Significance
Why the brain is uniquely sensitive to hypoxia and which cells are involved is incompletely understood. Here we identify that, upon ischemic stroke, in endothelial cells and neurons the reactive oxygen-forming NADPH oxidase 4 (NOX4) causes breakdown of the BBB and neuronal cell death. This mechanism is unique to the brain and not found in other forms of ischemia in the body. Genetic deletion of either cell type (endothelial or neuronal) or pharmacological inhibition of NOX4 leads to a significant reduction of infarct volume and direct neuroprotection. This mechanism explains the unique vulnerability of the hypoxic brain compared with other organs and provides a clear rationale for first-in-class neuroprotective therapies in stroke.
Keywords: NOX4, stroke, BBB, neurotoxicity, endothelium
Abstract
Ischemic injury represents the most frequent cause of death and disability, and it remains unclear why, of all body organs, the brain is most sensitive to hypoxia. In many tissues, type 4 NADPH oxidase is induced upon ischemia or hypoxia, converting oxygen to reactive oxygen species. Here, we show in mouse models of ischemia in the heart, brain, and hindlimb that only in the brain does NADPH oxidase 4 (NOX4) lead to ischemic damage. We explain this distinct cellular distribution pattern through cell-specific knockouts. Endothelial NOX4 breaks down the BBB, while neuronal NOX4 leads to neuronal autotoxicity. Vascular smooth muscle NOX4, the common denominator of ischemia within all ischemic organs, played no apparent role. The direct neuroprotective potential of pharmacological NOX4 inhibition was confirmed in an ex vivo model, free of vascular and BBB components. Our results demonstrate that the heightened sensitivity of the brain to ischemic damage is due to an organ-specific role of NOX4 in blood–brain-barrier endothelial cells and neurons. This mechanism is conserved in at least two rodents and humans, making NOX4 a prime target for a first-in-class mechanism-based, cytoprotective therapy in the unmet high medical need indication of ischemic stroke.
Ischemic and/or hypoxic disease states such as myocardial infarction (MI), peripheral artery disease, and stroke collectively represent the most frequent causes of death and disability (1). However, their underlying mechanisms and cellular interplay leading to organ damage and chronic loss of function are incompletely understood. Consequently, treatments of these conditions are currently restricted to the symptomatic removal of the occlusion (2) without also using therapies directed to preserve organ function (3).
One pathomechanism that has been put forward as a possible cause of postischemic cell death and organ dysfunction is oxidative stress (4, 5), i.e., the appearance of reactive oxygen species (ROS) in either unphysiological locations or at unphysiological levels. However, global ROS-scavenging antioxidants provide little or no protection in this scenario (6, 7), arguing against the oxidative stress hypothesis (8). A recent alternative approach to oxidative stress focuses on the disease-relevant enzymatic sources of ROS and their pharmacological inhibition (4, 5).
NADPH oxidases (NOX) are one of the main sources of ROS and, importantly, the only known enzyme family that has ROS formation as its sole known function (4). Of these, NOX4 appears most relevant as a target for therapy, as it is induced under ischemia or hypoxia in different cells and tissues (9–11) and may be a long-sought common denominator of ischemia-reperfusion (IR) injury (12). We here test this hypothesis in three mouse models of the most relevant ischemic disease states, all associated also with increased ROS formation: MI (13), peripheral artery disease (13), and stroke (14). We also validate the role of NOX4 in a relevant rat in vivo and in human in vitro models.
Results
Postischemic Injury Depends on NOX4 in Stroke but Not in MI or Hindlimb Ischemia.
To elucidate whether hypoxia-inducible NOX4-derived ROS is a systemic mechanism of ischemic tissue damage, we investigated three different in vivo mouse models related to three highly relevant ischemic disease conditions: MI and transient ischemia of the heart, peripheral artery disease, and stroke. First, relative Nox4 gene expression was evaluated in heart and hindlimb muscle in wild-type (WT) mice (8–16 wk) subjected to MI and hindlimb ischemia, respectively, and compared with nonischemic mice. Nox4 was up-regulated after ischemia in all these models, including both in vivo and in vitro models of brain ischemia (SI Appendix, Fig. S1).
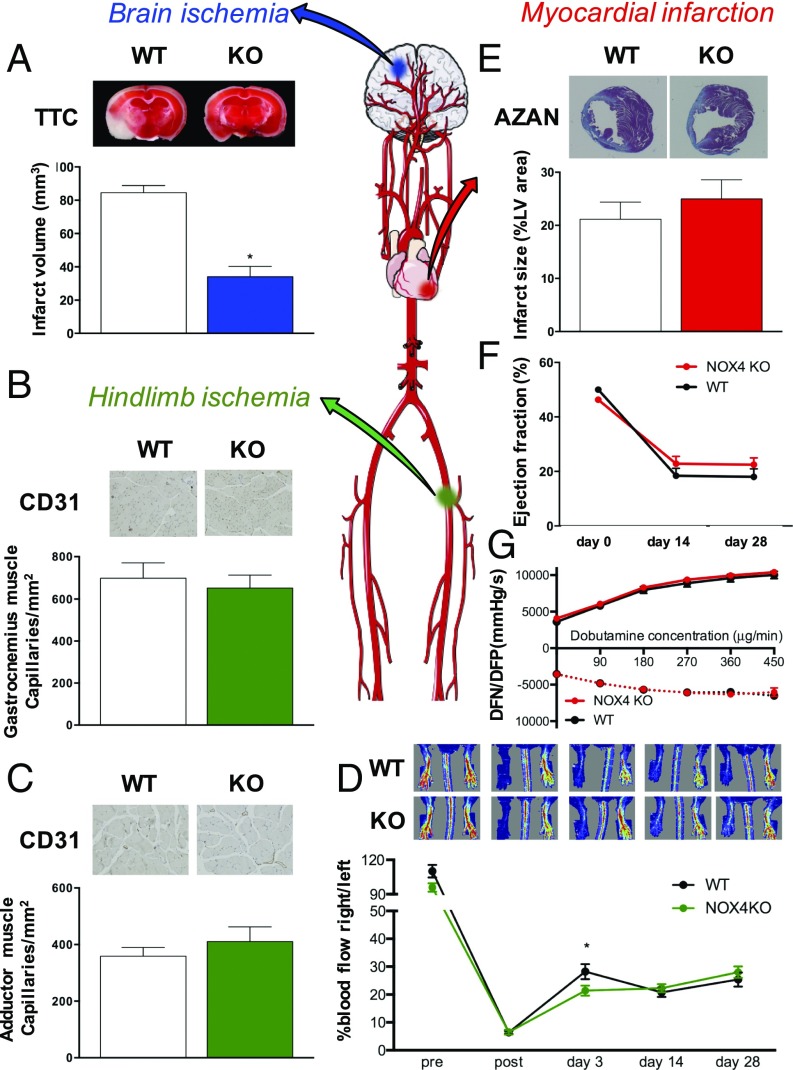
For stroke, 8- to 12-wk-old male global Nox4 KO mice were subjected to 1 h of transient middle cerebral artery occlusion (tMCAO) and 24 h of reperfusion. Consistent with our previous findings, Nox4 KO mice developed significantly smaller brain infarct sizes compared with the WT group (Fig. 1A). Likewise, permanent occlusion of the middle cerebral artery in Nox4 KO mice showed the same infarct size reduction as in the transient occlusion (9).
Fig. 1.
Role of NOX4 in brain ischemia (blue), hindlimb ischemia (green), and MI (red). (A) Infarct volume in Nox4 KO mice (blue) was reduced compared with the WT mice (*P < 0.05, n = 8). (B) Quantified number of capillaries in the ischemic gastrocnemius muscle was not different between Nox4 KO (green, n = 16) and WT mice (white, n = 22). (C) Quantified number of capillaries in the ischemic adductor muscle did not show a difference between Nox4 KO (green, n = 15) and WT mice (white, n = 19). (D) Blood flow restoration decreased in the Nox4 KO mice (green, n = 17) at day 3, an effect that was not seen in the long-term with Nox4 KO mice (green, n = 17) and WT mice (black, n = 23) (*P < 0.05), showing the same blood flow at 28 d after ischemia. (E) No significant differences in infarct size 4 wk after MI between Nox4 KO (red, n = 25) and WT mice (white, n = 26). (F) Ejection fraction decreased after MI with no significant change between Nox4 KO (red, n = 23) and WT mice (black, n = 21). (G) Left ventricular function was not different between Nox4 KO (red, n = 22) and WT mice (black, n = 20). Representative staining pictures are shown above each graph.
To investigate the role of NOX4 in hindlimb ischemia, which mimics human peripheral artery disease, sexually mature adult Nox4 KO and WT mice from 12 to 16 wk old were subjected to permanent ligation of the femoral artery. Angiogenesis (sprouting of new capillaries) occurs as a response to the ischemic muscle damage, mainly in the distal calf muscles (13), and was assessed via the capillary density marker, CD31, at day 28 as reported previously (11) and well within the reported range of 14 and 35 d postligation (15). No significant differences were observed in the gastrocnemius (Fig. 1B) or adductor muscle (Fig. 1C) in Nox4 KO mice versus WT littermates. Similarly, blood flow restoration was not modified by global Nox4 deficiency (Fig. 1D).
We also investigated the possible role of NOX4 in MI and IR of the heart after permanent and transient (45 min) occlusion of the left descending coronary artery in 8- to 12-wk-old Nox4 KO mice. Infarct sizes, assessed by AZAN stain 4 wk after MI or IR, were similar in Nox4 KO and WT mice (Fig. 1E and SI Appendix, Fig. S2A). Functionally, ejection fraction at 2 and 4 wk post-MI or post-IR were not different between Nox4 KO and WT mice (Fig. 1F and SI Appendix, Fig. S2B). Furthermore, a change in maximal and minimal first derivative of left ventricle (LV) pressure (LV dP/dtmax, min) in response to increasing doses of dobutamine is an excellent and sensitive measure of in vivo β-adrenergic receptor function. However, the contractility and relaxing capacity of post-MI hearts were not altered in Nox4 KO mice after MI (Fig. 1G) or IR (SI Appendix, Fig. S2C). To improve the temporal comparability of the heart data to the tMCAO model, short-term experiments with 45 min of ischemia and 24 h of reperfusion also were performed. Infarct sizes, as assessed by tetrazolium chloride (TTC) stain, did not differ between WT and NOX4 KO mice (SI Appendix, Fig. S2D). Also, the functional parameters, ejection fraction (SI Appendix, Fig. S2E), and contractility (SI Appendix, Fig. S2F) were similar in WT and Nox4 KO mice. Thus, although NOX4 was up-regulated in all three ischemic models, deletion of Nox4 did not modify outcomes after ischemia of the heart nor of the hindlimb. Deletion of Nox4, however, conveyed a major a protective in stroke, indicating a surprising highly organ-specific role of NOX4.
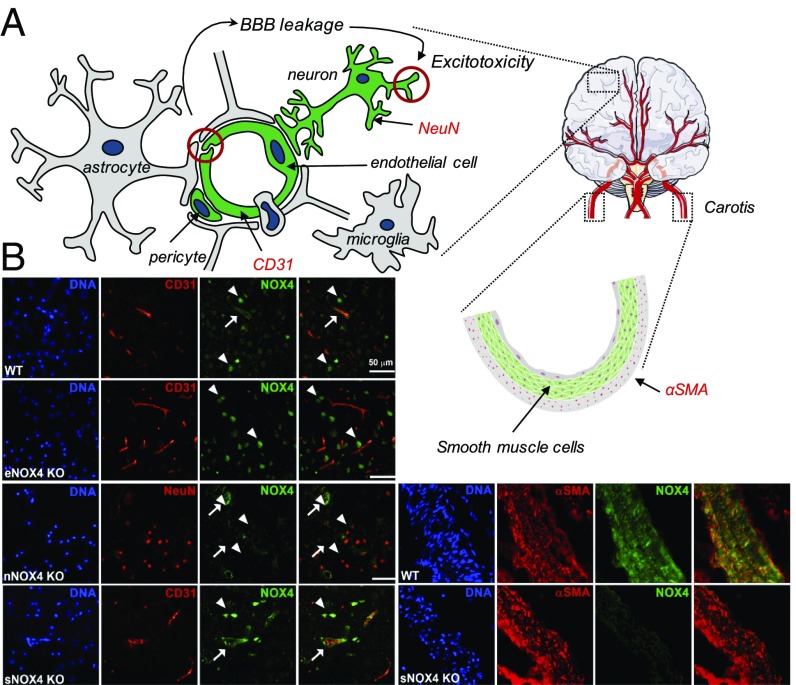
As a possible explanations for this, the blood–brain barrier (BBB) is a unique feature of the brain versus heart and skeletal muscle and, in ischemic cerebrovascular smooth muscle cells (SMCs), NOX4 impairs myogenic tone (15). We therefore investigated the contribution of both vascular cell types, endothelial and vascular smooth muscle. Moreover, as some neurons were also NOX4 immunopositive (9), we also investigated neuron-specific Nox4 KO mice (Fig. 2A).
Fig. 2.
Validation of cell-specific NOX4 deletion in mice in endothelial, neuronal, and SMCs. (A, Left) Main cellular components of the neurovascular unit are included in the scheme. NOX4 is predominantly expressed in some components of the neurovascular unit such as endothelial cells (green), neurons (green), and pericytes (green) rather than astrocytes (gray), macrophages (gray), and microglia (gray). In fact, NOX4 play an important role in both BBB leakage after stroke (red circle on left) and neuron-derived excitotoxicity (red circle on right). Neuronal (NeuN) and endothelial (CD31) makers are also included in red. (A, Right) Due to the lack of SMCs (green) in the brain, carotid artery tissue was needed for the cell-specific Nox4 KO mice validation. SMC-specific marker (αSMA) is in red. (B, Left, first row) Immunohistochemistry (IHC) of brain tissue from a WT animal shows NOX4 expression (green) in neurons (top and bottom arrowheads) and endothelial cells (middle arrow). (B, Left, second row) IHC shows no expression of endothelial NOX4 in eNox4 KO (see merged panels) while NOX4 is still expressed in neurons (arrows). (B, Left, third row) Neuronal NOX4 is not expressed (green, all arrows) where neurons are detected by the specific tissue marker NeuN (red). (B, Left, fourth row) sNOX4 KO presents NOX4 expression (green) in both neuronal (arrow) and endothelial cells (arrowhead). Since specific expression of NOX4 in SMCs is not possible to detect in brain tissue, carotid tissue is used. (B, Right, first row) Carotid artery sections from WT mice show SMCs (red) and NOX4 (green) expression. (B, Right, second row) Carotid tissue from sNOX4 KO animals show no expression of NOX4 (green) in SMCs in comparison with WT sections. DNA was visualized using Hoechst dye.
Cell-Specific Nox4 KO Mice Generation and Validation.
Endothelial cell-specific Nox4 KO mice were generated by crossing Nox4 floxed homozygous female mice with Tie2-Cre+ (endothelial cell-specific promotor) (16) male mice. Neuronal and SMC-specific Nox4 KO mice were generated using inducible CaMKII− Cre+ (17) and SMMHC− Cre+ (18) mice, respectively. While neuronal (nKO) and SMC NOX4 KO (sKO) are tamoxifen-inducible mice lines, endothelial NOX4 KO (eKO) mice bear a constitutive deletion. Therefore, both SMC (sWT) and neuronal WT (nWT) require tamoxifen injections before studying their KO phenotype, and consequently both share the tamoxifen-treated WT line as a control (n/sWT) (SI Appendix, Fig. S3). Costaining of NOX4 and CD31 showed the cell-specific absence of the NOX4 protein in endothelial cells in the brain of endothelial Nox4 KO (eKO) mice (Fig. 2B, Left, second row) but not in SMC-specific Nox4 KO (sKO) (Fig. 2B, Left, fourth row). Using a similar costaining approach of NOX4 and NeuN, no NOX4 could be detected in neurons of the neuronal Nox4 KO (nKO) mice (Fig. 2B, Left, third row). As no SMC could be stained in the brain, for control purposes, carotid tissue was used to confirm that SMC of the sKO indeed do not express NOX4 (Fig. 2B, Right). Collectively, this confirmed three cell-specific Nox4 KO mouse lines: eKO (constitutive), nKO (inducible), and sKO (inducible) (SI Appendix, Fig. S3). The Tie2 promoter is also active in bone marrow. However, Nox4 expression was beyond the detection limit in both WT and Tie2-Cre+ mice while β-actin (internal control) and Nox2 signals, the major immune cell NOX isoform, were strong and similar both in WT and Tie2-Cre+ mice. These data suggest that macrophages/bone marrow cells would not contribute to an eKO phenotype (SI Appendix, Table S1).
Endothelial and Neuronal, but Not Smooth Muscle, NOX4 Deficiency Improves Stroke Outcome.
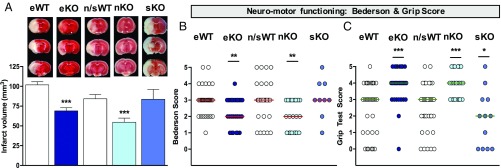
To examine the poststroke impact of cell-specific eKO, sKO, and nKO of NOX4, 8- to 12-wk-old mice were subjected to 1 h of tMCAO followed by 24 h of reperfusion. TTC-stained brain sections showed a significant reduction of infarct volume on day 1 after surgery in eKO and nKO compared with WT mice, but not in sKO mice (Fig. 3A). As a clinical parameter, two independent neurological outcome parameters were assessed, which measure global neurological and motor impairments, i.e., the Bederson score (Fig. 3B) and the Grip test (Fig. 3C). Both outcome scores were significantly improved in eKO and nKO but not in sKO mice, the latter showing even a slightly deleterious outcome in the Grip test (Fig. 3C). These data suggest a contribution of endothelial and neuronal but not SMC NOX4 in stroke.
Fig. 3.
Endothelial and neuronal, but not SMC-specific NOX4 KO reduces brain infarct and neurological scores. (A) eKO (dark blue) and nKO (light blue) show smaller infarct sizes compared with WT mice (white) (***P < 0.001, n = 23), sKO mice (cyan) did not show decreased infarcts (n = 12). Complete sets of brain slices from a representative animal (TTC staining) are shown above the graph. (B) Neurological outcome was improved in eKO and nKO in comparison with WT mice (**P < 0.01, n = 23). However, no protection on neurological function was observed in sKO (n = 12). (C) Motor function was also enhanced in eKO and nKO in comparison with WT mice (***P < 0.001, n = 23), but not in sKO mice (*P < 0.05). The n/sWT mice were treated with tamoxifen based on their respective n/sKO mice. The eWT mice were not treated since eKO have a constitutive deletion.
Differential Roles of Endothelial and Neuronal NOX4 in BBB Breakdown and Neurotoxicity.
Increased vascular permeability and subsequent neurotoxicity are considered key contributors to poststroke pathophysiology (19). Since the localizations of NOX4 in mice (Fig. 2B) and humans (19) match these two pathomechanisms, we hypothesized that NOX4 may contribute to both of them.
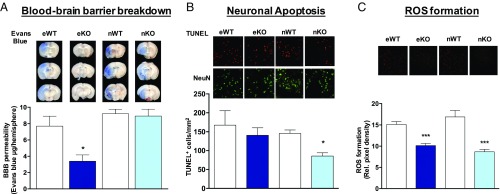
First, we assessed the role of NOX4 in BBB disruption by functional quantification of poststroke edema formation using extravasation of the vascular tracer, Evans blue, into the brain parenchyma. Evans Blue extravasation was significantly reduced in brains from eKO but not nKO mice, indicating a specific role of eNox4 in poststroke BBB stability (Fig. 4A). Second, we quantified neuronal cell death by costaining for the neuronal marker NeuN and a TUNEL detection kit. Apoptotic neurons were reduced in nKO (Fig. 4B) but not eKO mice (Fig. 4B). Necrotic cells were also assessed in both eKO and nKO mice, showing similar results, no effect in eKO, and reduction in nKO (SI Appendix, Fig. S4). Autophagy may be an alternative pathomechanism in brain ischemia (20). Indeed, in stroked eKO versus WT mice the autophagy marker, beclin-1, was significantly reduced (SI Appendix, Fig. S5). Thus, BBB protection and smaller infarct in eNOX4 KO are most likely linked to less autophagic stress and subsequently a reduction in cell demise (21), while nNox4 KO mice showed direct neuroprotection via an antiapoptotic mechanism.
Fig. 4.
Contribution of endothelial and neuronal NOX4 to BBB breakdown, neuronal apoptosis, and ROS formation. (A) BBB integrity as assessed by Evans blue extravasation was preserved in the eKO (dark blue) but not in the nKO (light blue) at day 1 after 1 h of tMCAO (*P < 0.05, n = 6). Complete set of brain slices from a representative animal are shown above the graph. (B) nKO presented fewer apoptotic cells in comparison with nWT (*P < 0.05, n = 5) while no effect was shown in eKO mice (n = 5). Representative staining panels are shown above the bar graph. (C) Both eKO and nKO mice showed decreased oxidative stress compared with their respective WT littermates (***P < 0.001, n = 5). Representative staining pictures are shown above the graph.
To test the link of both observations to the enzymatic activity of NOX4, i.e., ROS formation, we measured oxidative stress using dihydroethidium staining of cryosections. ROS generation was significantly reduced in the infarcted brains of both nKO and eKO mice compared with their corresponding controls (Fig. 4C). Thus, ROS formation appears to be a common denominator of both eNOX4 and nNOX4.
Neuroprotective Effect of Neuronal NOX4 Inhibition Is Confirmed by in Vivo and in Vitro Approaches in a Second Species.
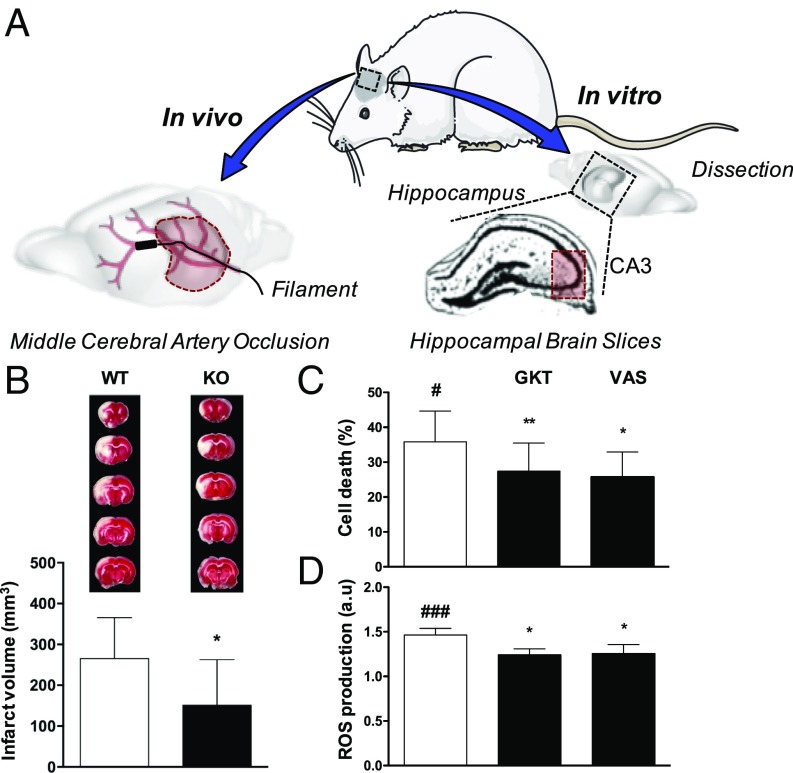
The stroke therapy academic industry roundtable (STAIR) criteria recommend that any result should be corroborated in another animal species (www.thestair.org/) (22). Until now only mice have been used to show that NOX4 inhibition reduces infarct volume and neurological deficits following cerebral ischemia (9). To further validate the role of NOX4 in stroke pathophysiology and outcome, we extended our studies to rats using both in vitro and in vivo models (Fig. 5A). We generated global Nox4 KO rats (SI Appendix, Fig. S6) and confirmed the absence of Nox4 DNA markers by genotyping (SI Appendix, Fig. S7) and brain, kidney, and lung mRNA markers by qPCR including β-actin as internal control (SI Appendix, Table S2). No phenotypic differences were observed in the Nox4 KO rat compared with its respective WT. Nox4 KO rats were then subjected to 90 min of tMCAO followed by 24 h of reperfusion. Consistent with our in vivo observations in the mouse model, Nox4 KO rats showed significantly smaller infarct volumes compared with the WT littermates (Fig. 5B). Importantly, replicating the protective effect of Nox4 deletion in a second species further strengthens the attractive hypothesis of NOX4 inhibition as a neuroprotective therapeutic approach for stroke patients.
Fig. 5.
Validation of NOX4 in a second animal species by using tMCAO in NOX4 KO rats and NOX4 inhibitors in hippocampal brain slices subjected to OGD. (A) Similarly to the mouse model, occlusion of the middle cerebral artery was performed in WT and Nox4 KO rats. Rat hippocampal brain slices were subjected to oxygen and glucose deprivation. After treatment, cell viability and ROS formation (CA3) were measured. (B) Infarct volume in Nox4 KO rats was decreased compared with the WT littermate [*P < 0.05, n = 11 (WT) and n = 9 (KO)]. Complete sets of brain slices from a representative animal (TTC staining) are shown above the graph. (C) Cell death (#P < 0.05) was significantly reduced in hippocampal brain slices treated with 0.1 μM GKT136901 and 10 μM VAS2870 as NOX inhibitors (*P < 0.05, **P < 0.01, n = 6). (D) ROS formation (###P < 0.001) was also decreased after NOX inhibition (*P < 0.05, n = 5).
We next used an in vitro rat hippocampal brain slice model mimicking ischemic conditions by oxygen and glucose deprivation (OGD), which allowed us (i) to corroborate our findings from the in vivo studies, using a translationally more relevant pharmacological approach and (ii) to further analyze nNOX4 function independently of any functional vascular component. Hippocampal brain slices were subjected for 15 min to OGD followed by 2 h of reoxygenation in the presence or absence of the NOX inhibitors GKT136901 (0.1 μM) or VAS2870 (10 μM). At the end of the experiment, hippocampal cell death was assessed by the MTT assay, and we observed that both NOX4 inhibitors were neuroprotective after an OGD period (Fig. 5C). Similarly, oxidative stress measured by the H2DCFDA probe was significantly reduced after 2 h of reoxygenation under NOX inhibitor treatment (Fig. 5D). To support these findings, hippocampal brain slices from Nox4 KO mice were subjected to OGD for 15 min followed by a 2-h reoxygenation period. As expected, cell death (SI Appendix, Fig. S8A) and ROS formation (SI Appendix, Fig. S8B) were significantly reduced in Nox4 KO mice compared with their respective WT littermates.
Once both rodent species (mouse and rat) showed direct neuroprotection and reduction of brain damage poststroke, we extended our findings to a human in vitro model of BBB. Primary cultures of human brain microvascular endothelial cells (HBMEC) were subjected to 6 h of hypoxia followed by 24 h of reoxygenation in the presence or absence of a NOX4 inhibition treatment. GKT136901 (1 μM) treatment significantly increased cell viability after a hypoxic period in comparison with nontreated cells (SI Appendix, Fig. S9), showing the possible potential of translating this therapy to humans. In agreement with our in vivo findings, similar in vitro ischemic conditions significantly increased cell permeability after 24 h of reoxygenation while treatment with GKT136901 (1 μM) prevented this detrimental effect as evidenced by a reduction in the diffusion of the dextran tracer (3 and 70 kDa) (SI Appendix, Fig. S10 A and B) and Evans Blue extravasation (SI Appendix, Fig. S10C) through a monolayer of HBMEC.
Using a combined preclinical meta-analysis, we previously established that NOX1 plays no role in brain ischemia (12). Nevertheless, GKT136901 has been described as a NOX1/NOX4 inhibitor (23) and may have acted also via NOX1. To test this possibility, Nox4 KO mice were subjected to 1 h tMCAO and treated 1 h poststroke with GKT136901 (10 mg/kg), showing no additional effect over nontreated Nox4 KO animals (SI Appendix, Fig. S11). Therefore, these data suggest that NOX1 plays no role in the action of GKT136901 and that NOX4-driven neurotoxicity can be pharmacologically prevented using isoform-specific NOX inhibitors that inhibit massive ROS production and oxidative damage. Hence, pharmacological modulation of NOX4 could lead to direct neuroprotection and improvement of BBB stability.
Discussion
Here we elucidate one mechanism: why, of all organs, the brain has the highest sensitivity to hypoxia/ischemia. The induction of NOX4, a primary source of ROS, in many tissues during hypoxia (9–11) suggested that NOX4 may be a uniform target for postischemic cytoprotective therapies. Surprisingly, however, we found the role of NOX4 in ischemia to be highly specific to the brain, i.e., in ischemic brain stroke, and not to be involved in lung ischemia (24), myocardial IR, or hindlimb ischemia. We have previously shown that the predominant localizations of NOX4 in the mouse and human brain, pre- and poststroke, are in endothelial cells and neurons (9). At the mRNA level, other cells have been described to express NOX4, which, however, does not translate to detectable levels at the protein level. By examining cell-specific Nox4 KO mice, we provide clear evidence on how endothelial and neuronal Nox4 have a major impact in brain vulnerability to ischemia. However, we cannot exclude that Nox4 minor expression in other cells may also contribute, in smaller percentages, to the observed phenotype. For example, pericytes have been suggested to play an important role in the formation and maintenance of the BBB, leading to a detrimental role in acute ischemia (25). Moreover, Nox4 has been recently considered as the major source of ROS in human pericytes (26). However, the fact that the endothelial and neuronal Nox4 KO mice add up completely to the global NOX4 KO phenotype would suggest that in our stroke model pericyte contribution is indeed rather minor and endothelial and neuronal cells constitute the main sources of NOX4 relevant to stroke damage.
One key explanation for the brain-specific role of NOX4 appears to reside in the BBB, which differentiates the brain from the heart and other organs (24). We identify endothelial NOX4, but not vascular smooth muscle nor neuronal NOX4, as the key source of ROS triggering to breakdown upon an ischemic stroke. In heart and vascular periphery such leakage and edema may also occur but is apparently less detrimental (25). Indeed, we find that NOX4 inhibition prevents BBB leakage in a human BBB in vitro model. It will be worth exploring whether in other organs with a blood–tissue barrier, e.g., the placenta (26) or mammary glands, a similar pathological relevance during ischemia or hypoxia is observed.
A second unexpected mechanism is that NOX4 can cause direct neuronal autotoxicity. Previously, NOX4 was thought to be primarily relevant in the vasculature. Neuronal autotoxicity in stroke, also termed excitotoxicity, was previously ascribed primarily to nitric oxide overproduction (27) and neurotrauma (28). However, both in vivo and also importantly in an ex vivo brain slice model free of BBB effects, neuronal NOX4 was clearly a major contributor to cellular autotoxicity upon ischemia or hypoxia. Pharmacological inhibition of NOX4 was also neuroprotective, providing proof-of-concept for the drugability of this component of NOX4’s actions as well.
Both cellular mechanisms explain why NOX4 inhibition is such a powerful experimental therapeutic approach in stroke (9). It directly prevents two damage-trigger mechanisms: the breakdown of the BBB and the subsequent neuronal autotoxicity.
To better estimate whether these mouse data may be translatable into the clinic as a first-in-class cytoprotective therapy in stroke, future experiments should confirm NOX4’s role in a second species and then translate it to humans. It is therefore reassuring that the essential role of NOX4 in stroke is preserved in a second rodent species using a Nox4 KO rat and by pharmacological intervention in a human brain microvascular endothelial cell model. Subsequent studies in large-animal stroke models and preparation for a clinical trial are ongoing (European Research Council–Proof of Concept Project 737586 SAVEBRAIN).
This study cannot elucidate why the substantial induction of NOX4 and subsequent ROS formation in heart and hindlimb and other organs (29) does not lead to tissue damage. However, this is reminiscent of the induction of NOX4 in the lung during hypoxia (9) and ischemia (26), respectively, which is also not detrimental. Previous studies looking at the short-term effects (after 24 h) showed either no (30) or a detrimental role (31) of NOX4 in heart ischemia. We are aware that Nox4 has been proposed to be one of the major sources of ROS in failing hearts (26), playing a key role in cardiac failure (32) and mitochondrial growth (33) and dysfunction in heart tissue (34). However, our findings do not indicate a short-term effect in heart ischemia. Likewise, looking at long-term outcomes in hindlimb and MI models, where the assessment was performed 4 wk after surgery, we detected no significant effect. With respect to the hindlimb model of ischemia, a variation of this model using an artery excision technique, which results in more inflammation and surrounding tissue damage, NOX4 is protective (11, 35, 36), which may involve NOX4’s anti-inflammatory role (36, 37). Using a pure ischemic hindlimb model, different KO strategy, and anesthesia, we find no role for NOX4.
In conclusion, we elucidate the superior sensitivity of the brain compared with other organs to ischemic damage at the cellular level by NOX4-dependent neuronal autotoxicity and BBB breakdown. Our findings also provide a clear rational for further development of NOX4 inhibitors as a first-in-class neuroprotective strategy in stroke.
Materials and Methods
Detailed methods are provided in SI Appendix, SI Materials and Methods.
Animals.
All animal experiments were approved by local state authorities (Regierung von Unterfranken) as well as by the German Animal Welfare Act, the Dutch law on animal experiments, and the Ethics Committee of the Faculty of Medicine, Universidad Autónoma de Madrid (Madrid).
Cell-Specific Nox4 KO Mice Generation.
Constitutive Nox4 KO mice and floxed Nox4 mice were generated as described (9). To generate endothelial-specific Nox4 KO (eNox4 KO) mice, female mice were bred with male mice that express the Cre recombinase gene under control of the endothelial-cell–specific Tie2 promotor (18). During breeding, only males that bear the Cre gene were selected for future breeding rounds, while females were not allowed to bear the Cre. For experiments Cre+ (eNox4 KO) males were used. SMC-specific and neuron-specific Nox4 KO mice were generated in an analogous way using SMMHC-CreERT2 mice (18) and CamKII Cre (17) mice, respectively.
Stroke Surgery (tMCAO Model).
The model was previously established as described for mice (9) and rat (38) surgery.
Brain Infarct Volume Measurements.
The ischemic lesion was measured 24 h after MCAO using TTC staining (38).
Murine Model of Hindlimb Ischemia.
The right femoral artery was permanently ligated. A suture (5-0 silk) was placed around the femoral artery in between the branching of the arteria epigastrica and the a. poplitea. These last two arteries were also ligated to prevent collateral flow and backflow, respectively. The wound was then closed with a 4-0 polysorb suture.
MI and IR of the Heart Models.
Using a left thoracotomy, the left descending coronary artery was ligated with a 6-0 polyprolene suture permanently for MI. For the transient ischemia of 45 min, a small polyethylene tube was inserted under the ligature, compressing the coronary artery, which was then removed after the ischemic period.
Hippocampal Brain Slices.
In vitro damage caused by oxygen–glucose deprivation/reoxygenation and the protection elicited by NOX inhibitors was studied in rat hippocampal slices. Brains from 2- to 3-mo-old male Sprague-Dawley rats (250–300 g) or Nox4 KO mice (2–3 mo) were isolated and subjected to OGD as previously described (39).
HBMEC Culture Subjected to Hypoxia.
HBMECs (Cell Systems) were cultured to ∼90% confluence. Cell medium was replaced for non-FBS–containing medium (2 mL/well) following 6 h of hypoxia (94.8% N2, 0.2% O2, and 5% CO2). The hypoxia period was followed by 24 h of reperfusion in the presence or absence of 1 μM GKT136901 (Genkyotech) as treatment.
Statistics.
All mice and in vitro data are expressed as mean ± SEM. Rat in vivo experiments are expressed as mean ± SD. Using the GraphPad Prism 5.0 software package statistical differences were determined by Student’s two-tailed t test and Mann–Whitney test experiments. For repeated measurements, a two-way ANOVA was used. Statistical comparisons between groups were performed using one-way ANOVA. The number of animals necessary to detect a standardized effect size on infarct volumes ≥0.2 was determined via a priori sample-size calculation. P values < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
Tie2 Cre mice were kindly provided by Prof. Yanagisawa (University of Texas Southwestern); SMMHC-Cre-ERT2 mice by Prof. Offermanns (Max-Planck-Institute Bad Neuheim) and Prof. P. Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire/University of Strasbourg Institute for Advanced Study); CamKII Cre mice by Dr. Plesnila and Dr. Schneider (Institute for Stroke and Dementia, Ludwig-Maximilians-Universität München). We thank A. M. Shah for providing the NOX4 antibody; Dr. K. Radermacher and Dr. K. Wingler for help in organizing animal creation, breeding, and shipment; and D. Urlaub, H. Menzel, J. Debets, A. Strzelecka-Brouns, and H. van Essen for technical help. This study was supported by European Research Council Advanced Investigator Grant 294683 RadMed (to H.H.H.W.S.); the Deutsche Forschungsgemeinschaft (C.K.); Fondo de Investigaciones Sanitarias (Instituto de Salud Carlos III/Fondo Europeo de Desarrollo Regional) (Programa Miguel Servet: CP14/00008, PI16/00735); Fundación Mutua Madrileña (J.E.); and Spanish Ministry of Economy and Competence Ref. SAF2015-63935R (to M.G.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705034114/-/DCSupplemental.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radermacher KA, et al. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid Redox Signal. 2013;18:1418–1427. doi: 10.1089/ars.2012.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stankowski JN, Gupta R. Therapeutic targets for neuroprotection in acute ischemic stroke: Lost in translation? Antioxid Redox Signal. 2011;14:1841–1851. doi: 10.1089/ars.2010.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casas AI, et al. Reactive oxygen-related diseases: Therapeutic targets and emerging clinical indications. Antioxid Redox Signal. 2015;23:1171–1185. doi: 10.1089/ars.2015.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dao VT-V, et al. Pharmacology and clinical drug candidates in redox medicine. Antioxid Redox Signal. 2015;23:1113–1129. doi: 10.1089/ars.2015.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt HHHW, et al. Antioxidants in translational medicine. Antioxid Redox Signal. 2015;23:1130–1143. doi: 10.1089/ars.2015.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C. Neuroprotection for stroke: Current status and future perspectives. Int J Mol Sci. 2012;13:11753–11772. doi: 10.3390/ijms130911753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diebold I, et al. The hypoxia-inducible factor-2alpha is stabilized by oxidative stress involving NOX4. Antioxid Redox Signal. 2010;13:425–436. doi: 10.1089/ars.2009.3014. [DOI] [PubMed] [Google Scholar]
- 9.Kleinschnitz C, et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8:e1000479. doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doerries C, et al. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res. 2007;100:894–903. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- 11.Craige SM, et al. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleikers PWM, et al. A combined pre-clinical meta-analysis and randomized confirmatory trial approach to improve data validity for therapeutic target validation. Sci Rep. 2015;5:13428. doi: 10.1038/srep13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madeddu P, et al. Murine models of myocardial and limb ischemia: Diagnostic end-points and relevance to clinical problems. Vascul Pharmacol. 2006;45:281–301. doi: 10.1016/j.vph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, et al. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couffinhal T, et al. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 16.Kisanuki YY, et al. Tie2-Cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 17.Casanova E, et al. A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis. 2001;31:37–42. doi: 10.1002/gene.1078. [DOI] [PubMed] [Google Scholar]
- 18.Wirth A, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 19.Krause K-H. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis. 2004;57:S28–S29. [PubMed] [Google Scholar]
- 20.Rami A, Kögel D. Apoptosis meets autophagy-like cell death in the ischemic penumbra: Two sides of the same coin? Autophagy. 2008;4:422–426. doi: 10.4161/auto.5778. [DOI] [PubMed] [Google Scholar]
- 21.Adhami F, Schloemer A, Kuan C-Y. The roles of autophagy in cerebral ischemia. Autophagy. 2007;3:42–44. doi: 10.4161/auto.3412. [DOI] [PubMed] [Google Scholar]
- 22.Macleod MR, et al. Good laboratory practice: Preventing introduction of bias at the bench. Stroke. 2009;40:e50–e52. doi: 10.1161/STROKEAHA.108.525386. [DOI] [PubMed] [Google Scholar]
- 23.Altenhöfer S, Radermacher KA, Kleikers PWM, Wingler K, Schmidt HHHW. Evolution of NADPH oxidase inhibitors: Selectivity and mechanisms for target engagement. Antioxid Redox Signal. 2015;23:406–427. doi: 10.1089/ars.2013.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weissmann N, et al. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat Commun. 2012;3:649. doi: 10.1038/ncomms1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura A, et al. Detrimental role of pericyte Nox4 in the acute phase of brain ischemia. J Cereb Blood Flow Metab. 2015;36:1143–1154. doi: 10.1177/0271678X15606456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroda J, et al. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinschnitz C, et al. NOS knockout or inhibition but not disrupting PSD-95-NOS interaction protect against ischemic brain damage. J Cereb Blood Flow Metab. 2016;36:1508–1512. doi: 10.1177/0271678X16657094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stover JF, et al. NOSTRA Investigators Nitric oxide synthase inhibition with the antipterin VAS203 improves outcome in moderate and severe traumatic brain injury: A placebo-controlled randomized phase IIa trial (NOSTRA) J Neurotrauma. 2014;31:1599–1606. doi: 10.1089/neu.2014.3344. [DOI] [PubMed] [Google Scholar]
- 29.Mittal M, et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 30.Braunersreuther V, et al. Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury. J Mol Cell Cardiol. 2013;64:99–107, and erratum (2014) 66:189. doi: 10.1016/j.yjmcc.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Matsushima S, et al. Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia-inducible factor-1α and upregulation of peroxisome proliferator-activated receptor-α. Circ Res. 2013;112:1135–1149. doi: 10.1161/CIRCRESAHA.111.300171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroda J, Sadoshima J. NADPH oxidase and cardiac failure. J Cardiovasc Transl Res. 2010;3:314–320. doi: 10.1007/s12265-010-9184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maejima Y, Kuroda J, Matsushima S, Ago T, Sadoshima J. Regulation of myocardial growth and death by NADPH oxidase. J Mol Cell Cardiol. 2011;50:408–416. doi: 10.1016/j.yjmcc.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ago T, et al. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M, et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray SP, et al. Reactive oxygen species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling. Arterioscler Thromb Vasc Biol. 2016;36:295–307. doi: 10.1161/ATVBAHA.115.307012. [DOI] [PubMed] [Google Scholar]
- 37.Schürmann C, et al. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur Heart J. 2015;36:3447–3456. doi: 10.1093/eurheartj/ehv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bederson JB, et al. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 39.Egea J, et al. Neuroprotection afforded by nicotine against oxygen and glucose deprivation in hippocampal slices is lost in alpha7 nicotinic receptor knockout mice. Neuroscience. 2007;145:866–872. doi: 10.1016/j.neuroscience.2006.12.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.