely summarizes your opinion on minimal requirements in the EV field? ° Minimal requirements are important, and I support MISEV ° Minimal requirements are important, and MISEV does not go far enough to uphold standards ° Minimal requirements are important, but MISEV is too restrictive ° Minimal requirements such as MISEV are an unnecessary imposition on the EV field ° I don’t have strong feelings about minimal requirements 6. If standards are to be adopted, which of the following is preferable? ° ISEV, with a large and diverse membership, should continue to take the lead in establishing minimal requirements ° Minimal requirements are better established by national societies or regional collaborative networks ° Leading experts or laboratories should develop and publish their own standards, and the best (highest IF or most cited) publications will eventually become the de facto standards in the field ° I have no preference 7. An update to MISEV is planned … how should it proceed? ° The ISEV board and JEV editors should update MISEV as they see fit. ° The ISEV board should also recruit ISEV members who are not on the board to assist in updating MISEV ° The ISEV community as a whole should be involved in updating MISEV, with invitations to contribute to specific points; community-wide voting is not needed ° The ISEV community should be involved not only in contributing/feedback, but also in voting to ratify specific points 8. Please comment on sections/types of information should be retained in future MISEV updates (check all that are applicable) ° minimal requirements to claim the presence of EVs in isolates ° general characterization of EVs ° characterization of single EVs ° studies of functional activities of EVs ° recommendations for controls ° other/comments on the above 9. Needed additions to MISEV include: ° Advice on pros and cons of different isolation methods ° Advice on protein markers to use for different types of EVs ° Advice on reliable antibodies for the EV protein markers, for different applications (WB, flow cytometry, immunohistochemistry, EM, etc.) ° Other (please specify) 10. If you would like to be involved in drafting or reviewing an update to MISEV, please provide your email address. Note: There will be additional opportunities to join the effort if you prefer not to disclose here. (Free form, optional) 11. If you would like to be involved in drafting or reviewing an update to MISEV, please describe your expertise in the EV field (e.g., published articles, unpublished work, specific areas of interest) (short answer, optional) 12. The following ISEV members should be consulted on MISEV updates (free form, optional) 13. If required, I/my laboratory are likely willing and able to devote a reasonable amount of resources to evaluate specific methods/reagents in the laboratory as needed to support a new MISEV release or other field-wide evaluation (yes/no) 14. Please share any other comments you may have (free form, optional) |
Respondents and their engagement with the MISEV2014 guidelines (Questions 1–3)
Eighty-two per cent of respondents had read the MISEV2014 guidelines, whereas 18% had not. Fifty-six per cent of all respondents had published on EVs in 2015 or 2016 (i.e. after MISEV2014 appeared), whereas 44% had not. A slightly larger percentage of recent authors had read MISEV2014 (87%) compared with those who had not published on EVs recently (74%, Figure 1(a)). Seventy-one per cent of recently publishing authors indicated that they followed and/or cited MISEV2014 in their work (Figure 1(b)).
Figure 1.
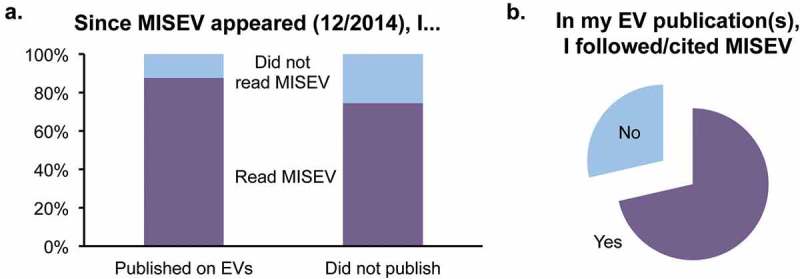
Community engagement with MISEV.
Perceived importance of guidelines (Questions 4 and 5)
Since the MISEV2014 authors have received a wide range of praise and critical comments on the guidelines, we sought to determine how the ISEV community viewed EV reporting or experimental guidelines in general and MISEV2014 specifically. When the question was phrased, “Do you think that minimal reporting and/or methodologic requirements are important for the EV field?” (Question 4), only one respondent answered “no” (Figure 2(a)). There was thus near-unanimity among respondents that requirements are needed.
A diversity of underlying opinions became apparent, though, through Question 5, a multiple-choice question about MISEV2014 in particular. Almost all respondents saw requirements as “important”, and a majority (53%) supported MISEV2014 as is. However, 25% considered MISEV2014 to be too restrictive, whereas 16% said it was not stringent enough (Figure 2(b)). Four per cent thought that MISEV2014 was an unnecessary imposition on the field. In conclusion, most survey participants are in favour of standards, with a majority of respondents supporting MISEV2014. It is clear, though, that sizeable portions of the ISEV constituency might favour updates and changes to MISEV2014.
Figure 2.
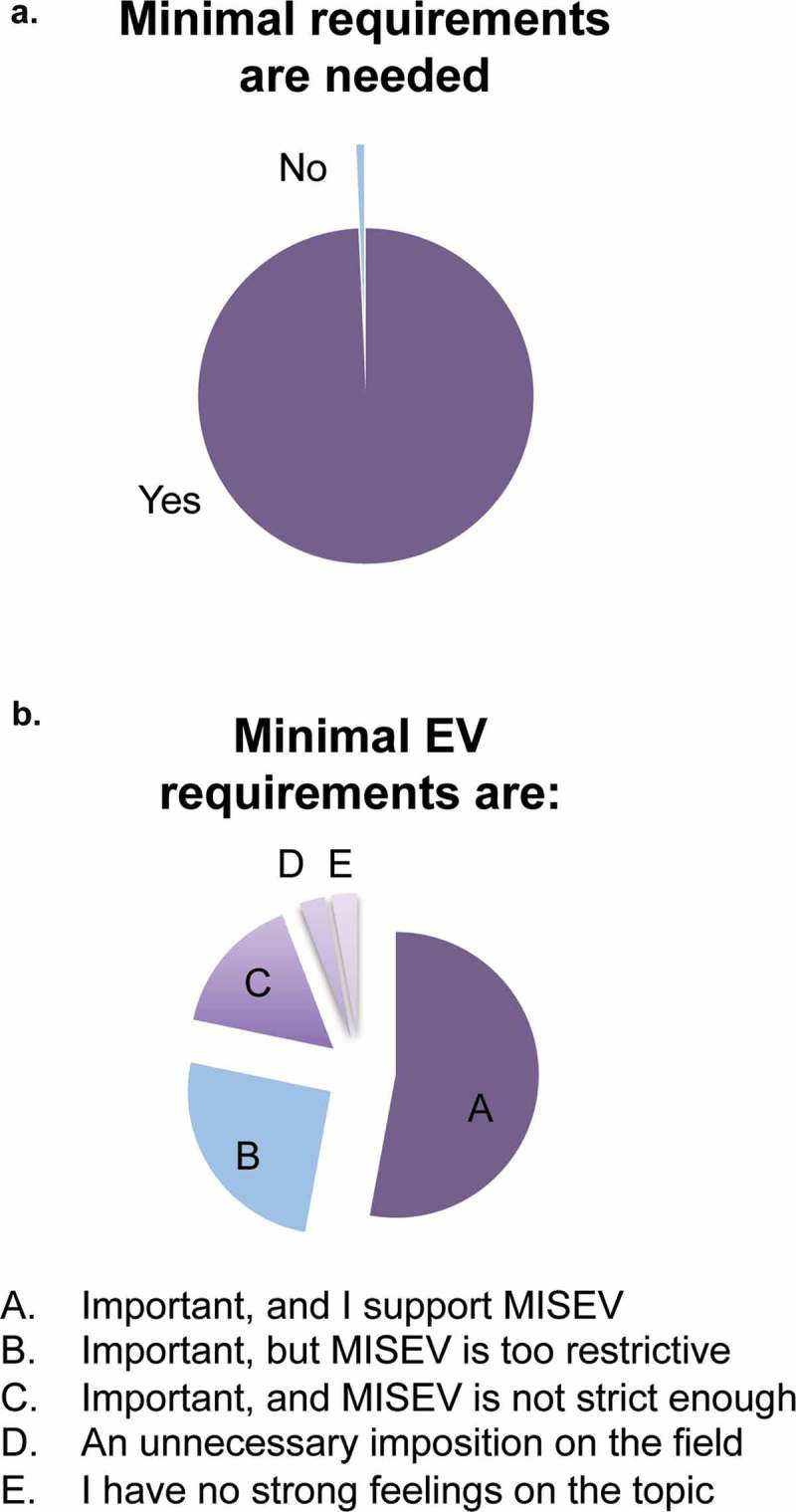
Opinions about minimal requirements in the EV field.
Who should update MISEV2014 (Questions 6 and 7)?
As for ISEV’s role in updates to MISEV2014, approximately 80% of respondents named ISEV, “with its large and diverse membership”, as the ideal body to issue requirements (Figure 3(a)). Other responses included “no preference” and nods to local or regional societies. Around 10% of respondents suggested that individual laboratories or scientists should publish requirements and that those with the highest impact factors or citations would become the de facto standards. The overwhelming emphasis on ISEV, however, places a responsibility on the society – leadership and membership – to advance standardization efforts for the field.
One unambiguous outcome of the survey was that ISEV board members and JEV editors are not encouraged to update MISEV2014 alone. This approach was endorsed by only 4% of respondents (Figure 3(b)). Thirty-five per cent of participants asked the ISEV board to include ISEV members who are not on the board, and 62% felt that the entire ISEV community should be invited to participate. Of the latter group, approximately three in four said that community voting would not be necessary, whereas one in four favoured community-wide voting on specific points.
Figure 3.
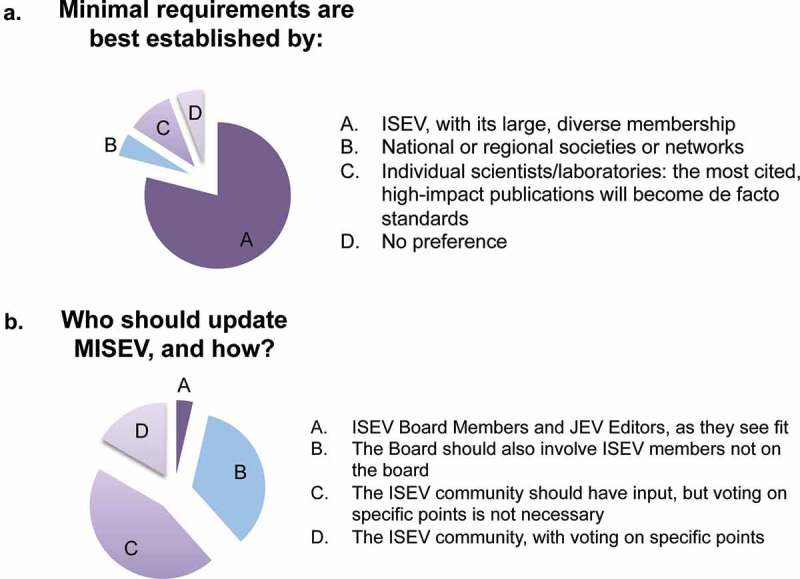
Who should establish and update minimal requirements?
Assembling a team for ongoingstandardization efforts
A key goal of the MISEV2014 survey was to begin the process of identifying EV experts in addition to the previous MISEV2014 authorship and the ISEV Board of Directors who are able and willing to contribute to future community efforts. Sixty-eight respondents volunteered by providing their email addresses. Only two of these respondents were MISEV2014 authors or members of the ISEV Board of Directors. Fifty-six of the volunteers gave details of their areas of expertise, including publications in many cases. Additionally, 20 respondents suggested other EV experts who, in their view, should be involved in future updates to MISEV. These names included 22 who were not authors of the previous MISEV2014 publication and are not on the current ISEV board. Only minimal overlap between the “volunteers” and “volunteered” was observed. Thus, in total, approximately 85 unique individuals were identified as possible contributors. As stated in the survey, additional calls for volunteers will be made in the future.
What topics should be retained or added (Questions 8 and 9)?
The majority of respondents were in favour of keeping all current components of MISEV2014, with 85% or more supporting retention of minimal requirements to claim presence of EVs in isolates, general characterization of EVs and suggestions for controls. Smaller majorities endorsed retention of single EV guidelines and recommendations for functional studies (Figure 4(a)).
What should or could be added in future MISEV updates? For Question 9, three choices were provided (specific recommendations on isolation methods, specific antibodies for detection and markers of multiple subtypes), along with an “other” comments box. While the way in which the survey was constructed prevented selection of more than one choice, the responses including the free-form comments made it clear that all selections were popular (Figure 4(b)). Updated recommendations on isolation methods were requested, including assessment of the proliferating commercial kit-based options to isolate EVs. Recommendations for judging the purity of vesicles were emphasized: while the presence of EVs is often shown, the presence and percentage of contaminants continue to be often ignored. Respondents suggested that contaminants could be categorized further based on EV sources (biofluids, tissues, cell-culture conditioned medium). Some felt that lists of reliable antibodies to detect EV positive or negative protein markers would be helpful, as well as guidelines on RNA, lipids and other EV components. Further guidelines on isolation method for functional studies were also raised, since isolation methods with different resultant purities may alter the functional roles of EV in vivo. Issuing from this problem, recommendations on experimental measurement of the activity of impurities were deemed important to place the functional roles of EVs in proper context.
Figure 4.
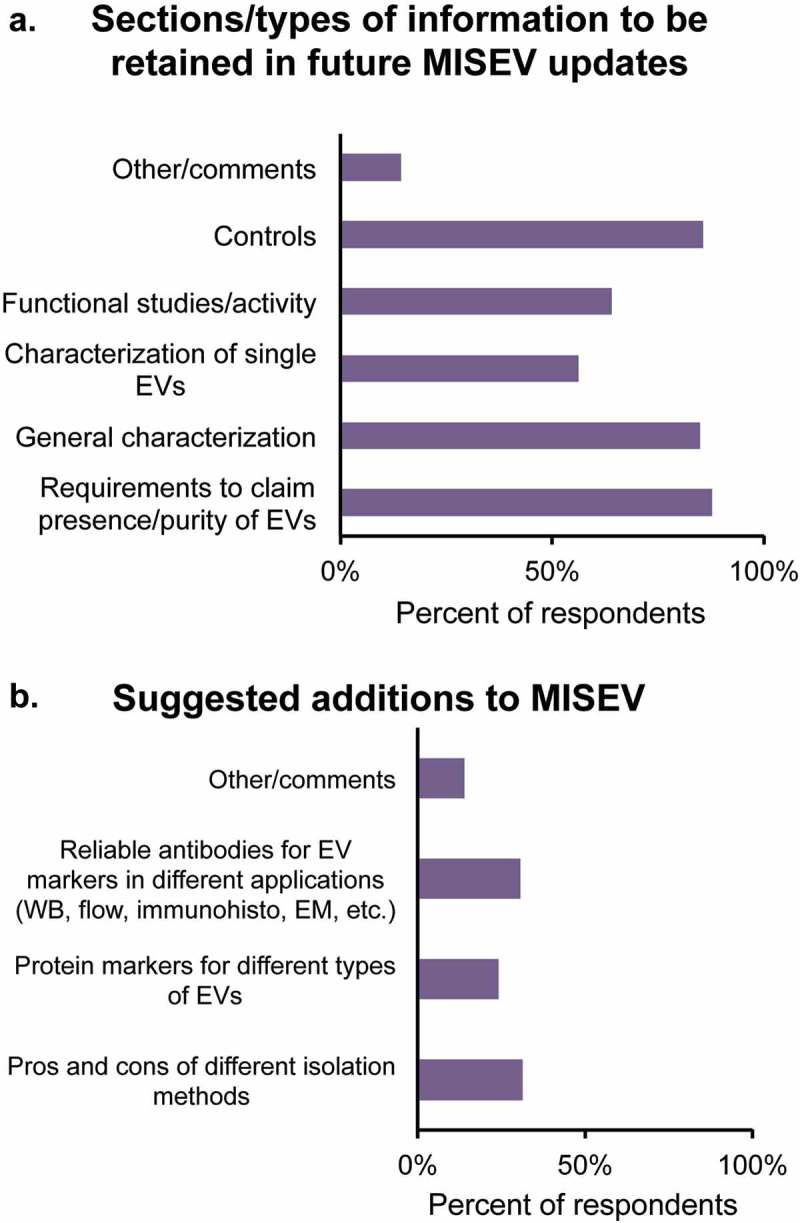
MISEV: suggested retentions and additions.
Positive and negative markers and contamination
Examining the free-form comments for Questions 8 and 9, multiple responses addressed the problem of contamination and positive and negative markers. How does one recognize and quantitate the various possible contaminants? Can we arrive at a consensus purity measurement? How can activity of non-EV components be excluded in functional studies? What is a negative marker? Is it found exclusively in non-EV contaminants, or can it also be present, if at reduced relative amounts, in EVs? Should positive EV markers merely be found in EVs, or must they also be enriched?
Other suggestions for additions
Several commenters stressed the need to accommodate studies of EVs from rare cell types, low-abundance body fluids and other exceptions, and not simply take a “one-size-fits-all” approach. Recommendations on different subtypes of EVs were an item of interest for several respondents, as were newer or newly applied methods for EV separation. Other comments suggested topics such as storage of EVs, assessment of stability and omics analysis specific to EVs.
Final comments box
A final comments box in the survey was used by <7% of respondents. Most of these free-form responses were positive general comments on MISEV. One comment suggested that, for a MISEV update, a comprehensive review of methodologies might be useful. Several commenters emphasized that any future MISEV release should continue to allow for exceptions: cases where the requirements might be waived or recognized as inapplicable. Further action from the community to spread the word about EV minimal standards to journals and journal editors was also encouraged.
Where do we go from here? Building bridges to enhance reproducibility in research
Revealing and centralizing methodologic details are part of successful standardization. To this end, we recognize with enthusiasm the EV-TRACK knowledgebase that was announced in Nature Methods [6] and in the accompanying letter to the editor [5]. We strongly encourage EV researchers to join the EV-TRACK initiative (www.evtrack.org), to use its tools to help guide effective research design and evaluation, and to obtain an EV-METRIC for each manuscript. We also hope the EV-METRIC will be included in submissions to the Journal of Extracellular Vesicles and to other journals, as well.
How will MISEV be updated?
In addition to exciting initiatives like EV-TRACK, minimal requirements in the field must also be updated regularly. This will be a large task as the field grows, and input is sought from a wider swath. We were interested to read a “guidelines to guidelines” recently published by Daniel J. Klionsky [7], noted autophagy researcher and the driving force behind the well-known autophagy guidelines. These guidelines, published in 2008 [8] and updated in 2012 [9] and 2016 [10], now include several thousand authors who reviewed the text and offered changes in a carefully guided manner. Klionsky’s staged writing process and a distributed author invitation plan ensure efficient progress but broad embrace of the global research body. In our view, the type of community consensus and buy-in fostered by this approach is exactly what we feel would be most valuable to EV researchers in an upcoming manifestation of the MISEV guidelines and also consistent with what the MISEV survey respondents indicated about their willingness and desire to be involved.
Regarding uncertainty
Standardization through MISEV updates is important, but of course uncertainty will remain that cannot be removed through minimal requirements alone. On many specific questions in EV research, including, for example, some quite basic matters of pre-analytical variables, the jury is still out. Careful comparisons to resolve these questions are conducted and published less often than might be optimal. This is because of the resources they require, resources that could otherwise be channelled into evaluating biological hypotheses that are more likely to secure funding. Importantly – and perhaps surprisingly to those who are less familiar with our highly collaborative field – 55% of respondents to the MISEV survey said that they or their laboratories could “devote reasonable resources” to evaluations, if these were to become necessary for MISEV updates. The language was kept intentionally vague, but “evaluations” could mean anything from literature reviews to technical/experimental comparisons. It is encouraging that such a large percentage of participants indicated a willingness to engage in coordinated activities. As needed, a Standardization Committee of ISEV could be formed to establish working groups on topics that have not been fully addressed and thus focus the substantial energies of the community.
Summary
Overall, the response to the ISEV survey on MISEV2014 produced three core findings: the value of MISEV2014, the need for regular updates that involve the wider community and the strong commitment of the ISEV community to advance the field through standardization efforts.
Acknowledgements
The contributions of all respondents to the MISEV2014 survey are gratefully acknowledged. The drafting authors would like to mention funding sources: US NIH DA040385 (KWW and CT), US NIH AG057430 (KWW) and US DoD W81XWH-16-1-0736 (CS).
Biography
KWW, CT, AF and MHM prepared the survey. KWW and CS analysed the results and wrote the manuscript. CT provided writing and planning assistance. All authors read and approved the final manuscript and provided feedback.
Funding Statement
This work was supported by the National Institute on Aging [AG057430];National Institute on Drug Abuse [DA040385];U.S. Department of Defense (US) [W81XWH-16-1-0736];
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1]. Lotvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles [Internet]. 2014;3:26913 Available from http://www.ncbi.nlm.nih.gov/pubmed/25536934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem [Internet]. 2009. February 28;55(4):611–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19246619 [DOI] [PubMed] [Google Scholar]
- [3]. Brazma A, Hingamp P, Quackenbush J, et al. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet [Internet]. 2001. December 01;29(4):365–371. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11726920 [DOI] [PubMed] [Google Scholar]
- [4]. Lee JA, Spidlen J, Boyce K, et al. MIFlowCyt: the minimum information about a flow cytometry experiment. Cytom Part A [Internet]. 2008. October;73A(10):926–930. [cited 2017 Sep 16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18752282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. van Deun J, Hendrix A.. Is your article EV-TRACKed? J Extracell Vesicles. Forthcoming 2017;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. van Deun J, Mestdagh P, Agostinis P, et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods [Internet]. 2017. February 28;14(3):228–232. [cited 2017 Mar 1]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28245209 [DOI] [PubMed] [Google Scholar]
- [7]. Klionsky DJ. Developing a set of guidelines for your research field: a practical approach. Mol Biol Cell [Internet]. 2016. March 1;27(5):733–738. [cited 2017 Sep 16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26915690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy [Internet]. 2008. February;4(2):151–175. [cited 2017 Sep 16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18188003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Klionsky D, Agholme L, Agnello M, et al. Guidelines for the use and interpretation of assays for monitoring autophagy Autophagy. 2012;8(4):445–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). doi.org [Internet]. 2016. January 2 p. 1–222. (1) [cited 2017 Sep 21]. Available form: https://www.ncbi.nlm.nih.gov/pubmed/26799652 [DOI] [PMC free article] [PubMed] [Google Scholar]


