Significance
Although the epidermis of the skin is the first tissue to manifest a zinc deficiency, the mechanisms underlying zinc-mediated epidermal formation are largely unknown. We demonstrated that the zinc transporter ZIP10, which is highly expressed in the outer root shelf of hair follicles, is essential for epidermal formation. Ablating Zip10 caused epidermal hypoplasia by down-regulating the transcriptional activity of p63, whereas ZIP10-mediated zinc influx promoted p63 transactivation to induce epidermal morphogenesis. Our results establish the physiological relevance of ZIP10 in epidermal development.
Keywords: zinc transporter, ZIP10, skin epidermis, hair follicle, development
Abstract
Skin tissues, in particular the epidermis, are severely affected by zinc deficiency. However, the zinc-mediated mechanisms that maintain the cells that form the epidermis have not been established. Here, we report that the zinc transporter ZIP10 is highly expressed in the outer root sheath of hair follicles and plays critical roles in epidermal development. We found that ZIP10 marked epidermal progenitor cell subsets and that ablating Zip10 caused significant epidermal hypoplasia accompanied by down-regulation of the transactivation of p63, a master regulator of epidermal progenitor cell proliferation and differentiation. Both ZIP10 and p63 are significantly increased during epidermal development, in which ZIP10-mediated zinc influx promotes p63 transactivation. Collectively, these results indicate that ZIP10 plays important roles in epidermal development via, at least in part, the ZIP10–zinc–p63 signaling axis, thereby highlighting the physiological significance of zinc regulation in the maintenance of skin epidermis.
The epidermis constitutes a rigid, stratified barrier that protects the body from dehydration and infections (1). In mice, the epidermis begins forming around embryonic day 8.5 (E8.5) (2). At E9.5, epidermal lineages expressing keratin 5 and 14 can be detected in the basal layer and periderm (3). The spinous and granular layers of the mature epidermis begin appearing at E14.5, when hair follicle specification begins (3). From E14.5 onward, epidermal progenitor cells proliferate vigorously to support epidermal development and terminal differentiation. Differentiated, cornified epidermal layers with barrier function are present by E17.5, just before birth (3).
This epidermal development requires the coordinated function of several zinc-binding proteins including enzymes and transcription factors (TFs) to orchestrate the various programs (4, 5). The master epidermal regulator p63 (6) triggers epithelial stratification through the altered balance of expression of its two isoforms, an N-terminal transcriptional activation (TA) domain-containing isoform and a truncated (ΔN) isoform (2, 5). Both isoforms contain a DNA-binding domain (DBD) with a zinc-binding site (6) that incorporates zinc for proper sequence-specific DNA binding (7). Competing metals can alter p63 function (7), implicating the possible requirement of a zinc atom to fine-tune p63 transcriptional activity.
Zinc homeostasis in mammalian cells is tightly regulated by zinc-transporting proteins (8) classified as zinc transporters (ZnTs) or Zrt- and Irt-like proteins (ZIPs) (9). The ZIP family, which has at least 14 members, imports extracellular or luminal zinc into the cytoplasm in mammals (10). ZIP members are expressed in specific tissues and act through rather selective signaling pathways. For example, ZIP13 is expressed mainly in connective tissues and is required for their development, whereas pathogenic ZIP13 mutations are found in a new type of Ehlers–Danlos syndrome (11–14). The intestinal zinc transporter ZIP4 is related to acrodermatitis enteropathica (AE), in which zinc deficiency causes skin sensitization and severe epidermal-barrier dysfunction (15, 16). Loss-of-function ZIP4 mutations cause a failure in zinc influx through the intestine, resulting in severe skin problems (17, 18). Additionally, recent data suggest that ZIP7 fine-tunes endoplasmic reticular condition for supporting protein disulfide isomerase activity in mesenchymal stem cells (19). Although our understanding of the roles of zinc in various cellular phenomena is improving, the molecular relationship between zinc homeostasis and the cells forming the skin epidermis remains largely unknown.
Here, we establish a critical link between ZIP10 and skin development, revealing a molecular mechanism underlying the requirement of zinc for developing the skin epidermis, and the highlight the clinical impact of ZIP10 as a potential therapeutic target for skin diseases.
Results
ZIP10 Is Predominantly Expressed in Epidermal Hair Follicles.
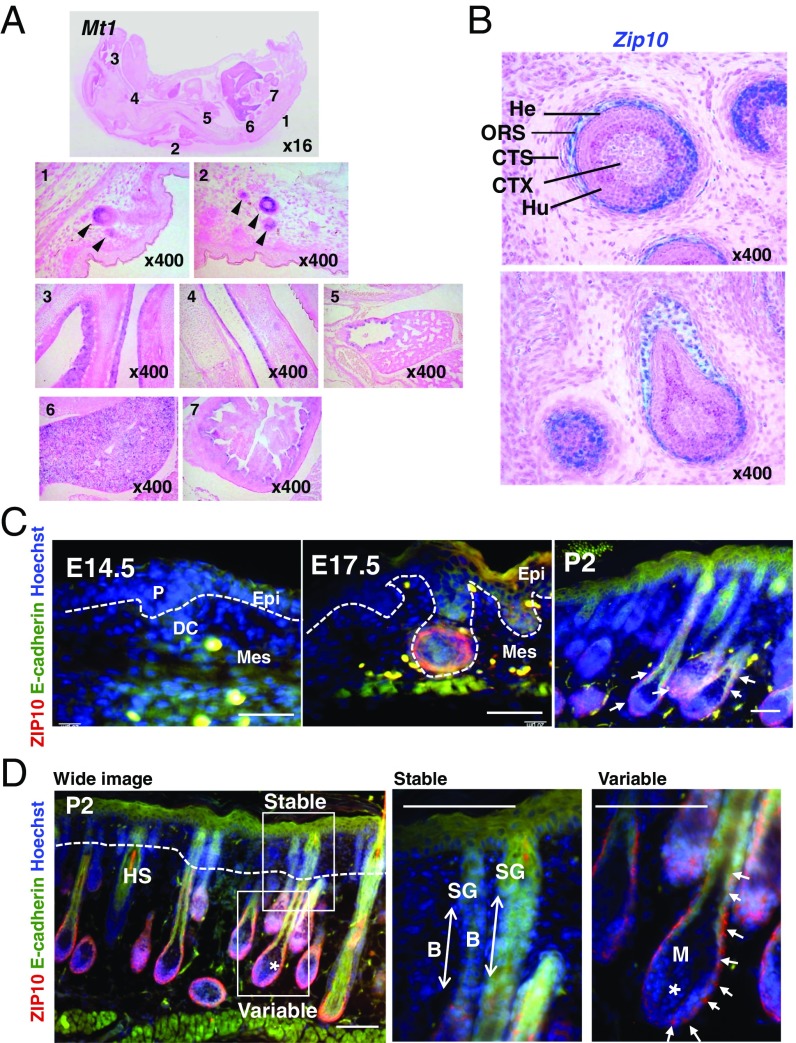
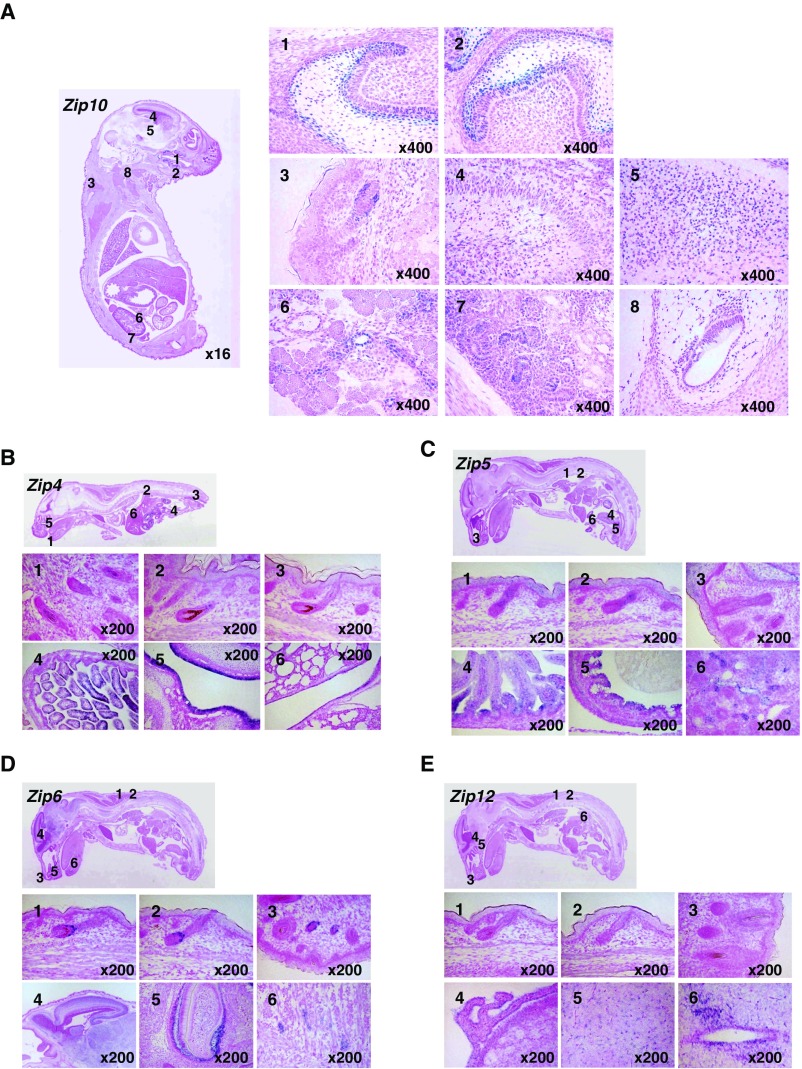
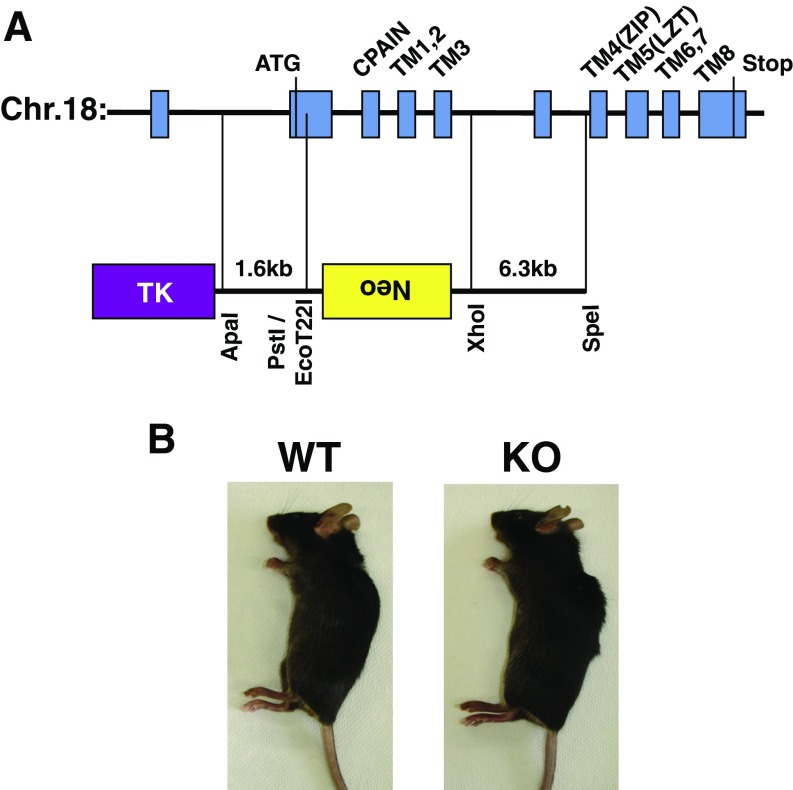
Zinc is reportedly enriched in skin areas, especially in hair follicles (20, 21). Although skin epidermis constitutes the primary tissue affected by zinc deficiency, the molecular mechanisms by which zinc contributes to the development of the skin epidermis are poorly understood. To investigate the precise area in which zinc is enriched during embryogenesis, we first analyzed the expression of zinc-induced metallothionein 1 (MT1) mRNA by in situ hybridization using whole sections of E18.5 mice, revealing that Mt1 was highly expressed in the early hair peg and in organs such as the lung, liver, and intestine (Fig. 1A). Next, we analyzed the expression level of the ZIP LIV-1 subfamily, which localizes to the mammalian plasma membrane, to identify zinc transporter(s) that influx zinc in these tissues. Among the ZIP family members, Zip10 was predominantly expressed in the epithelial tissues, including the outer root sheath (ORS), the lower part of Huxley’s layer in the hair follicles, and the tooth germ at E17.5 (Fig. 1B and Fig. S1A). Immunocytochemistry revealed that the ZIP10 protein was present in the early hair peg during epidermal development (Fig. 1C, E17.5). At postnatal day 2, ZIP10 was mainly expressed in the variable components of the hair follicle and was also present in hair matrix cells and in the stable components of the hair follicle (Fig. 1D). Zip4, a causative gene for AE, was mainly expressed in the intestine as previously reported (22) (Fig. S1B). Zip5 was expressed in the intestine and kidney (Fig. S1C), whereas Zip12 was found in the choroid plexus, medulla oblongata, and spinal cord (Fig. S1E). Although Zip6 was also observed in hair follicles (Fig. S1D), it was expressed rather topically such as in the upper hair bulge region (Fig. S1D). Moreover, Zip6-deficient (KO) mice had no apparent skin abnormalities (Fig. S2). Together, these findings indicated that ZIP10 might serve as a predominant zinc transporter to influx zinc into the epidermis-forming cells, and thus may be involved in the development of the epithelium of the skin and related appendages.
Fig. 1.
ZIP10 expression in the epidermis. (A) In situ hybridization in the E18.5 mouse embryo showed Mt1 expression in the (1, 2) dorsal skin, (3) nasal cavity, (4) trachea, (5) lung, (6) liver, and (7) intestine. (B) Zip10 was expressed in the E17.5 mouse embryo in the whiskers. The defined lineages are Henle’s (He) and Huxley’s (Hu) layer in the internal root sheath, the outer root sheath (ORS), the connective-tissue sheath (CTS), and the cortex of the shaft (CTX). (C and D) Immunocytochemistry for ZIP10 (red) and E-cadherin (green) in skin from mouse embryos or postnatal day 2 (P2) mice. B, bulge region; DC, dermal condensate; Epi, epithelium; HS, hair shaft; M, hair matrix; Mes, mesenchyme; P, placode; SG, sebaceous gland; *, dermal papilla. (Scale bar, 100 µm.)
Fig. S1.
mRNA expression of LIV-1 subfamily members. (A) Zip10 was expressed in the E17.5 mouse embryo in the (1, 2) teeth, (3) dorsal skin, (4) hippocampus, (5) thalamus, (6) pancreas, (7) kidney, and (8) inner ear. (B) Zip4 was expressed in the newborn mouse in the (1) whisker, (2, 3) dorsal skin, (4) intestines, (5) nasal cavity, and (6) trachea. (C) Zip5 was expressed at E18.5 in the (1, 2) dorsal skin, (3) whisker, (4) small intestines, (5) large intestines, and (6) kidney. (D) Zip6 was expressed at E18.5 in the (1, 2) dorsal skin, (3) whisker, (4) brain, (5) teeth, (6) and tongue. (E) Zip12 was expressed at E18.5 in the (1, 2) dorsal skin, (3) whisker, (4, 5) brain, and (6) spinal cord.
Fig. S2.
Zip6-KO mice have a normal skin phenotype. (A) Genomic DNA region deleted to generate Zip6-KO mice. ZIP6 N terminus, including the PAL motif, was targeted (55). (B) Photographs showing that the Zip6-KO mice had a normal phenotype.
ZIP10 Marks Epidermal Progenitor Cell Subsets.
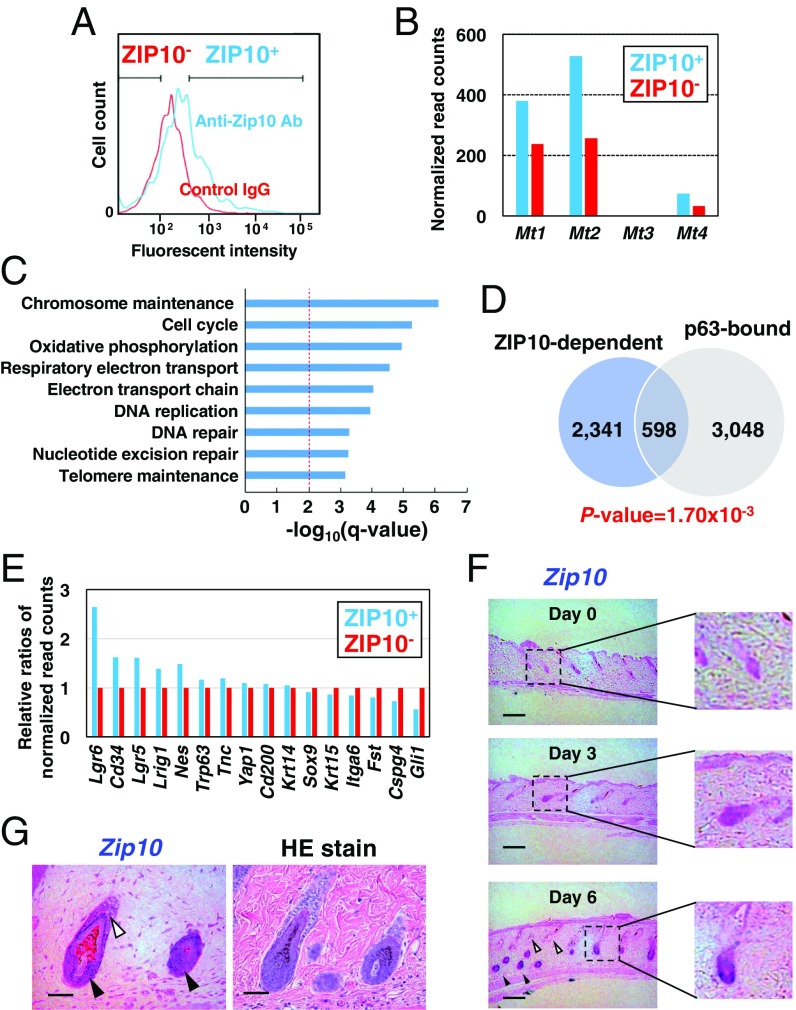
To characterize the ZIP10-positive (ZIP10+) cells in the hair follicles, we sorted the ZIP10+ and ZIP10− cells and performed mRNA sequencing (Fig. 2A and Table S1). Mt1, Mt2, and Mt4, members of the Mt family that are indicators for intracellular zinc levels (8), were up-regulated in the ZIP10+ cells, suggesting that zinc was enriched in these cells (Fig. 2B). Of the differentially expressed genes (DEGs) identified, 1,763 were up-regulated and 1,108 were down-regulated in ZIP10+ cells compared with ZIP10− cells (Table S2 A and B). The genes up-regulated in ZIP10+ cells were mainly involved in processes related to epidermal development, such as DNA repair, telomere maintenance, and the cell cycle (Fig. 2C and Table S3 A and B). Given that these epidermal processes are critically governed by p63 (23), a master TF for epithelial development, we hypothesized that there might be an overlap between genes that depend on ZIP10 and p63. In fact, a significant number of the DEGs (23%, P = 1.70 × 10−3) between ZIP10+ and ZIP10− cells were known p63 targets despite comparable TP63 expression levels in both cells (24) (Fig. 2 D and E). Moreover, a comparative analysis of gene expression changes between ZIP10-dependent and p63-dependent genes showed a significant correlation (Fig. S3). These results support our hypothesis that ZIP10 and p63 may tightly cooperate for epidermal formation.
Fig. 2.
ZIP10 marks lgr6high and glilow hair follicle progenitor cells. (A) ZIP10+ cells from the hair follicles of 2-wk-old WT mice were sorted by flow cytometry using an anti-ZIP10 antibody. (B) Normalized read counts of Mt1, Mt2, Mt3, and Mt4 in ZIP10+ and ZIP10− cells. (C) Biological processes represented by the up-regulated genes in ZIP10high cells. Bars represent enrichment scores, given as −log10(q value), for the pathways shown. The red dotted line indicates the cutoff of q value = 0.01. (D) Overlap in gene sets between the ZIP10-dependent genes and the previously identified p63 target genes (24). (E) Relative ratios of normalized read counts of known epidermal stem cell markers in ZIP10+ compared with ZIP10− cells. (F) In situ hybridization revealed that shaving induced Zip10 expression in the hair peg (filled arrowheads: early stage; open arrowheads: aged) and (G) that Zip10 was induced in the ORS (open arrowhead) and the hair matrix (filled arrowheads) after shaving.
Fig. S3.
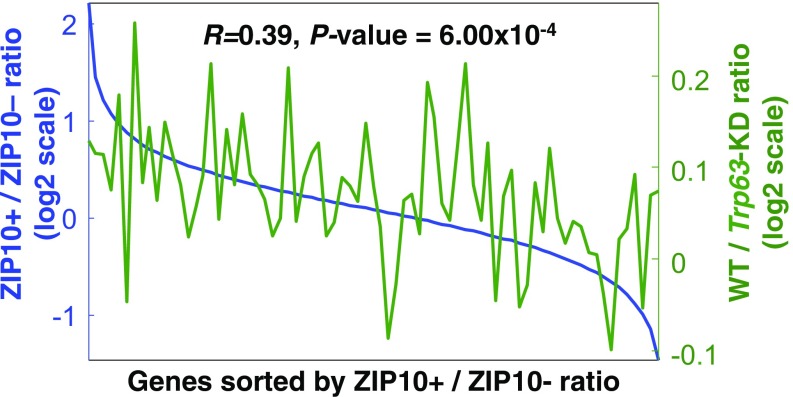
Changes in gene expression between ZIP10 dependency and p63 dependency. Comparative analysis of the gene expression profiles between the log2-fold differences in ZIP10+/ZIP10− cells and in WT/Trp63-KD skin. The gene expression microarrays for WT and Trp63-KD skin were obtained from GEO (GSE3108). The x axis shows the sorted genes according to the magnitude of log2-fold changes in ZIP10+ versus ZIP10− cells. The Left y axis indicates the average log2-fold changes in ZIP10+ versus ZIP10− cells for individual bins of the sorted genes (100 genes/bin); the Right y axis indicates the average log2-fold changes in WT versus Trp63-KD skin for the genes in the individual bins defined by the ZIP10 comparison.
Hair follicles contain diverse stem cell subsets that contribute to epidermal homeostasis and regeneration (25). We next investigated the expression of epidermal stem cell marker genes (25, 26) in ZIP10+ cells. Lgr6, CD34, and Lgr5 were highly expressed, whereas less Gli1 was detected in the ZIP10+ cells (Fig. 2E), indicating that ZIP10 marks lgr6high and glilow epidermal progenitor cell subsets. Serial stem cells commit to becoming progenitor cells to regenerate the epidermis when the hair cycle restarts or the epidermis is injured (25). Therefore, we tested whether shaving the skin, which stimulates epidermal regeneration, could induce Zip10 expression. In situ hybridization analysis revealed that Zip10 was significantly induced in newly generated hair follicles (Fig. 2 F and G). Together, these data indicated that ZIP10 is functionally associated with the generation and regeneration of the skin epidermis in vivo.
Zip10flox/floxkeratin14-Cre Mice Have Epidermal Hypoplasia.
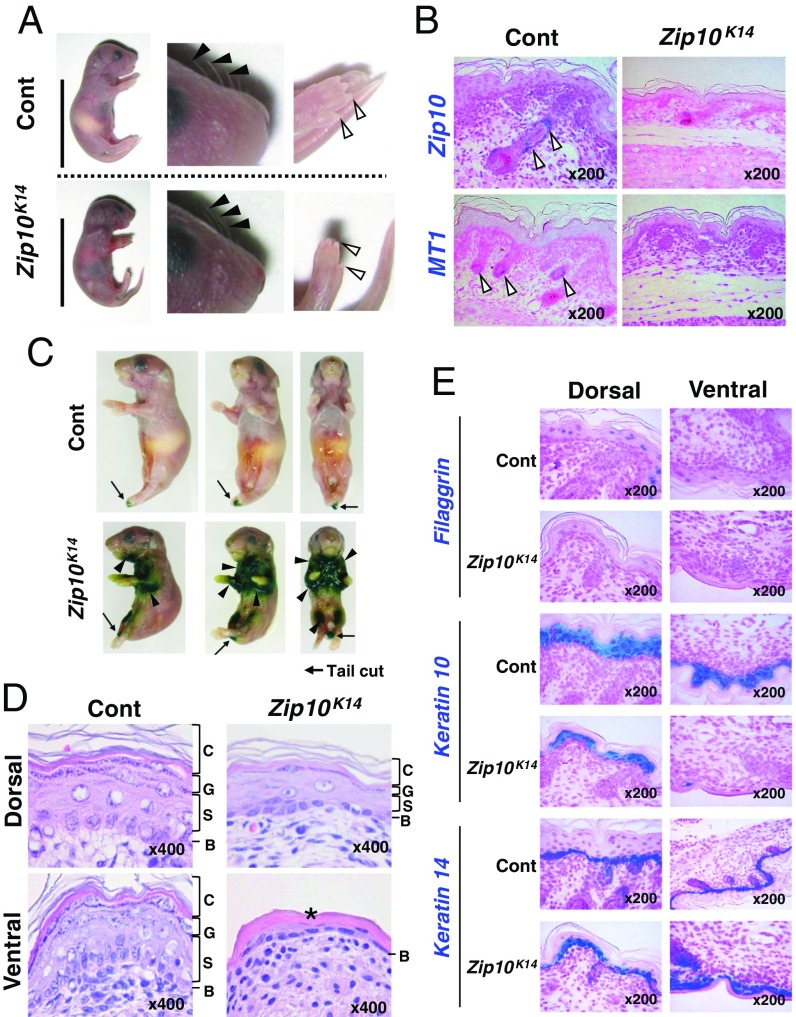
To elucidate the role of ZIP10 roles in the epidermis, we crossed Zip10flox/flox mice with keratin14 (K14)-Cre transgenic mice to generate Zip10flox/floxkeratin14-Cre (Zip10K14) mice. Zip10K14 newborn mice had scarlet skin with thin and feeble hair (Fig. 3A) and died within a few days, possibly owing to total body dehydration (27) or for undefined reasons. The reduced Mt1 expression in the hair follicles of Zip10K14 newborn mice implied the zinc deficiency compared with control mice (Fig. 3B). X-gal staining revealed that the skin barrier function was substantively impaired; in particular, the ventral skin shows severe dysgenesis in comparison with the dorsal skin, indicating that epidermal proliferation and differentiation does not progress properly from the middle developmental stage at E10–E14 (Fig. 3C). Indeed, hematoxylin and eosin (H&E) staining and in situ hybridization analysis using epidermal differentiation markers indicated that the loss of ZIP10 caused epidermal dysgenesis (Fig. 3D). The dorsal skin of Zip10K14 mice undergoes epidermal stratification, although each epidermal layer appears atrophic with thinning of the granular layer compared with control epidermis (Fig. 3D). The ventral skin of Zip10K14 mice contains no spinous or granular layer, as in the epidermis of the early developmental stage (Fig. 3D). Notably, the fact that keratin 14 is comparable in both mutant and control epidermis (Fig. 3E) supports that the epidermal formation is normally initiated, whereas the previous results indicate that progression from the middle developmental stage is impaired. For example, low expression of differentiation markers such as filaggrin and keratin 10 illustrate the defects of epidermal differentiation (Fig. 3E), revealing that ZIP10 has a critical role in epidermal development.
Fig. 3.
Zip10flox/floxK14-Cre mice show polar epidermal hypoplasia. (A) P1 Zip10K14 mice had thin whiskers (filled arrowheads) but normal limb development (open arrowheads). (Scale bar, 2 cm.) (B) In situ hybridization showed that Zip10 and Mt1 were expressed in the skin from P1 control (Cont) (open arrowheads) but not P1 Zip10K14 mice. (C) Skin barrier analysis by X-gal staining revealed that the skin barrier was impaired in P1 Zip10K14 mice (filled arrowheads); arrows indicate a tail cut. (D) H&E staining revealed dorsal epidermal hypoplasia and ventral embryonic epidermis (asterisk) in P1 Zip10K14 mice. B, basal layer; C, cornified layer; G, granular layer; S, spinous layer. (E) In situ hybridization showed reduced filaggrin and keratin 10 expression but normal keratin 14 expression in Zip10K14 mice.
ZIP10 Supports p63 Functions During Epidermal Development.
ZIP10 physically and functionally correlates with p63 function.
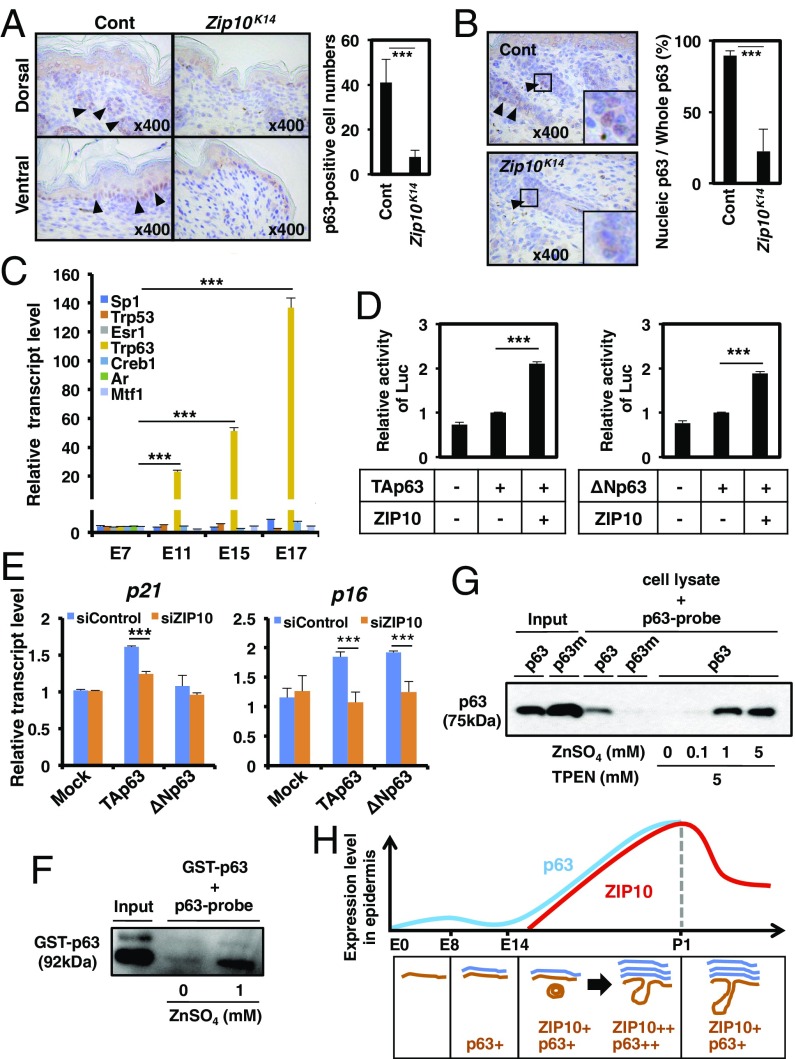
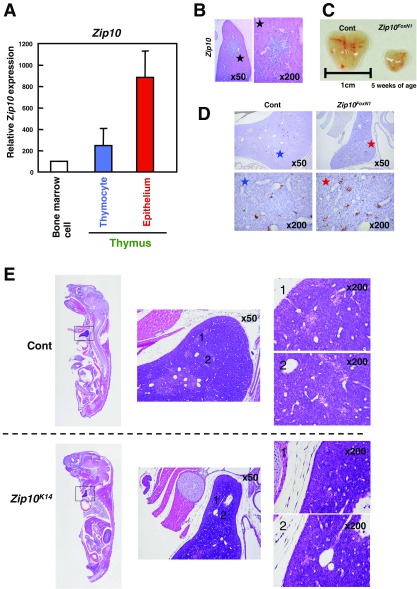
Given that ZIP10-dependent gene expression level nicely correlates with p63-mediated gene expression (Fig. 2D and Fig. S3) and the epidermal hypoplasia in Zip10K14 mice was recapitulated in the abnormality caused by the loss of Trp63 (28), we next investigated the functional association between ZIP10 and p63. The Trp63 gene encodes the p63 protein, a zinc-binding TF that marks epidermal progenitor cells and regulates the cellular programs that form the epidermis (29, 30). In the Zip10K14 epidermis, the number of p63-positive (p63+) epidermal progenitor cells was significantly reduced in both the hair follicle and the interfollicular region, and hair follicles were sparse (Fig. 4A) as the Trp63-null mice (31). In addition, whereas p63 localizes to the nucleus in the normal epidermis, it localized to the cytosol in the Zip10K14 epidermis (Fig. 4B). Notably, the importance of p63 in epithelial cell proliferation was also demonstrated by using thymus-specific Trp63-deficient (Trp63FoxN1) mice (30), in which we observed that Zip10 was highly expressed in the medulla of the thymus (Fig. S4 A and B). To determine whether ZIP10 is also essential in the proliferation of progenitor cells, we generated thymus-specific Zip10-deficient (Zip10FoxN1) mice by crossing FoxN1-Cre transgenic mice with Zip10flox/flox mice. Zip10FoxN1 mice had a hypoplastic thymus with increased TUNEL+ cells (Fig. S4 C and D), similar to the thymus in Trp63-null (30) and Zip10K14 mice (Fig. S4E). These data indicate that ZIP10 is essential for biological events involving p63 in epithelium tissues.
Fig. 4.
ZIP10 facilitates p63 transactivation in epidermal development. (A) Immunohistochemistry showed fewer p63-expressing cells (brown) in the dorsal and ventral epidermis in newborn (P1) Zip10K14 compared with control mice, and (B) abnormal p63 cellular localization in Zip10K14 mice. Arrowheads indicate p63-expressing cells. Five different parts of two skin specimens derived from each mouse were analyzed (n = 5, ***P < 0.001). (C) Real-time PCR of Trp63 across embryo development. The data are representative of three independent experiments (***P < 0.001). (D) ZIP10 overexpression increases the transactivation of the p63 proteins. H1299 cells stably expressing the TAp63 or ΔNp63 protein were transfected with pPG13-Luc for 24 h and subjected to luciferase assay. The data are representative of three independent experiments (***P < 0.001). (E) ZIP10 depletion down-regulated p16 and p21 expression in HeLa cells. HeLa cells were treated with siZIP10 for 48 h and then transfected with plasmids encoding p63; the mRNA levels were quantified by real-time PCR. The data are representative of three independent experiments (***P < 0.001). (F) p63 protein bound to its DNA-binding sequence (p63-probe) was directly modulated by zinc. The GST-tagged recombinant p63 proteins (GST-p63) were mixed with biotinylated p63 binding sequence and avidin-agarose beads. The protein bound to the complex of p63 binding sequence and the avidin-agarose beads were subjected to Western blotting analysis and detected by anti-p63 antibody. (G) Cell lysates derived from HEK293 cells transfected with either p63 or p63 mutant (p63m) plasmids were mixed with biotinylated p63 binding sequence and avidin-agarose beads with or without ZnSO4 and TPEN at the indicated concentrations. The protein bound to the complex of p63 binding sequence and avidin-agarose beads was subjected to Western blotting analysis and detected by anti-p63 antibody. (H) ZIP10, whose expression is elevated in the progenitor cells at ORS of hair follicles during epidermal morphogenesis, contributes to the activities of elevated p63 by supplying zinc. This allows p63 to properly bind to DNA for initiating the gene expression necessary for epithelium morphogenesis, such as cell proliferation and stratification.
Fig. S4.
ZIP10 is involved in the development of thymus epithelium. (A) Quantification of Zip10 mRNA levels by real-time PCR using mouse thymocytes and epithelial cells (n = 2). (B) In situ hybridization revealed that Zip10 was expressed in the medulla of the thymus in newborn control mice. (C) The thymus was smaller in Zip10FoxN1 than in control mice. (D) A TUNEL assay showed more TUNEL+ cells (brown) in the Zip10FoxN1 thymus than in the control thymus. (E) H&E staining revealed thymic hypoplasia in Zip10K14 mice.
ZIP10 mediates zinc homeostasis as assessed by molecular and pathway analyses.
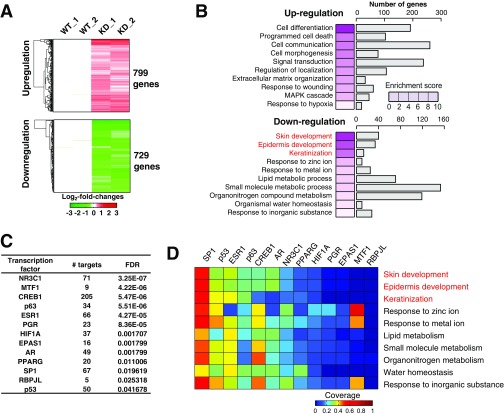
To reveal the further molecular association between the ZIP10 and p63 related to epidermal formation, we performed microarray analysis with mRNAs isolated from human wild-type (WT) and ZIP10-knockdown (KD) keratinocytes. We identified 799 up-regulated and 729 down-regulated genes in ZIP10-KD keratinocytes compared with control cells (Fig. S5A and Table S2 C and D). The up-regulated genes in ZIP10-KD keratinocytes were involved in the processes of cell death, morphogenesis, and signal transduction (Fig. S5B and Table S3 C and D). The down-regulated genes in ZIP10-KD keratinocytes were mainly involved in skin development and keratinization, indicating that ZIP10 depletion is responsible for disturbing epidermal formation, as was observed in the Zip10K14 epidermis (Fig. S5B and Table S3 C and D). Based on the fact that zinc homeostasis may affect the transactivation of zinc-dependent TFs such as p63, and to clarify which TFs are responsive to ZIP10 depletion in keratinocytes, we performed TF enrichment analysis for the down-regulated genes in ZIP10-KD keratinocytes using MetaCore software from GeneGo (Thomson Reuters). We identified 13 key TFs (NR3C1, MTF1, CREB1, p63, ESR1, PGR, HIF1A, EPAS1, AR, PPARG, SP1, RBPJL, and p53) that target a significant number of genes [false discovery rate (FDR) < 0.05] (Fig. S5C). Among these TFs, SP1, p53, ESR1, p63, CREB1, and AR regulate a significant number of genes involved in epidermal development and homeostasis (Fig. S5D). Notably, only Trp63, a mouse homolog of the human p63 gene, is markedly increased during epidermal development (over 100-fold) (Fig. 4C). These data indicate that ZIP10 supports the up-regulation of p63 activity to direct epidermal development.
Fig. S5.
Genome-wide gene expression analysis reveals that ZIP10 is important for transactivation in human epidermal keratinocytes. (A) Heatmap for the up-regulated and down-regulated genes in ZIP10-KD human epidermal keratinocytes. Mean-centered values (log2 scale) for two WT replicates were subtracted from the intensities for each WT (WT_1 and WT_2) and ZIP10-KD (KD_1 and KD_2) samples. Color bars represent the gradient of log2-fold changes in each comparison. (B) Gene ontology biological processes (GOBPs) represented by the up-regulated and down-regulated genes in ZIP10-KD human epidermal keratinocytes. Bars represent the number of genes and color key (left side of each bar) represents enrichment scores, expressed as −log10(P value). GOBPs with red letters are the processes related to epidermis development. (C) Significant TFs (FDR < 0.05) that have significant number of targets in 729 down-regulated genes by ZIP10-KD. (D) Heatmap representing the proportion (coverage) of the number of genes targeted by each TF in C among the down-regulated genes mapped to each enriched GOBPs. GOBPs with red letters are the processes related to epidermis development.
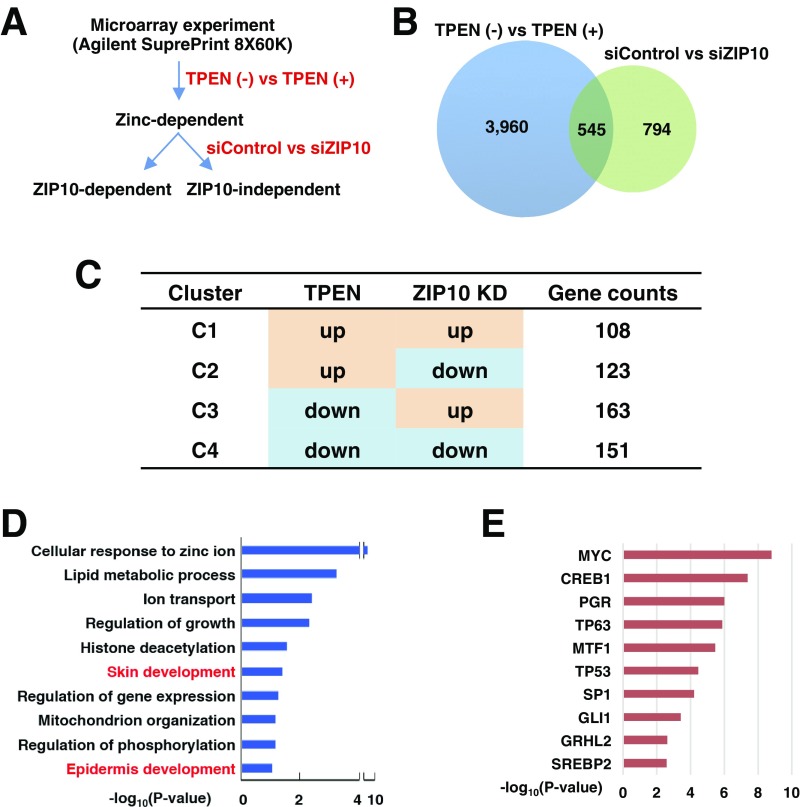
Next, we addressed the association between ZIP10, zinc, and p63 (Fig. S6A). First, zinc-dependent genes were identified by the comparison of N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine (TPEN)-treated and untreated cells, and then ZIP10-dependent genes were obtained from among the zinc-dependent genes by comparing ZIP10-KD with WT cells (Fig. S6B). We found that 545 genes demonstrated common changes in expression by either TPEN treatment or ZIP10-KD, whereas 151 genes commonly down-regulated in TPEN-treated and ZIP10-KD keratinocytes (Fig. S6 B and C), which belong to cluster 4 (C4 in Fig. S6C), are thought to be directly regulated by ZIP10-mediated zinc. We further investigated these 151 genes by performing TF enrichment and pathway enrichment analysis (Fig. S6 D and E). The genes in C4 were found to still be significantly involved in epidermal development (Fig. S6D). Furthermore, p63 was indicated as one of the most significant factors targeting the genes in C4 (Fig. S6E). Taken together, these data support that ZIP10-mediated zinc homeostasis is crucial for the p63 function during epidermal development.
Fig. S6.
Genome-wide gene expression analysis reveals that the ZIP10–zinc signal is important for p63 transactivation in human epidermal keratinocytes. (A) Experimental scheme to obtain the genes directly affected by ZIP10-mediated zinc influx. (B) Overlap between the DEGs obtained by TPEN treatment [TPEN (−) vs. TPEN (+)] and ZIP10-KD (siControl vs. siZIP10). (C) Expression pattern (C1–C4) of the overlapped genes by TPEN treatment and ZIP10-KD. (D) Gene ontology biological processes (GOBPs) represented by the genes in C4. (E) Top 10 significant TFs that have significant numbers of targets among 151 commonly down-regulated genes (C4 in C) by treatment of TPEN and ZIP10-KD.
The ZIP10–zinc signal is required for p63 transactivation.
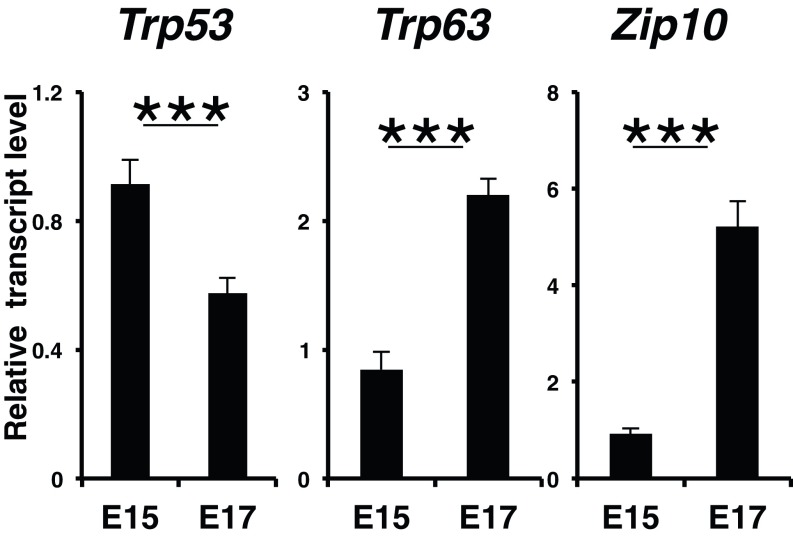
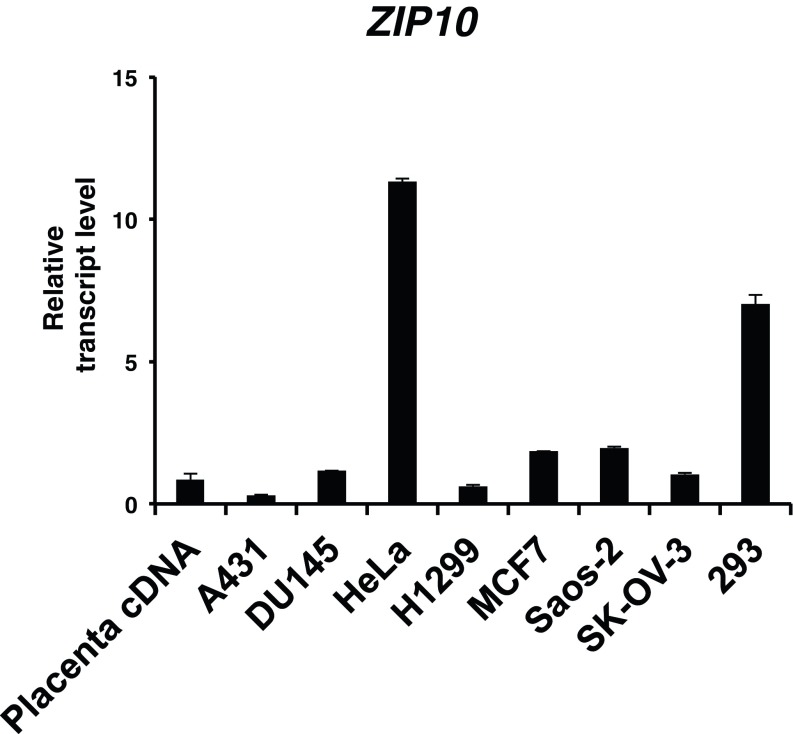
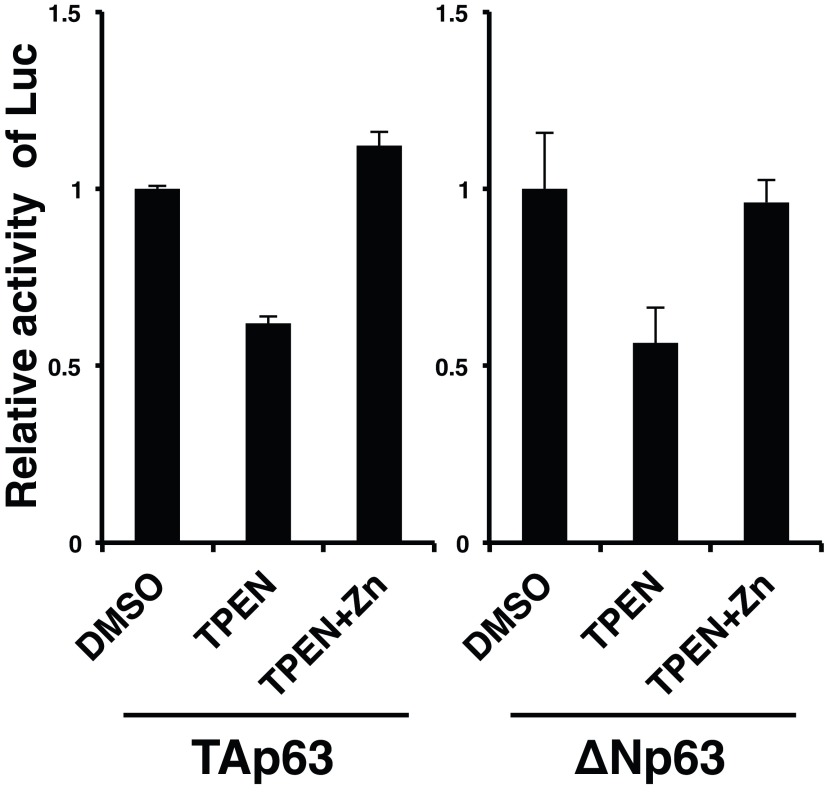
Epidermal progenitor cell proliferation and terminal differentiation progress vigorously from E14.5 to just before birth (23). Zip10 and Trp63 expression increased dramatically during this period (Figs. 1C and 4C and Fig. S7), which prompted us to determine whether ZIP10 could support the p63 activity. We used H1299 cells, which express little detectable p63 protein (32), stably complemented with two p63 isoforms, either TAp63 or ΔNp63. The ectopic expression of ZIP10 up-regulated p63 transactivation (Fig. 4D). To investigate whether ZIP10 depletion could affect the expression of p63 target genes, cell lines with high expression of ZIP10 were selected (Fig. S8) and then treated with the ZIP10 siRNA. The siRNA depletion of ZIP10 down-regulated the expression of p63 target genes (Fig. 4E). Similarly, depleting intracellular zinc by chelation with TPEN reduced p63 transactivation, whereas this reduction was rescued by zinc treatment (Fig. S9), suggesting that both ZIP10 and zinc participate in p63 regulation.
Fig. S7.
Zip10 and Trp63 are increased from E15 to E17. Real-time PCR revealed that the mRNA levels of Zip10 and Trp63 but not Trp53 increased from E15 to E17. The data are representative of three independent experiments (***P < 0.001).
Fig. S8.
Expression of ZIP10 in various cell lines. Quantification of ZIP10 mRNA levels by real-time PCR reveals the highest expression in the HeLa cell line.
Fig. S9.
Zinc treatment restored the transcriptional activities of the p63 proteins in TPEN-treated H1299 cells. H1299 cells stably expressing the p63 TAp63 or ΔNp63 isoforms were transfected with pPG13-Luc. The cells were treated with TPEN combined with 5 μM ZnSO4 for 24 h and examined by luciferase assay. The data are representative of three independent experiments.
Finally, we examined whether zinc directly promotes p63 protein binding to the p63 binding sequence. DNA pull-down assay revealed that zinc addition increased DNA binding of p63 recombinant protein (Fig. 4F). Furthermore, ectopically expressed p63 protein in cells efficiently recruited the p63 binding sequence but not p63 mutant protein totally lacking zinc binding sites (16) (Fig. 4G). TPEN treatment decreased p63 binding to its target DNA sequence, which was restored by the zinc supplement in a dose-dependent manner (Fig. 4G). These results clearly indicated that ZIP10-mediated zinc delivery is critical for proper p63 transactivation.
Discussion
In the present study, we show that the hair follicle-localized zinc transporter ZIP10 is essential for epidermis formation. During epidermal development, ZIP10 supplies zinc to p63 to promote its activity. Therefore, the ZIP10–zinc–p63 signaling axis is indispensable for epidermal morphogenesis (Fig. 4H).
ZIP10 Is a Key Regulator of Zinc Homeostasis During Epidermal Development.
The involvement of ZIP10 in various systems has been reported as follows: ZIP10 is highly expressed in invasive and metastatic breast cancer cell lines, implying its association with their characteristic features (33). ZIP10 is involved in the epithelial to mesenchymal transition by inhibiting glycogen synthase kinases (GSKs) and down-regulating E-cadherin in the NMuMG cell model, and the zebrafish embryo model in which ZIP10 forms a heteromer with ZIP6 to regulate the embryonic development (34–36). These data suggest that ZIP10 plays crucial roles in these cell lines and fish; however, the in vivo physiological relevance of ZIP10 in mammals is largely unknown. Our recent data using a mouse model reveal that ZIP10 facilitates antiapoptotic signaling during early B-cell development and controls the B-cell receptor signaling strength (37, 38). To investigate another role of ZIP10 in vivo, we assessed Zip10 expression with in situ hybridization using mouse whole-body sections, demonstrating that Zip10 was enriched in ORS of hair follicles (Fig. 1B). Considering these findings together with the observation that the most down-regulated genes in the ZIP10-KD cells belonged to the “epidermal development” group that reflects epidermis-specific events (Fig. S5B), we thus focused on the role of ZIP10 in epidermal development. In fact, ZIP10 was highly expressed in the hair follicle bulb and ORS cells (Fig. 1), peaked during epidermal formation, as did p63, and was induced again by shaving (Figs. 1 and 2F). The ZIP10 pattern of expression was correlated with that of MT1 (20, 21) (Fig. 1). The zinc-inducible protein MT1, which is observed in hair matrix cells of the bulb and in ORS cells (21) along with ZIP10 (Fig. 1), increases when stimulated with proliferation agents (20, 21), implying that zinc constitutes an important metal ion for the proliferation of epidermal progenitors. Notably, although ZIP1, ZIP6, and ZIP10 were expressed in epidermal cells and ZIP6 was also expressed in hair follicles (Fig. S1), neither Zip1-KO nor Zip6-KO mice exhibit apparent epidermal abnormalities (39) (Fig. S2). These findings indicate that ZIP10 is a key regulator of zinc homeostasis during epidermal development.
ZIP10 Regulates Epidermal Progenitor Cells.
We found that ZIP10+ cells highly express Lgr6 but less Gli1 (Fig. 2E), suggesting that ZIP10 may regulate the function of LGR6+ hair follicle stem cells. Hair follicles contain a heterogeneous pool of stem cells (25) that contributes critically and distinctly to the rebuilding of epidermal structures such as the interfollicular epidermis, hair follicles, and sebaceous glands after a skin injury (40). LGR6+ stem cells can generate all of these cell lineages, whereas the GLI1+ stem cells do not contribute to sebaceous glands (3, 25). Lgr6 expression appears at E14.5, peaks around postnatal day 7–15, and then gradually decreases (3), thus sharing the time course features of Zip10 (Fig. 1). Considering that Zip10K14 mice exhibit severe hypoplasia in stratified epithelia, diminished hair follicles numbers, and that epidermal loss by shaving markedly induces Zip10 expression in WT hair follicle cells (Figs. 2F and 3), ZIP10 activity may support the maintenance of hair follicle stem cells (Figs. 3 and 4).
Involvement of ZIP10 in p63 Transactivation.
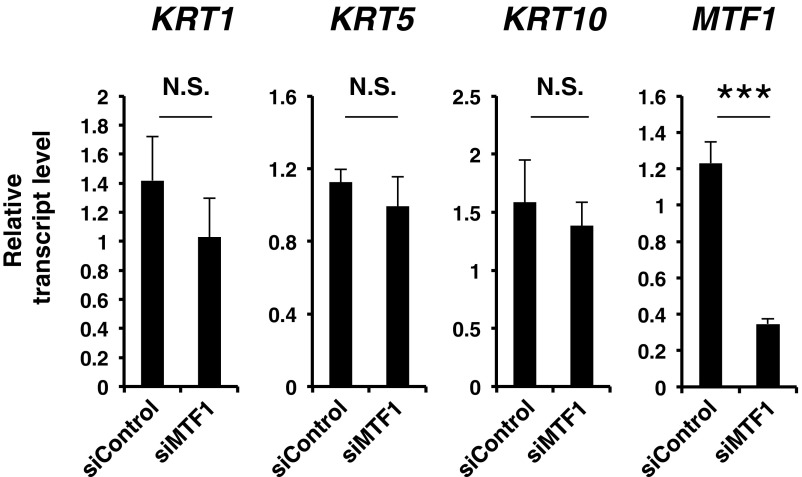
It is of particular interest that p63 requires ZIP10 for its transactivation (Fig. 4D). p63 is a master TF for epidermal development: Ablating p63 severely disturbs the development of stratified epithelia and epithelial appendages and is embryonic lethal (28, 41), suggesting that p63 is crucial for epithelial stem cells. Among p63 isotypes, TAp63 is expressed in early embryonic epidermal development to trigger the commitment to stratification (2, 42), whereas ΔNp63 is predominantly expressed during late epidermal development and is mainly expressed in basal cells in the adult epidermis to maintain the proliferative potential of epidermal progenitor cells (2). Given that Zip10K14 mice were born with apparent appendages (Fig. 3A) and comparable level of keratin 14 marking epidermal basal cells (Fig. 3E), which are not reported on p63-null mice (41), these results indicate that p63 is active in the early epidermal developmental stage of ∼E14. However, when stratification occurs, p63 expression is dramatically elevated along with the requirement of ZIP10 for directing zinc homeostasis toward p63, leading to efficient epidermal morphogenesis. The potential exists that MTF-1, a zinc-responsive TF (43), may direct the gene regulation for epidermis formation via ZIP10. However, MTF1 is comparably expressed during epidermal development (Fig. 4C), which is inconsistent with p63 and ZIP10 (Figs. 1C and 4C), and we could not find significant changes in keratin expression in MTF1-KD cells (Fig. S10), suggesting low probability of MTF-1 involvement in gene regulation for epidermis formation.
Fig. S10.
MTF1 depletion shows comparable keratin gene expression. Normal human keratinocytes were treated with siMTF1 for 72 h, and the mRNAs were isolated. The mRNA levels were quantified by real-time PCR. The data are representative of three independent experiments (***P < 0.001).
ZIP10–Zinc–p63 Axis for Epidermal Formation.
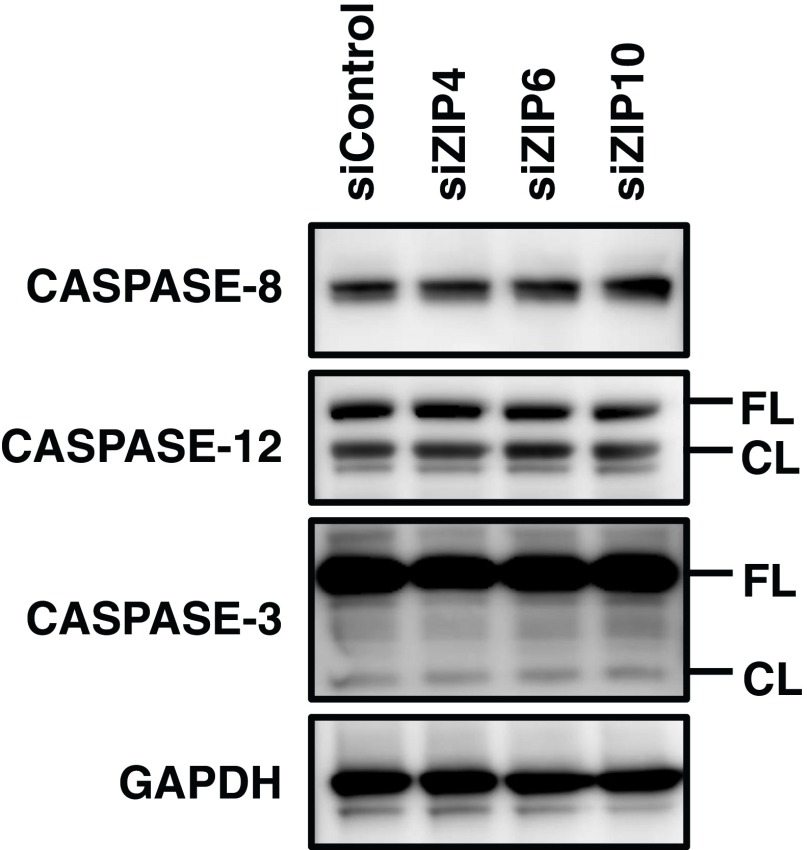
p63, which belongs to the p53 family, in fact possesses zinc-binding residues that are required for normal transactivation. A crystal structure analysis of p63 revealed many pathogenic mutations in its zinc-binding motif or in positions that could affect zinc binding (6). Zinc binding stabilizes p53 DBD architecture, whereas zinc chelation disrupts it, indicating that zinc is essential for folding the WT conformation for sequence-specific DNA binding (44). Therefore, it might also occur that zinc capturing in the equivalent domains facilitates p63 function. The loss of ZIP10 interferes with p63 functions in keratinocytes but does not activate Caspase-3, unlike its effect in B cells (37) (Fig. S11), indicating that the molecules influenced by ZIP10 may differ in each cell type. Our study thus yields insights into the relevance of ZIP10-mediated zinc signals in regard to p63 function, suggesting that further exploration of the role(s) of ZIP10, p63, and the intermediary molecules will help to understand the pathophysiology of the molecular basis of the epidermis, and of the severe dermatitis caused by zinc deficiency.
Fig. S11.
Zinc transporter depletion does not induce caspase activation. Normal human keratinocytes were treated with each siRNA for 72 h, and the proteins were isolated, after which the CASPASE proteins were analyzed by Western blotting. CL, cleaved; FL, full-length. GAPDH is shown as a loading control.
In conclusion, we established an essential role of ZIP10 in skin epidermal development and showed that ZIP10-mediated intracellular zinc homeostasis governs epidermal development, at least in part, by supporting p63 transactivation. Our findings provide an insight into the relevance of zinc homeostasis in dermatological processes. Further exploration of the questions that remain unanswered regarding the molecular mechanisms involving the ZIP10–zinc–p63 axis will help elucidate the critical role of zinc homeostasis in skin formation and regeneration.
Materials and Methods
Detailed descriptions of all of the materials and methods are provided in SI Materials and Methods. Differences between two groups were analyzed by Student’s two-tailed t test. All procedures including mouse handling were conducted according to guidelines approved by the RIKEN Institutional Animal Care and Use Committee (K24-007).
SI Materials and Methods
Mice.
Zip10flox mice were generated as previously described (37). K14-Cre mice were purchased from The Jackson Laboratory, and FoxN1-Cre mice were kindly donated by Georg Holländer, University of Oxford, Oxford. These mice were crossed with Zip10flox mice to generate conditional knockouts. Zip6-KO mice were generated as previously described (45). Briefly, a targeting vector was created to eliminate the genomic region encompassing exons 3–4 of Zip6 by inserting a neo-cassette into the region between exons 2 and 5 and deleting the intervening exons (Fig. S2A). This vector was introduced into R1 ES cells, and cloned homologous recombinants were selected with antibiotics and verified by genotyping. We developed chimeric mice with the targeted ES cell clones and interbred the offspring to obtain homozygous mice. The heterozygous mice were phenotypically normal, and crosses between heterozygotes produced homozygous mutant mice according to Mendelian expectations.
Cell Culture and Materials.
H1299, HeLa, and 293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) with 10% FBS and antibiotics, at 37 °C. A 3×FLAG-tagged ZIP10 was constructed as previously described (37, 38). Chin Ha Jung, Seoul National University, Seoul, Republic of Korea kindly donated pcDNA4-HisMax vectors expressing TAp63α or ΔNp63α. TPEN (Sigma) and Doxorubicin (Sigma) were dissolved in dimethyl sulfoxide and stored at −80 °C before use.
Immunohistochemistry and in Situ Hybridization.
Immunostaining was performed as previously described (14, 46, 47). Briefly, mouse embryos and whole skin were fixed in 4% paraformaldehyde overnight at 4 °C. Paraffin sections were stained with H&E or incubated with primary antibodies to ZIP10 (37), E-cadherin (1:400; Takara), or p63 (1:100; Chemicon). Primary antibodies were detected using Alexa Fluor 488 donkey anti-mouse IgG (IgG; H+L) (1:500; Life Technologies), Alexa Fluor 488 goat anti-rat IgG (H+L) (1:500; Life Technologies), Alexa Fluor 488 donkey anti-rabbit IgG (H+L) (1:500; Life Technologies), Alexa Fluor 594 donkey anti-mouse IgG (H+L) (1:500; Life Technologies), Alexa Fluor 594 donkey anti-rabbit IgG (1:500; Invitrogen), or Alexa Fluor 594 goat anti-rabbit IgG (H+L) (1:500; Life Technologies) for 1 h at room temperature. TUNEL assays were performed according to the manufacturer’s instructions (Roche). In situ hybridization was carried out using Genostaff methods, and probes are available from Genostaff (Tokyo): mouse Slc39a4/Zip4 (MP-S-009), mouse Slc39a5/Zip5 (MP-S-026), mouse Slc39a6/Zip6 (MP-S-010), mouse Slc39a10/Zip10 (MP-S-012), mouse Slc39a12/Zip12 (MP-S-027), mouse Keratin 10 (MP-K-005), mouse Keratin 14 (MP-K-006), mouse Metallothionein 1 (MP-M-004), mouse Filaggrin (MP-F-016). All fluorescence microscopy images were obtained with an LSM 700 or LSM 780 confocal microscope (Carl Zeiss) or an Axio Scan.Z1 (Carl Zeiss).
Western Blots and Fluorescence Microscopy.
Cells were collected in 1% Nonidet P-40 containing 0.05 M Tris⋅HCl (pH 7.5), 0.15 M NaCl, and 0.01 M MgCl2, and centrifuged at 15,000 × g for 5 min. The supernatant was boiled for 5 min in SDS-polyacrylamide gel electrophoresis (SDS/PAGE) sample buffer containing 0.125 M Tris⋅HCl (pH 6.8), 20% glycerol, 4% SDS, 10% 2-mercaptoethanol, and 0.004% bromophenol blue, and was loaded onto a 5–20% gradient gel. For fluorescence microscopy, cells were cultured on Lab-Tek chamber slides (Nunc), fixed with 4% paraformaldehyde in PBS, permeabilized with 0.1% Triton X-100 in PBS containing 1% BSA for 5 min, and incubated with each antibody. Fluorescence was detected after secondary staining with the Alexa Fluor 488-conjugated F(ab′)2 fragment of goat anti-mouse IgG (Invitrogen) and the Alexa Fluor 594-conjugated F(ab′)2 fragment of goat anti-rabbit IgG (Invitrogen).
Quantitative Real-Time PCR.
cDNA was synthesized using ReverTra Ace (Toyobo). We used the TaqMan Gene Expression Assay (Applied Biosystems) following the manufacturer’s instructions to determine the mRNA levels of Trp53 (Mm01731290_g1), Trp63 (Mm00495793_m1), Zip10 (Hs00393794_m1, Mm00554174_m1), Sp1 (Mm00489039_m1), Esr1 (Mm00433149_m1), Creb1 (Mm00501607_m1), Ar (Mm00442688_m1), and MTF1 (Hs00232306_m1).
Isolation of ZIP10-Expressing Cells from Skin.
The ventral skin excised from 1-wk-old C57BL/6 mice was minced with a scalpel and incubated overnight with Dispase II (2.4 U/mL; Roche), Collagenase (50 U/mL; Worthington Biochemical Corporation), and 10% FBS in DMEM (Invitrogen) at 4 °C. Next, the tissues were pipetted vigorously to dissociate the cells, which were filtered through a 40-μm cell strainer (Greiner Bio-One) and incubated with anti-ZIP10 polyclonal antibody or normal rabbit IgG for 30 min on ice, and then with Alexa Fluor 488-conjugated anti-rabbit IgG for 15 min on ice. The cells were also stained with 7-AAD (BD), and ZIP10+ and ZIP10− cells were sorted using a FACSAria II (BD Biosciences).
DNA Pull-Down Assay.
DNA pull-down assay was performed as previously described (48). Briefly, HEK293 cells, transfected with either p63 or p63 mutant cDNA plasmids for 48 h, were lysed. Either the cell lysates (10 g of protein) or the recombinant GST-tagged p63 protein (0.6 g) (Sigma) in 200 μL of NETN buffer were incubated with biotinylated DNA probe of p63 binding sequence (200 pmol) and 20 μL of streptavidin-agarose beads. The protein–DNA complex was subjected to SDS/PAGE followed by immunoblotting with an anti-p63 antibody (Santa Cruz). The following biotinylated, double-stranded p63 DNA probes were used: biotin-5′-TTGAAACATGTCTGGACATGTCCTGGA-3′ as sense; and biotin-5′-TCCAGGACATGTCCAGACATGTTTCAA-3′ as antisense.
mRNA-Sequencing Experiment.
We obtained total RNAs from ZIP10+ and ZIP10− cells. The RNA-sequencing library was constructed from the total RNA (10 ng) using a NEBNext Ultra RNA Library Prep Kit for Illumina (New England Biolabs). The prepared libraries were sequenced using an Illumina HiSeq 1500 in 50-base single-end mode. The GEO accession number for the RNA sequence is GSE83099.
Analysis of mRNA-Sequencing Data.
We removed the adapter sequences (TrueSeq universal and index adapters) and then trimmed the ends of the adapter-free reads for which PHRED scores were lower than 20 using Cutadapt software (49). The remaining reads were then aligned to the mouse reference genome (UCSC mm9) using the TopHat aligner (50). After aligning, we quantified the gene expression using Cufflinks (51) and further normalized the expression values [reads per kilobase of transcript per million mapped reads (RPKM)] using quantile normalization for more robust between-sample comparisons. The genes with an RPKM ≥ 1 for at least one of two samples (ZIP10+ and ZIP10− cells) were defined as “expressed genes” and were used for downstream analysis. Among the protein-coding genes expressed, those with a change greater than twofold were identified as differentially expressed genes (DEGs).
Gene Expression Microarray Experiment.
Duplicate experiments were performed for WT and ZIP10-KD and also for TPEN-untreated and TPEN-treated human keratinocytes. Total RNA was isolated from tissues using Qiazol (Qiagen) following the manufacturers’ protocols. Reverse transcription reactions were performed using a High-Capacity cDNA synthesis kit (ABI). Quantitative RT-PCR was performed with SYBR green fluorescent dye using an ABI 7500 Fast Real-Time PCR system. Relative mRNA expression was determined by relative standard curve methods using 18S as an internal control to normalize samples. The samples were then hybridized onto the array (Agilent SurePrint G3 Human GE v2 8×60K). The gene expression dataset was deposited at the Gene Expression Omnibus database (accession nos. GSE83098 and GSE99204).
Analysis of the Gene Expression Microarray.
We used gmedian signals as raw intensities for downstream analysis. We processed the raw intensities as previously described (52). In brief, we first normalized log2 intensities of all probes by quantile normalization and determined whether each probe was expressed or not using a Gaussian mixture modeling. The expressed probes were used for downstream analysis to obtain the DEGs. We identified the DEGs in the comparison of WT and ZIP10-KD cells, and also TPEN-untreated and TPEN-treated cells using the previously reported integrative statistical hypothesis testing method computing adjusted P value by combining t test and the median ratio test (53). The genes with adjusted P value smaller than 0.05 and the log2-median ratios larger than 95% of null distribution were selected as DEGs.
Functional Enrichment Analysis.
To identify biological processes and pathways represented by the DEGs, we performed functional enrichment analysis using the ConsensusPathDB and DAVID bioinformatics software (54). The terms with value of q < 0.01 for ConsensusPathDB and the terms with value of P < 0.05 for DAVID were defined as representative biological processes or pathways.
Transcription Factor Analysis.
To identify the relevant transcription factors (TFs) targeting the significant number of genes among the focused sets of DEGs, we used MetaCore software from Genego (https://portal.genego.com/). The TFs with false discovery rate (FDR) <0.05 and the number of targets >2 were selected as significant TFs.
Luciferase Assay.
A reporter plasmid that includes the regulatory region of PG13 upstream of the luciferase genes was kindly donated by Chin Ha Jung. H1299 cells were seeded in 12-well plates and transfected with the appropriate plasmids along with a β-galactosidase–expressing plasmid for normalizing the transfection efficiency. Luciferase assays were performed as instructed by the manufacturer (Promega).
Supplementary Material
Acknowledgments
We are grateful for support from Drs. Toshio Hirano, Kazuo Koyasu, Masaru Taniguchi, and Kou Kaneko. This work was supported by KAKENHI (26462324 and 17H04011) of the Ministry of Education, Culture, Sports, Science and Technology; the Sumitomo Foundation; the Naito Foundation; the Nestlé Nutrition Council Japan; the Mitsubishi Foundation; the Vehicle Racing Commemorative Foundation; and the Takeda Science Foundation (to T.F.). D.H. was supported by the Institute for Basic Science (IBS-R013-G1) from the National Research Foundation funded by the Korean government (Ministry of Science, Information and Communications Technology, and Future Planning).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo [mRNA sequencing data (accession no. GSE83099) and gene expression microarray data (accession nos. GSE83098 and GSE99204)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710726114/-/DCSupplemental.
References
- 1.Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- 2.Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snippert HJ, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 4.Botchkarev VA, Gdula MR, Mardaryev AN, Sharov AA, Fessing MY. Epigenetic regulation of gene expression in keratinocytes. J Invest Dermatol. 2012;132:2505–2521. doi: 10.1038/jid.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai X, Segre JA. Transcriptional control of epidermal specification and differentiation. Curr Opin Genet Dev. 2004;14:485–491. doi: 10.1016/j.gde.2004.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Gorlatova N, Kelman Z, Herzberg O. Structures of p63 DNA binding domain in complexes with half-site and with spacer-containing full response elements. Proc Natl Acad Sci USA. 2011;108:6456–6461. doi: 10.1073/pnas.1013657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tichý V, Navrátilová L, Adámik M, Fojta M, Brázdová M. Redox state of p63 and p73 core domains regulates sequence-specific DNA binding. Biochem Biophys Res Commun. 2013;433:445–449. doi: 10.1016/j.bbrc.2013.02.097. [DOI] [PubMed] [Google Scholar]
- 8.Fukada T, Kambe T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics. 2011;3:662–674. doi: 10.1039/c1mt00011j. [DOI] [PubMed] [Google Scholar]
- 9.Kambe T, Hashimoto A, Fujimoto S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol Life Sci. 2014;71:3281–3295. doi: 10.1007/s00018-014-1617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hara T, et al. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J Physiol Sci. 2017;67:283–301. doi: 10.1007/s12576-017-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bin BH, et al. Molecular pathogenesis of spondylocheirodysplastic Ehlers-Danlos syndrome caused by mutant ZIP13 proteins. EMBO Mol Med. 2014;6:1028–1042. doi: 10.15252/emmm.201303809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bin BH, et al. Biochemical characterization of human ZIP13 protein: A homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers-Danlos syndrome. J Biol Chem. 2011;286:40255–40265. doi: 10.1074/jbc.M111.256784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bin BH, Hojyo S, Ryong Lee T, Fukada T. Spondylocheirodysplastic Ehlers-Danlos syndrome (SCD-EDS) and the mutant zinc transporter ZIP13. Rare Dis. 2014;2:e974982. doi: 10.4161/21675511.2014.974982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukada T, et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS One. 2008;3:e3642. doi: 10.1371/journal.pone.0003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Küry S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 16.Bin BH, et al. An acrodermatitis enteropathica-associated Zn transporter, ZIP4, regulates human epidermal homeostasis. J Invest Dermatol. 2017;137:874–883. doi: 10.1016/j.jid.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 17.Kambe T, Andrews GK. Novel proteolytic processing of the ectodomain of the zinc transporter ZIP4 (SLC39A4) during zinc deficiency is inhibited by acrodermatitis enteropathica mutations. Mol Cell Biol. 2009;29:129–139. doi: 10.1128/MCB.00963-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bin BH, et al. Requirement of zinc transporter SLC39A7/ZIP7 for dermal development to fine-tune endoplasmic reticulum function by regulating protein disulfide isomerase. J Invest Dermatol. 2017;137:1682–1691. doi: 10.1016/j.jid.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Hanada K, Sawamura D, Hashimoto I, Kida K, Naganuma A. Epidermal proliferation of the skin in metallothionein-null mice. J Invest Dermatol. 1998;110:259–262. doi: 10.1046/j.1523-1747.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- 21.Karasawa M, et al. Localization of metallothionein in hair follicles of normal skin and the basal cell layer of hyperplastic epidermis: Possible association with cell proliferation. J Invest Dermatol. 1991;97:97–100. doi: 10.1111/1523-1747.ep12478393. [DOI] [PubMed] [Google Scholar]
- 22.Dufner-Beattie J, et al. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol Chem. 2003;278:33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- 23.Koster MI, Roop DR. The role of p63 in development and differentiation of the epidermis. J Dermatol Sci. 2004;34:3–9. doi: 10.1016/j.jdermsci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 24.McDade SS, et al. Genome-wide analysis of p63 binding sites identifies AP-2 factors as co-regulators of epidermal differentiation. Nucleic Acids Res. 2012;40:7190–7206. doi: 10.1093/nar/gks389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solanas G, Benitah SA. Regenerating the skin: A task for the heterogeneous stem cell pool and surrounding niche. Nat Rev Mol Cell Biol. 2013;14:737–748. doi: 10.1038/nrm3675. [DOI] [PubMed] [Google Scholar]
- 26.Ghadially R. 25 years of epidermal stem cell research. J Invest Dermatol. 2012;132:797–810. doi: 10.1038/jid.2011.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elias PM. Stratum corneum defensive functions: An integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 28.Yang A, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 29.Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006;20:3185–3197. doi: 10.1101/gad.1463206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senoo M, Pinto F, Crum CP, McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 31.Su X, et al. TAp63 prevents premature aging by promoting adult stem cell maintenance. Cell Stem Cell. 2009;5:64–75. doi: 10.1016/j.stem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayan BS, et al. Cleavage of the transactivation-inhibitory domain of p63 by caspases enhances apoptosis. Proc Natl Acad Sci USA. 2007;104:10871–10876. doi: 10.1073/pnas.0700761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007;98:692–697. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brethour D, et al. A ZIP6-ZIP10 heteromer controls NCAM1 phosphorylation and integration into focal adhesion complexes during epithelial-to-mesenchymal transition. Sci Rep. 2017;7:40313. doi: 10.1038/srep40313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong BY, et al. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol Hum Reprod. 2014;20:1077–1089. doi: 10.1093/molehr/gau066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor KM, et al. Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem J. 2016;473:2531–2544. doi: 10.1042/BCJ20160388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyai T, et al. Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc Natl Acad Sci USA. 2014;111:11780–11785. doi: 10.1073/pnas.1323549111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hojyo S, et al. Zinc transporter SLC39A10/ZIP10 controls humoral immunity by modulating B-cell receptor signal strength. Proc Natl Acad Sci USA. 2014;111:11786–11791. doi: 10.1073/pnas.1323557111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK. Mouse ZIP1 and ZIP3 genes together are essential for adaptation to dietary zinc deficiency during pregnancy. Genesis. 2006;44:239–251. doi: 10.1002/dvg.20211. [DOI] [PubMed] [Google Scholar]
- 40.Blanpain C, Simons BD. Unravelling stem cell dynamics by lineage tracing. Nat Rev Mol Cell Biol. 2013;14:489–502. doi: 10.1038/nrm3625. [DOI] [PubMed] [Google Scholar]
- 41.Mills AA, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 42.Koster MI, Kim S, Huang J, Williams T, Roop DR. TAp63alpha induces AP-2gamma as an early event in epidermal morphogenesis. Dev Biol. 2006;289:253–261. doi: 10.1016/j.ydbio.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 43.Andrews GK. Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals. 2001;14:223–237. doi: 10.1023/a:1012932712483. [DOI] [PubMed] [Google Scholar]
- 44.Méplan C, Richard MJ, Hainaut P. Metalloregulation of the tumor suppressor protein p53: Zinc mediates the renaturation of p53 after exposure to metal chelators in vitro and in intact cells. Oncogene. 2000;19:5227–5236. doi: 10.1038/sj.onc.1203907. [DOI] [PubMed] [Google Scholar]
- 45.Sedivy JM, Joyner AL. Gene Targeting. W. H. Freeman and Company; New York: 1992. pp. 1–146. [Google Scholar]
- 46.Bin BH, et al. Fibronectin-containing extracellular vesicles protect melanocytes against ultraviolet radiation-induced cytotoxicity. J Invest Dermatol. 2016;136:957–966. doi: 10.1016/j.jid.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Toyoshima KE, et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun. 2012;3:784. doi: 10.1038/ncomms1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukada T, Tonks NK. Identification of YB-1 as a regulator of PTP1B expression: Implications for regulation of insulin and cytokine signaling. EMBO J. 2003;22:479–493. doi: 10.1093/emboj/cdg067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–12. [Google Scholar]
- 50.Trapnell C, Pachter L, Salzberg SL. TopHat: Discovering splice junctions with RNA-seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trapnell C, et al. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong HS, et al. Transcriptional regulatory networks underlying the reprogramming of spermatogonial stem cells to multipotent stem cells. Exp Mol Med. 2017;49:e315. doi: 10.1038/emm.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang D, et al. A data integration methodology for systems biology: Experimental verification. Proc Natl Acad Sci USA. 2005;102:17302–17307. doi: 10.1073/pnas.0508649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 55.Kitamura H, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.