Significance
Activation of the cGAMP-cGAS-STING pathway has recently been shown to mediate virus- or bacteria-induced activation of the innate immune response. Here we report that this pathway also plays an important role in obesity-induced inflammation and metabolic dysfunction, beyond its well-characterized roles in innate immune surveillance. We have also identified adipose disulfide-bond A oxidoreductase-like protein as a key regulator of mitochondrial integrity and function, which protects mice from obesity-induced inflammation and insulin resistance by suppressing mtDNA release-induced activation of the cGAS-cGAMP-STING pathway. Our study suggests that targeting the cGAS-cGAMP-STING pathway in adipose tissue may be an effective approach to ameliorating obesity-induced chronic inflammation and its associated metabolic diseases.
Keywords: obesity, inflammation, insulin resistance, DsbA-L, cGAS
Abstract
Chronic inflammation in adipose tissue plays a key role in obesity-induced insulin resistance. However, the mechanisms underlying obesity-induced inflammation remain elusive. Here we show that obesity promotes mtDNA release into the cytosol, where it triggers inflammatory responses by activating the DNA-sensing cGAS-cGAMP-STING pathway. Fat-specific knockout of disulfide-bond A oxidoreductase-like protein (DsbA-L), a chaperone-like protein originally identified in the mitochondrial matrix, impaired mitochondrial function and promoted mtDNA release, leading to activation of the cGAS-cGAMP-STING pathway and inflammatory responses. Conversely, fat-specific overexpression of DsbA-L protected mice against high-fat diet-induced activation of the cGAS-cGAMP-STING pathway and inflammation. Taken together, we identify DsbA-L as a key molecule that maintains mitochondrial integrity. DsbA-L deficiency promotes inflammation and insulin resistance by activating the cGAS-cGAMP-STING pathway. Our study also reveals that, in addition to its well-characterized roles in innate immune surveillance, the cGAS-cGAMP-STING pathway plays an important role in mediating obesity-induced metabolic dysfunction.
Obesity has reached epidemic proportions globally, and is associated with various metabolic diseases such as type II diabetes, cardiovascular disease, and many types of cancer. Numerous studies have shown that chronic sterile inflammation in white adipose tissue (WAT), a major depot for chemical energy storage in the form of triglyceride (TG) and for hormone and cytokine production in response to nutritional and environmental changes, plays a key role in mediating obesity-induced insulin resistance and its associated metabolic diseases (1, 2). However, the mechanisms underlying obesity-induced inflammation remain to be completely elucidated.
Mitochondria are regarded as “end-function” organelles, which receive various signals from cells to produce ATP and regulate energy homeostasis in response to environmental and physiological changes. However, accumulating evidence suggests that mitochondria are also places of information integration and release to maintain cell homeostasis (3–6). Under stress conditions, damaged mitochondria can release a number of inflammation-promoting signals such as reactive oxygen species (ROS), mitochondria-derived peptides, Ca2+, cytochrome c, and some yet-to-be-characterized signals in response to the altered cellular changes (6, 7). Thus, mitochondrial dysfunction-triggered activation of inflammatory signaling pathways could be a potential mechanism underlying obesity-induced insulin resistance.
The cGMP-AMP (cGAMP) synthase (cGAS; also known as MB21D1) has recently been identified as a cytosolic DNA sensor of pathogen-derived DNA that activates the type I IFN response by synthesizing the eukaryotic secondary messenger 2′3′-cGAMP in response to viral and microbial infections (8, 9). cGAMP binds to the adaptor protein STING (also known as TMEM173), leading to the activation of a protective, antiviral signaling cascade in innate immune cells (4, 9). A recent finding suggests that, in addition to pathogen-derived DNA from microbial infection, the cGAS-cGAMP-STING pathway could also be activated by cytosolic mitochondrial DNA (mtDNA) (4, 10, 11). Unlike nuclear DNAs, which are highly packaged by enriched histones, mtDNAs lack protective histones and are particularly susceptible to attack by mitochondrial damaging factors such as ROS (12). Under certain pathophysiological conditions such as autoimmune diseases, increased mitochondrial stress leads to ROS overproduction as well as mtDNA oxidation and packaging disturbance, triggering mtDNA release to the cytoplasm (3, 4, 13, 14). However, whether obesity induces mtDNA release and activates the cGAS-cGAMP-STING signaling pathway and, if yes, whether the pathway contributes to obesity-induced sterile inflammation and insulin resistance, remain unexplored.
In the current study, we show that obesity induces mtDNA release, which triggers the activation of the cGAS-cGAMP-STING pathway and a consequent increase in chronic sterile inflammatory response in adipose tissue. Obesity-induced activation of the cGAS-cGAMP-STING pathway could be prevented by overexpression of disulfide bond A oxidoreductase-like protein (DsbA-L), a protein originally identified from the mitochondrial matrix of rat liver (15). On the other hand, adipose tissue-specific ablation of DsbA-L led to mitochondrial dysfunction and increased mtDNA release, triggering the activation of the cGAS-cGAMP-STING pathway, increased inflammation, and exacerbated obesity-induced insulin resistance. Our study has uncovered a signaling mechanism underlying the link between obesity-induced mitochondrial dysfunction and inflammation.
Results
Obesity Triggers Activation of the cGAS-cGAMP-STING Pathway Through mtDNA Release in Adipose Tissues.
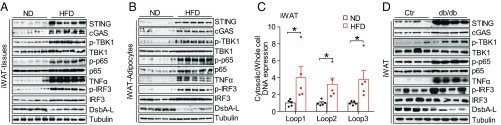
The expression levels of cGAS and STING were markedly increased in inguinal WAT (iWAT) and epididymal WAT (eWAT) of high-fat diet (HFD)-induced obese mice, which were associated with down-regulation of DsbA-L (Fig. 1A and Fig. S1A). Concurrent with the activation of the cGAS-cGAMP-STING pathway, obesity greatly increased the phosphorylation of TBK1, NF-κB p65, and IRF3, as well as the expression of TNF-α, in fat tissue (Fig. 1A and Fig. S1A). Given that adipose tissue not only contains adipocytes but also macrophages and other cells, we examined the activation of the cGAS-cGAMP-STING pathway in purified adipocytes, F4/80+ macrophages (MΦs), and the macrophage-negative stromal vascular fraction (SVF-MΦ-Neg). We found that HFD feeding greatly activates the cGAS-cGAMP-STING pathway not only in adipocytes (Fig. 1B and Fig. S1B) but also in MΦ and SVF-MΦ-Neg fractions (Fig. S1 C–G), suggesting that activation of the cGAS-cGAMP-STING pathway in both adipocytes and macrophages may contribute to HFD-induced inflammation.
Fig. 1.
mtDNA release activates the cGAS-cGAMP-STING pathway in adipose tissues of obese mice. (A) Immunoblot analysis for expression of STING, cGAS, TNF-α, and DsbA-L and the phosphorylation of TBK1 at Ser172, IRF3 at Ser396, and NF-κB p65 at Ser536 in iWAT from normal chow diet- and high-fat diet-fed C57BL/6 mice. (B) Immunoblot analysis of purified adipocytes from iWAT of ND- and HFD-fed C57BL/6 mice. (C) Cytosolic mtDNA content in freshly purified adipocytes from iWAT of ND- and HFD-fed C57BL/6 mice; n = 5. (D) Immunoblot analysis of iWAT from db/db mice and their control mice. Data are presented as mean ± SEM. *P < 0.05.
To determine whether obesity-induced activation of the cGAS-cGAMP-STING pathway is due to enhanced mtDNA release into the cytosol, we purified total DNA from the cytosolic fraction of adipocytes freshly isolated from normal diet (ND)- or HFD-fed mice. The purity of the cytosolic fraction was confirmed by cell-fractionation studies, which showed no contamination with the mitochondrial marker Complex IV, the endoplasmic reticulum (ER) marker Erp57, and the nuclear markers Lamin A and Tert (Fig. S1 H and I). mtDNA levels were significantly increased in the cytosol of adipocytes from HFD-induced obese mice compared with control mice (Fig. 1C and Fig. S1J). Enhanced cGAS-cGAMP-STING signaling and reduced DsbA-L expression were also observed in eWAT and iWAT from db/db mice compared with their control littermates (Fig. 1D and Fig. S1K). Taken together, these findings suggest that activation of the cGAS-cGAMP-STING pathway by mtDNA release could be a potential mechanism underlying obesity-induced inflammation.
Adipose Tissue-Specific Disruption of DsbA-L Exacerbates High-Fat Diet-Induced Obesity and Insulin Resistance.
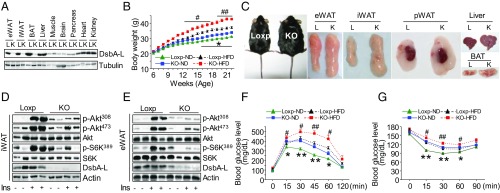
The negative association between DsbA-L expression and cGAS-cGAMP-STING pathway activation in obese adipose tissue suggests that DsbA-L deficiency may play a role in diet-induced activation of the cGAS-cGAMP-STING pathway. To test this possibility, we generated fat-specific DsbA-L knockout mice (DsbA-LfKO) by crossing DsbA-L floxed mice (16) with adiponectin-Cre mice (Fig. 2A). Fat-specific knockout of DsbA-L had no significant effect on food intake in mice fed either an ND or an HFD (Fig. S2 A and B). However, DsbA-LfKO mice gained more body weight than the wild-type control mice under both ND- and HFD-feeding conditions (Fig. 2B). Consistent with these findings, DsbA-LfKO mice displayed increased fat pad and fat mass (Fig. 2C and Fig. S2 C and D) but showed no significant difference in fasting glucose levels (Fig. 2F), bone mineral density (Fig. S2E), or lean mass (Fig. S2 C and D) compared with their control littermates fed an ND or HFD. Hematoxylin and eosin (H&E) staining revealed that white adipocyte size (Fig. S2 F and G) but not number (Fig. S2H) was greatly increased in the DsbA-LfKO mice. In addition, larger multilocular lipid droplets were observed in brown adipose tissue (BAT) of the DsbA-LfKO mice compared with the Loxp control mice (Fig. S2F). DsbA-L deficiency had no significant effect on liver morphology in mice fed an ND but greatly promoted a fatty liver in mice fed an HFD (Fig. 2C and Fig. S2F). Consistent with these findings, fat-specific knockout of DsbA-L inhibited insulin signaling in adipose tissue and promoted glucose and insulin intolerance in mice fed either an ND or an HFD (Fig. 2 D–G). These findings uncover a key role of adipose DsbA-L in the regulation of whole-body glucose and lipid homeostasis.
Fig. 2.
Fat tissue-specific DsbA-L knockout mice display increased fat mass and insulin resistance. (A) Immunoblot analysis for expression of DsbA-L in different tissue homogenates of male DsbA-LfKO (K) and Loxp (L) control mice. (B) Body weight of male DsbA-LfKO and Loxp control mice during ND and HFD feeding; n = 12. (C) Representative photographs of eWAT, iWAT, perirenal WAT (pWAT), liver, and BAT from DsbA-LfKO and Loxp control mice fed HFD. (D and E) Immunoblot analysis for Akt phosphorylation at Thr308 and Ser473 and S6K phosphorylation at Thr389 in (D) iWAT and (E) eWAT from HFD-fed DsbA-LfKO (KO) and Loxp control mice after i.p. injection of 1 U/kg insulin for 5 min. (F) Glucose tolerance tests in DsbA-LfKO and Loxp control mice fed either ND or HFD. Loxp/KO-ND groups, n = 8; Loxp-HFD group, n = 15; KO-HFD group, n = 12. (G) Insulin tolerance tests in DsbA-LfKO and Loxp control mice fed either ND or HFD; Loxp groups, n = 6; KO groups, n = 9. *, Loxp vs. KO mice fed ND; #, Loxp vs. KO mice fed HFD. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01. #P < 0.05, ##P < 0.01.
DsbA-L Deficiency Impairs Mitochondrial Function and Promotes mtDNA Release-Induced Activation of the cGAS-cGAMP-STING Pathway and Inflammatory Response.
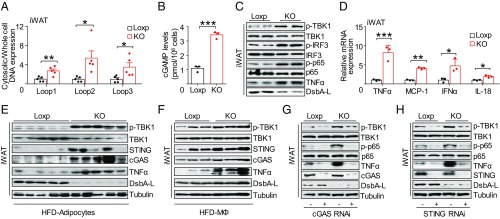
Given that DsbA-L is localized in mitochondria (15, 17), we sought to determine whether disrupting DsbA-L expression in adipose tissue affects mitochondrial function. We found that DsbA-L deficiency significantly increased mitochondrial ROS levels (Fig. S3A), concurrent with a significant decrease in mitochondrial ATP levels and membrane potential (Fig. S3 B and C). Consistent with diminished mitochondrial function, primary adipocytes from DsbA-LfKO mice exhibited lower basal and maximal respiration, as well as reduced spare respiratory capacity, compared with primary adipocytes isolated from control mice (Fig. S3 D and E), indicating that oxidative phosphorylation was impaired in DsbA-L–deficient adipocytes. Interestingly, there was a significant increase in mtDNA release into the cytosol of adipocytes derived from iWAT and eWAT of DsbA-LfKO mice compared with control littermates (Fig. 3A and Fig. S4A). Increased mtDNA release was also observed in cultured DsbA-L–deficient primary white adipocytes (Fig. S4B), indicating that DsbA-L has a cell-autonomous effect on mtDNA release. Concurrent with increased mtDNA release, 2′3′-cGAMP levels and the phosphorylation of TBK1, IRF3, and NF-κB p65 were all markedly increased in DsbA-L–deficient adipocytes (Fig. 3 B and C). Consequently, DsbA-L deficiency greatly increased mRNA expression of inflammatory genes such as TNF-α, MCP-1, IFN-α, and IL-18 in cultured primary adipocytes (Fig. 3D), concurrent with enhanced secretion of inflammatory cytokines in serum of DsbA-LfKO mice (Fig. S4C). The activation of the cGAS-cGAMP-STING signaling pathway was also observed in adipocytes freshly isolated from DsbA-LfKO mice fed either an ND or HFD (Fig. 3E and Fig. S4 D–F). We also observed a slight increase of the cGAS-cGAMP-STING signaling pathway in MΦ and SVF-MΦ-Neg fractions of the DsbA-LfKO mice (Fig. 3F and Fig. S4 G–I), indicating cross-talk between adipocytes and adipose-resident macrophages. Suppressing cGAS or STING expression in DsbA-L knockout primary adipocytes by shRNA inhibited the phosphorylation of TBK1 and NF-κB p65 and reduced TNF-α gene expression (Fig. 3 G and H). In addition, treating DsbA-L–deficient adipocytes with amlexanox, a selective inhibitor of TBK1 (18), significantly diminished phosphorylation of IRF3 and TNF-α expression (Fig. S4J). These results provide strong evidence that activation of the cGAS-cGAMP-STING signaling pathway plays a contributing role in triggering inflammatory responses in adipose tissue of the fat-specific DsbA-L knockout mice.
Fig. 3.
DsbA-L deficiency promotes mtDNA release-induced activation of the cGAS-cGAMP-STING pathway and inflammatory response. (A) Cytosolic mtDNA content in freshly purified adipocytes from iWAT of DsbA-LfKO and Loxp control mice; n = 5. (B and C) 2′3′-cGAMP levels (B) and immunoblot analysis (C) in primary adipocytes from DsbA-LfKO and Loxp control mice. (D) mRNA expression of inflammatory genes in primary adipocytes from DsbA-LfKO and Loxp control mice. (E) Immunoblot analysis of purified adipocytes from iWAT of DsbA-LfKO and Loxp control mice fed HFD. (F) Immunoblot analysis of F4/80+ macrophages from DsbA-LfKO and Loxp control mice fed HFD; each blot represents the average level of two mice. (G and H) Immunoblot analysis of primary adipocytes from DsbA-LfKO and Loxp control mice transiently expressing cGAS-shRNA (G) or STING-shRNA (H) and their control plasmids. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
To further confirm if mtDNA release mediates DsbA-L deficiency-induced activation of the cGAS-cGAMP-STING pathway, we treated primary adipocytes with a low dose (150 to 450 ng/mL) of ethidium bromide (EtBr), a well-known mtDNA-depleting compound that prevents mtDNA replication and transcription without much effect on genomic DNA (10, 19). EtBr treatment dramatically inhibited mtDNA expression (Fig. S5A) and significantly reduced the phosphorylation of TBK1, NF-κB p65, and IRF3 as well as TNF-α expression in DsbA-L–deficient primary adipocytes (Fig. S5B). Similar results were also obtained with another mtDNA-depletion compound, dideoxycytidine (ddC), an mtDNA polymerase-γ inhibitor that has no effect on the activity of nuclear DNA polymerases (10, 20) (Fig. S5 C and D). Taken together, these results strongly suggest that mtDNA is the major mediator of DsbA-L deficiency-induced activation of the cGAS-cGAMP-STING signaling pathway and its downstream inflammatory responses in adipocytes.
Overexpression of DsbA-L Protects Against mtDNA-Induced Activation of the cGAS-cGAMP-STING Signaling Pathway Through an Adiponectin- and ER Localization-Independent Mechanism.
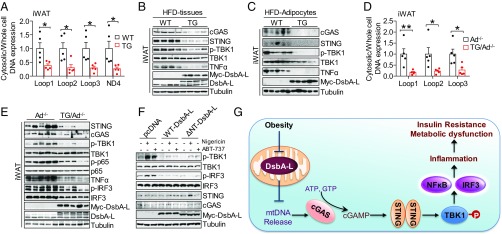
We previously found that fat-specific overexpression of DsbA-L (DsbA-LfTG) protected mice from HFD-induced obesity and inflammation (21). We speculated that overexpressing DsbA-L might suppress HFD-induced inflammation through inhibition of the mtDNA release-activated cGAS-cGAMP-STING signaling pathway. Consistent with this view, HFD-induced mtDNA release was significantly suppressed in adipocytes from eWAT and iWAT of DsbA-LfTG mice compared with control mice (Fig. 4A and Fig. S6A), which correlated with reduced activation of the cGAS-cGAMP-STING pathway in both adipose tissue and purified adipocytes of HFD-fed mice (Fig. 4 B and C) as well as decreased serum levels of inflammatory cytokines such as TNF-α, MCP-1, IL-18, IL-1β, IP-10, IL-5, RANTES, MIP-1α, and IL-17A in DsbA-LfTG mice compared with control mice (Fig. S6B). Reduced activation of the cGAS-cGAMP-STING pathway was also observed in purified adipocytes from DsbA-LfTG mice fed an ND (Fig. S6C) but not in MΦ and SVF-MΦ-Neg fractions of the mice (Fig. S6 D and E). Treating primary adipocytes with nigericin or ABT-737, two compounds known to stimulate mtDNA release (11, 22), significantly increased mtDNA release (Fig. S7 A and B), cGAMP levels (Fig. S7C), phosphorylation of TBK1 and NF-κB p65, and the expression of inflammatory genes (Fig. S7 D–F). The stimulatory effects of nigericin and ABT-737 on mtDNA release, cGAMP levels, and the expression of inflammatory genes, however, were all significantly suppressed in cells overexpressing DsbA-L (Fig. S7). DsbA-L has been reported to regulate adiponectin multimerization and function (17, 21, 23). Consistent with this result, we found that fat-specific knockout of DsbA-L significantly reduced the serum levels and multimerization of adiponectin (Fig. S8). Given that adiponectin has been reported to exert antiinflammatory function (24, 25), we asked if some effects of DsbA-L on the mtDNA release-activated cGAS-cGAMP-STING pathway are mediated by adiponectin action. To test this, we examined the protective effect of DsbA-L in fat-specific DsbA-L transgenic mice lacking adiponectin (DsbA-LfTG/Ad−/−) (21). We found that HFD-induced mtDNA release and activation of the cGAS-cGAMP-STING pathway were significantly reduced in DsbA-LfTG/Ad−/− mice compared with adiponectin knockout mice (Ad−/−) (Fig. 4 D and E and Fig. S9), indicating that the protective effect of DsbA-L on the mtDNA release-activated cGAS-cGAMP-STING pathway is adiponectin-independent. Since DsbA-L is localized not only in mitochondria but also in the ER (17), we examined whether ER localization is necessary for DsbA-L to inhibit cGAS-cGAMP-STING signaling. Transient overexpression of an ER localization-defective mutant of DsbA-L (DsbA-LΔNT), which is unable to promote adiponectin multimerization (17), prevented nigericin- and ABT-737–induced activation of phosphorylation of TBK1 and IRF3 in adipocytes (Fig. 4F). Taken together, these results suggest that mitochondrial localization plays a major role in DsbA-L suppressing mtDNA release-induced activation of the cGAS-cGAMP-STING pathway.
Fig. 4.
DsbA-L protects mtDNA-induced activation of cGAS-cGAMP-STING signaling through an adiponectin- and ER localization-independent mechanism. (A) Cytosolic mtDNA content in freshly purified adipocytes from iWAT of DsbA-LfTG mice and wild-type control mice fed HFD. (B and C) Immunoblot analysis of iWAT tissue (B) or purified adipocytes (C) from DsbA-LfTG and wild-type control mice fed HFD. (D) Cytosolic mtDNA content in freshly purified adipocytes from iWAT of Ad−/− mice and DsbA-LfTG/Ad−/− mice fed HFD. (E) Immunoblot analysis of iWAT from Ad−/− mice and DsbA-LfTG/Ad−/− mice fed HFD. (F) Immunoblot analysis of adipocytes transiently overexpressing myc-tagged wild-type DsbA-L, ∆NT-mutated DsbA-L, and the control plasmid (pcDNA) for 24 h, followed with or without 4 μM nigericin or 10 μM ABT-737 treatment for 12 h. (G) A proposed model for obesity-induced activation of the cGAS-cGAMP-STING pathway. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01.
Discussion
Obesity triggers a state of chronic, low-grade inflammation in insulin target tissues such as liver, muscle, and fat, leading to an overproduction and secretion of cytokines and chemokines that cause insulin resistance (1). While numerous studies strongly suggest that inflammation plays a major role in obesity-induced insulin resistance and various metabolic diseases, the precise mechanisms underlying obesity-induced inflammation remain uncertain. In the current study, we show that obesity induces mtDNA release in adipose tissue, which activates the cGAS-cGAMP-STING signaling pathway. In innate immune cells, activation of the cGAS-cGAMP-STING pathway triggers the type I IFN response, which has evolved as a major protective immune defense mechanism for the detection and suppression of microbial infection (9, 14). However, overactivation of the cGAS-cGAMP-STING pathway, which leads to an overproduction of harmful proinflammatory cytokines, has been found in some autoimmune disease patients (26–28). Our study shows that the cGAS-cGAMP-STING pathway is activated in the adipose tissue of obese mice. In addition, we demonstrate that inhibition of this signaling pathway reduces obesity-induced inflammation and improves metabolic homeostasis (Fig. 4 and Fig. S6). Notably, we also observed an activation of the cGAS-cGAMP-STING pathway in F4/80+ macrophages and the SVF in obese mice (Fig. S1 C–G), indicating a potential involvement of these cells in HFD-induced inflammation. Taken together, these results uncover a mechanism underlying obesity-induced inflammation and insulin resistance and identify novel therapeutic targets for treating obesity-induced inflammation and metabolic dysfunction.
In this study, we have uncovered an important role of DsbA-L in regulating mitochondrial integrity and function. DsbA-L was originally isolated from the mitochondrial matrix (15, 29). The role of DsbA-L in mitochondria, however, remains unknown. We found that disrupting DsbA-L expression in adipocytes significantly impaired mitochondrial function and increased mtDNA release into the cytosol, where it activated the cGAS-cGAMP-STING pathway (Fig. 3 and Figs. S3 and S4). Conversely, fat-specific overexpression of DsbA-L significantly alleviated obesity-induced mtDNA release and activation of the cGAS-cGAMP-STING pathway (Fig. 4 and Fig. S6). Activation of the cGAS-cGAMP-STING pathway leads to the activation of downstream kinases TBK1 and IKK, which phosphorylate and activate IRF3 and NF-κB, triggering the expression of type I interferons and inflammatory cytokines such as TNF-α, IL-1β, IL-18, MCP-1, RANTES, IL-6, and so forth (9, 30). Consistently, DsbA-L deficiency in adipocytes significantly increased inflammatory gene expression and cytokine secretion (Fig. 3D and Fig. S4C), whereas fat-specific overexpression of DsbA-L suppressed HFD-induced inflammation (Fig. S6B). These results suggest an important contribution of the adipocyte cGAS-cGAMP-STING pathway to systemic inflammatory events. In addition, we also observed a slight increase of cGAS-cGAMP-STING signaling in MΦ and SVF-MΦ-Neg fractions from DsbA-LfKO mice fed either an ND or HFD (Fig. 3F and Fig. S4 G–I), indicating cross-talk between adipocytes and adipose-resident immune cells. Together with the findings that the cGAS-cGAMP-STING pathway is also activated in macrophage and SVF fractions of HFD-fed obese mice (Fig. S1 C–G), our results suggest that activation of the cGAS-cGAMP-STING pathway in adipose-resident macrophages may also play an important role in obesity-induced inflammation and metabolic dysfunction. Future studies using macrophage-specific DsbA-L and cGAS knockout mice will be necessary to test this hypothesis.
The precise mechanism by which DsbA-L protects against diet-induced mitochondrial dysfunction and mtDNA release remains unclear. DsbA-L is a 25-kDa protein originally identified from the mitochondrial matrix and named GST-kappa (15). However, subsequent analysis of the complete amino acid sequence revealed that GST-kappa had little sequence similarity to any other members of the GST family (29, 31) but shares high sequence and secondary structure homology to Escherichia coli disulfide-bond A oxidoreductase DsbA (32, 33). Nevertheless, GST-kappa does not have the classic CXXC motif, which is involved in disulfide-bond formation in DsbA and other oxidoreductases, and thus GST-kappa does not promote protein disulfide-bond formation in vitro (17, 21, 23). For these reasons, we renamed this protein DsbA-like protein, or DsbA-L (23). We previously found that DsbA-L is also localized in the ER and plays an important role in adiponectin multimerization and function (23, 34). However, DsbA-L by itself is not sufficient to promote adiponectin multimerization in the presence of oxidative glutathione and trimeric adiponectin by in vitro assay (23), suggesting that DsbA-L may function as a chaperone, rather than acting as an oxidoreductase to directly catalyze intermolecular disulfide-bond formation in the ER. One possible mechanism by which DsbA-L improves mitochondrial function may be that this protein also functions as a chaperone in mitochondria, where it facilitates the correct folding, localization, and/or interaction of important mitochondrial proteins involved in mtDNA replication, transcription, or structural integrity to maintain mtDNA homeostasis. Thus, DsbA-L deficiency, especially under stress conditions such as obesity, may perturb mtDNA biosynthesis and/or disturb mtDNA stability, resulting in mtDNA release into the cytosol. Another possible mechanism underlying DsbA-L deficiency-induced mtDNA release may be the overproduction of ROS. The electron transport chain (ETC) is the major site for ROS production, and DsbA-L deficiency greatly increased mitochondrial ROS levels (Fig. S3A). mtDNA is especially susceptible to attack by ROS due to its close proximity to the ETC and the lack of protective histones (35, 36). In fact, oxidized mtDNAs have been shown to be released into the cytosol, where they activate downstream events such as cGAS-cGAMP-STING–dependent activation of TBK1 and inflammasome formation (3, 13, 30, 37). Therefore, increased ROS, which could be due to reduced ROS scavenging or impaired mitochondrial oxidative respiration in DsbA-L–deficient adipocytes, could account for the increased mtDNA release in obesity- and DsbA-L–deficient adipocytes.
Mitochondria are critical for cell function due to their essential roles in the production of ATP, the energy currency of the cell, and the regulation of whole-body energy homeostasis. Mitochondrial dysfunction is associated with not only metabolic diseases such as obesity, insulin resistance, and type II diabetes but also with cardiovascular diseases, aging, neuron degenerative diseases, immune dysfunction, and cancer (38–41). Here we observed an increase in mtDNA release-induced activation of the cGAS-cGAMP-STING pathway in HFD-fed C57BL/6 mice. Although it remains unclear whether mitochondrial stress-induced mtDNA release is a consequence or a cause of diet-induced obesity, DsbA-L deficiency at least clearly demonstrates that mitochondrial dysregulation can cause mtDNA release-induced activation of the cGAS-cGAMP-STING pathway and inflammation in adipocytes, thereby exacerbating obesity and causing insulin resistance.
In summary, our study demonstrates that obesity promotes mtDNA release into the cytosol, where it engages the DNA-sensing cGAS-cGAMP-STING pathway. We identify DsbA-L as a key player in preserving mitochondrial homeostasis since its deficiency triggers mtDNA release-induced activation of the cGAS-cGAMP-STING signaling pathway, thereby activating sterile chronic inflammation and subsequently leading to insulin resistance and metabolic dysfunction (Fig. 4G). Our study provides evidence showing that the DNA-sensing cGAS-cGAMP-STING pathway plays a critical role in metabolism and energy homeostasis, beyond its well-characterized roles in immune surveillance. Further characterization of this pathway in metabolic tissues will help broaden our understanding of the pathogenesis of obesity, and promote the development of new pharmacological tools to treat obesity and its related metabolic diseases.
Materials and Methods
Animals.
All animal experiments were performed according to the procedures approved by University of Texas Health at San Antonio (UTHSA)’s Animal Care and Use Committee. Fat-specific DsbA-L knockout mice (DsbA-LfKO) were generated by crossing DsbA-L Loxp mice (16) with adiponectin-Cre mice (Jackson Laboratory; stock no. 010803). Male C57BL/6 mice (Jackson Laboratory) age 8 wk were fed a 60% HFD (Research Diets) or normal chow diet for 16 wk and used to perform Western blot and mtDNA release experiments. Male homozygous Leprdb mice (db/db) age 8 wk and their control heterozygous mice were obtained from the Jackson Laboratory and used for Western blot analysis. Male DsbA-LfKO mice and LoxP control littermates were fed a normal chow diet or 45% HFD (Research Diets) for all of the following experiments.
Cell Study and Adipocyte Isolation.
The 3T3-L1 cell line was purchased from ATCC. Primary stromal vascular fractions and adipocytes from murine epididymal white fat and inguinal white fat depots were digested in isolation buffer containing 4% BSA and 1.5 mg/mL collagenase A (Roche) for 25 min at 37 °C with gentle agitation. The cell suspension was filtered through a 100-μm filter and then centrifuged at 700 × g for 3 min to separate floating adipocytes from the SVF pellet. Purified adipocytes were washed in PBS twice for further experiments. The SVF was cultured and differentiated to adipocytes as described previously (23). Details of all other experimental procedures can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Yidong Bai (Department of Cell Systems & Anatomy, UTHSA) for his kind help with the Seahorse study. We also thank the efforts of the UTHSA institutional Mass Spectrometry Laboratory and support from NIH Grant P30 CA54174. This work was supported by NIH R01 Grants DK76902 (to F.L.), R01 DK102965 (to L.Q.D.), R01 DK093587 (to Y.X.), and R01 DK101379 (to Y.X.), National Basic Research Program of China 2014CB910501 (to F.L.), and National Natural Science Foundation of China 20907027 (to J.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708744114/-/DCSupplemental.
References
- 1.Saltiel AR. New therapeutic approaches for the treatment of obesity. Sci Transl Med. 2016;8:323rv2. doi: 10.1126/scitranslmed.aad1811. [DOI] [PubMed] [Google Scholar]
- 2.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimada K, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West AP, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Feng M, Guan W. Mitochondrial DNA sensing by STING signaling participates in inflammation, cancer and beyond. Int J Cancer. 2016;139:736–741. doi: 10.1002/ijc.30074. [DOI] [PubMed] [Google Scholar]
- 6.López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13:106–118. doi: 10.1016/j.mito.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Tschopp J. Mitochondria: Sovereign of inflammation? Eur J Immunol. 2011;41:1196–1202. doi: 10.1002/eji.201141436. [DOI] [PubMed] [Google Scholar]
- 8.Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell. 2014;54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 10.Rongvaux A, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White MJ, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehrke N, et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39:482–495. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Barber GN. STING: Infection, inflammation and cancer. Nat Rev Immunol. 2015;15:760–770. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris JM, Meyer DJ, Coles B, Ketterer B. A novel glutathione transferase (13-13) isolated from the matrix of rat liver mitochondria having structural similarity to class theta enzymes. Biochem J. 1991;278:137–141. doi: 10.1042/bj2780137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, et al. Hepatic DsbA-L protects mice from diet-induced hepatosteatosis and insulin resistance. FASEB J. 2017;31:2314–2326. doi: 10.1096/fj.201600985R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu M, et al. Endoplasmic reticulum (ER) localization is critical for DsbA-L protein to suppress ER stress and adiponectin down-regulation in adipocytes. J Biol Chem. 2015;290:10143–10148. doi: 10.1074/jbc.M115.645416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reilly SM, et al. An inhibitor of the protein kinases TBK1 and IKK-ε improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19:313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashiguchi K, Zhang-Akiyama QM. Establishment of human cell lines lacking mitochondrial DNA. Methods Mol Biol. 2009;554:383–391. doi: 10.1007/978-1-59745-521-3_23. [DOI] [PubMed] [Google Scholar]
- 20.Kaguni LS. DNA polymerase gamma, the mitochondrial replicase. Annu Rev Biochem. 2004;73:293–320. doi: 10.1146/annurev.biochem.72.121801.161455. [DOI] [PubMed] [Google Scholar]
- 21.Liu M, et al. Fat-specific DsbA-L overexpression promotes adiponectin multimerization and protects mice from diet-induced obesity and insulin resistance. Diabetes. 2012;61:2776–2786. doi: 10.2337/db12-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong Z, et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164:896–910. doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu M, et al. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc Natl Acad Sci USA. 2008;105:18302–18307. doi: 10.1073/pnas.0806341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Y, Liu M. Adiponectin: A versatile player of innate immunity. J Mol Cell Biol. 2016;8:120–128. doi: 10.1093/jmcb/mjw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8:93–100. doi: 10.1093/jmcb/mjw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci USA. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao D, et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc Natl Acad Sci USA. 2015;112:E5699–E5705. doi: 10.1073/pnas.1516465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray EE, Treuting PM, Woodward JJ, Stetson DB. Cutting edge: cGAS is required for lethal autoimmune disease in the Trex1-deficient mouse model of Aicardi-Goutières syndrome. J Immunol. 2015;195:1939–1943. doi: 10.4049/jimmunol.1500969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pemble SE, Wardle AF, Taylor JB. Glutathione S-transferase class kappa: Characterization by the cloning of rat mitochondrial GST and identification of a human homologue. Biochem J. 1996;319:749–754. doi: 10.1042/bj3190749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang C, Wei X, Wei Y. Mitochondrial DNA in the regulation of innate immune responses. Protein Cell. 2016;7:11–16. doi: 10.1007/s13238-015-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morel F, et al. Gene and protein characterization of the human glutathione S-transferase kappa and evidence for a peroxisomal localization. J Biol Chem. 2004;279:16246–16253. doi: 10.1074/jbc.M313357200. [DOI] [PubMed] [Google Scholar]
- 32.Nebert DW, Vasiliou V. Analysis of the glutathione S-transferase (GST) gene family. Hum Genomics. 2004;1:460–464. doi: 10.1186/1479-7364-1-6-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ladner JE, Parsons JF, Rife CL, Gilliland GL, Armstrong RN. Parallel evolutionary pathways for glutathione transferases: Structure and mechanism of the mitochondrial class kappa enzyme rGSTK1-1. Biochemistry. 2004;43:352–361. doi: 10.1021/bi035832z. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, et al. DsbA-L alleviates endoplasmic reticulum stress-induced adiponectin downregulation. Diabetes. 2010;59:2809–2816. doi: 10.2337/db10-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cahill A, et al. Effects of alcohol and oxidative stress on liver pathology: The role of the mitochondrion. Alcohol Clin Exp Res. 2002;26:907–915. [PMC free article] [PubMed] [Google Scholar]
- 36.Kong Y, Trabucco SE, Zhang H. Oxidative stress, mitochondrial dysfunction and the mitochondria theory of aging. Interdiscip Top Gerontol. 2014;39:86–107. doi: 10.1159/000358901. [DOI] [PubMed] [Google Scholar]
- 37.Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123:951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 40.O’Rourke B. Metabolism: Beyond the power of mitochondria. Nat Rev Cardiol. 2016;13:386–388. doi: 10.1038/nrcardio.2016.95. [DOI] [PubMed] [Google Scholar]
- 41.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu M, et al. Grb10 promotes lipolysis and thermogenesis by phosphorylation-dependent feedback inhibition of mTORC1. Cell Metab. 2014;19:967–980. doi: 10.1016/j.cmet.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernández-Vizarra E, et al. Isolation of mitochondria for biogenetical studies: An update. Mitochondrion. 2010;10:253–262. doi: 10.1016/j.mito.2009.12.148. [DOI] [PubMed] [Google Scholar]
- 44.Paulin R, et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab. 2014;20:827–839. doi: 10.1016/j.cmet.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.