Abstract
Background
The purpose of this study is to examine the expression levels of lymphatic endothelial markers in colorectal cancer and to explore the correlation between the expression levels of markers and lymph node status.
Methods
Forty-seven paired fresh tumor tissues and para-cancerous tissues were collected from colorectal cancer patients who received surgical treatment between August 2015 and March 2016 in Cancer Hospital, Chinese Academy of Medical Sciences. Real-time quantitative PCR (RTQ–PCR) was used to check the expression levels of LYVE–1, VEGFR–3, Podoplanin, and Prox–1 in tumor and para-cancerous tissues.
Results
The positive expression rates of LYVE–1, VEGFR–3, Podoplanin, and Prox–1 in tumor tissues were 100, 93.6, 100, and 91.4%, but 100, 100, 100, and 87.2% in para-cancerous tissues. Comparing with para-cancerous tissues, tumor tissues had significantly lower expression levels of LYVE–1 (P < 0.001) and VEGFR–3 (P = 0.013) and higher levels of Podoplanin (P = 0.016) and Prox–1 (P = 0.078). There was no correlation between lymph node status and the expression level of LYVE–1 in tumor tissues (P = 0.354) or par-cancerous tissues (P = 0.617); similar results were found for VEGFR–3 (P = 0.631, 0.738), Podoplanin (P = 0.490, 0.625), and Prox–1 (P = 0.503, 0.174). Meanwhile, there was no correlation between N-staging and the expression level of LYVE–1 in tumor tissues (P = 0.914) or para-cancerous tissues (P = 0.784); similar results were found for VEGFR–3 (P = 0.493, 0.955), Podoplanin (P = 0.199, 0.370), and Prox–1 (P = 0.780, 0.234).
Conclusions
There was no correlation between expression levels of lymphatic endothelial markers and lymph node status; LYVE–1, VEGFR–3, Podoplanin, and Prox–1 could not be used for predicting the lymph node status or N-staging of colorectal cancer.
Keywords: LYVE–1, VEGFR–3, Podoplanin, Prox–1, Colorectal cancer, RTQ–PCR
Background
Lymphatic system markers which have been used for studying the mechanism of lymphatic metastasis for several kinds of malignant tumors recently include lymphatic endothelial markers and lymphatic endothelial growth factors, and the lymphatic endothelial markers mainly include lymphatic vessel endothelial hyaluronic acid receptor–1 (LYVE–1), vascular endothelial growth factor receptor 3 (VEGFR–3), Podoplanin, and Prox–1 [1, 2]. The correlation between the lymphatic endothelial markers and lymphatic metastasis in some malignant tumors such as gastric cancer, breast cancer, ovarian cancer, and so on have been studied by some centers, and there is no consensus yet whether lymphatic endothelial markers can be used for predicting the N-staging of carcinoma [3–7]. In this study, we used real-time quantitative PCR (RTQ–PCR) to examine the expression of the four kinds of lymphatic endothelial markers in colorectal cancer; meanwhile, we analyzed the difference of expression levels in tumor tissue and para-cancerous tissue and we also analyzed the correlation between the expression levels of LYVE–1, VEGFR–3, Podoplanin, or Prox–1 and lymph node status.
Methods
Tissue collection
Forty-seven paired fresh tumor tissues and para-cancerous tissues (2-cm tissue adjacent to cancer) were collected from colorectal cancer patients who received surgical treatment between August 2015 and March 2016 in Cancer Hospital, Chinese Academy of Medical Sciences. All tissues were collected within half an hour after the removal of specimens. RNA was extracted using the standard RNAzol procedure. cDNA was subsequently synthesized using a reverse transcription kit according to the manufacturer’s instructions.
Primers
Primers of four markers were synthesized by Sangon Biotech (Shanghai) Co., Ltd: LYVE–1 forward primer: 5′–AAGAATGAAGCTGCTGGGTTT–3′, LYVE–1 reverse primer: 5′–GACATAGCAAAATCCAAGACCA–3′; VEGFR–3 forward primer: 5′–AGGGAGACGCCCTTTCATG–3′, VEGFR–3 reverse primer: 5′–GAGGGCTCTTTGGTCAAGCA–3′; Podoplanin forward primer: 5′–CACGGAGAAAGTGGATGGAGA–3′, Podoplanin reverse primer: 5′–GCCGATGGCTAGTAAGACCC–3′; Prox–1 forward primer: 5′–AAAGCAAAGCTCATGTTTTTTTATA–3′, Prox–1 reverse primer: 5′–GTAAAACTCACGGAAATTGCTAAA–3′.
RTQ–PCR
Bio–Rad CFX96 real-time PCR system was used for RTQ–PCR; relative quantitative analysis was proceeded according to the SYBR Green I method. Reaction system was as follows: SsoFastTM EvaGreen supermix 10 μl, forward primer of target gene 2 μl (2 μM), reverse primer 2 μl (2 μM), deionized water 4 μl, and cDNA 2 μl. RTQ–PCR conditions were as follows: 40 cycles, pre-degeneration 95 °C for 30′, degeneration 95 °C for 10′, and annealing extension 60 °C for 10′.
Statistical analysis
Statistical analyses were performed using statistical software package SPSS version 16.0. A P value less than 0.05 was considered to be statistically significant. Student’s t test was used for the analysis of continuous variables, and one-way ANOVA was used for the analysis between groups.
Results
Twenty-eight of the 47 patients were males and 19 were females in this study. Their ages ranged from 34 to 84 with the median age of 56.3. The number of patients with ascending colon cancer, transverse colon cancer, descending colon cancer, sigmoid colon cancer, and rectal cancer were 7, 2, 1, 8, and 29, respectively. Lymph node involvement was detected in 27 patients. The general parameters of patients were shown in Table 1.
Table 1.
The general parameters of patients in this study
| Parameters | |
|---|---|
| Gender, case | |
| Male | 29 |
| Female | 18 |
| Age, year, mean (range) | 56.3 (34–84) |
| Location of tumors, case | |
| Cecum | 0 |
| Ascending colon | 7 |
| Transverse colon | 2 |
| Descending colon | 1 |
| Sigmoid colon | 8 |
| Rectum | 29 |
| Tumor size, cm, mean (range) | 4.8 (2.2–14.0) |
| Type of pathology, case | |
| Adenocarcinoma | 44 |
| Mucinous adenocarcinoma | 1 |
| Signet ring cell carcinoma | 2 |
| Degree of differentiation, case | |
| Well | 3 |
| Moderate | 34 |
| Poor | 10 |
| Depth of invasion, case | |
| T2 | 2 |
| T3 | 38 |
| T4a | 7 |
| N-staging, case | |
| N0 | 20 |
| N1a | 7 |
| N1b | 5 |
| N2a | 8 |
| N2b | 7 |
| TNM staging, case | |
| IIa | 18 |
| IIb | 2 |
| IIIb | 18 |
| IIIc | 9 |
Different expressions of the four markers between tumor tissues and para-cancerous tissues
Expression rates in tumor tissues and para-cancerous tissues
Both of the positive expression rates of LYVE–1 in tumor tissues and para-cancerous tissues were 100%, and the same results could be found for Podoplanin. A 93.6% positive expression rate of VEGFR–3 was found in tumor tissue, but 100% in para-cancerous tissue. Prox–1 had a positive expression rate of 91.4% in tumor tissue and 87.2% in para-cancerous tissue.
Expression levels in tumor tissues and para-cancerous tissues
Expression level of LYVE–1 in para-cancerous tissues was significantly higher than that in tumor tissues (P < 0.001), and a higher expression level VEGFR–3 was also found in para-cancerous tissues compared with that in tumor tissues (P = 0.013), whereas a different result was found for Podoplanin, the higher expression level was checked in tumor tissues (P = 0.016). For Prox–1, there were no significantly different expression levels in between tumor tissues and para-cancerous tissues (Fig. 1).
Fig. 1.
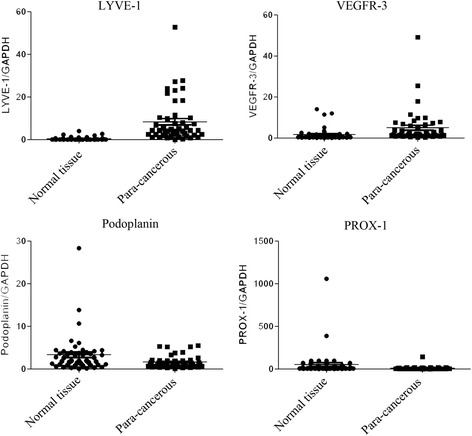
Expression levels of markers in tumor tissues and para-cancerous tissues. Both LYVE-1 and VEGFR-3 had a significantly higher expression level in para-cancerous tissues than that in tumor tissues (LYVE-1: P < 0.001; VEGFR-3: P = 0.013), while Podoplanin had a lower expression level in para-cancerous tissues than in tumor tissues (P = 0.016); no significantly different expression levels between tumor tissues and para-cancerous tissues was found for Prox–1
The correlation between lymph node status and expression levels of markers
The expression level of LYVE–1 in tumor tissues of patients with lymph node involvement was similar with that in patients without lymph node involvement (P = 0.354), and the similar results were found for VEGFR–3 (P = 0.631), Podoplanin (P = 0.490), and Prox–1 (P = 0.503). Similarly, the expression level of LYVE–1 in para-cancerous tissues of N(+) staging patients was similar with that in N(−) staging patients (P = 0.617); the similar results were found for VEGFR–3 (P = 0.738), Podoplanin (P = 0.625), and Prox–1 (P = 0.174) (Fig. 2).
Fig. 2.
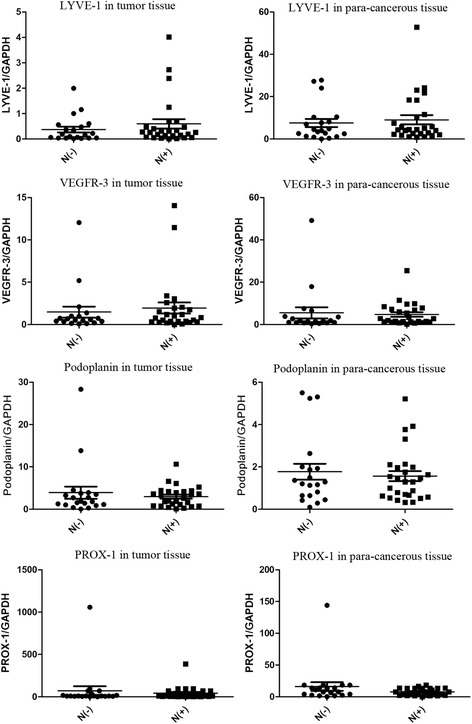
Correlation between lymph node status and expression levels of markers in tumor tissues or para-cancerous tissues. Status of lymph node did not influence the expression level of the four makers; no significantly different expression levels were found in tumor tissues and no obvious differences in para-cancerous tissues. (In tumor tissues: LYVE-1, P = 0.354; VEGFR–3, P = 0.631; Podoplanin, P = 0.490; Prox–1, P = 0.503; in para-cancerous: LYVE-1, P = 0.617; VEGFR–3, P = 0.738; Podoplanin, P = 0.625; Prox–1, P = 0.174)
The correlation between N-staging and expression level of markers
The difference of expression level of LYVE–1 in tumor tissues of patients with N1a, N1b, N2a, and N2b staging was not found (P = 0.914); same results were found for VEGFR–3 (P = 0.493), Podoplanin (P = 0.199), and Prox–1 (P = 0.780). No difference was found for LYVE–1 in para-cancerous tissues (P = 0.784); similarly, no differences for VEGFR–3 (P = 0.955), Podoplanin (P = 0.370), and Prox–1 (P = 0.234) (Fig. 3).
Fig. 3.
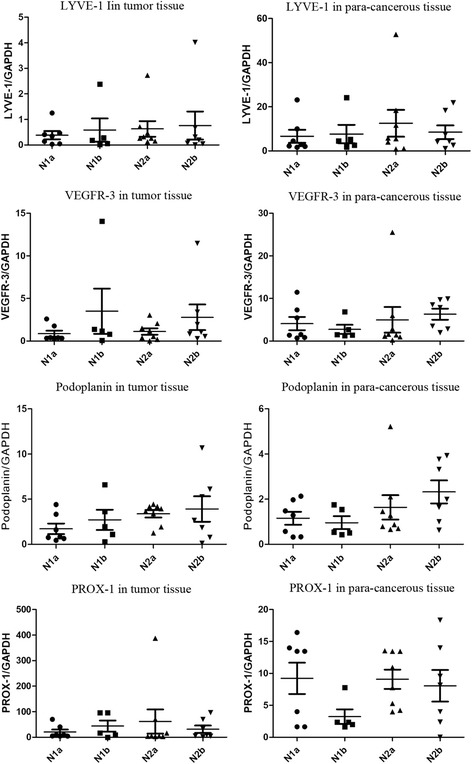
Correlation between N-staging and expression levels of markers in tumor tissues or para-cancerous tissues. The expression levels of these four makers in tumor tissues or para-cancerous tissues were not impacted by N-staging of tumor. (In tumors tissues: LYVE-1, P = 0.914; VEGFR–3, P = 0.493; Podoplanin, P = 0.199; Prox–1, P = 0.780; in para–cancerous: LYVE-1, P = 0.784; VEGFR–3, P = 0.955; Podoplanin, P = 0.370; Prox–1, P = 0.234)
Discussion
The discovery of lymphatic system markers is promoting the research progress of metastatic mechanism. The correlation between lymphatic endothelial markers and lymphatic metastasis had been reported by several centers during the last decade. However, there is no consensus yet whether lymphatic endothelial markers can be used for predicting lymphatic status.
LYVE–1, as a new hyaluronic acid receptor, was found in 1999 [8]. It mainly expressed in lymphatic endothelial cells [9, 10]. Some studies showed that lymphatic endothelial cells of malignant tumors had specific expression of LYVE–1 [11]. Expression of LYVE–1 in gastric cancer was studied by Ozmen et al. [12]; his results showed that the expression level of LYVE–1 in cancer tissues was obviously higher than that in para-cancerous tissues, and an increased expression level was related with the increased proportion of lymph node involvement. In 2006, Gao et al. [13] designed a study to examine the expression of LYVE–1 in colorectal cancer tissues and normal tissues; his results showed that the expression level of LYVE–1 in cancer tissues was higher than that in normal tissues although no significant difference was found. A PCR study designed by Lu et al. [14] confirmed that LYVE–1 mainly existed in margins of tumor.
Among all lymphatic endothelial markers, VEGFR–3 was discovered earliest. VEGFR–3 has vital function during the process of generation and development of vascular system [15, 16]. A study designed by Kawakami et al. [5] found that the positive rate of VEGFR–3 in colorectal cancer tissues was 79.2%; meanwhile, his study confirmed that there was no correlation between the expression level of VEGFR–3 in cancer tissues and lymph node involvement; however, different conclusion was made by some other professors: some studies showed that an increased rate of lymph nodes involvement was related to the higher expression level of VEGFR–3 in colorectal cancer tissues [17, 18].
Initially, the Podoplanin was found on the surface of glomerular podocytes, and its expression in lymphatic endothelial cells was firstly reported in 1999 [19]. Podoplanin mainly exists in small lymphatic vessels, but it does not express in big lymphatic vessels with smooth muscle. Braun et al. [20] found that Podoplanin was a sensitive factor for predicting the involvement of lymph nodes in invasive breast cancer; the study of Wada et al. [21] demonstrated that the expression level of Podoplanin was related to the involvement rate of lymph nodes and lymphovascular invasion in T1 stage colorectal cancer.
Prox–1, as one kind of nuclear transcription factors, is the homologous gene of prospero got from Drosophila melanogaster; it has the functions of regulating cell mitosis and inducing the differentiation of lymphatic endothelial cells [22]. Parr et al. [23] found that colorectal cancer tissues had a higher expression level of Prox–1; the study results of Agarwal et al. [7] showed that breast cancer with lymph node involvement had a higher expression level of Prox–1 in cancer tissues.
Some similar or different results were found based on our study; all of the four markers had the high positive expression rates not only in tumor tissues but also in para-cancerous cancer tissues, and the expression levels of LYVE–1 and VEGFR–3 was higher in para-cancerous tissues than that in tumor tissues, whereas the higher expression levels of Podoplanin and Prox–1 was found in tumor tissues. There was no correlation between lymph node status and the expression level of LYVE–1 in tumor tissues or para-cancerous tissues; similar results were found for VEGFR–3, Podoplanin, and Prox–1. Meanwhile, no correlation between N-staging and the expression level of LYVE–1 in tumor tissues or para-cancerous tissues; similar results were found for VEGFR–3, Podoplanin, and Prox–1.
Conclusions
There was no correlation between lymph node status and expression of LYVE–1, VEGFR–3, Podoplanin, or Prox–1; these four lymphatic endothelial markers could not be used for predicting the lymph node status or N-staging of colorectal cancer.
Acknowledgements
The authors thank Hu Jun-jie, Zeng Wei-gen, and Shi Lei for collecting the data; their supports are the key factor for the completion of the manuscript.
Funding
None
Availability of data and materials
Not applicable
Abbreviations
- LYVE–1
Lymphatic vessel endothelial hyaluronic acid receptor–1
- VEGFR–3
Vascular endothelial growth factor receptor 3
Authors’ contributions
HQ and ZXM provided the concept; ZXM and WHY were the major contributors in writing the manuscript; HQ and HWX analyzed and interpreted the patient data; all authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xing-mao Zhang, Email: zhangboshi_12@163.com.
Wen-xiao Han, Email: llx_cyyy@126.com.
Hong-ying Wang, Email: zhou_haitao1@163.com.
Qiang He, Phone: +861085231501, Phone: 13811094044, Email: heqiang_cy@163.com.
References
- 1.Sundar SS, Zhang H, Brown P, Manek S, Han C, Kaur K, et al. Role of lymphangiogenesis in epithelial ovarian cancer. Br J Cancer. 2006;94:1650–1657. doi: 10.1038/sj.bjc.6603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundar SS, Ganesan TS. Role of lymphangiogenesis in cancer. J Clin Oncol. 2007;25:4298–4307. doi: 10.1200/JCO.2006.07.1092. [DOI] [PubMed] [Google Scholar]
- 3.Hall FT, Freeman JL, Asa SL, Jackson DG, Beasley NJ. Intratumoral lymphatics and lymph node metastases in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129:716–719. doi: 10.1001/archotol.129.7.716. [DOI] [PubMed] [Google Scholar]
- 4.Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, et al. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315–1320. [PubMed] [Google Scholar]
- 5.Kawakami M, Furuhata T, Kimura Y, Yamaguchi K, Hata F, Sasaki K, et al. Quantification of vascular endothelial growth factor-C and its receptor-3 messenger RNA with real-time quantitative polymerase chain reaction as a predictor of lymph node metastasis in human colorectal cancer. Surgery. 2003;133:300–308. doi: 10.1067/msy.2003.45. [DOI] [PubMed] [Google Scholar]
- 6.Yamanashi T, Nakanishi Y, Fujii G, Akishima-Fukasawa Y, Moriya Y, Kanai Y, et al. Podoplanin expression identified in stromal fibroblasts as a favorable prognostic marker in patients with colorectal carcinoma. Oncology. 2009;77:53–62. doi: 10.1159/000226112. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal B, Saxena R, Morimiya A, Mehrotra S, Badve S. Lymphangiogenesis does not occur in breast cancer. Am J Surg Pathol. 2005;29:1449–1455. doi: 10.1097/01.pas.0000174269.99459.9d. [DOI] [PubMed] [Google Scholar]
- 8.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, Ganss R, et al. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol. 2006;209:67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 10.Wu M, Du Y, Liu Y, He Y, Yang C, Wang W, et al. Low molecular weight hyaluronan induces lymphangiogenesis through LYVE-1-mediated signaling pathways. PLoS One. 2014;9:e92857. doi: 10.1371/journal.pone.0092857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunnick GH, Jiang WG, Gomez KF, Mansel RE. Lymphangiogenesis quantification using quantitative PCR and breast cancer as a model. Biochem Biophys Res Commun. 2001;288:1043–1046. doi: 10.1006/bbrc.2001.5869. [DOI] [PubMed] [Google Scholar]
- 12.Ozmen F, Ozmen MM, Ozdemir E, Moran M, Seckin S, Guc D, et al. Relationship between LYVE-1, VEGFR-3 and CD44 gene expressions and lymphatic metastasis in gastric cancer. World J Gastroenterol. 2011;17:3220–3228. doi: 10.3748/wjg.v17.i27.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao F, Lu YM, Cao ML, Liu YW, He YQ, Wang Y. Expression and quantification of LYVE-1 in human colorectal cancer. Clin Exp Med. 2006;6:65–71. doi: 10.1007/s10238-006-0097-4. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Yang Q, Du Y, Feng G, Yang C. Expression analysis of lymphangiogenic factors in human colorectal cancer with quantitative RT-PCR. Cancer Investig. 2007;25:393–396. doi: 10.1080/07357900701508934. [DOI] [PubMed] [Google Scholar]
- 15.Pajusola K, Aprelikova O, Korhonen J, Kaipainen A, Pertovaara L, Alitalo R, et al. FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res. 1992;52:5738–5743. [PubMed] [Google Scholar]
- 16.Galland F, Karamysheva A, Pebusque MJ, Borg JP, Rottapel R, Dubreuil P, et al. The FLT4 gene encodes a transmembrane tyrosine kinase related to the vascular endothelial growth factor receptor. Oncogene. 1993;8:1233–1240. [PubMed] [Google Scholar]
- 17.Okita NT, Yamada Y, Takahari D, Hirashima Y, Matsubara J, Kato K, et al. Vascular endothelial growth factor receptor expression as a prognostic marker for survival in colorectal cancer. Jpn J Clin Oncol. 2009;39:595–600. doi: 10.1093/jjco/hyp066. [DOI] [PubMed] [Google Scholar]
- 18.Smith NR, Baker D, James NH, Ratcliffe K, Jenkins M, Ashton SE, et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin Cancer Res. 2010;16:3548–3561. doi: 10.1158/1078-0432.CCR-09-2797. [DOI] [PubMed] [Google Scholar]
- 19.Roy S, Chu A, Trojanowski JQ, Zhang PJ. D2-40, a novel monoclonal antibody against the M2A antigen as a marker to distinguish hemangioblastomas from renal cell carcinomas. Acta Neuropathol. 2005;109:497–502. doi: 10.1007/s00401-005-0999-3. [DOI] [PubMed] [Google Scholar]
- 20.Braun M, Flucke U, Debald M, Walgenbach-Bruenagel G, Walgenbach KJ, Holler T, et al. Detection of lymphovascular invasion in early breast cancer by D2-40 (podoplanin): a clinically useful predictor for axillary lymph node metastases. Breast Cancer Res Treat. 2008;112:503–511. doi: 10.1007/s10549-007-9875-2. [DOI] [PubMed] [Google Scholar]
- 21.Wada H, Shiozawa M, Katayama K, Okamoto N, Miyagi Y, Rino Y, et al. Systematic review and meta-analysis of histopathological predictive factors for lymph node metastasis in T1 colorectal cancer. J Gastroenterol. 2015;50:727–34. [DOI] [PubMed]
- 22.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parr C, Jiang WG. Quantitative analysis of lymphangiogenic markers in human colorectal cancer. Int J Oncol. 2003;23:533–539. doi: 10.3892/ijo.23.2.533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable


