Significance
Zika virus (ZIKV) infection has been associated with multiple pathologies of the central nervous system (CNS) including microcephaly, Guillain-Barré syndrome, lissencephaly, the loss of white and gray matter volume and acute myelitis. Using organotypic brain slice cultures, we determined that ZIKV replicates across different embryonic developmental stages, and viral infection can disrupt proper brain development leading to congenital CNS complications. These data illustrate that all lineages of ZIKV tested are neurotropic, and that infection may disrupt neuronal migration during brain development. The results expand our understanding of neuropathologies associated with congenital Zika virus syndrome.
Keywords: flavivirus, Zika virus, neurotropism, microcephaly, cortical malformations
Abstract
Fetal infection with Zika virus (ZIKV) can lead to congenital Zika virus syndrome (cZVS), which includes cortical malformations and microcephaly. The aspects of cortical development that are affected during virus infection are unknown. Using organotypic brain slice cultures generated from embryonic mice of various ages, sites of ZIKV replication including the neocortical proliferative zone and radial columns, as well as the developing midbrain, were identified. The infected radial units are surrounded by uninfected cells undergoing apoptosis, suggesting that programmed cell death may limit viral dissemination in the brain and may constrain virus-associated injury. Therefore, a critical aspect of ZIKV-induced neuropathology may be defined by death of uninfected cells. All ZIKV isolates assayed replicated efficiently in early and midgestation cultures, and two isolates examined replicated in late-gestation tissue. Alteration of neocortical cytoarchitecture, such as disruption of the highly elongated basal processes of the radial glial progenitor cells and impairment of postmitotic neuronal migration, were also observed. These data suggest that all lineages of ZIKV tested are neurotropic, and that ZIKV infection interferes with multiple aspects of neurodevelopment that contribute to the complexity of cZVS.
Microcephaly is a neurodevelopmental disorder that is clinically characterized by dramatic physical reduction of head circumference (1–3). A variety of etiologies can lead to microcephaly, including vertical transmission of the TORCH microbes: Toxoplasma gondii, rubella, cytomegalovirus, herpes simplex virus, or other pathogens, such as coxsackievirus, varicella zoster virus, HIV, human T-lymphotropic virus, or Treponema. Infection with the arbovirus Zika virus (ZIKV) has been associated with the development of congenital cortical malformations, including microcephaly (4), defining the virus as a new TORCH agent (5).
The reduced head circumference seen with microcephaly is a consequence of reduced brain volume, also manifested in a thinner neocortex. A healthy mature cerebral cortex is composed of six distinct layers of neurons. Most cells within these layers arise from a single layer of apical–basal polarized cells that line the cerebral ventricle (6). This single layer of cells undergoes multiple rounds of symmetric mitotic divisions, which largely define the ultimate size of the brain. These cells increasingly undergo asymmetric divisions at which point the cells are referred to as radial glial progenitors (RGPs) (7). The asymmetric divisions create two postmitotic daughter cells, with one migrating basally out from the proliferative ventricular zone (VZ) toward the developing cortical plate (CP) of the neocortex. The other cell remains as an RGP attached at the ventricular surface, and the collective progeny of this RGP defines a “radial column.” A signature feature of the RGP is its elongated basal fiber that links the VZ to the opposing pial surface. Migration of the postmitotic cells into the cortical plate of the developing neocortex occurs along the RGP process (8). The late stages of neocortical development establish the gyri, sulci, and cytoarchitecture of the mature brain. Mutations in genes critical for these processes all may interfere with the proper number of neurons and layers, resulting in a thinner neocortex, consistent with autosomal recessive microcephaly (reviewed in ref. 9).
Zika virus, a (+) RNA arbovirus in the Flaviviridae family, is a reemerging human pathogen. Originally isolated from a febrile sentinel macaque in 1947, the first reported human infections occurred in Nigeria during the early 1950s (10, 11). Initially it was thought that Zika virus infection did not result in clinical disease, but was experimentally neurotropic in mice (10, 11). If symptoms of viral infection were observed in humans, they were mild, including rash, joint pain, and fever (12). During the 2007 and 2013 outbreaks in isolated Pacific islands, adult ZIKV infection was associated with neurological dysfunction such as Guillain-Barré syndrome, a disorder in which the immune system attacks the peripheral nervous system, and other central nervous system (CNS) complications (13). Once ZIKV spread to the Western Hemisphere, it was recognized to be responsible for the increase of children born with microcephaly and cortical malformations in Brazil and Colombia (14). By late 2016, ZIKV infection was known to cause many neurological abnormalities outside of microcephaly, including lissencephaly, loss of brain volume, pachygria, and ventriculomegaly (15), which all comprise congenital Zika virus syndrome (cZVS) (4).
Several animal models of ZIKV infection, including mice and nonhuman primates, have recently been established (16). These models illustrate many features of ZIKV-induced pathology, including viremia and neuronal tissue tropism. The inability to genetically manipulate nonhuman primates restricts their use for understanding cZVS. Organoid culture models provide limited information about virus infection of target cells within the context of the developing tissue and organ, since some cell types are missing and there is little to no immune system or vascular representation. Furthermore, many organoid cultures used to understand ZIKV biology only develop neural identity, not tissue organization (17, 18). Organoid cultures do not allow for the study of neuropathologies in the late developing brain, are difficult to consistently manufacture, and are characterized by immature neuronal connectivity; therefore, they are far more simplistic than the brain (19).
Organotypic brain slice cultures are widely used to understand neuronal connectivity and neurodegenerative disorders (20–22) and have enriched our understanding of autosomal recessive microcephaly and brain development (23, 24). Furthermore, organotypic brain slice cultures from embryonic mice are a genetically amenable system to study cZVS and the ability of the virus to infect neural cells (neurotropism), as the virus inoculum is placed directly on target cells of the cultures. This system fully separates neurotropism from neuroinvasion, virus entry into the CNS from distant sites of the body. Consequently, observations made in organotypic brain slice cultures generated from embryonic mice expand our understanding of viral infection beyond established animal models and organoid cultures.
In this study the effects of ZIKV infection on the embryonic mouse brain were determined using organotypic brain slice cultures. All ZIKV isolates tested from 1947 to the present replicated in early and midgestation embryonic brain tissue [embryonic days 13 (E13) and 15 (E15), respectively], while two isolates replicated in brain slice cultures from E19 embryos. Virus replication led to increased apoptosis within the cultures and impaired neuronal migration into the cortical plate. The results suggest that infection with different isolates of ZIKV can cause severe brain developmental abnormalities. These data begin to illuminate how ZIKV-associated neuropathologies develop.
Results
Zika Virus Neurovirulence Is Independent of Genetic Lineage.
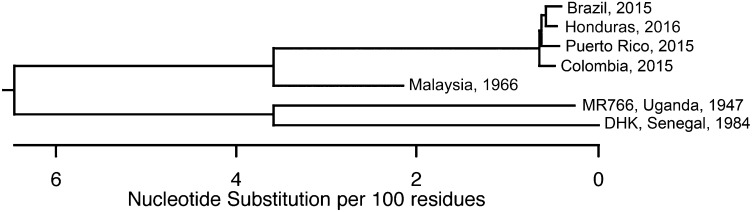
It has been suggested that neurovirulence was acquired as ZIKV spread from Africa through Asia and the Pacific to South America (25). Analysis of the genome sequences of isolates from 1947 to the present defines two phylogenetic groups (Fig. 1). Neurologic disease has been associated with the Asian lineage of viruses (25). To determine if neurotropism is a recently acquired property, replication of virus isolates from both phylogenetic groups was assayed in organotypic brain slice cultures from E15 mouse embryos. Neurogenesis at this stage corresponds to weeks 13/14 in human gestation, the beginning of the second trimester when there is maximal migration of the postmitotic neurons into the cortical plate (26, 27). Indirect immunofluorescence (IF) microscopy of brain slice cultures 3 d postinfection (dpi) with the 2015 Puerto Rican ZIKV isolate revealed virus-specific antigen [ZIKV-envelope (ZIKV-E)] throughout the developing neocortex and midbrain (Fig. 2A). ZIKV-E was visualized in cells that line the ventricular surface of the developing brain, with staining extending throughout the radial columns of RGP progenitor cells and their neuronal progeny (Fig. 2B). Although the slices were uniformly exposed to ZIKV, the ZIKV-E signal demonstrated focal areas of infection. This observation aligns with previous reports that human and mouse neuronal stem cells are a site of ZIKV infection (28, 29). Furthermore, viral antigen was detected throughout the cortical plate to the pial surface in brain slices infected with a Malaysian isolate from 1996 (Fig. S1).
Fig. 1.
Phylogenetic tree of ZIKV isolates used in this work. Entire genome sequences of seven ZIKV isolates examined in this study were aligned using the ClustalW module of DNAstar. Results reveal the evolutionary relationship between viral isolates.
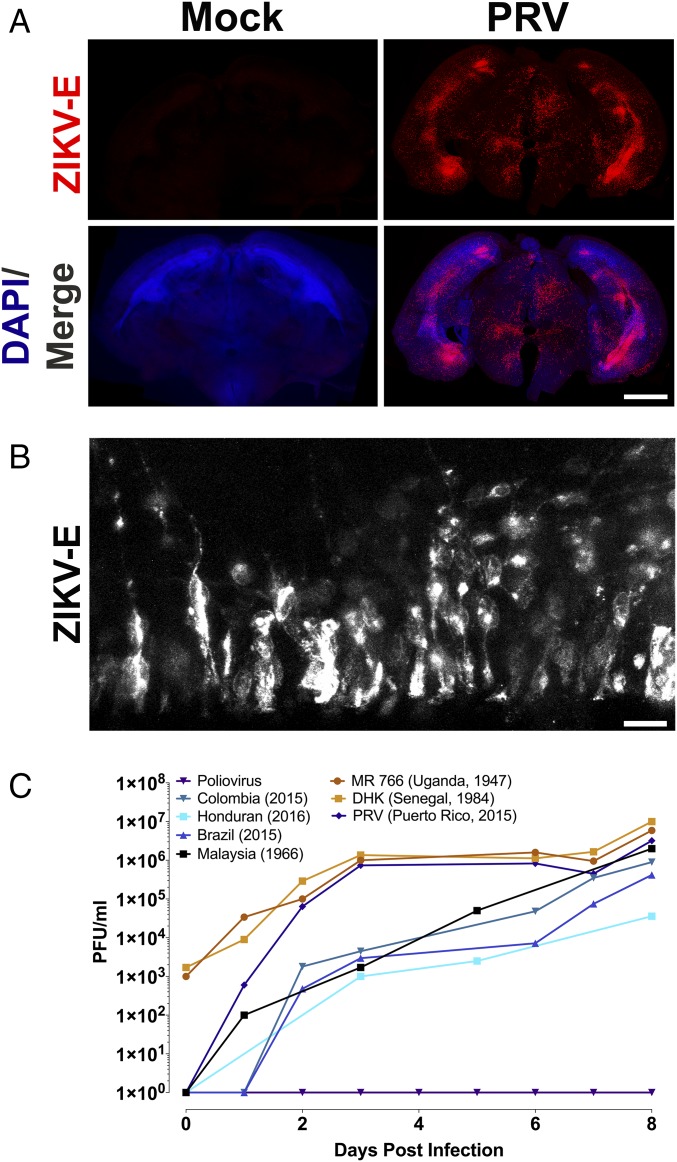
Fig. 2.
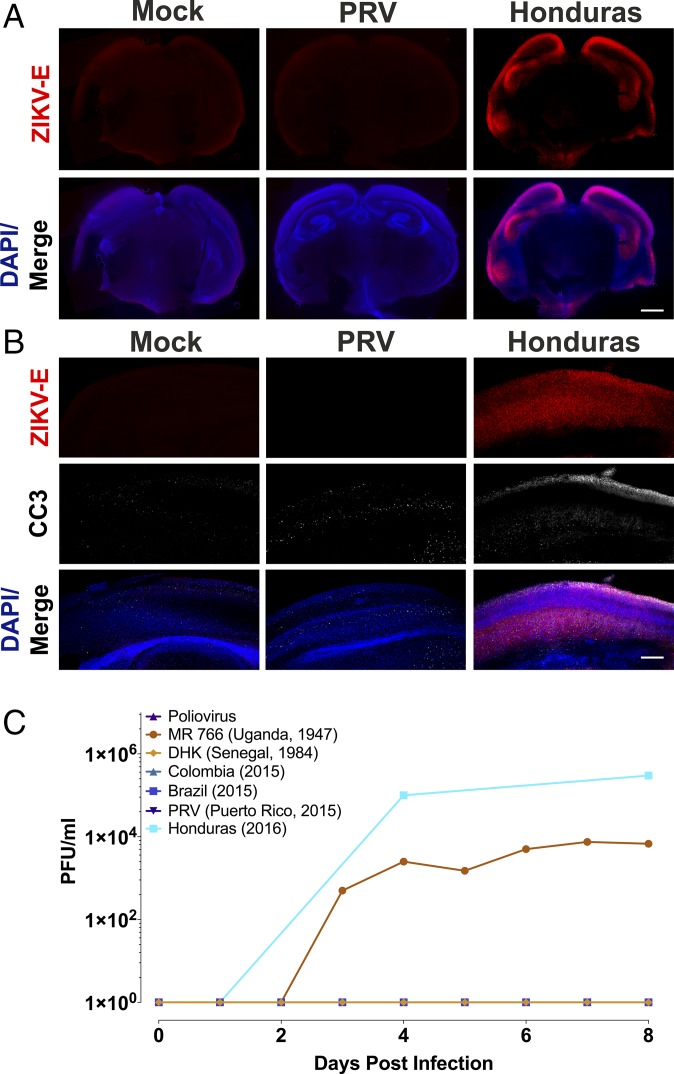
Representative tissue distribution and replication of ZIKV isolates in organotypic mouse brain slices. (A) Brain slice cultures from E15 embryos were infected with 105 pfu of the ZIKV PRV isolate or uninfected and at 3 dpi, were fixed and stained with pan-flavivirus antibody against the E glycoprotein (ZIKV-E) and DAPI. ZIKV-E signal demonstrated ZIKV infection in focal areas, including the developing neocortex and midbrain. (Scale bar: 500 μm.) (B) Higher magnification (60× objective) of the ventricular surface of infected brain slice culture, stained with pan-flavivirus ZIKV-E antibody. The location of these cells along the ventricle in the ventricular zone, and their morphology with apical and basal processes all support that these ZIKV-E positive cells are radial glia. (Scale bar: 10 μm.) (C) Time course of replication of multiple isolates of ZIKV in E15 brain slice cultures. Results are representative of three independent experiments.
To determine if infection of E15 brain slice cultures was limited to isolates from Puerto Rico and Malaysia, slices were infected with 105 pfu of five additional ZIKV isolates from 1947 to the present: African (MR766), Senegalese, Brazilian, Colombian, and Honduran (Fig. 2C). At different times after infection, culture supernatants were harvested and virus production was determined by plaque assay. By 8 dpi, infectious virus was detected in cultures infected with each of the isolates (Fig. 2C). Poliovirus was used as a negative control, as the related flavivirus dengue was previously shown not to replicate in embryonic brain slices (30). Poliovirus did not replicate in brain slice cultures, as the mice used do not produce the poliovirus receptor, CD155 (Fig. 2C).
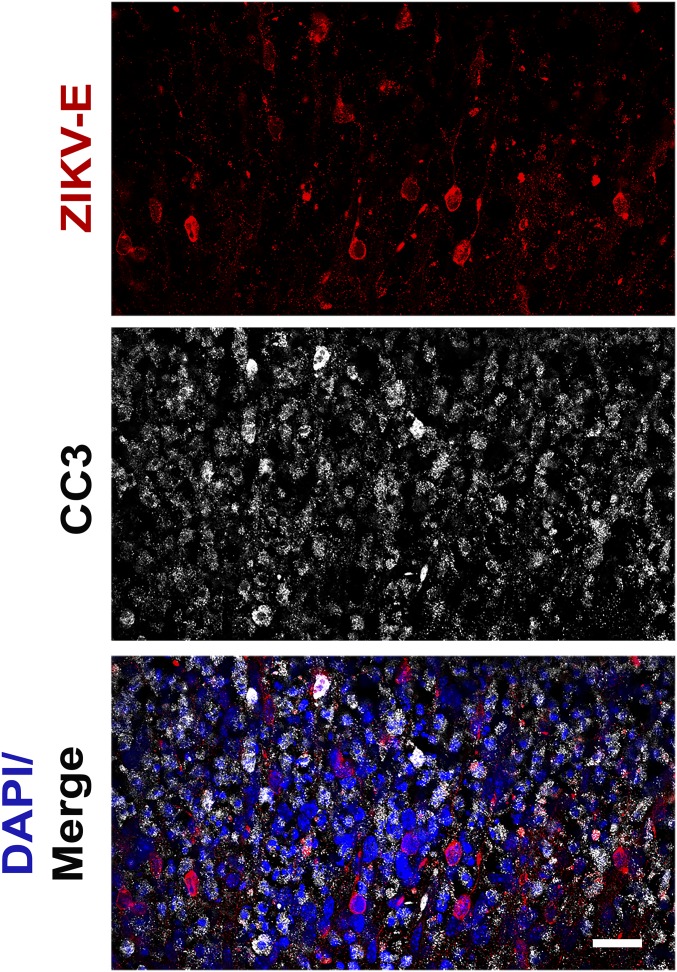
In previous reports ZIKV infection of human neural progenitor cells produced from induced pluripotent stem cells revealed increased apoptotic cell death (29). Therefore, IF analysis was used to detect cleaved caspase 3 in the infected brain slice cultures. There was a significant increase in apoptosis observed in organotypic slice cultures infected with each ZIKV isolate compared with uninfected cultures (Fig. S2). Induction of caspase 3 cleavage occurred mostly within the cells that surround the virus-infected cell (Fig. 3), which has also been suggested by the results of other studies (30, 31). These regions of apoptosis were confined to areas marked by ZIKV-E staining, as seen in the neocortex of an organotypic slice infected with the Malaysian isolate (Fig. S1).
Fig. 3.
ZIKV infection of organotypic mouse brain slices leads to increased apoptosis. Brain slice cultures from E15 embryos were infected with 105 pfu of the indicated ZIKV isolates or poliovirus and at 8 dpi, were fixed and stained with pan-flavivirus antibody against the E glycoprotein (ZIKV-E), antibody to cleaved caspase 3 (CC3), and DAPI. Examination of PRV ZIKV-infected slices reveals that the vast majority (91.13%, 0.132 SD) of ZIKV-E positive cells are negative for CC3. (Scale bar: 10 μm.)
These findings demonstrate that ZIKV infects the embryonic mouse brain during midgestation, that the virus is neurotropic independent of its genetic lineage (African or Asian), and that infection leads to apoptosis. It is not possible to correlate the growth capacity of different ZIKV isolates with induction of apoptosis without knowledge of the cell number in each slice culture. The results also establish organotypic embryonic brain slice cultures as a useful system to investigate ZIKV-induced brain malformations.
Developmental Restraints of Zika Virus Infection Revealed with Embryonic Organotypic Brain Slice Cultures.
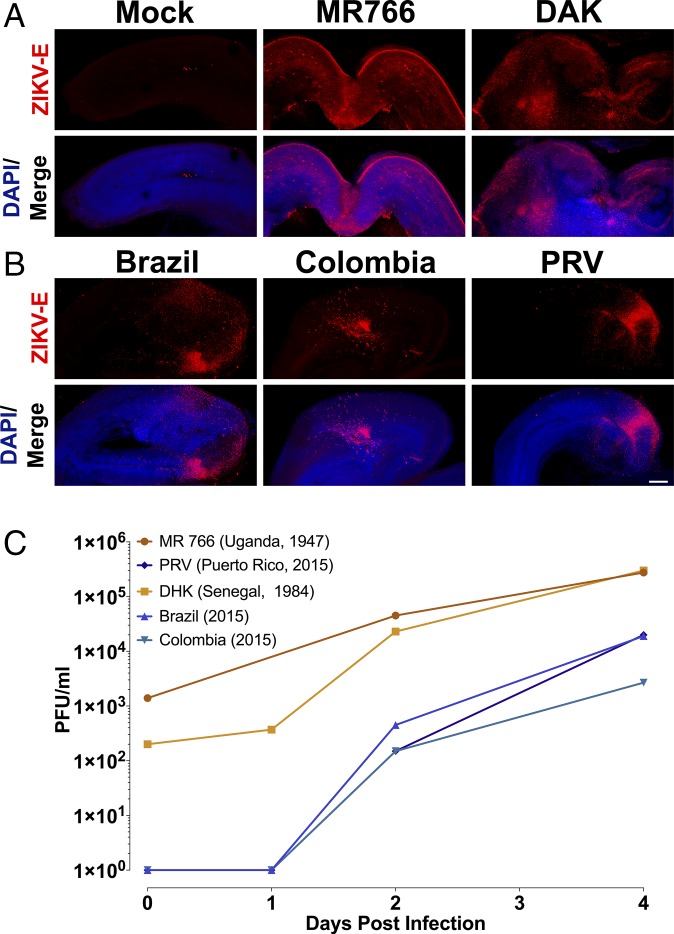
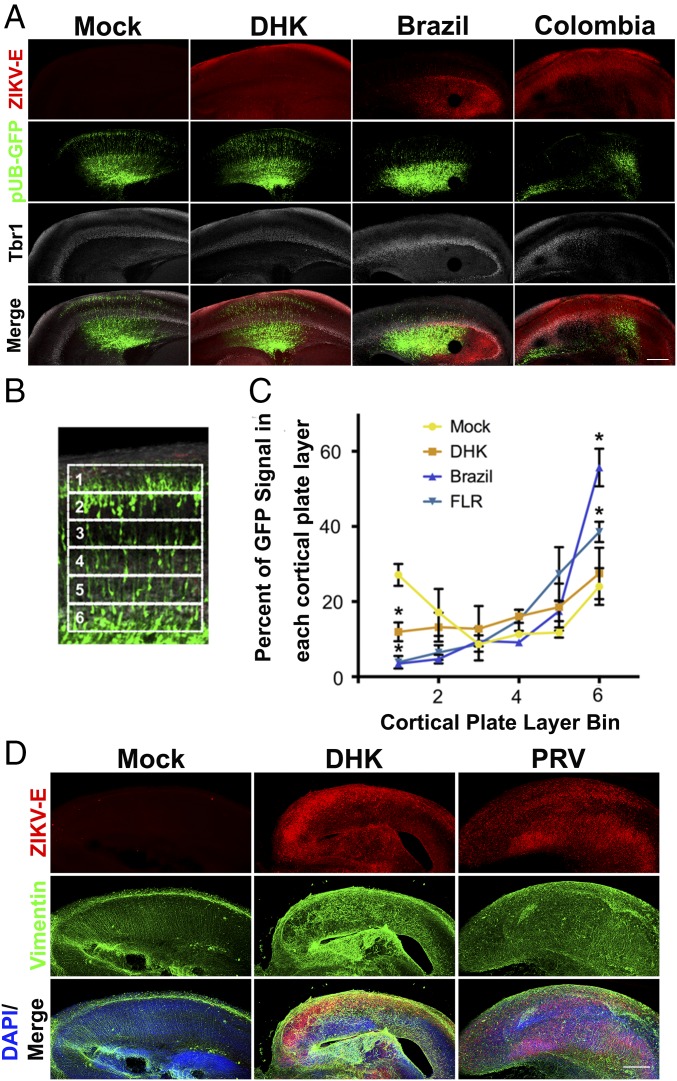
The results of epidemiological studies initially suggested that the greatest risks for developing cZVS are associated with maternal ZIKV infection during the first and second trimester (32, 33). Neurological dysfunction has been associated with virus infection during the third trimester, or shortly after birth, albeit less frequently (34–38). To test whether ZIKV neurotropism is regulated by gestational age, organotypic brain slice cultures were generated from embryonic mice at a range of developmental stages. Brain slices from E13 embryos, which are developmentally comparable to the middle of the first trimester of human neurogenesis, supported viral replication of all five ZIKV isolates examined, as determined by expression of a virus-specific antigen within focal areas of the neocortex (Fig. 4 A and B) and by virus production (Fig. 4C).
Fig. 4.
ZIKV replicates in early gestation neural tissue. (A and B) Brain slice cultures from E13 embryos were infected with 105 pfu of the indicated ZIKV isolates and at 8 dpi, were fixed and stained with pan-flavivirus antibody against the E glycoprotein (ZIKV-E) and DAPI. All ZIKV isolates tested infected E13 brain slices. (Scale bar: 250 μm.) (C) Time course of replication of multiple isolates of ZIKV in E13 brain slice cultures. Results are representative of three independent experiments.
Brain slice cultures derived from E19 embryos, which mirror the late stage of neurogenesis during human gestation, supported productive infection with the Honduran and the prototype MR766 isolates (Fig. 5A). During infection with the Honduran isolate, ZIKV-E protein was observed only in the neocortex of E19 slices, not within the proliferative zone as seen in E15-infected slices (Fig. 5B). These observations mirror those seen during ZIKV infection of late-term organoids and in postnatal mice (23, 28, 39). Other ZIKV isolates, including PRV, did not infect E19 brain slice cultures (Fig. 5B). Identifying the stage in replication that is inhibited in E19 brain slice cultures, and the viral determinants, will provide mechanistic insight into the control of ZIKV replication during early embryonic development.
Fig. 5.
Replication of two ZIKV isolates in late gestation neural tissue. (A and B) Brain slice cultures from E19 embryos were infected with 105 pfu of the indicated ZIKV isolates and at 8 dpi, were fixed and stained with pan-flavivirus antibody against the E glycoprotein (ZIKV-E), cleaved caspase 3 (CC3), and DAPI. Unlike E13 and E15 organotypic slices, E19 slices supported the infection and replication of only the MR766 and Honduran isolates. Staining for ZIKV-E after infection with the Honduran isolate demonstrated viral infection throughout the developing neocortex, with little staining in other brain regions. (Scale bar: 500 and 250 μm in A and B.) (C) Time course of replication of multiple isolates of ZIKV in E19 brain slice cultures. Poliovirus, DHK, Colombia, and PRV isolates did not replicate as the lines are superimposed and not readily visible. Results are representative of three independent experiments.
Effects of ZIKV Infection on Neocortical Layer Formation.
The spectrum of neuronal malformations associated with clinical cZVS is no longer limited to microcephaly. It includes brain calcifications, lissencephaly, ventriculomegaly, cerebellar hypoplasia, and brainstem dysfunction, leading to clinical symptoms, including seizures and spasticity (4). The wide spectrum of neuropathology seen in patients with cZVS suggests that multiple aspects of brain development may be affected, including neuronal migration. To gain insight as to whether ZIKV replication may alter this behavior, GFP-encoding DNA was electroporated ex utero into the radial glial progenitor cells (40) that line the ventricular surface of E15 brains before generation of the organotypic cultures, and infected with ZIKV 24 h later. IF of organotypic cultures from these brains revealed that ZIKV infection impeded neuronal redistribution as reflected by a lower percentage of electroporated cells reaching the most superficial layers of the neocortex 4 dpi (Fig. 6 B and C). All of the ZIKV isolates tested impaired neuronal migration into the cortical plate (Fig. 6 A–C). These migratory neuronal precursors did not exhibit overt defects in their cellular morphology, such as misdirected leading processes or a multipolar morphology (Fig. 6B), prompting an analysis of the RGP basal processes that serve as a scaffold for the migrating neuronal precursors.
Fig. 6.
Neuronal migration is impaired during ZIKV infection. (A) Plasmid encoding GFP DNA was electroporated into E19 brains ex utero before production of slice cultures. Twenty-four hours later, the slice cultures were infected with 105 pfu of the indicated ZIKV isolates and at 4 dpi, were fixed and stained with pan-flavivirus antibody against the E glycoprotein (ZIKV-E), antibody against cortical layers 2–4 marker Tbr1, and DAPI. ZIKV infection appeared to limit neuronal precursor migration into the most superficial layers (corresponding to layers 2/3). (Scale bar: 250 µm.) (B) The cortical plate was divided into six regions of equal size, and the percentage of GFP fluorescent intensity in each sector over the whole six sectors was calculated. (C) Distribution of GFP positive cells within each sector for mock and ZIKV-infected slices. The number 1 is the pial surface, and 6 is the deepest cortical plate layer. ZIKV infection significantly reduced the number of neuronal precursors migrating into the outer neocortex (bin 1) and significantly increased the number of neuronal precursors remaining in the inner neocortex (bin 6). Data represented as mean ± SEM for each condition in each bin in C. Unpaired t test was used to determine significance (*P < 0.05, n = 3 embryonic brains across different replicate experiments). (D) Sections from A were stained with antibody against the E glycoprotein (ZIKV-E) and antibody against vimentin to mark the radial glia progenitor (RGP) basal processes, which are the fibers upon which bipolar neurons migrate, and with DAPI. ZIKV infection perturbed the RGP scaffold compared with control slices. (Scale bar: 250 μm for A and D.)
Microscopic analysis of the infected organotypic slices also revealed that ZIKV infection perturbs the basal projections of the radial glial progenitor cells, marked by vimentin staining (Fig. 6D). Intact radial glial fibers (Fig. 6D, mock) are necessary for proper neuronal migration into the cortical plate. These data support the hypothesis that ZIKV infection can cause structural changes to the basal process of the radial glial progenitor cells and, thereby, impair neuronal migration.
Discussion
Congenital ZIKV syndrome is a significant public health challenge. Microcephalic children may be born to both symptomatic or asymptomatic infected mothers, complicating proper diagnosis and treatment. ZIKV-associated neuronal dysfunction may develop postnatally, and there remains a lack of understanding of the long-term cognitive and physical disabilities associated with cZVS (41). Many models of ZIKV infection have been established but none have or can systematically examine the consequences of viral infection across pre- and postnatal brain development (16, 30, 42).
Mouse Brain Slices as a Model System.
The results reported here demonstrate that organotypic brain slice cultures are ideal for studying ZIKV neurotropism. Infection of mouse embryonic brain slice cultures with ZIKV leads to identification of the sites of infection, and visualization of both cell death and impaired neuronal migration into the cortical plate. Although production of organotypic brain slices severs some axonal connections, these cultures nevertheless maintain many aspects of in vivo brain biology, including functional local synaptic circuitry with preserved brain architecture, vascularization, and immune composition, and can remain viable for at least 8 d in culture. They can be generated from the exact stage of embryonic development desired. Organoid cultures are unable to faithfully mimic the cellular and structural complexity of the brain, including the presence of a cortical plate, and are heterogeneous with respect to cell type composition and structure, which influences the reproducibility of any findings (19).
Was ZIKV Always Neurotropic?
Two hypotheses have been suggested to explain the recent epidemic emergence of ZIKV and previously unrecognized neurotropism and congenital disease (25). One possibility is that mutations in the viral genome were selected that enhance transmission and expand tropism of the virus. Alternatively, the virus might have been randomly introduced into large immunologically naïve populations, and the increased numbers of cases revealed previously undetected syndromes. All ZIKV isolates examined, from 1947 to the present, replicated in early and midgestation embryonic brain slice cultures (E13 and E15, respectively), demonstrating that ZIKV neurotropism is not a newly acquired characteristic. This observation suggests that the earliest human ZIKV infections could have led to neuronal pathology that was too rare for a clear association to be established, especially in areas with poor health infrastructure. Our findings do not rule out the possibility that mutations within the ZIKV genome have been selected that enhance viral neuroinvasion.
Effect of ZIKV Infection on Neocortical Layer Formation.
The spectrum of neuronal malformations associated with clinical cZVS is no longer limited to microcephaly. It includes brain calcifications, abnormal neuronal migration, cerebellar hypoplasia, lissencephaly, ventriculomegaly, and brainstem dysfunction, leading to clinical symptoms including seizures and spasticity (4). Many of these pathologies are believed to result from deficiencies in early–mid brain development. The initial findings of our study determined that productive ZIKV infection can occur at the beginning of corticogenesis. Consequently, we hypothesized that ZIKV infection could impair postmitotic neuronal migration and/or facilitate premature delamination of cells along the ventricular membrane, resulting in a thinner brain cortex. ZIKV infection impaired neuronal migration into the putative neocortex, although no abnormalities were noted in the morphology of the migrating neurons themselves. The morphology of the RGP basal processes was impaired with ZIKV infection, which no longer provided a linear scaffold for migration of neuronal precursors. A similar scaffold disorganization was observed in ZIKV-infected organotypic human fetal brain slices (42) and is an important contributor to other neurodevelopmental diseases (43). It is unclear if the disarray of the RGP basal processes led to the neuronal migration abnormalities found in ZIKV-infected slices, and/or if other mechanisms such as increased apoptosis or changes intrinsic to the migrating neuronal precursors led to their impaired migration. Nevertheless, our data describing virus-associated brain development defects should aid in our understanding of how cZVS develops.
Several virus phenotypes, including impairment of neuronal migration into the cortical plate and induction of apoptosis, were observed in slices infected with all ZIKV isolates. The observed cell death was predominantly seen in cells that surrounded ZIKV-infected cells. Neurotoxicity of ZIKV could result directly from apoptosis of the infected cell as a means of limiting infectious virus production, or by the release of a cytotoxic factor promoting apoptosis of neighboring cells and impairing virus dissemination. The variation of observed apoptosis between isolates suggests that infection with ZIKV from Asia and the Americas may lead to a greater density of cell death than those from Africa. Inability to determine cell number in the slice prevents our ability to calculate the multiplicity of infection; therefore, no association between replication efficiency and apoptotic density can be made. Apoptosis and aberrant neuronal migration both likely contributed to reduced cortical density and the development of congenital Zika virus syndrome.
Cell Type and Developmental Stage Specificity.
The greatest risks for developing cZVS are associated with ZIKV infection during the first and second trimesters (32, 33). Our results show that two of seven ZIKV isolates can replicate in brain slice cultures generated from E19 embryos, equivalent to the third trimester of human gestation. For example, the PRV isolate, which replicated in E13 and E15 brain slice cultures, did not replicate in E19 cultures. This observation suggests that the changing cellular environment during embryonic development influences viral neurotropism. Fewer than 10 amino acids distinguish PRV from MR766 and the Honduran isolate, but which is the genetic determinant of replication in E19 brain slice cultures will require further study. Recently it was shown that the PRV isolate replicated in the brain of 1-d-old mice inoculated s.c. (44). The discrepancy with our results is unexplained, but could involve the genetic background of the mouse or the virus.
Additional neurodevelopmental deficits associated with cZVS have been identified since the first reports of the association of ZIKV infection with microcephaly. These observations are consistent with our understanding that a decrease in neuron number due to either inhibition of RGP proliferation or apoptosis reduces brain size. When apoptosis predominates, areas of programmed cell death contribute not only to an overall microcephalic phenotype, but facilitate the development of neocortical defects such as intracerebral calcifications. Additionally, the neuronal migration defects observed in areas of ZIKV-envelope staining are more typical of the neuropathology associated with lissencephaly. Together these data suggest that multiple mechanisms may contribute to the neuropathology in children diagnosed with cZVS, and that the neurotropism of ZIKV virus is a characteristic of isolates from Africa, Asia, and the Americas.
Materials and Methods
Detailed materials and methods are available in SI Materials and Methods.
Ethics Statement.
All experiments were performed in accordance with guidelines from the Guide for the Care and Use of Laboratory Animals of the NIH (45). Protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Columbia University School of Medicine (assurance no. AC-AAAR5408).
Cells and Viruses.
Vero cells were grown in Dulbecco’s modified Eagle medium (Invitrogen), 10% FCS (HyClone), and 1% penicillin–streptomycin (Invitrogen). Zika viruses (ZIKVs) MR766 (Uganda) (10), DAK (Senegal), FLR (Colombia), PRVABC59 (Puerto Rico) (46), P6-706 (Malaysia), and R103451 (Honduras) were obtained from BEI Resources. Brazilian isolate (ZIKV-Paraiba/2015) was kindly provided by Lucia Gama (The Johns Hopkins School of Medicine). All viruses were propagated and assayed in Vero cells. Viral titers were determined by plaque assay.
Virus Infections.
Organotypic brain slice cultures were infected with 105 pfu of ZIKV isolates: MR766 African, DHK Senegal, Brazil, Colombia, PRV, and Honduras, or poliovirus (P1/Mahoney). Virus was allowed to adsorb to the slices for 1 h at 37 °C. The inoculum was removed, and the slices were washed two times in 1× PBS. Infected slices were cultured in cortical culture medium (CCM) for 8 d. A total of 500 μL of culture medium was removed and replenished at the described points postinfection.
Supplementary Material
Acknowledgments
We thank the members of the R.B.V. laboratory; Drs. Alex Baffett, Hynek Wichterle, Franck Polleux, Gregg Gundersen, Yosef Sabo, Elisa Canepa, Stav Kameny, and Leslie Vosshall for technical expertise and feedback; and Drs. Connie Cepko and Lucia Gama for providing reagents. This project was supported by NIH GM105536 (to R.B.V.), National Institute of Neurological Disorders and Stroke F30NS095577 (to D.J.D.), and NIH AI121944 and AI102597 (to V.R.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714624114/-/DCSupplemental.
References
- 1.Barkovich AJ, et al. A classification scheme for malformations of cortical development. Neuropediatrics. 1996;27:59–63. doi: 10.1055/s-2007-973750. [DOI] [PubMed] [Google Scholar]
- 2.Mochida GH. Genetics and biology of microcephaly and lissencephaly. Semin Pediatr Neurol. 2009;16:120–126. doi: 10.1016/j.spen.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mochida GH, Walsh CA. Molecular genetics of human microcephaly. Curr Opin Neurol. 2001;14:151–156. doi: 10.1097/00019052-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Driggers RW, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016;374:2142–2151. doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- 5.Steele RW. Zika virus: An explosive pandemic and a new TORCH agent. Clin Pediatr (Phila) 2016;55:698–700. doi: 10.1177/0009922816638660. [DOI] [PubMed] [Google Scholar]
- 6.Rakic P. Elusive radial glial cells: Historical and evolutionary perspective. Glia. 2003;43:19–32. doi: 10.1002/glia.10244. [DOI] [PubMed] [Google Scholar]
- 7.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 8.Rakic P. Neurons in rhesus monkey visual cortex: Systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 9.Faheem M, et al. Molecular genetics of human primary microcephaly: An overview. BMC Med Genomics. 2015;8(Suppl 1):S4. doi: 10.1186/1755-8794-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 11.MacNamara FN. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 12.Lazear HM, Diamond MS. Zika virus: New clinical syndromes and its emergence in the Western Hemisphere. J Virol. 2016;90:4864–4875. doi: 10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ECDC . Rapid Risk Assessment: Zika Virus Infection Outbreak, French Polynesia. European Centre for Disease Prevention and Control; Stockholm: 2014. [Google Scholar]
- 14.Kleber de Oliveira W, et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy–Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:242–247. doi: 10.15585/mmwr.mm6509e2. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention . Zika Virus. Centers for Disease Control and Prevention; Atlanta: 2016. [Google Scholar]
- 16.Morrison TE, Diamond MS. Animal models of Zika virus infection, pathogenesis, and immunity. J Virol. 2017;91:e00009-17. doi: 10.1128/JVI.00009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancaster MA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabriel E, et al. Recent Zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell. 2017;20:397–406.e5. doi: 10.1016/j.stem.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Qian X, Nguyen HN, Jacob F, Song H, Ming GL. Using brain organoids to understand Zika virus-induced microcephaly. Development. 2017;144:952–957. doi: 10.1242/dev.140707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gähwiler BH, Hefti F. Guidance of acetylcholinesterase-containing fibres by target tissue in co-cultured brain slices. Neuroscience. 1984;13:681–689. doi: 10.1016/0306-4522(84)90088-5. [DOI] [PubMed] [Google Scholar]
- 21.Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: A technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- 22.Cho S, Wood A, Bowlby MR. Brain slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Curr Neuropharmacol. 2007;5:19–33. doi: 10.2174/157015907780077105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doobin DJ, Kemal S, Dantas TJ, Vallee RB. Severe NDE1-mediated microcephaly results from neural progenitor cell cycle arrests at multiple specific stages. Nat Commun. 2016;7:12551. doi: 10.1038/ncomms12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baffet AD, et al. Cellular and subcellular imaging of motor protein-based behavior in embryonic rat brain. Methods Cell Biol. 2016;131:349–363. doi: 10.1016/bs.mcb.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Weaver SC. Emergence of epidemic Zika virus transmission and congenital Zika syndrome: Are recently evolved traits to blame? MBio. 2017;8:e02063-16. doi: 10.1128/mBio.02063-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: A review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- 27.Rakic P, Cameron RS, Komuro H. Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol. 1994;4:63–69. doi: 10.1016/0959-4388(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 28.Qian X, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang H, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brault JB, et al. Comparative analysis between flaviviruses reveals specific neural stem cell tropism for Zika virus in the mouse developing neocortex. EBioMedicine. 2016;10:71–76. doi: 10.1016/j.ebiom.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simonin Y, et al. Zika virus strains potentially display different infectious profiles in human neural cells. EBioMedicine. 2016;12:161–169. doi: 10.1016/j.ebiom.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brasil P, et al. Zika virus infection in pregnant women in Rio de Janeiro–Preliminary report. N Engl J Med. 2016 doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacheco O, et al. Zika virus disease in Colombia: Preliminary report. N Engl J Med. 2016 doi: 10.1056/NEJMoa1604037. [DOI] [PubMed] [Google Scholar]
- 34.Soares de Souza A, et al. Fetal infection by Zika virus in the third trimester: Report of 2 cases. Clin Infect Dis. 2016;63:1622–1625. doi: 10.1093/cid/ciw613. [DOI] [PubMed] [Google Scholar]
- 35.Nunes AT, et al. Comparative proteome analysis reveals that blood and sugar meals induce differential protein expression in Aedes aegypti female heads. Proteomics. 2016;16:2582–2586. doi: 10.1002/pmic.201600126. [DOI] [PubMed] [Google Scholar]
- 36.Soares de Oliveira-Szejnfeld P, et al. Congenital brain abnormalities and Zika virus: What the radiologist can expect to see prenatally and postnatally. Radiology. 2016;281:203–218. doi: 10.1148/radiol.2016161584. [DOI] [PubMed] [Google Scholar]
- 37.Melo AS, et al. Congenital Zika virus infection: Beyond neonatal microcephaly. JAMA Neurol. 2016;73:1407–1416. doi: 10.1001/jamaneurol.2016.3720. [DOI] [PubMed] [Google Scholar]
- 38.van der Linden V, et al. Description of 13 infants born during October 2015-January 2016 with congenital Zika virus infection without microcephaly at birth–Brazil. MMWR Morb Mortal Wkly Rep. 2016;65:1343–1348. doi: 10.15585/mmwr.mm6547e2. [DOI] [PubMed] [Google Scholar]
- 39.Huang WC, Abraham R, Shim BS, Choe H, Page DT. Zika virus infection during the period of maximal brain growth causes microcephaly and corticospinal neuron apoptosis in wild type mice. Sci Rep. 2016;6:34793. doi: 10.1038/srep34793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imayoshi I, et al. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science. 2013;342:1203–1208. doi: 10.1126/science.1242366. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention . Congenital Zika Virus Infection Initial Evaluation. Centers for Disease Control and Prevention; Atlanta: 2017. [Google Scholar]
- 42.Onorati M, et al. Zika virus disrupts phospho-TBK1 localization and mitosis in human neuroepithelial stem cells and radial glia. Cell Rep. 2016;16:2576–2592. doi: 10.1016/j.celrep.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carabalona A, et al. A glial origin for periventricular nodular heterotopia caused by impaired expression of Filamin-A. Hum Mol Genet. 2012;21:1004–1017. doi: 10.1093/hmg/ddr531. [DOI] [PubMed] [Google Scholar]
- 44.Manangeeswaran M, Ireland DD, Verthelyi D. Zika (PRVABC59) infection is associated with T cell infiltration and neurodegeneration in CNS of immunocompetent neonatal C57Bl/6 mice. PLoS Pathog. 2016;12:e1006004. doi: 10.1371/journal.ppat.1006004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
- 46.Lanciotti RS, Lambert AJ, Holodniy M, Saavedra S, Signor LdelC. Phylogeny of Zika virus in Western Hemisphere, 2015. Emerg Infect Dis. 2016;22:933–935. doi: 10.3201/eid2205.160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.