Significance
Virus cross-infection is an important topic in understanding the course of virus dissemination and evolution. Viruses may spread between the same host species or into taxonomically distinct organisms. The occurrences of cross-kingdom viral infection for certain virus groups are suggested by the current virus taxonomic data. In particular, several plants and fungal viruses show close phylogenetic relationships, but productive transmission of virus between plant and fungal hosts in nature has not been directly demonstrated. Here, we describe the natural infection of Rhizoctonia solani fungus by a plant virus, cucumber mosaic virus (CMV). We further demonstrate that R. solani can acquire and transmit CMV during plant infection. Our findings are evidence of cross-kingdom virus transmission from the plant to fungus.
Keywords: plant virus, fungus, transmission, cross-kingdom
Abstract
The transmission of viral infections between plant and fungal hosts has been suspected to occur, based on phylogenetic and other findings, but has not been directly observed in nature. Here, we report the discovery of a natural infection of the phytopathogenic fungus Rhizoctonia solani by a plant virus, cucumber mosaic virus (CMV). The CMV-infected R. solani strain was obtained from a potato plant growing in Inner Mongolia Province of China, and CMV infection was stable when this fungal strain was cultured in the laboratory. CMV was horizontally transmitted through hyphal anastomosis but not vertically through basidiospores. By inoculation via protoplast transfection with virions, a reference isolate of CMV replicated in R. solani and another phytopathogenic fungus, suggesting that some fungi can serve as alternative hosts to CMV. Importantly, in fungal inoculation experiments under laboratory conditions, R. solani could acquire CMV from an infected plant, as well as transmit the virus to an uninfected plant. This study presents evidence of the transfer of a virus between plant and fungus, and it further expands our understanding of plant–fungus interactions and the spread of plant viruses.
Viruses, as obligate parasites that infect cellular organisms, strictly depend on compatibility with the cellular components for viral multiplication in the host (1–3). Viruses usually spread from one host to another in the environment but, depending on the host range and transmission pathways, certain viruses may be limited to a few or only specific host species, while other viruses could infect various organisms belonging to the same biological class/phylum (4). In fact, a number of viruses are known to infect organisms from different taxonomic kingdoms, usually involving an insect as one of the host species. This is exemplified by some plant viruses belonging to the families Bunyaviridae, Rhabdoviridae, Reoviridae, and genus Tenuivirus that infect and propagate in their insect vectors (5, 6), as well as a recent report on a fungal virus that infects an insect and uses it as a transmission vector (7). Furthermore, cross-kingdom infections can be experimentally established for some virus–host systems in the laboratory (8–13), suggesting that more viruses may cross the kingdom barrier in nature if the appropriate conditions, such as close association/contact and compatibility with the potential novel host, are available to facilitate such an event. Indeed, the occurrence of horizontal virus transfers across kingdoms is one of the theories suggested to account for the presence of many viruses that are taxonomically related but exist in organisms belonging to different taxonomic kingdoms (14–16).
Virtually all flowering plants are exposed to attack by pathogenic fungi (17). Necrotrophic fungal pathogens kill host tissue and absorb nutrients from the dead host cells, while biotrophic and hemibiotrophic fungal plant pathogens colonize the living host, and some commonly develop a specialized organ for uptake of sugars and other nutrients (18–21). To promote infection and disease development, fungal pathogens secrete or inject effector proteins that interfere with the host immunity and other physiological processes (22–24). Interestingly, numerous studies recently reported the occurrences of horizontal transfer of small RNAs, which are usually in the form of micro-RNAs or small interfering RNAs, from fungi and also other microbes to plants, and vice versa (25–27), showing that the infecting fungus and plant could exchange their genetic materials. Fungal virus (mycovirus) infections widely occur in fungi, including in plant pathogenic fungi. Several mycoviruses have been characterized to reduce the virulence of their fungal hosts (28). Notably, some mycoviruses have relatively close sequence identities to plant viruses (29–32). These observations give rise to speculation about the transmission of viruses between plant and fungi in the not so distant past. Nevertheless, no contemporary cross-infection of a plant virus to a phytopathogenic fungal host has been described.
Rhizoctonia solani Kühn [teleomorph: Thanatephorus cucumeris (Frank) Donk] is a soil-borne plant pathogenic fungus that has a wide host range, including field crops, vegetables, ornamental plants, alfalfa, shrubs, and fruit trees, and spreads throughout the world (33). The disease symptoms may vary depending on the host plants, but the most common symptoms are root or stem rot, stem canker, and damping-off of seedlings (34). R. solani is a basidiomycete fungus that does not produce asexual spores (conidia) and exists primarily as vegetative mycelium and/or sclerotia (resting bodies) in nature (35). Several studies have shown that R. solani hosts diverse mycoviruses, including some of the novel viruses that do not belong to any established virus families or genera (36–41).
In this study, we discovered an infection of a plant virus, cucumber mosaic virus (CMV), in an R. solani strain isolated from potato plants (Solanum tuberosum L.) in the field. CMV is a positive single-stranded RNA [(+)ssRNA] virus belonging to the genus Cucumovirus in the family Bromoviridae (42). CMV is known to have the largest host range of any plant virus and is vectored mainly by aphid insects in a nonpropagative manner (43, 44). CMV has a tripartite genome composed of RNA1, RNA2, and RNA3, each encapsidated within isometric virus particles (44). Furthermore, we were able to demonstrate the transmission of CMV from plant to R. solani, as well as the opposite direction from R. solani to plant under laboratory conditions. We discuss the significance of our findings in the context of understanding the host range expansion, spread, and evolution of plant viruses in nature.
Results
Discovery of a CMV Isolate Infecting an R. solani Strain.
To investigate the occurrence of mycoviruses in R. solani, we screened 29 R. solani strains isolated from potato plants grown in several fields in Inner Mongolia Province of China for the presence of double-stranded RNA (dsRNA), which is a molecular marker for RNA virus infection. Eleven strains were found to contain dsRNAs with diverse sizes, and seven fungal strains representing each distinct dsRNA pattern were selected for further analysis (SI Appendix, Fig. S1A). Those dsRNA-containing strains showed similar colony morphology (SI Appendix, Fig. S1B). Sequence analysis of the intergenic spacer region (ITS) of rRNA indicated that those R. solani strains belong to the hyphal anastomosis group (AG)-3 (45). The dsRNAs extracted from those seven strains were pooled and then subjected to next-generation sequencing (NGS). BLASTp searches using the contig sequences assembled from the NGS reads revealed a number of mycovirus-related sequences. Surprisingly, in addition to these, three contigs (DN711_c0_gl–DN711_c0_g3) showed high sequence identity with RNA1, 2, 3 of CMV (SI Appendix, Table S1). The CMV sequences identified in R. solani (hereafter referred to as CMV-Rs) show a typical CMV genome organization in which RNA1 encodes 1a (replicase component), RNA2 encodes 2a (replicase component) and 2b (RNA-silencing suppressor), and RNA3 encodes 3a (movement protein, MP) and coat protein (CP) (Fig. 1A). BLAST analysis indicated that the CMV-Rs sequences are most closely identical (>98% identity) to those of CMV isolates from East Asia countries that belong to the subgroup Ia.
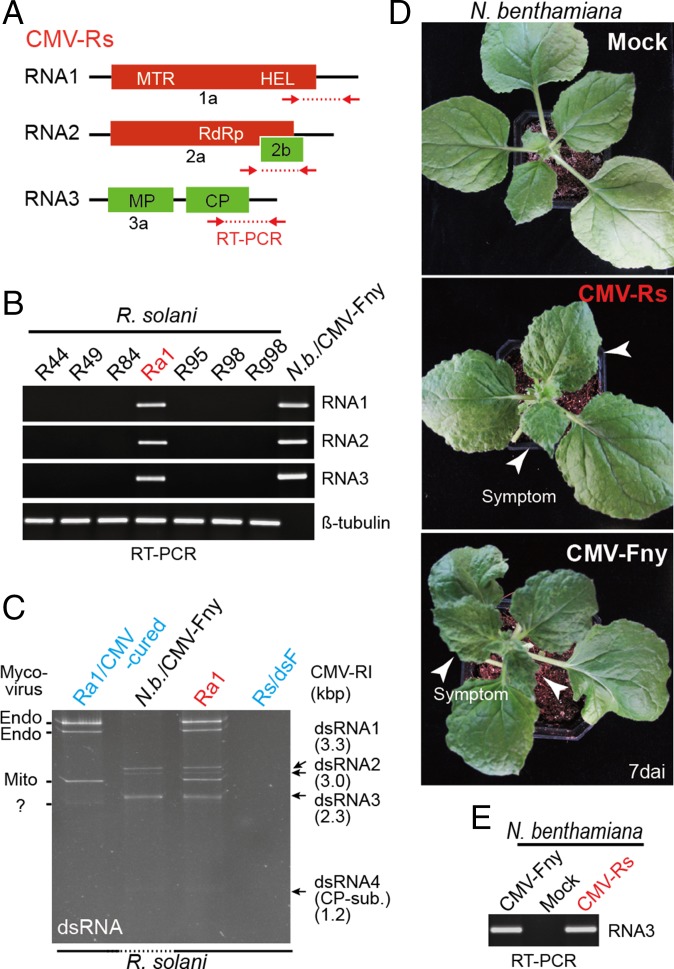
Fig. 1.
CMV infection in R. solani. (A) Schematic representation of genome structure of CMV-infecting R. solani (not to scale). Colored boxes and lines indicate the reading frame (ORF) and untranslated regions (UTR), respectively. (B and E) RT-PCR detection of CMV in R. solani strains (B) or N. benthamiana plants (E). ssRNA fraction extracted from R. solani strains or N. benthamiana leaves were used for RT-PCR using primer sets specific for CMV RNA 1, 2, 3 and R. solani beta-tubulin. PCR products were run on agarose gel electrophoresis and stained with ethidium bromide (EtBr). (C) dsRNA profiles of R. solani strain infected with CMV or cured. dsRNAs were extracted from total RNAs of fungi, run on polyacrylamide gel electrophoresis, and stained with EtBr. dsRNA extracted from leaves of CMV-infected N. benthamiana plants was included in the gel electrophoresis (Nb/CMV-Fny). (D) Virus symptom expressions in N. benthamiana plants mechanically rub-inoculated with CMV-Fny virions or total RNA extracted from R. solani carrying CMV-Rs. Plants were photographed at 7 dai. CP-sub, CP subgenomic RNA; N.b., N. benthamiana.
RT-PCR and sequence analysis of the amplification products confirmed that CMV-Rs is present in one R. solani strain designated as Ra1 (Fig. 1B). Consistently, this strain, but not the cured strain (Ra1/CMV-cured), contains some dsRNA segments (3.3, 3.0, 2.3, and 1.2 kbp) that resemble the pattern of dsRNAs extracted from the leaves of CMV-infected plants (Fig. 1C). RT-PCR analysis using primer sets specific for the virus-related sequences showed that the Ra1 strain also contains at least three (+)ssRNA mycoviruses related to endornaviruses and mitoviruses (Fig. 1C and SI Appendix, Table S1). The complete analysis of mycoviruses identified from our current screening on R. solani strains will be published elsewhere.
To validate that CMV-Rs is indeed a plant virus, the total RNA extracted from Ra1 (CMV-Rs–carrying strain) was used as inoculum for mechanical rub-inoculation of the leaves of Nicotiana benthamiana plants. Starting from 7 days after inoculation (dai), inoculated plants showed a typical mosaic symptom, which is similar to the symptom induced by a subgroup I CMV isolate (Fny) (46, 47) in N. benthamiana plants (Fig. 1D), and virus accumulations in upper leaves of inoculated plants were confirmed by RT-PCR (Fig. 1E). These results strongly suggest that CMV-Rs is a plant virus that has crossed the host-specificity barrier and infects phytopathogenic fungus R. solani.
Introduction of CMV into R. solani and Other Fungi.
We then questioned whether R. solani could also host other CMV isolates. To answer this, the CMV-Fny isolate, which has 95% amino acid sequence identity to CMV-Rs in 1a proteins (SI Appendix, Fig. S2), was introduced into dsRNA-free R. solani strain (Rs/dsF) by transfection of fungal protoplasts with purified virus particles. After transfection and regeneration of R. solani protoplasts, CMV-Fny accumulation was detected by either RNA or Western blotting (Fig. 2 A and B) and dsRNA analysis (Fig. 2C), indicating that R. solani supports the replication of another CMV isolate. Moreover, CMV-Fny was stably maintained in this R. solani transfectant during successive cultures (SI Appendix, Fig. S3). Nevertheless, CMV-Fny infection did not affect the morphology and growth of R. solani on potato dextrose agar (PDA) medium (Fig. 2D).
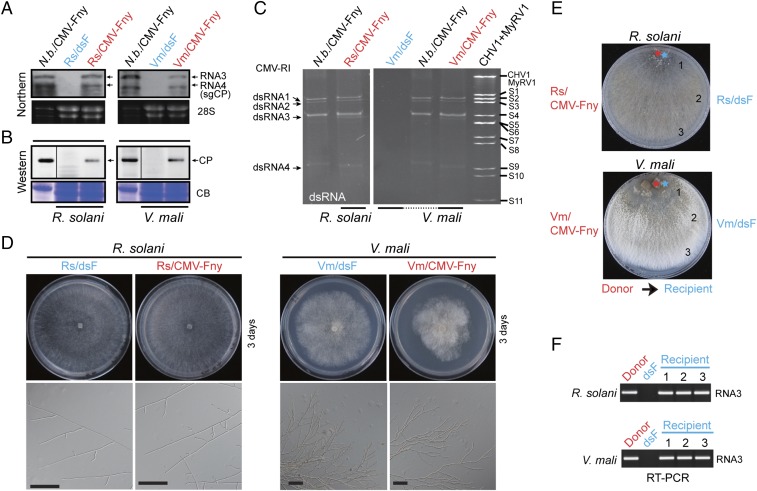
Fig. 2.
The introduction of CMV into fungal protoplasts. (A) RNA blot analysis of CMV RNA3 accumulation in R. solani and V. mali strains regenerated from protoplasts transfected with CMV-Fny virions. An RNA sample derived from CMV-Fny–infected leaves of N. benthamiana plants was included in the analysis. The RNA gel was stained with ethidium bromide, and 28S rRNA is shown as a loading control (28S). (B) Western blot analysis of CMV CP accumulation in fungal strains and leaves described in A. Vertical lines indicate that two separate lanes in the same blot were spliced together. To confirm protein loading amount, coomassie blue-stained total proteins (CB) run on separated gels are shown as loading controls (Bottom). (C) dsRNA profiles of R. solani and V. mali strains infected with CMV. dsRNA extracted from leaves of CMV-infected N. benthamiana plants was included in the gel electrophoresis (Nb/CMV-Fny). dsRNA of Cryphonectria hypovirus 1 (CHV1) and mycoreovirus 1 (MyRV1) were used as the size marker. (D) Colony and hyphal morphology of R. solani and V. mali strains infected with CMV-Fny on PDA medium. Fungi were grown for 3 d and photographed. (Bars, 100 μm.) (E) Coculturing of virus-infected and virus-free fungal strains on PDA medium for investigating virus horizontal transfer through hyphal fusion. The transmission of the virus from donor to recipient fungi is indicated by arrows. Number (1–3) indicates the positions where the mycelia of fungal recipients were taken for subsequent culture. Plates were photographed at 6 d after coculture. (F) RT-PCR detection of CMV RNA3 accumulation in fungal recipients described in E. N.b., N. benthamiana.
As mycoviruses are commonly transmitted horizontally via hyphal anastomosis and vertically through sporulation (48), we investigated if CMV also undergoes such transmissions in R. solani. First, in a horizontal transfer experiment, where Rs/dsF and CMV-Fny–infected R. solani (Rs/CMV-Fny) strains were cocultured side-by-side on a PDA medium, CMV-Fny was efficiently transmitted to Rs/dsF through hyphal fusion (Fig. 2 E and F). A similar result was obtained when the Ra1 strain was used as a donor in a hyphal fusion experiment (SI Appendix, Fig. S4). Because R. solani does not produce asexual spores (conidia), we examined CMV transmission through basidiospores (sexual spores). Of 100 basidiospore progenies of Ra1, none was found to contain CMV-Rs, whereas the presence of endornaviruses was consistently detected in the progenies (SI Appendix, Fig. S5). Thus, unlike endornaviruses, CMV is not transmitted through sexual spores.
To investigate the ability of CMV to replicate in other fungi, CMV-Fny virions were transfected to protoplasts of three phytopathogenic ascomycete fungi, Valsa mali, Cryphonectria parasitica, and Fusarium graminearum, which are known as the causal agents of apple canker, chestnut blight, and wheat head blight diseases, respectively. CMV-Fny accumulation was detected in V. mali (Fig. 2 A–C), and this virus was stably maintained and transmitted horizontally via hyphal anastomosis in the transfectant (Fig. 2E and SI Appendix, Fig. S3). CMV-Fny–infected V. mali developed more aerial hyphae than virus-free strain (Vm/dsF) on PDA medium (Fig. 2D), and in a virulence assay on apple fruits and apple twigs, Vm/CMV-Fny produced bigger lesions than Vm/dsF (SI Appendix, Fig. S6). In contrast, a very low level of CMV-Fny accumulation was detected by RT-PCR in the first-generation transfectants of C. parasitica and F. graminearum, and later, the virus was undetectable after subsequent subcultures (SI Appendix, Fig. S7), indicating that these two fungi are not suitable hosts for CMV.
CMV Transferred from Plant to R. solani.
Our finding of CMV infection in a field strain of R. solani raises the important question of whether CMV could be transferred from plant to R. solani during infection in the natural environment. To explore this possibility, we examined virus acquisition by R. solani under laboratory conditions. In this experiment, potato and N. benthamiana plants infected with CMV-Fny were then inoculated with Rs/dsF in the lower stem. After allowing R. solani to colonize the stem, the fungus was then reisolated from the plants, cultured, and subjected for virus detection using RT-PCR (Fig. 3A). Strikingly, Rs/dsF induced much more severe stem rot disease in CMV-infected than in virus-free potato and N. benthamiana plants, showing that CMV infection enhances the plant susceptibility to R. solani. At 14 or 4 d after R. solani inoculation in potato and N. benthamiana plants, respectively, the stems of CMV-infected plants were heavily rotted (damping off), and the plants were collapsed, while virus-free plants retained vigor (Fig. 3B and SI Appendix, Fig. S8A). By RT-PCR, we found that 38% and 28% of fungal strains isolated from virus-infected potato and N. benthamiana plants, respectively, were carrying CMV-Fny (Fig. 3 C and D and SI Appendix, Fig. S8B), demonstrating that R. solani could acquire CMV during infection.
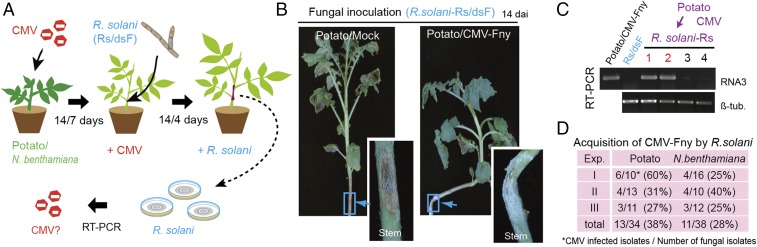
Fig. 3.
Acquisition of CMV by R. solani. (A) A cartoon illustration describing the experimental procedure for investigating CMV acquisition by R. solani. (B) Stem rot disease in virus-free and CMV-infected potato plants inoculated with virus-free R. solani (Rs/dsF). (Insets) Close-up views of the inoculated stems. (Magnification: 4.5×.) Plants were photographed at 14 d after fungal inoculation. (C) RT-PCR detection of CMV RNA3 accumulation in R. solani strains isolated from inoculated stem of potato plants. (D) Efficiency of CMV-Fny acquisition by R. solani from potato and N. benthamiana plants. Exp, experiment; tub, tubulin.
R. solani Transmits CMV to the Plant.
Next, we examined whether CMV could be transmitted to the plant by inoculating CMV-infected R. solani to the stem of potato or N. benthamiana plants, and then the virus accumulation in upper leaves was analyzed. Interestingly, severe stem rot disease was induced by Rs/CMV-Fny and Ra1 infections, leading to plant collapse on day 12 (potato) or 4 (N. benthamiana) after inoculation, while virus-free Rs/dsF caused only mild stem rot, and the plants remained erect (Fig. 4A and SI Appendix, Fig. S9A), similar to previous observations (Fig. 3B and SI Appendix, Fig. S8A). At this point, the plants infected with Ra1 and Rs/CMV-Fny did not show any obvious virus mosaic symptoms, but CMV accumulations were detected by RT-PCR in the uppermost leaves of around half of the potato plants and all N. benthamiana plants (Fig. 4 B and C and SI Appendix, Fig. S9B). When the same uppermost leaves were placed on medium, no growth of R. solani colony was observed (SI Appendix, Fig. S10), suggesting that the fungus did not systemically spread to the upper leaves. This result shows that R. solani could transmit CMV to the plant during infection.
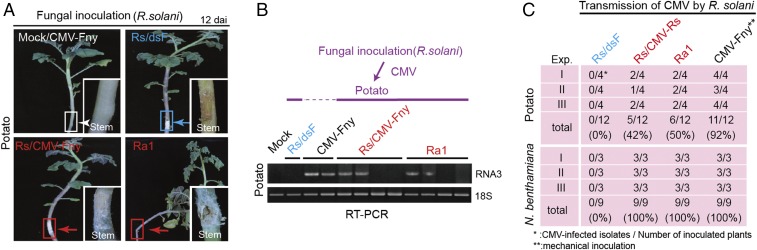
Fig. 4.
Transmission of CMV from R. solani to the plant. (A) Development of stem rot disease in potato plants infected with virus-free (dsF) and virus-infected R. solani strains. (Insets) Close-up views of the inoculated stems. (Magnification: 3×.) Plants were photographed at 12 dai. (B) Detection of CMV RNA3 accumulation in uppermost leaves of potato plants by RT-PCR. (C) Efficiency of CMV transmission by R. solani to the potato and N. benthamiana plants. Exp, experiment.
Discussion
In light of the potential application for the biological control of crop fungal diseases, extensive efforts have been made to identify and characterize viruses infecting phytopathogenic fungi (28, 48). To our knowledge, natural infection of a plant virus in a fungus has not been reported previously. A plant virus, tobacco mosaic virus [TMV, genus Tobamovirus, a monopartite (+)ssRNA genome] or its relatives has long been known to associate with some fungi, such as rust and powdery mildew, but the direct evidence of virus replication in fungal cells was not reported (49, 50). Many mycoviruses and plant viruses share similar characteristics, especially in terms of replication and coding strategies of their genome, but notably, a number of mycoviruses are capsidless, and an extracellular phase is generally absent or unknown in the mycoviruses life cycle, while the majority of plant viruses are spread by biological vectors, which are mainly arthropods (51–53). The compatibility between plant virus and fungus as a host was first demonstrated experimentally by replication of brome mosaic virus [BMV, genus Bromovirus, a tripartite (+)ssRNA genome] in yeast Saccharomyces cerevisiae (12), and later, a similar yeast system has been successfully applied to replication of other plant viruses (9). More recently, TMV was shown to replicate in ascomycete fungus Colletotrichum acutatum (8). Likewise, we have demonstrated that two quite divergent filamentous fungi, R. solani (a basidiomycete) and V. mali (a ascomycete), are compatible hosts for CMV multiplication (Fig. 2). Thus, many phytopathogenic fungi may be able to support the multiplication of plant viruses, in particular, those belonging to the alpha-like (+)ssRNA virus superfamily such as CMV, TMV, and BMV (14). Considering the high probability of coinfection of an individual plant with phytopathogenic fungi and plant viruses in nature, we anticipate that the cross-infections of a plant virus to fungi are not merely isolated cases and could occur more frequently.
The observation that R. solani could acquire and transmit CMV during infection presents a finding that parasitic materials could be translocated bidirectionally between plant and pathogenic fungus during infection. Unlike plant biotrophic fungi, such as rust and powdery mildew fungi that commonly absorb nutrients from the host cells using feeding organs called haustoria without penetrating the plant plasma membrane, R. solani is a necrotrophic pathogen that actively decomposes the host cells for uptake of nutrients. This possibly allows the flow of bigger molecules, such as virus particles that are present in the plant cell contents into fungal cells. The previous report showed that necrotrophic ascomycete fungus Sclerotinia sclerotiorum could be infected by an ssDNA mycovirus, Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV-1, genus Gemycircularvirus), when virions were applied extracellularly to hyphae, but the mechanism of SsHADV-1 entry into fungal cells remains unclear (54). Similarly, TMV enters and replicates in C. acutatum (hemibiotrophic) through the addition of virus particles to liquid medium (8). It has been suggested that TMV enters the cell through cell membrane damage that occurs during hyphae grow or through pinocytosis (55). Nevertheless, it is also possible that CMV is translocated between the plant and fungus in the form of naked RNA or ribonucleoprotein. It is hypothesized that small-RNA molecules are transferred from plant to fungus through an exosomal pathway (56). Further studies are needed to elucidate the mechanism by which CMV translocates between plant and fungus.
A question arises whether R. solani is a potential vector transmission of CMV. In fact, a number of plant viruses are transmitted by zoosporic soil-borne fungi (chytrids) or plasmodiophorids (protists) to the roots (51, 57). Nonetheless, zoosporic vectors are not known to be the host of virus multiplication, and the association of the virus with a vector is mediated by direct attachment of virus particles to the surface of vector or facilitated by viral-encoded protein through unknown mechanisms (51, 58). Moreover, those soil-borne vectors are not considered serious pathogenic threats to the crop plant. R. solani is also a soil-borne fungus that infects roots, stems, and underground plant organs (34). The efficiency of CMV acquisition and transmission by R. solani should be tested under field conditions, and extensive screening of R. solani strains for the CMV infection would reveal the actual prevalence of viruliferous fungus in the field. Compared with virus transmission by common soil-borne vectors, transmission of a plant virus by a phytopathogenic fungus may pose a greater threat in the sense that simultaneous infection of two different pathogens could elicit more serious damage to the plant (59). Interestingly, our experiment showed synergistic effects of CMV infection on R. solani virulence in potato and N. benthamiana plants (Figs. 3B and 4A and SI Appendix, Figs. S8 and S9). CMV 2b protein, which interferes with basal plant defense-signaling pathways (60, 61), might contribute to this synergism. CMV infection does not affect the growth and morphology of R. solani (Fig. 2D); thus, in terms of pathogenicity, acquiring CMV seems to benefit R. solani.
Plant and fungal partitiviruses (bipartite dsRNA virus, picornavirus-like superfamily)/endornaviruses [alpha-like (+)ssRNA virus superfamily] show close phylogenetic relationships (31, 32). Likewise, some mycoviruses classified as members of the family Flexiviridae [alpha-like (+)ssRNA virus superfamily], which previously contains only plant virus members, were also found (62–65). Considering the compatibility of certain plant alpha-like (+)ssRNA viruses with fungal hosts as demonstrated by this and previous studies, future investigations of viruses infecting fungi may further uncover the occurrence of past and ongoing transmissions of this virus group into the fungal hosts. Collectively, our data therefore supports the view that the transmission of viruses between plant and fungal hosts contributes to the evolution and genetic diversities of plant and fungal viruses. Mycovirus with a close phylogenetic relationship to CMV has so far not been found in fungi. A long-term study is necessary to monitor CMV adaptation and evolution within the fungal host. Lastly, from an ecological perspective, cross-infection of plant viruses to fungus could extend the reservoir of plant viruses in the environment. Intriguingly, some plant viruses including CMV were recently found in some invertebrates unrelated to plant-virus insect vectors by metatranscriptomic analyses (66). Taken together, these observations imply that certain plant viruses may actually spread beyond their known conventional hosts.
Experimental Procedures
Fungal Strains, Virus Isolates, and Plant Materials.
A total of 319 R. solani strains were isolated from potato tubers with black scurf disease randomly collected from different potato-planting areas located in the middle and western parts of Inner Mongolia Province of China from year 2008–2015. All fungal strains were maintained at laboratory. For identification of R. solani strains, fungal DNA was isolated using standard phenol–chloroform extraction and ethanol precipitation and used for PCR amplification of the ITS1 and ITS2 regions of ribosomal RNA (67). The amplified ITS sequences were subjected to Nucleotide BLAST search. Twenty-nine fungal strains were selected for dsRNA screening. CMV-Rs sequences have been submitted to NCBI with the accession number, MG025947 (RNA1), MG025948 (RNA2), and MG025949 (RNA3). The CMV-cured Ra1 strain is obtained by a single basidiospore isolation. C. parasitica strain EP155 infected with Cryphonectria hypovirus 1 or mycoreovirus 1 and virus-free were generous gifts from Nobuhiro Suzuki (Okayama University, Japan). V. mali YL strain was isolated from an apple orchard in Shaanxi Province, China. F. graminearum PH1 strain was provided by Zhonghua Ma (Zhejiang University, China). CMV-Fny virions were provided by X. B. Wang (China Agricultural University, China). CMV isolates were maintained in N. benthamiana plants and used for inoculation.
All fungal strains were grown on PDA medium for 3–6 d at 24–26 °C for morphological observation or on cellophane-covered PDA medium for RNA, DNA, and protein extractions. Potato cultivar Shepody was used in this study and provided by Bolin Liu (Northwest A&F University, China). Potato and N. benthaniana plants were grown in a growth room at 22 ± 2 °C with a photoperiod of 16 h/8 h (day/night).
Virus and Fungal Inoculation.
For mechanical virus inoculation, virions, total RNAs, or CMV-infected leaves homogenated in 0.1 M phosphate buffer (pH 7.0) were used as the inoculums and rubbed onto carborundum-dusted leaves of potato and N. benthamiana plants. For inoculation of R. solani to potato and N. benthamiana plants, lower stems were wounded using sterilized toothpicks, and mycelia-containing gel plugs (around 0.5 × 1 cm), which were picked up from the edge of a 3-d old culture colony, were placed on the wounded area. The inoculated part of the stem was wrapped with parafilm for 24 h. Inoculated plants were grown at 22 ± 2 °C, 70–80% humidity, and a photoperiod of 16 h/8 h (day/night).
Virus Acquisition by R. solani.
To investigate CMV acquisition by R. solani, potato and N. benthamiana plants were first mechanically rub-inoculated with CMV (12–16 plants were inoculated in each experiment), and, after virus infection was confirmed by RT-PCR (at 14 and 7 dai in potato and N. benthamiana plants, respectively), virus-free R. solani was then inoculated to the lower stems. Fourteen (potato) or four (N. benthamiana) dai, R. solani was retrieved from the inoculated plants with the procedures as follow; small pieces (roughly 0.2 × 0.5 cm) of stem tissue colonized by the fungus were cut and washed using 75–80% ethanol solutions and followed by several rinses with sterilized water. The stem tissue pieces (about 15 pieces from one stem) were then put on PDA medium containing streptomycin, ampicillin, and tetracycline (50 µg/mL). After the fungi had grown, colonies were subcultured on fresh PDA medium. R solani strains were reconfirmed by PCR amplification and sequence analysis of the ITS regions.
Next-Generation Sequencing and Bioinformatics Analysis.
Preparation of the cDNA library for next-generation sequencing was performed using NEBNext UltraTM RNA Library Prep Kit for Illumina (New England Biolabs) and sequenced on the Illumina HiSEq 4000 platform (Illumina). Raw reads were cleaned by removing adapter sequences, low-quality bases (PHRED quality scores ≤5) were trimmed with a Trimmomatic package with default parameter, and truncated reads smaller than 35 bp were discarded. All clean reads were then assembled using the de novo assembly program Trinity (trinityrnaseq.github.io) with K-mer value = 25. All assembled transcripts were subjected to BLASTx searches with a cutoff of E ≤ 1e-5.
Fungal protoplast isolation, virus transfection, RNA extraction, RT-PCR detection, RNA blot analysis, and Western blot analysis methods were described in SI Appendix, SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Drs. N. Suzuki, Z. Ma, B. Liu, and X. Wang for research materials; Dr. C. Han for helpful discussions; and Dr. A. J. Gibbs for valuable comments on the manuscript. This work was supported in part by National Key Research and Development Program of China Grant 2017YFD0201100; National Natural Science Foundation of China Grants 31260416 and 31550110222; 111 program for crop breeding for disease resistance and genetic improvement, Science Foundation of Shaanxi Grant 2016KW-069 (to L. Sun); and Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research Grants 15K07312, 16H06436, and 17H01463 (to H.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714916114/-/DCSupplemental.
References
- 1.Harak C, Lohmann V. Ultrastructure of the replication sites of positive-strand RNA viruses. Virology. 2015;479-480:418–433. doi: 10.1016/j.virol.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagy PD, Pogany J. The dependence of viral RNA replication on co-opted host factors. Nat Rev Microbiol. 2011;10:137–149. doi: 10.1038/nrmicro2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto M, Neriya Y, Yamaji Y, Namba S. Recessive resistance to plant viruses: Potential resistance genes beyond translation initiation factors. Front Microbiol. 2016;7:1695. doi: 10.3389/fmicb.2016.01695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pommerville J. Alcamo’s Fundamentals of Microbiology: Body Systems. Jones & Bartlett Publ; Burlington, MA: 2012. [Google Scholar]
- 5.Whitfield AE, Falk BW, Rotenberg D. Insect vector-mediated transmission of plant viruses. Virology. 2015;479-480:278–289. doi: 10.1016/j.virol.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Hogenhout SA, Ammar D, Whitfield AE, Redinbaugh MG. Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol. 2008;46:327–359. doi: 10.1146/annurev.phyto.022508.092135. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, et al. Fungal DNA virus infects a mycophagous insect and utilizes it as a transmission vector. Proc Natl Acad Sci USA. 2016;113:12803–12808. doi: 10.1073/pnas.1608013113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mascia T, et al. Gene silencing and gene expression in phytopathogenic fungi using a plant virus vector. Proc Natl Acad Sci USA. 2014;111:4291–4296. doi: 10.1073/pnas.1315668111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagy PD. Yeast as a model host to explore plant virus-host interactions. Annu Rev Phytopathol. 2008;46:217–242. doi: 10.1146/annurev.phyto.121407.093958. [DOI] [PubMed] [Google Scholar]
- 10.Nagy PD, Pogany J, Lin J-Y. How yeast can be used as a genetic platform to explore virus-host interactions: From ‘omics’ to functional studies. Trends Microbiol. 2014;22:309–316. doi: 10.1016/j.tim.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Nerva L, Varese GC, Falk BW, Turin M. Mycoviruses of an endophytic fungus can replicate in plant cells: Evolutionary implications. Sci Rep. 2017;7:1908. doi: 10.1038/s41598-017-02017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janda M, Ahlquist P. RNA-dependent replication, transcription, and persistence of brome mosaic virus RNA replicons in S. cerevisiae. Cell. 1993;72:961–970. doi: 10.1016/0092-8674(93)90584-d. [DOI] [PubMed] [Google Scholar]
- 13.Selling BH, Allison RF, Kaesberg P. Genomic RNA of an insect virus directs synthesis of infectious virions in plants. Proc Natl Acad Sci USA. 1990;87:434–438. doi: 10.1073/pnas.87.1.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koonin EV, Dolja VV, Krupovic M. Origins and evolution of viruses of eukaryotes: The ultimate modularity. Virology. 2015;479-480:2–25. doi: 10.1016/j.virol.2015.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolja VV, Koonin EV. Common origins and host-dependent diversity of plant and animal viromes. Curr Opin Virol. 2011;1:322–331. doi: 10.1016/j.coviro.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roossinck MJ. Plant virus ecology. PLoS Pathog. 2013;9:e1003304. doi: 10.1371/journal.ppat.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knogge W. Fungal infection of plants. Plant Cell. 1996;8:1711–1722. doi: 10.1105/tpc.8.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staples RC. Nutrients for a rust fungus: The role of haustoria. Trends Plant Sci. 2001;6:496–498. doi: 10.1016/s1360-1385(01)02126-4. [DOI] [PubMed] [Google Scholar]
- 19.Szabo LJ, Bushnell WR. Hidden robbers: The role of fungal haustoria in parasitism of plants. Proc Natl Acad Sci USA. 2001;98:7654–7655. doi: 10.1073/pnas.151262398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laluk K, Mengiste T. 2010. Necrotroph attacks on plants: Wanton destruction or covert extortion? Arabidopsis Book, e0136.
- 21.Stone J. Necrotroph. Encycl Plant Pathol. 2001;2:676–677. [Google Scholar]
- 22.Lo Presti L, et al. Fungal effectors and plant susceptibility. Annu Rev Plant Biol. 2015;66:513–545. doi: 10.1146/annurev-arplant-043014-114623. [DOI] [PubMed] [Google Scholar]
- 23.Koeck M, Hardham AR, Dodds PN. The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell Microbiol. 2011;13:1849–1857. doi: 10.1111/j.1462-5822.2011.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye W, Ma W. Filamentous pathogen effectors interfering with small RNA silencing in plant hosts. Curr Opin Microbiol. 2016;32:1–6. doi: 10.1016/j.mib.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Baulcombe DC. VIGS, HIGS and FIGS: Small RNA silencing in the interactions of viruses or filamentous organisms with their plant hosts. Curr Opin Plant Biol. 2015;26:141–146. doi: 10.1016/j.pbi.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Weiberg A, Wang M, Bellinger M, Jin H. Small RNAs: A new paradigm in plant-microbe interactions. Annu Rev Phytopathol. 2014;52:495–516. doi: 10.1146/annurev-phyto-102313-045933. [DOI] [PubMed] [Google Scholar]
- 27.Han L, Luan Y-S. Horizontal transfer of small RNAs to and from plants. Front Plant Sci. 2015;6:1113. doi: 10.3389/fpls.2015.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie J, Jiang D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu Rev Phytopathol. 2014;52:45–68. doi: 10.1146/annurev-phyto-102313-050222. [DOI] [PubMed] [Google Scholar]
- 29.Pearson MN, Beever RE, Boine B, Arthur K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol Plant Pathol. 2009;10:115–128. doi: 10.1111/j.1364-3703.2008.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nibert ML, et al. 3D structures of fungal partitiviruses. Adv Virus Res. 2013;86:59–85. doi: 10.1016/B978-0-12-394315-6.00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roossinck MJ. Lifestyles of plant viruses. Philos Trans R Soc Lond B Biol Sci. 2010;365:1899–1905. doi: 10.1098/rstb.2010.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roossinck MJ. Persistent Plant Viruses: Molecular Hitchhikers or Epigenetic Elements? Viruses: Essential Agents of Life. Springer; Dordrecht, The Netherlands: 2012. pp. 177–186. [Google Scholar]
- 33.Anderson NA. The genetics and pathology of Rhizoctonia solani. Annu Rev Phytopathol. 1982;20:329–347. [Google Scholar]
- 34.Agrios GN. Plant Pathology. Academic; London: 2005. [Google Scholar]
- 35.Sneh B, Jabaji-Hare S, Neate S, Dijst G. Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control. Springer Sci & Business Media; Dordrecht, The Netherlands: 2013. [Google Scholar]
- 36.Zheng L, Zhang M, Chen Q, Zhu M, Zhou E. A novel mycovirus closely related to viruses in the genus Alphapartitivirus confers hypovirulence in the phytopathogenic fungus Rhizoctonia solani. Virology. 2014;456-457:220–226. doi: 10.1016/j.virol.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 37.Zheng L, Liu H, Zhang M, Cao X, Zhou E. The complete genomic sequence of a novel mycovirus from Rhizoctonia solani AG-1 IA strain B275. Arch Virol. 2013;158:1609–1612. doi: 10.1007/s00705-013-1637-3. [DOI] [PubMed] [Google Scholar]
- 38.Zhong J, Chen C-Y, Gao B-D. Genome sequence of a novel mycovirus of Rhizoctonia solani, a plant pathogenic fungus. Virus Genes. 2015;51:167–170. doi: 10.1007/s11262-015-1219-4. [DOI] [PubMed] [Google Scholar]
- 39.Bartholomäus A, et al. Identification of a novel mycovirus isolated from Rhizoctonia solani (AG 2-2 IV) provides further information about genome plasticity within the order Tymovirales. Arch Virol. 2017;162:555–559. doi: 10.1007/s00705-016-3085-3. [DOI] [PubMed] [Google Scholar]
- 40.Bartholomäus A, et al. Deep sequencing analysis reveals the mycoviral diversity of the virome of an avirulent isolate of Rhizoctonia solani AG-2-2 IV. PLoS One. 2016;11:e0165965. doi: 10.1371/journal.pone.0165965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das S, Falloon RE, Stewart A, Pitman AR. Molecular characterisation of an endornavirus from Rhizoctonia solani AG-3PT infecting potato. Fungal Biol. 2014;118:924–934. doi: 10.1016/j.funbio.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 42.King AM, Lefkowitz E, Adams MJ, Carstens EB. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier; London: 2011. [Google Scholar]
- 43.Jacquemond M. Cucumber mosaic virus. Adv Virus Res. 2012;84:439–504. doi: 10.1016/B978-0-12-394314-9.00013-0. [DOI] [PubMed] [Google Scholar]
- 44.Palukaitis P, Roossinck MJ, Dietzgen RG, Francki RI. Cucumber mosaic virus. Adv Virus Res. 1992;41:281–348. doi: 10.1016/s0065-3527(08)60039-1. [DOI] [PubMed] [Google Scholar]
- 45.Carling DE. Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control. Springer; Dordrecht, The Netherlands: 1996. Grouping in Rhizoctonia solani by hyphal anastomosis reaction; pp. 37–47. [Google Scholar]
- 46.Rizzo TM, Palukaitis P. Nucleotide sequence and evolutionary relationships of cucumber mosaic virus (CMV) strains: CMV RNA 1. J Gen Virol. 1989;70:1–11. doi: 10.1099/0022-1317-70-1-1. [DOI] [PubMed] [Google Scholar]
- 47.Rizzo TM, Palukaitis P. Nucleotide sequence and evolutionary relationships of cucumber mosaic virus (CMV) strains: CMV RNA 2. J Gen Virol. 1988;69:1777–1787. doi: 10.1099/0022-1317-69-8-1777. [DOI] [PubMed] [Google Scholar]
- 48.Ghabrial SA, Suzuki N. Viruses of plant pathogenic fungi. Annu Rev Phytopathol. 2009;47:353–384. doi: 10.1146/annurev-phyto-080508-081932. [DOI] [PubMed] [Google Scholar]
- 49.Nienhaus F. Tobacco mosaic virus strains extracted from conidia of powdery mildews. Virology. 1971;46:504–505. doi: 10.1016/0042-6822(71)90054-7. [DOI] [PubMed] [Google Scholar]
- 50.Yarwood C, Hecht-Poinar E. Viruses from rusts and mildews. Phytopathology. 1973;63:1111–1115. [Google Scholar]
- 51.Bragard C, et al. Status and prospects of plant virus control through interference with vector transmission. Annu Rev Phytopathol. 2013;51:177–201. doi: 10.1146/annurev-phyto-082712-102346. [DOI] [PubMed] [Google Scholar]
- 52.Ghabrial SA, Castón JR, Jiang D, Nibert ML, Suzuki N. 50-plus years of fungal viruses. Virology. 2015;479-480:356–368. doi: 10.1016/j.virol.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 53.Hull R. Plant Virology. Academic; London: 2013. [Google Scholar]
- 54.Yu X, et al. Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc Natl Acad Sci USA. 2013;110:1452–1457. doi: 10.1073/pnas.1213755110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mascia T, Gallitelli D, Palukaitis P. Something new to explore: Plant viruses infecting and inducing gene silencing in filamentous fungi. Mob Genet Elements. 2014;4:e29782. doi: 10.4161/mge.29782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nowara D, et al. HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell. 2010;22:3130–3141. doi: 10.1105/tpc.110.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andika IB, Kondo H, Sun L. Interplays between soil-borne plant viruses and RNA silencing-mediated antiviral defense in roots. Front Microbiol. 2016;7:1458. doi: 10.3389/fmicb.2016.01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams MJ, Antoniw JF, Mullins JG. Plant virus transmission by plasmodiophorid fungi is associated with distinctive transmembrane regions of virus-encoded proteins. Arch Virol. 2001;146:1139–1153. doi: 10.1007/s007050170111. [DOI] [PubMed] [Google Scholar]
- 59.Guerret MG, Barbetti MJ, You MP, Jones RA. Effects of temperature on disease severity in plants of subterranean clover infected singly or in mixed infection with Bean yellow mosaic virus and Kabatiella caulivora. J Phytopathol. 2016;164:608–619. [Google Scholar]
- 60.Ji L-H, Ding S-W. The suppressor of transgene RNA silencing encoded by Cucumber mosaic virus interferes with salicylic acid-mediated virus resistance. Mol Plant Microbe Interact. 2001;14:715–724. doi: 10.1094/MPMI.2001.14.6.715. [DOI] [PubMed] [Google Scholar]
- 61.Lewsey MG, et al. Disruption of two defensive signaling pathways by a viral RNA silencing suppressor. Mol Plant Microbe Interact. 2010;23:835–845. doi: 10.1094/MPMI-23-7-0835. [DOI] [PubMed] [Google Scholar]
- 62.Martelli GP, Adams MJ, Kreuze JF, Dolja VV. Family flexiviridae: A case study in virion and genome plasticity. Annu Rev Phytopathol. 2007;45:73–100. doi: 10.1146/annurev.phyto.45.062806.094401. [DOI] [PubMed] [Google Scholar]
- 63.Xie J, et al. Characterization of debilitation-associated mycovirus infecting the plant-pathogenic fungus Sclerotinia sclerotiorum. J Gen Virol. 2006;87:241–249. doi: 10.1099/vir.0.81522-0. [DOI] [PubMed] [Google Scholar]
- 64.Howitt RL, Beever RE, Pearson MN, Forster RL. Genome characterization of Botrytis virus F, a flexuous rod-shaped mycovirus resembling plant ‘potex-like’ viruses. J Gen Virol. 2001;82:67–78. doi: 10.1099/0022-1317-82-1-67. [DOI] [PubMed] [Google Scholar]
- 65.Howitt RL, Beever RE, Pearson MN, Forster RL. Genome characterization of a flexuous rod-shaped mycovirus, Botrytis virus X, reveals high amino acid identity to genes from plant ‘potex-like’ viruses. Arch Virol. 2006;151:563–579. doi: 10.1007/s00705-005-0621-y. [DOI] [PubMed] [Google Scholar]
- 66.Shi M, et al. Redefining the invertebrate RNA virosphere. Nature. 2016;540:539–543. doi: 10.1038/nature20167. [DOI] [PubMed] [Google Scholar]
- 67.Fiers M, et al. Genetic diversity of Rhizoctonia solani associated with potato tubers in France. Mycologia. 2011;103:1230–1244. doi: 10.3852/10-231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.