Significance
G protein-coupled receptors (GPCRs) have long been considered to function primarily at the plasma membrane. Consequently, most drugs are designed to target GPCRs at the cell surface. Ligand-bound GPCRs undergo clathrin- and dynamin-dependent endocytosis. It is uncertain whether GPCRs in endosomes control complex pathophysiological processes in vivo and are a viable therapeutic target. We report that the CGRP receptor signals from endosomes to regulate activity of pain-transmitting neurons in the spinal cord. Lipid-conjugated CGRP receptor antagonists accumulate in endosomes, selectively inhibit endosomal signals, and block sustained excitation of spinal neurons and persistent nociception. The results suggest that GPCRs in endosomes, in addition to those at the cell surface, control ongoing pathophysiological processes in vivo and identify GPCRs in endosomes as a new target for therapy.
Keywords: G protein-coupled receptors, endocytosis, neuropeptides, pain, nociception
Abstract
G protein-coupled receptors (GPCRs) are considered to function primarily at the plasma membrane, where they interact with extracellular ligands and couple to G proteins that transmit intracellular signals. Consequently, therapeutic drugs are designed to target GPCRs at the plasma membrane. Activated GPCRs undergo clathrin-dependent endocytosis. Whether GPCRs in endosomes control pathophysiological processes in vivo and are therapeutic targets remains uncertain. We investigated the contribution of endosomal signaling of the calcitonin receptor-like receptor (CLR) to pain transmission. Calcitonin gene-related peptide (CGRP) stimulated CLR endocytosis and activated protein kinase C (PKC) in the cytosol and extracellular signal regulated kinase (ERK) in the cytosol and nucleus. Inhibitors of clathrin and dynamin prevented CLR endocytosis and activation of cytosolic PKC and nuclear ERK, which derive from endosomal CLR. A cholestanol-conjugated antagonist, CGRP8–37, accumulated in CLR-containing endosomes and selectively inhibited CLR signaling in endosomes. CGRP caused sustained excitation of neurons in slices of rat spinal cord. Inhibitors of dynamin, ERK, and PKC suppressed persistent neuronal excitation. CGRP8–37–cholestanol, but not unconjugated CGRP8–37, prevented sustained neuronal excitation. When injected intrathecally to mice, CGRP8–37–cholestanol inhibited nociceptive responses to intraplantar injection of capsaicin, formalin, or complete Freund’s adjuvant more effectively than unconjugated CGRP8–37. Our results show that CLR signals from endosomes to control pain transmission and identify CLR in endosomes as a therapeutic target for pain. Thus, GPCRs function not only at the plasma membrane but also in endosomes to control complex processes in vivo. Endosomal GPCRs are a drug target that deserve further attention.
G protein-coupled receptors (GPCRs) have long been considered to function primarily at the plasma membrane, where they interact with ligands in the extracellular fluid and couple to heterotrimeric G proteins that convey signals within the cell. Consequently, most therapeutic drugs are designed to target GPCRs at the cell surface. Ligand-bound GPCRs interact with β-arrestins (βARRs), which desensitize G protein signaling, mediate receptor endocytosis, and thereby rapidly terminate plasma membrane signaling (1). The conventional view that GPCRs signal only from the plasma membrane has been challenged by reports that GPCRs can continue to signal from endosomes by G protein- and βARR-mediated mechanisms (2–9). However, the contribution of endosomal signaling of GPCRs to the control of complex pathophysiological processes in vivo is uncertain, and whether endosomal GPCRs are a viable therapeutic target is far from clear.
GPCRs in endosomes can generate signals in subcellular compartments (2, 6–8). Compartmentalized signaling involves GPCR association with signaling and regulatory proteins that determine the subcellular location of signals. By these mechanisms, a large number of different GPCRs can specifically control cellular functions using a small number of effectors. The importance of GPCR compartmentalized signaling for integrated responses requires further investigation.
Calcitonin gene-related peptide (CGRP) is expressed throughout the nervous system (10). The CGRP receptor comprises calcitonin receptor-like receptor (CLR), a GPCR, and receptor activity modifying protein 1 (RAMP1), a single transmembrane protein that imparts ligand specificity and ensures CLR targeting to the cell surface. Noxious stimuli evoke CGRP release from the terminals of primary sensory neurons in the dorsal horn of the spinal cord and in peripheral tissues. CGRP activates CLR/RAMP1 on spinal neurons to induce nociception and on peripheral arterioles to cause neurogenic inflammation. CGRP and CLR are targets for migraine pain (10). Although CGRP stimulates endocytosis of CLR/RAMP1 (11), the contribution of CLR/RAMP1 endocytosis to pain transmission is uncertain, and whether CLR in endosomes is a therapeutic target for pain is unknown. We have recently found that the neurokinin 1 receptor (NK1R) signals from endosomes to mediate substance P (SP)-induced nociception (12). We now describe a major role for endosomal CLR in nociception and identify CLR in endosomes as a therapeutic target.
Results
CGRP Stimulates Clathrin- and Dynamin-Dependent Endocytosis of CLR.
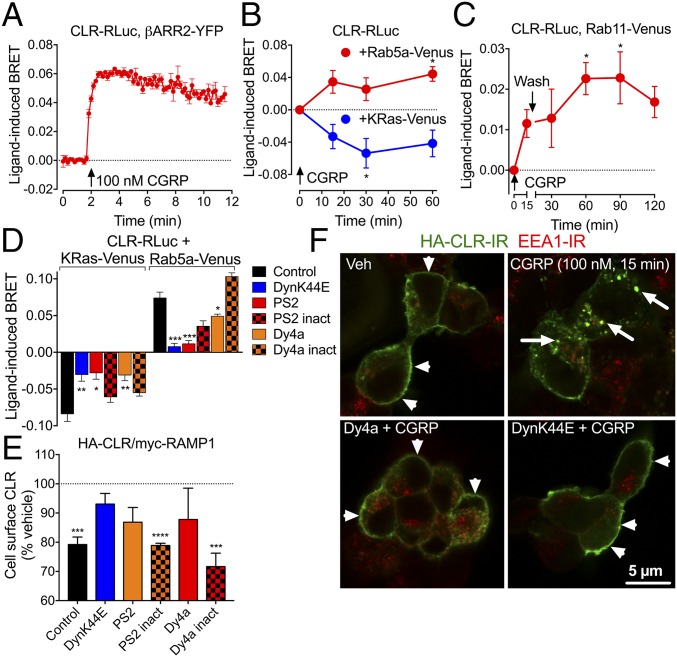
We used Bioluminescence Resonance Energy Transfer (BRET) to quantify the proximity of CLR to βARR2, an adaptor for clathrin-mediated endocytosis, and to resident proteins of the plasma membrane (KRas), early endosomes (Rab5a), and recycling endosomes (Rab11) of HEK293 cells coexpressing CLR and RAMP1 (12, 13). CGRP (100 nM, continuous) increased CLR-RLuc/βARR2-YFP BRET (EC50, 2 nM; pEC50, 8.74 ± 0.18) (Fig. 1A and Fig. S1A). CGRP decreased CLR-RLuc/KRas-Venus BRET and increased CLR-RLuc/Rab5a-Venus BRET (Fig. 1B). After incubation with CGRP (15 min) and washout, there was an increase in CLR-RLuc/Rab11-Venus BRET (Fig. 1C). Dominant-negative dynamin K44E (DynK44E), the dynamin inhibitor Dyngo4a (Dy4a, 30 μM) (14), and the clathrin inhibitor PitStop2 (PS2, 30 μM) (15) prevented CLR removal from the plasma membrane and inhibited trafficking to early endosomes (Fig. 1D). There was no effect of inactive Dy4a or PS2 analogs. We confirmed these results using two other assays of endocytosis. Cell-surface ELISA using antibodies to extracellular epitopes (HA-CLR, myc-RAMP1) showed that CGRP (100 nM, continuous) induced rapid removal of both receptor components from the plasma membrane (Fig. S1B). DynK44E, Dy4a, and PS2, but not inactive analogs, inhibited CLR removal (Fig. 1E). CGRP (100 nM, 15 min) induced CLR trafficking from the plasma membrane to early endosomes identified by early endosome antigen 1 (EEA1) immunoreactivity (IR) (Fig. 1F and Fig. S1C). Dy4a and DynK44E inhibited endocytosis. Expression of Rab5a-Venus did not appreciably alter the appearance of EEA1-positive early endosomes, supporting the suitability of the BRET approach to study CLR endocytosis (Fig. S1D).
Fig. 1.
CLR endocytosis. (A–D) BRET assays of CLR-RLuc8 and βARR2-YFP (A), Rab5a-Venus and KRas-Venus (B and D), and Rab11-Venus (C) proximity in HEK cells. (D) CLR-RLuc8 and Rab5a-Venus or KRas-Venus BRET (100 nM CGRP, 15 min). (E) Cell-surface HA-CLR ELISA in HEK cells (100 nM CGRP, 15 min). n = 3 experiments. (F) Confocal images of HA-CLR-IR and EEA1-IR in HEK cells. Dy4a, dynamin inhibitor; DynK44E, dominant-negative dynamin; inact, inactive analog; PS2, clathrin inhibitor. n = 3–6 experiments. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.0001 to basal or control. ANOVA, Sidak’s test.
CLR Endocytosis Mediates a Subset of Signals in Subcellular Compartments.
To study CGRP signaling in subcellular compartments, we expressed in HEK cells HA-CLR/myc-RAMP1 and genetically encoded Förster Resonance Energy Transfer (FRET) biosensors for plasma membrane and cytosolic cAMP (pmEpac2 and cytoEpac2, respectively), plasma membrane and cytosolic protein kinase C (PKC; pmCKAR and cytoCKAR), and cytosolic and nuclear extracellular signal-regulated kinase (ERK; cytoEKAR and nucEKAR) (12, 13, 16). Biosensors are targeted to subcellular compartments and are reversibly modified by second messengers, kinases, and phosphatases. Single-cell high-content imaging was used to study signaling kinetics in subcellular compartments of living cells.
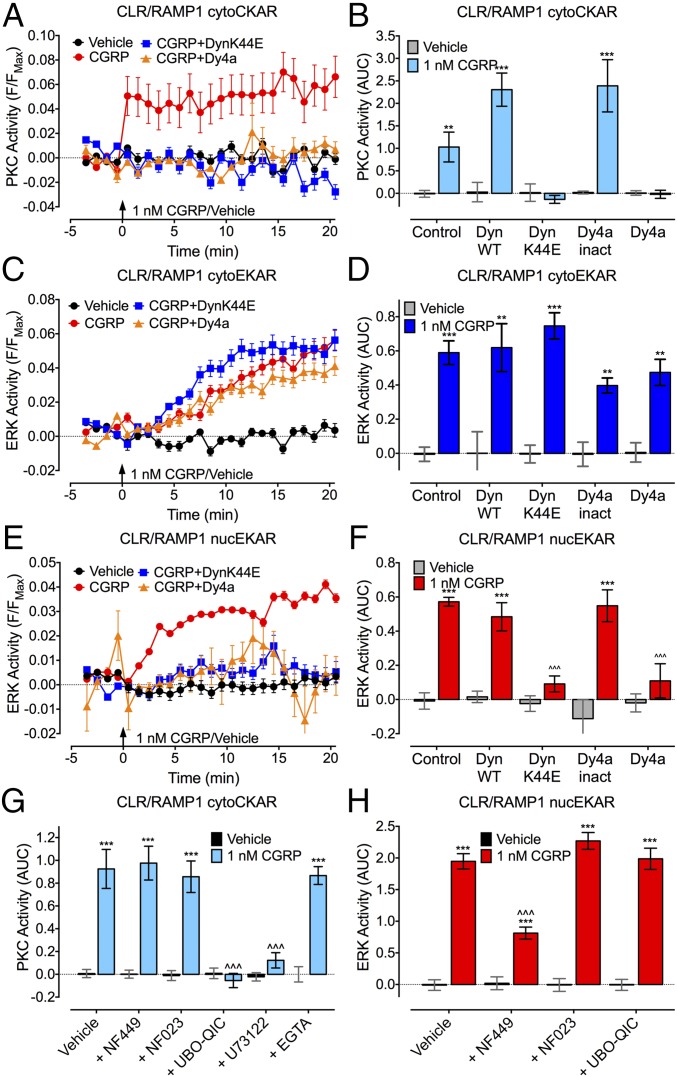
CGRP (1 nM, continuous) induced a rapid and sustained increase in plasma membrane and cytosolic cAMP (Fig. S2 A–C), in agreement with CLR coupling to Gαs and adenylyl cyclase (10). CGRP induced a rapid and sustained activation of PKC in the cytosol but not at the plasma membrane (Fig. S2 D–F) and a gradual and sustained activation of cytosolic and nuclear ERK (EC50, 50–70 nM) (Figs. S2 G–I and S3). DynK44E or Dy4a, but not wild-type (WT) dynamin or inactive Dy4a, abolished CGRP-induced activation of cytosolic PKC and nuclear ERK but not cytosolic ERK (Fig. 2 A–F).
Fig. 2.
CLR compartmentalized signaling. FRET assays of CGRP-induced activation of cytosolic PKC (cytoCKAR, A, B, and G), cytosolic ERK (cytoEKAR, C and D), and nuclear ERK (nucEKAR, E, F, and H) in individual HEK-HA-CLR/myc-RAMP1 cells. (A, C, and E) Kinetics; (B, D, and F–H) area under curve (AUC). Cells were treated with inhibitors of dynamin (DynK44E, Dy4a), Gαs (NF449), Gαi (NF023), Gαq (UBO-QIC), phospholipase Cβ (U73122), or with a chelator of intracellular Ca2+ (EGTA-AM). n = 29–401 cells, n = 3 experiments. **P < 0.01, ***P < 0.001 to vehicle; ∧∧∧P < 0.001 to CGRP control. ANOVA, Tukey’s test.
NF449 (10 µM, Gαs inhibitor) suppressed activation of nuclear ERK but not cytosolic PKC, whereas NF023 (10 µM, Gαi inhibitor) had no effect (Fig. 2 G and H). UBO-QIC (100 nM, Gαq inhibitor) blocked activation of cytosolic PKC but not nuclear ERK. U73122 (1 µM, phospholipase Cβ inhibitor), but not EGTA-acetoxymethyl (EGTA-AM) (100 µM, a membrane-permeant chelator of intracellular Ca2+), also inhibited activation of cytosolic PKC.
Thus, dynamin-dependent endocytosis of CLR mediates activation of nuclear ERK and cytosolic PKC but not cytosolic ERK. Nuclear ERK activation requires Gαs, whereas cytosolic PKC activation depends on Gαq but not Ca2+ mobilization.
A Cholestanol-Conjugated CLR Antagonist Inhibits CGRP Signaling in Endosomes.
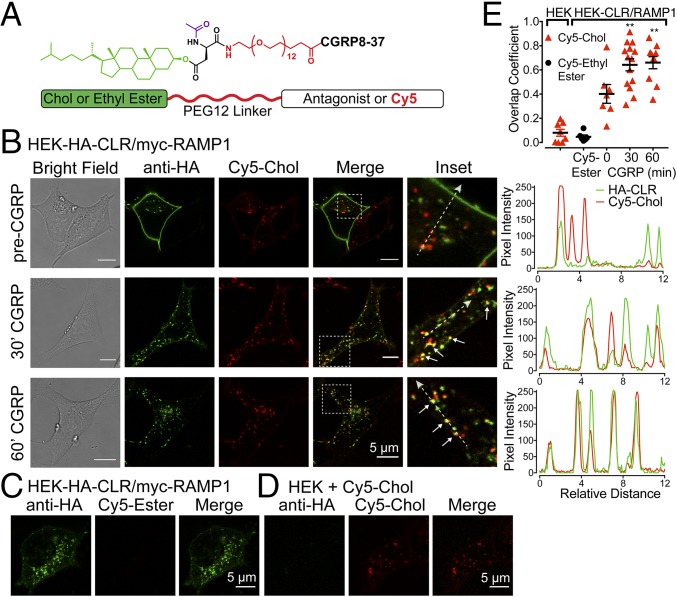
Conjugation to the membrane lipid cholestenol (Chol) promotes endosomal delivery and retention of peptidase inhibitors and NK1R antagonists, which enhances therapeutic efficacy (12, 17). We used a similar approach to deliver a membrane-impermeant CLR antagonist, CGRP8–37, to CLR in endosomes. Tripartite probes were synthesized comprising the following: Chol, which promotes membrane insertion and anchoring, or ethyl ester (control), which does not insert into membranes; a flexible polyethylene glycol-12 (PEG) linker to promote antagonist presentation in an aqueous environment; and a cargo of either cyanine 5 (Cy5) for localization or CGRP8–37 (Fig. 3A and Fig. S4).
Fig. 3.
Tripartite probes. (A) Probe structure. (B–D) Confocal images of live HEK cells. (B and C) HEK-HA-CLR/myc-RAMP1 cells were incubated with Cy5-Chol (B) or Cy5-Ethyl Ester (C). CLR was labeled with HA-Alexa488 antibody. Cells were stimulated with CGRP (50 nM, 30 or 60 min) to induce endocytosis. Insets (white boxes) show magnified regions and colocalization (arrows). Traces (Left) show relative overlap of pixel intensities for HA-CLR and Cy5-Chol along dashed lines. (D) Untransfected HEK cells incubated with Cy5-Chol. (E) Overlap coefficient for HA-CLR and Cy5-Chol or Cy5-Ethyl Ester. n = 6–14 cells, n = 4 experiments. **P < 0.01 to 0 min. ANOVA, Dunnett’s test.
To examine probe delivery to endosomes containing CLR, HEK-HA-CLR/myc-RAMP1 cells were incubated with Cy5-Chol or Cy5-Ethyl Ester (60 min, 37 °C), washed, and incubated with Alexa488–anti-HA antibody (40 min) to label cell-surface CLR. Live cells were imaged by confocal microscopy (37 °C). To induce CLR endocytosis, cells were stimulated with CGRP (50 nM) 3 h after initial exposure to probes. Before exposure to CGRP, Cy5-Chol was concentrated in endosomes, CLR was at the cell surface (Fig. 3B), and Cy5-Ethyl Ester remained extracellular (Fig. 3C). Alexa488–anti-HA antibody did not bind to untransfected HEK cells, confirming specificity (Fig. 3D). After incubation with CGRP (30 and 60 min), CLR and Cy5-Chol were colocalized in endosomes with overlapping pixel intensities (Fig. 3B and Movie S1). The CLR and Cy5-Chol overlap coefficient significantly increased after incubation with CGRP (Fig. 3E). Thus, Chol conjugation delivers probes to endosomes containing CLR.
CGRP8–37–Chol inhibited CGRP (1 nM)-induced cAMP formation in HEK-HA-CLR/myc-RAMP1 cells with an identical potency to unconjugated CGRP8–37 (pEC50: CGRP8–37, 6.17 ± 0.22; CGRP8–37-Chol, 6.36 ± 0.14; Fig. S5A).
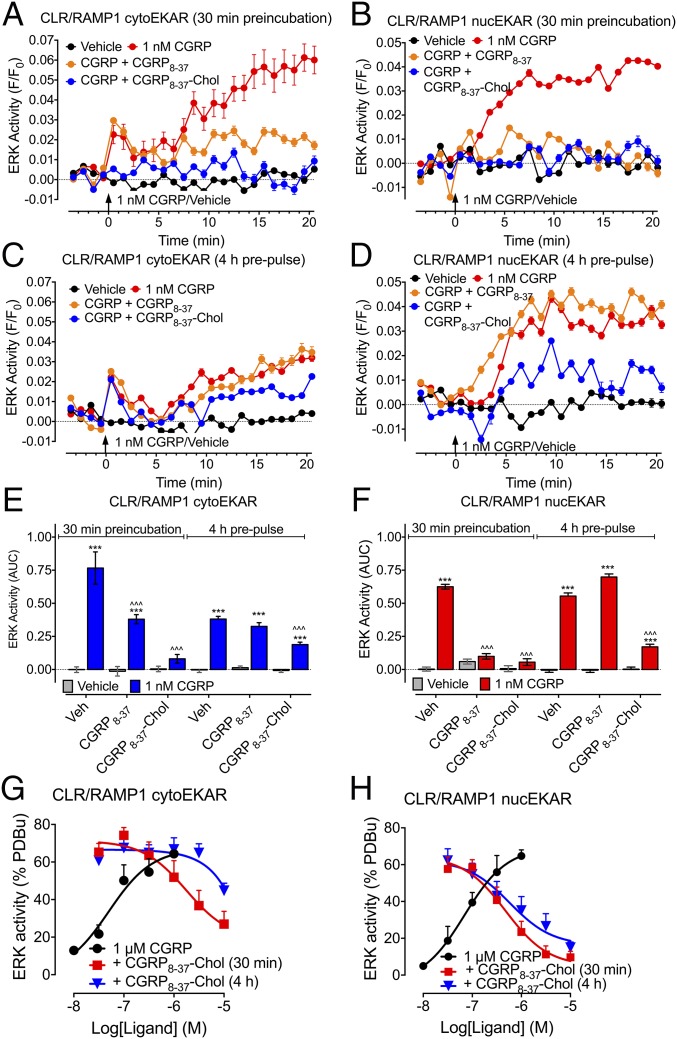
To examine the capacity of CGRP8–37–Chol and CGRP8–37 to inhibit CLR signaling at the plasma membrane and in endosomes, we incubated HEK-HA-CLR/myc-RAMP1 cells with antagonists for 30 min, washed, and examined CGRP signaling immediately after washing (when Chol-conjugated probes were at the plasma membrane) or after 4 h (when probes were in endosomes). CGRP stimulation of cytosolic ERK (derived from plasma membrane CLR) or nuclear ERK (derived from endosomal CLR) was measured.
When assayed immediately after 30 min of preincubation, both CGRP8–37 and CGRP8–37–Chol inhibited CGRP-stimulated activation of cytosolic and nuclear ERK (Fig. 4 A, B, E, and F). When cells were pulse-incubated with antagonists for 30 min, washed, and then stimulated with CGRP 4 h later, only CGRP8–37–Chol was capable of inhibiting nuclear ERK (Fig. 4 C–F). Using the population-based FRET assay, we demonstrated that while CGRP8–37–Chol had similar potency in inhibiting nuclear and cytosolic ERK after 30 min of preincubation (pIC50: cytosolic ERK, 5.57 ± 0.37; nuclear ERK, 6.23 ± 0.23), CGRP8–37–Chol more potently inhibited nuclear ERK (pIC50, 6.24 ± 0.34) than cytosolic ERK (pIC50, <5) when the cells were pulse-incubated with the antagonists (Fig. 4 G and H and Fig. S6). A probe lacking CGRP8–37 (PEG-Biotin-Chol) had no effect on ERK activation, which excludes nonspecific disruption of signaling by Chol or PEG. In cells that were pulse-incubated with CGRP8–37–Chol, CGRP still stimulated CLR endocytosis 4 h later, as shown by the decrease in CLR-RLuc/KRas-Venus BRET and the increase in CLR-RLuc/Rab5a-Venus BRET (Fig. S5B). The results show that Chol conjugation provides a mechanism for selective and sustained antagonism of endosomal CLR signaling.
Fig. 4.
Tripartite antagonism of CLR. ERK activity was assessed in individual HEK-HA-CLR/myc-RAMP1 cells expressing FRET biosensors for cytosolic ERK (cytoEKAR, A, C, and E) or nuclear (nucEKAR, B, D, and F) ERK. Cells were preincubated with vehicle, CGRP8–17, or CGRP8–37–Chol for 30 min and washed. CGRP-stimulated ERK activity was assessed immediately after washing (A and B, 30 min preincubation) or 4 h after washing (C and D, 4 h prepulse). (A–D) Kinetics. (E and F) Area under curve (AUC). (G and H) Effects of graded concentrations of CGRP8–37–Chol on cytosolic (G) and nuclear (H) ERK signaling in populations of HEK-HA-CLR/myc-RAMP1 cells. (A–F) n = 159–417 cells, n = 3 experiments; (G and H) n = 4–9 experiments. ***P < 0.001 to vehicle; ∧∧∧P < 0.001 to antagonist vehicle control. ANOVA, Tukey’s test.
CLR Signaling in Endosomes Mediates Nociceptive Transmission.
To determine whether spinal neurons express functional CLR, we examined CGRP signaling in neurons isolated from the dorsal horn of the rat spinal cord. CGRP increased [Ca2+]i in 52 ± 17% (942 neurons, 15 rats) of neurons (Fig. S7A). Preincubation with CGRP8–37 (1 µM, 30 min) abolished CGRP signals, which confirms expression of CLR (Fig. S7B). Neuronal excitation was examined by cell-attached patch clamp recordings from lamina I neurons in slices of rat spinal cord. Transient exposure to CGRP- (1 μM, 2 min) stimulated firing of action potentials that was sustained for at least 20 min after washout (Fig. 5 A–C). CGRP-responsive neurons also responded to SP (1 μM), supporting coexpression of CLR and NK1R in second-order spinal neurons.
Fig. 5.
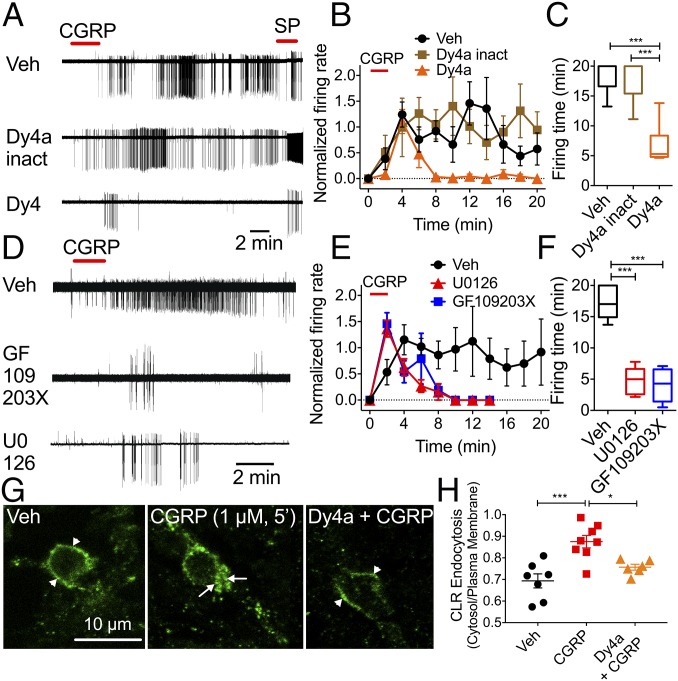
CLR endocytosis and excitation of spinal neurons. (A–F) CGRP-induced activation of lamina I neurons in rat spinal cord slices. Dy4a, dynamin inhibitor; GF109203X, PKC inhibitor; U0126, MEK inhibitor. (A and D) Representative traces. (B and E) Firing rate normalized to 2 min. (C and F) Firing duration to last action potential. n = 5–8 neurons per group; n = 19 rats. *P < 0.05, ***P < 0.001. ANOVA, Sidak’s multiple comparisons test (firing rate), or Dunn’s multiple comparisons test (firing time). (G) Confocal images of CLR-IR. Arrow, endosomes. Arrowhead, plasma membrane. (H) Quantification of CLR endocytosis. n = 6–8 neurons per group, n = 3 rats. *P < 0.05, ***P < 0.001. ANOVA, Tukey’s test.
To determine whether CLR signaling from endosomes contributes to CGRP-induced excitation, we incubated spinal cord slices with Dy4a or inactive Dy4a (30 µM) or vehicle 10 min before CGRP challenge. Dy4a did not affect the immediate CGRP-induced excitation but prevented the sustained response (Fig. 5 A–C). We preincubated tissue with U0126 (MEK inhibitor) and GF109203X (PKC inhibitor) (1 μM, 30–45 min) to examine the underlying signaling mechanisms. U0126 reduced the CGRP-stimulated firing time of lamina I neurons by 72.4 ± 5.1% (U0126, 17.37 ± 1.2 min; control, 4.8 ± 0.9 min; P < 0.0001, n = 6 neurons for U0126 and n = 5 neurons for control, seven rats) and reduced the average number of spontaneous action potentials by 86.6 ± 11.6% compared with controls (Fig. 5 D–F). GF109203X reduced CGRP-stimulated firing time by 76.5 ± 5.3% (GF109203X, 17.37 ± 1.2 min; control 4.07 ± 0.9 min; P < 0.0001, n = 8 neurons for GF109203X and n = 5 neurons for control, nine rats) and reduced the average number of CGRP-induced action potentials by 98.8 ± 0.4% compared with controls. CGRP (1 µM, 5 min) induced endocytosis of CLR-IR in spinal neurons (Fig. 5 G and H). Dy4a inhibited CGRP-induced endocytosis of CLR-IR.
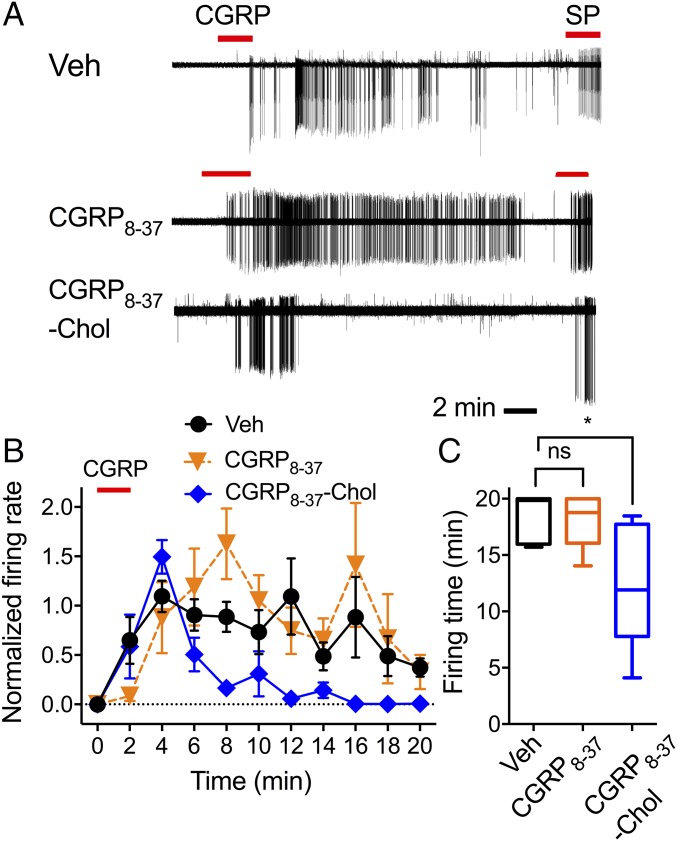
To obtain direct support for the concept that CLR signaling in endosomes mediates sustained excitation of spinal neurons, we preincubated spinal cord slices with vehicle, CGRP8–37, or CGRP8–37–Chol (1 µM, 60 min); washed; and challenged with CGRP 60 min later. In vehicle-treated slices, CGRP caused rapid onset action potential discharge that was sustained after washout (Fig. 6). CGRP8–37 did not affect this response. However, as observed with a dynamin inhibitor, CGRP8–37–Chol abolished sustained CGRP-evoked firing without affecting initial responses.
Fig. 6.
Tripartite antagonism of CGRP-induced excitation of spinal neurons. Spinal cord slices were incubated with vehicle (Veh), CGRP8–37, or CGRP8–37–Chol for 60 min; washed; and challenged with CGRP 60 min later. (A) Representative traces. (B) Firing rate normalized to 2 min. (C) Firing duration to last action potential. n = 5–7 neurons per group, n = 21 rats. *P < 0.05. ANOVA, Sidak’s test (firing rate), or Dunn’s test (firing time). ns, not significant.
The results support the hypothesis that endosomal CLR signaling mediates persistent excitation of spinal neurons. CLR in endosomes activates PKC and ERK, which control neuronal excitation.
CLR Signaling in Endosomes Mediates Nociception.
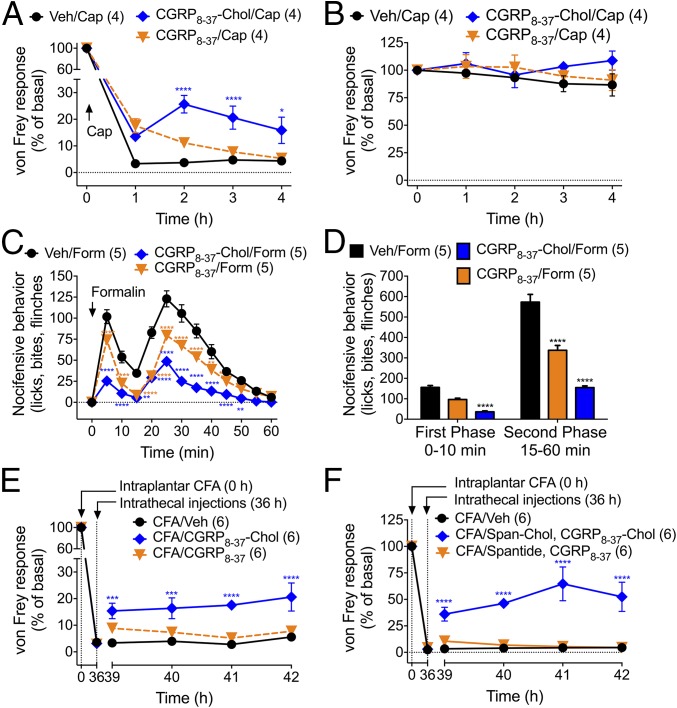
Does endosomal delivery enhance the antinociceptive efficacy of CLR antagonists? To evaluate this possibility, we administered vehicle, CGRP8–37, or CGRP8–37–Chol (5 µL, 10 µM) to mice by intrathecal injection (L3/L4) 3 h before intraplantar injection of capsaicin (5 µg, 10 µL). This time allows accumulation of tripartite probes in endosomes of spinal neurons (12). We examined mechanical nociception by stimulation of the plantar surface of the paw using von Frey filaments. In vehicle-treated mice, capsaicin caused mechanical allodynia of the ipsilateral paw that was sustained for 4 h (Fig. 7A). Intrathecal CGRP8–37 had a transient antinociceptive effect at 1 h, whereas CGRP8–37–Chol induced a larger antinociceptive effect that was sustained for 4 h. CGRP8–37 and CGRP8–37–Chol did not affect withdrawal responses of the contralateral (noninjected) paw (Fig. 7B). CGRP8–37–Chol blunted the noninflammatory (first phase) and inflammatory (second phase) of the nocifensive response to intraplantar formalin (4%, 10 µL) more effectively than CGRP8–37 (Fig. 7 C and D). When injected 36 h after intraplantar injection of complete Freund’s adjuvant (CFA, 2 mg·mL−1, 10 µL), which causes a long-lasting inflammatory hyperalgesia, CGRP8–37–Chol but not CGRP8–37 reversed the mechanical hyperalgesia (Fig. 7E).
Fig. 7.
Tripartite antagonism of nociception. Antagonists were injected intrathecally 3 h before intraplantar injection of capsaicin (Cap, A and B) or formalin (Form, C and D) or 36 h after CFA (E and F). von Frey withdrawal responses to stimulation of the planar surface of the injected paws (A, E, and F) or noninjected paws (B) and nocifensive behavior (C and D) were assessed. Number of mice used in each group is indicated in parentheses. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 to basal or vehicle control. ANOVA, Dunnett’s test.
Painful stimuli also induce the release of SP from the central terminals of primary sensory neurons in the dorsal horn, where SP induces NK1R endocytosis in second-order neurons and central transmission (12). To target the endosomal NK1R, we conjugated the NK1R antagonist spantide (Span) to Chol. When coadministered, Span–Chol (5 µL, 50 µM) and CGRP8–37–Chol (5 µL, 10 µM) caused a marked (∼75%) reversal of CFA-induced mechanical hyperalgesia, whereas the combination of unconjugated Span and CGRP8–37 had no effect (Fig. 7F).
Discussion
Our results support the hypothesis that complex pathophysiological events, such as nociceptive transmission in the spinal cord, are not solely mediated by activation of GPCRs at the plasma membrane. We propose that GPCRs in endosomes generate sustained signals in subcellular compartments that underlie complex pathophysiological processes in vivo and that endosomal receptors are a valid but neglected therapeutic target.
By using FRET biosensors targeted to subcellular compartments, we found that CGRP stimulates cAMP formation in the cytosol and plasma membrane, activates cytosolic and nuclear ERK, and activates PKC only in the cytosol. These signals were maintained in the continued presence of CGRP, suggesting sustained CLR activation. The observation that inhibitors of endocytosis prevent activation of cytosolic PKC and nuclear ERK suggests that these signals arise from persistent endosomal CLR signaling. In contrast, endocytic inhibitors did not affect CGRP-induced activation of cytosolic ERK, which likely originates from cell-surface CLR (Fig. S8). Cytosolic PKC activation depends on Gαq and is independent of Ca2+ mobilization. In contrast, nuclear ERK activation requires activation of Gαs. Thus, both Gαq and Gαs mediate CLR signaling from endosomes (Fig. S8). These findings support reports that Gαq and Gαs mediate endosomal signaling of other GPCRs (2, 5, 12, 18). CLR in endosomes could interact with many signaling partners, including G proteins. Additional studies are required to determine the composition of CLR signaling complexes and to elucidate the pathways by which G proteins in endosomes induce the sustained activation of cytosolic PKC and nuclear ERK.
Protease-activated receptor-2 (4), NK1R (3, 12), β2-adrenergic receptor (5), parathyroid receptor-1 (2), PAC1 receptor (7), dopamine D1 receptor (19), and receptors for glucagon-like peptide 1 (20), luteinizing hormone (21), and thyroid stimulating hormone (18) can signal from endosomes. Evidence of endosomal signaling derives from studies of model cells treated with inhibitors of endocytosis. These approaches provide mechanistic information but offer limited insight into the contribution of endosomal GPCR signaling for control of complex processes in intact tissues or animals. The observation that inhibitors of dynamin, ERK, and PKC suppress CGRP-induced excitation of spinal neurons suggests that endosomal CLR signaling is necessary for nociceptive transmission. Inhibitors of endocytosis also inhibit SP-induced excitation of spinal neurons and PACAP-induced excitability of cardiac neurons and can suppress nociception (7, 12). Thus, endocytosis of several GPCRs may be required for their actions on neuronal function.
We used Chol-conjugated probes, which accumulated in early endosomes containing CLR, to specifically evaluate the function of CLR in endosomes. After transient incubation and recovery, CGRP8–37–Chol prevented CGRP-induced activation of nuclear ERK, which originates from endosomal CLR, but did not affect activation of cytosolic ERK, which derives from CLR at the plasma membrane, or inhibit CLR endocytosis. Thus, CGRP8–37–Chol selectively inhibits endosomal CLR signaling. CGRP8–37–Chol inhibited CGRP-evoked excitation of spinal neurons, whereas unconjugated, membrane-impermeant CGRP8–37 was inactive. After intrathecal injection, CGRP8–37–Chol inhibited and reversed nociception more efficaciously than the unconjugated antagonist. The capacity of CGRP8–37–Chol to specifically antagonize endosomal CLR signaling and sustained excitation of spinal neurons, and to cause prolonged antinociception, reveals the importance of endosomal signaling for nociception and illustrates the therapeutic utility of endosomally directed drugs. Combined lipidated CLR and NK1R antagonists could be especially effective antinociceptive drugs. The results also support a role for CGRP, released from the central projections of peptidergic nociceptors, and CLR/RAMP1 on second order spinal neurons in mechanical nociception (10).
There are several limitations to our study. We investigated CLR signaling in HEK293 cells. It will be necessary to determine whether CLR trafficking also regulates signaling in spinal neurons. The findings that inhibitors of dynamin and endosomal CGRP signaling (CGRP8–37–Chol, MEK, PKC inhibitors) attenuate CGRP-induced excitation of neurons in spinal cord slices suggests that this is the case. However, the effects of endocytosis inhibitors on neuronal excitation require cautious interpretation because clathrin and dynamin mediate trafficking of many receptors and ion channels that regulate excitation of neurons (22, 23). Although we did not investigate the mechanisms by which endosomal CLR causes sustained excitation of spinal neurons, PKC and ERK may regulate the activity of ion channels and the transcription of genes that control excitation (12). PKC can also mediate CLR desensitization (24).
Our findings may be relevant to the development of CLR antagonists for the treatment of migraine (10). The vasodilator actions of CGRP are likely mediated by an adenylyl cyclase, cAMP, and PKA pathway that operates at the plasma membrane to activate ATP-sensitive K+ channels that lead to relaxation. Inhibiting this pathway could compromise blood supply to other organs, such as the heart. The specific targeting of endosomal signaling may provide a novel strategy to limit this side effect. Whether the efficacy of small-molecule CLR antagonists depends on their capacity to antagonize endosomal CLR signaling is unknown. GPCRs are the largest class of signaling proteins, control many pathophysiological processes, and are the target of 30% of therapeutic drugs. Thus, our findings that GPCRs signal from endosomes in vivo and are targets for therapy may have far-reaching consequences.
Materials and Methods
Animals.
The Institutional Animal Care and Use Committees of Monash University and the University of Sydney approved all experiments on animals.
Reagents.
Tripartite probe synthesis, cDNAs, and HEK cell transfection have been described (11–13).
CLR Signaling and Endocytosis in Cell Lines.
Compartmentalized signaling was analyzed by FRET (12, 13, 16). Endocytosis was studied by BRET, cell-surface ELISA, and immunofluorescence (12, 13). Cy5-Chol and HA-CLR-IR colocalization was quantified (25).
Electrophysiology, CLR Endocytosis, and [Ca2+]i Assays in Spinal Neurons.
Spontaneous currents were recorded from lamina I neurons in slices of rat spinal cord by cell-attached patch electrodes (26). CLR was localized by immunofluorescence (27). The plasma membrane/cytosolic pixel intensity ratio was determined to assess CLR-IR endocytosis (12). Lamina I–III dorsal horn neurons from 1- to 2-d-old neonatal rats were cultured for 6–8 d before [Ca2+]i assays (28).
Nociception.
Nociception was studied in mice (12). Investigators were blinded to test agents.
Statistics.
Results are mean ± SEM. Differences were assessed using Student’s t test (two comparisons) or one- or two-way ANOVA, as indicated in the figure legends.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant NS102722; NHMRC Grants 63303, 1049682, and 1031886; ARC Centre of Excellence in Convergent Bio-Nano Science and Technology, and Takeda Pharmaceuticals Inc. (to N.W.B.). M.L.H. is a NHMRC RD Wright Career Development Fellow; M.C. is a Monash Fellow.
Footnotes
Conflict of interest statement: Research in the N.W.B. laboratory is funded in part by Takeda Pharmaceuticals.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706656114/-/DCSupplemental.
References
- 1.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 2.Cheloha RW, Gellman SH, Vilardaga J-P, Gardella TJ. PTH receptor-1 signalling-mechanistic insights and therapeutic prospects. Nat Rev Endocrinol. 2015;11:712–724. doi: 10.1038/nrendo.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeFea KA, et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc Natl Acad Sci USA. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeFea KA, et al. Beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irannejad R, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irannejad R, von Zastrow M. GPCR signaling along the endocytic pathway. Curr Opin Cell Biol. 2014;27:109–116. doi: 10.1016/j.ceb.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May V, Parsons RL. G protein-coupled receptor endosomal signaling and regulation of neuronal excitability and stress responses: Signaling options and lessons from the PAC1 receptor. J Cell Physiol. 2017;232:698–706. doi: 10.1002/jcp.25615. [DOI] [PubMed] [Google Scholar]
- 8.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: A legitimate platform for the signaling train. Proc Natl Acad Sci USA. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomsen ARB, et al. GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell. 2016;166:907–919. doi: 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol Rev. 2014;94:1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padilla BE, et al. Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and beta-arrestins. J Cell Biol. 2007;179:981–997. doi: 10.1083/jcb.200704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen DD, et al. Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci Transl Med. 2017;9:eaal3447. doi: 10.1126/scitranslmed.aal3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen DD, et al. Endothelin-converting enzyme 1 and β-arrestins exert spatiotemporal control of substance P-induced inflammatory signals. J Biol Chem. 2014;289:20283–20294. doi: 10.1074/jbc.M114.578179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson MJ, Deane FM, Robinson PJ, McCluskey A. Synthesis of Dynole 34-2, Dynole 2-24 and Dyngo 4a for investigating dynamin GTPase. Nat Protoc. 2014;9:851–870. doi: 10.1038/nprot.2014.046. [DOI] [PubMed] [Google Scholar]
- 15.Robertson MJ, et al. Synthesis of the Pitstop family of clathrin inhibitors. Nat Protoc. 2014;9:1592–1606. doi: 10.1038/nprot.2014.106. [DOI] [PubMed] [Google Scholar]
- 16.Halls ML, Poole DP, Ellisdon AM, Nowell CJ, Canals M. Detection and quantification of intracellular signaling using FRET-based biosensors and high content imaging. Methods Mol Biol. 2015;1335:131–161. doi: 10.1007/978-1-4939-2914-6_10. [DOI] [PubMed] [Google Scholar]
- 17.Rajendran L, et al. Efficient inhibition of the Alzheimer’s disease beta-secretase by membrane targeting. Science. 2008;320:520–523. doi: 10.1126/science.1156609. [DOI] [PubMed] [Google Scholar]
- 18.Calebiro D, et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotowski SJ, Hopf FW, Seif T, Bonci A, von Zastrow M. Endocytosis promotes rapid dopaminergic signaling. Neuron. 2011;71:278–290. doi: 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuna RS, et al. Glucagon-like peptide-1 receptor-mediated endosomal cAMP generation promotes glucose-stimulated insulin secretion in pancreatic β-cells. Am J Physiol Endocrinol Metab. 2013;305:E161–E170. doi: 10.1152/ajpendo.00551.2012. [DOI] [PubMed] [Google Scholar]
- 21.Lyga S, et al. Persistent cAMP signaling by internalized LH receptors in ovarian follicles. Endocrinology. 2016;157:1613–1621. doi: 10.1210/en.2015-1945. [DOI] [PubMed] [Google Scholar]
- 22.Heymann JA, Hinshaw JE. Dynamins at a glance. J Cell Sci. 2009;122:3427–3431. doi: 10.1242/jcs.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 24.Pin SS, Bahr BA. Protein kinase C is a common component of CGRP receptor desensitization induced by distinct agonists. Eur J Pharmacol. 2008;587:8–15. doi: 10.1016/j.ejphar.2008.02.083. [DOI] [PubMed] [Google Scholar]
- 25.Manders EEM, Verbeek FJ, Aten JA. Measurement of co-localization of objects in dual-colour confocal images. J Microsc. 1993;169:375–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 26.Imlach WL, Bhola RF, May LT, Christopoulos A, Christie MJ. A positive allosteric modulator of the adenosine A1 receptor selectively inhibits primary afferent synaptic transmission in a neuropathic pain model. Mol Pharmacol. 2015;88:460–468. doi: 10.1124/mol.115.099499. [DOI] [PubMed] [Google Scholar]
- 27.Cottrell GS, et al. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol. 2005;490:239–255. doi: 10.1002/cne.20669. [DOI] [PubMed] [Google Scholar]
- 28.Zhao P, et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J Biol Chem. 2014;289:27215–27234. doi: 10.1074/jbc.M114.599712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.