Chemical ecology is the study of the chemical languages, cues, and mechanisms controlling interactions among living beings, including communication among individuals of the same species and between organisms and their environment. Organisms use chemicals to lure their mates, associate with symbionts, deter enemies, and fend off pathogens (1). Since the identification of the silkworm moth sex pheromone almost six decades ago (2), chemical ecologists have been deciphering hundreds of these “Rosetta Stones” (3) by using bioassay-guided protocols. This conventional chemical ecology approach is based on an invasive process of extracting secretions from chemical signal (semiochemical) senders (e.g., female moths), separating extracts into fractions, using receivers (e.g., male moths) to assist in the identification of active ingredients and, finally, by elucidating chemical structures and synthesis. The state-of-the-art techniques in chemical ecology have reduced analysis to even single individuals in many cases, but it is still too invasive for studying endangered or vulnerable species. In PNAS (4), a multidisciplinary group of scientists from China, Italy, and France apply tools of reverse chemical ecology (5) to study chemical communication in the giant panda, Ailuropoda melanoleuca, a vulnerable species endemic to China.
The giant panda has an obligate bamboo diet and a carnivorous digestive system (6), which leads to a sedentary life with a limited reproduction rate, resulting in only a single offspring every other year. This mismatch of lifestyle and physiology coupled with fragmented habitats in its native environment in southwest China placed the giant panda on a list of endangered species until last year. Conservation campaigns would benefit from understanding at the molecular level how the giant pandas communicate among themselves and with the environment. Zhu et al. (4) use genomics, proteomics, functional analysis, and structural biology to get a better understanding of chemical communication and host–plant interactions in the giant panda. First, the authors analyze the panda genome to identify putative odorant-binding protein (OBP) genes. OBPs have been identified by two independent groups almost simultaneously in the sensillum lymph of moths (7) and bovine nasal mucous (8). Their role in olfaction is still a matter of considerable debate, but it has been well established that, at a minimum, they are carriers of odorants from the external environment (air) through the aqueous compartments surrounding odorant receptors. In insects, OBP expression is more restricted to the sensillum lymph surrounding receptors (9), but in vertebrates OBPs are also involved in transporting semiochemicals from the site of production to the external environment (10). In short, in vertebrates OBPs are involved in the delivery and uptake of semiochemicals. Zhu et al. (4) identify in the panda genome two OBPs, AimelOBP3 and AimelOBP5, of particular interest, given their sequence similarities to a salivary protein involved in releasing and detecting pig pheromone (10) and a human nose OBP (11), respectively. By using proteomics approaches and samples collected during regular health examinations, Zhu et al. (4) demonstrate that indeed these proteins are expressed in both the nasal mucus and saliva of the giant panda. Functional analysis showed that these two proteins have different binding preferences. AimelOBP5 has a binding preference for fatty acids and no binding to aldehydes and most plant volatile compounds, including those derived from bamboo. In contrast, AimelOPB3 displays a high affinity for long-chain unsaturated aldehydes and natural terpenoids. Although constituents of pheromones of the giant panda are yet to be identified, these findings suggest a possible parallel with the Asian elephant, which uses (Z)-7-dodecen-1-yl acetate, a common constituent of moth sex pheromone systems (12), as their own sex pheromone (13). Additionally, the high affinity of AimelOBP3 to the terpenoid cedrol is of particular interest, because this semiochemical is a key constituent of the bouquet emitted by spring bamboo shoots (14). Semiochemicals that attract the giant panda to bamboo (plant kairomone) may help conservation efforts by improving attraction and enticing the giant panda to feed on supplemental nutrition, such as “the panda bread” (15). On the other hand, panda-produced odorants, particularly male-emitted pheromones, may be invaluable in conservation efforts. One possible application is the use of male-produced odorants (pheromones) to enhance sexual motivation (15).
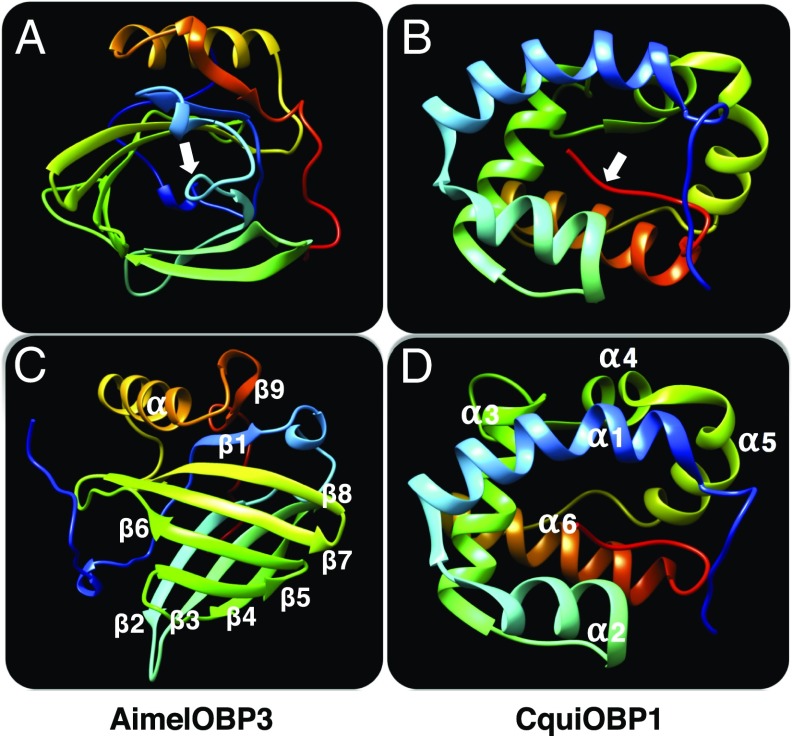
To gain more insights into the putative pheromones carried by AimelOBP3, the author studied the 3D structure of AimelOBP3. Like other vertebrate OBPs, AimelOBP3 belongs to the lipocalin family, with a β-barrel formed by eight-stranded, antiparallel β-sheets, which are rolled up in a cylindrical shape (Fig. 1A). An α-helix flanking the barrel is followed by a ninth β-sheet and an unstructured C terminus (Fig. 1C). A loop in the N terminus covers the binding pocket. Whereas insect OBPs require three disulfide bridges for proper folding (16), AimelOBP3 needs only one disulfide bond to link β-strand-4 to the C-terminal segment (16). Although functionally similar, insect OBPs have a completely different folding. As exemplified by an OBP from the southern house mosquito, Culex quinquefasciatus, CquiOBP1 (17), insect OBPs are helical-rich (Fig. 1B), with binding pockets covered by a C-terminal segment. Further analysis by Zhu et al. (4) shows that (Z)-11-tetradecen-1-yl acetate appears to be an excellent ligand for AimelOBP3, thus suggesting that the giant panda pheromone system might involve this or related aldehydes. Rome was not built in a day, nor does the article by Zhu et al. (4) have answers to all of the questions, but it does shed light on chemical communication of the giant panda and possible applications in conservation biology.
Fig. 1.
Structures of the odorant-binding proteins from the giant panda, AimelOBP3 (4), and the southern house mosquito, Cx. quinquefasciatus, CquiOBP1 (17). Whereas AimelOBP3 (A and C), like other vertebrate OBPs, show a lipocalin folding, insect OBPs like CquiOBP1 (B and D) are helical-rich. The binding cavity of the former is covered by a loop closer to the N terminus, whereas the binding pocket of the latter is covered by an unstructured segment in the C terminus (see arrows in A and B). Figure prepared with University of California, San Francisco Chimera software. Rainbow colored representations with N and C terminals in blue and red, respectively.
For the last six decades, chemical ecology has unraveled intricacies in animal communication and contributed significantly to improving the human condition. Sex pheromones and other semiochemicals have been used for surveillance (18), mass trapping,
Zhu et al. use genomics, proteomics, functional analysis, and structural biology to get a better understanding of chemical communication and host–plant interactions in the giant panda.
attract-and-kill (19), and mating disruption (20) strategies aimed at insect pests as well as insects of medical importance (21). The savings for the environment for reduced use of agricultural chemicals are enormous and the direct benefits to growers are tangible (22). Molecules that have a signaling or defensive value in nature, such as ivermectin, cyclosporine, FK-506, and taxol, prove to be useful to humans (1). Additionally, chemical ecology may serve conservation biology. Pheromone-based monitoring is already used for assessing the conservation status of many threatened species (23, 24). With their reverse and noninvasive approach, Zhu et al. (4) pave the way for chemical ecology to serve conservation biology by assisting in ongoing programs aimed at saving the fragile population of merely 1,864 giant pandas remaining in the wild (25).
Supplementary Material
Acknowledgments
The author’s research is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health Grants R01AI095514 and R21AI128931.
Footnotes
The author declares no conflict of interest.
See companion article on page E9802.
References
- 1.Eisner T, Meinwald J. National Academy of Sciences . Chemical Ecology: The Chemistry of Biotic Interaction. National Academy Press; Washington, DC: 1995. [Google Scholar]
- 2.Butenandt A, Beckmann R, Stamm D, Hecker E. Uber den Sexual-Lockstoff des Seidenspinners Bombyx mori—Reindarstellung und Konstitution. Z Naturforsch Pt B. 1959;14:283–284. [Google Scholar]
- 3.Leal WS. Deciphering the Rosetta Stone of insect chemical communication. American Entomologist. 2014;60:223–230. [Google Scholar]
- 4.Zhu J, et al. Reverse chemical ecology: Olfactory proteins from the giant panda and their interactions with putative pheromones and bamboo volatiles. Proc Natl Acad Sci USA. 2017;114:E9802–E9810. doi: 10.1073/pnas.1711437114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leal WS. 2005. Pheromone reception. The Chemistry of Pheromones and Other Semiochemicals II, Topics in Current Chemistry, ed Schulz S (Springer, Berlin), Vol 240, pp 1–36.
- 6.Nie YG, et al. Obligate herbivory in an ancestrally carnivorous lineage: The giant panda and bamboo from the perspective of nutritional geometry. Funct Ecol. 2015;29:26–34. [Google Scholar]
- 7.Vogt RG, Riddiford LM. Pheromone binding and inactivation by moth antennae. Nature. 1981;293:161–163. doi: 10.1038/293161a0. [DOI] [PubMed] [Google Scholar]
- 8.Pelosi P, Baldaccini NE, Pisanelli AM. Identification of a specific olfactory receptor for 2-isobutyl-3-methoxypyrazine. Biochem J. 1982;201:245–248. doi: 10.1042/bj2010245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leal WS. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 2013;58:373–391. doi: 10.1146/annurev-ento-120811-153635. [DOI] [PubMed] [Google Scholar]
- 10.Marchese S, Pes D, Scaloni A, Carbone V, Pelosi P. Lipocalins of boar salivary glands binding odours and pheromones. Eur J Biochem. 1998;252:563–568. doi: 10.1046/j.1432-1327.1998.2520563.x. [DOI] [PubMed] [Google Scholar]
- 11.Briand L, et al. Evidence of an odorant-binding protein in the human olfactory mucus: Location, structural characterization, and odorant-binding properties. Biochemistry. 2002;41:7241–7252. doi: 10.1021/bi015916c. [DOI] [PubMed] [Google Scholar]
- 12.Ando T, Inomata S, Yamamoto M. Lepidopteran sex pheromones. Top Curr Chem. 2004;239:51–96. doi: 10.1007/b95449. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen LE, Lee TD, Roelofs WL, Zhang A, Daves GD., Jr Insect pheromone in elephants. Nature. 1996;379:684. doi: 10.1038/379684a0. [DOI] [PubMed] [Google Scholar]
- 14.Chung MJ, Cheng SS, Lin CY, Chang ST. Profiling of volatile compounds of Phyllostachys pubescens shoots in Taiwan. Food Chem. 2012;134:1732–1737. doi: 10.1016/j.foodchem.2012.03.120. [DOI] [PubMed] [Google Scholar]
- 15.Bian X, et al. Exposure to odors of rivals enhances sexual motivation in male giant pandas. PLoS One. 2013;8:e69889. doi: 10.1371/journal.pone.0069889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leal WS, Nikonova L, Peng G. Disulfide structure of the pheromone binding protein from the silkworm moth, Bombyx mori. FEBS Lett. 1999;464:85–90. doi: 10.1016/s0014-5793(99)01683-x. [DOI] [PubMed] [Google Scholar]
- 17.Mao Y, et al. Crystal and solution structures of an odorant-binding protein from the southern house mosquito complexed with an oviposition pheromone. Proc Natl Acad Sci USA. 2010;107:19102–19107. doi: 10.1073/pnas.1012274107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suckling DM. Monitoring for surveillance and management. In: Allison JD, Carde RT, editors. Pheromone Communication in Moths: Evolution, Behavior, and Application. Univ of California Press; Oakland, CA: 2016. pp. 337–347. [Google Scholar]
- 19.Cork A. Pheromones as management tools mass trapping and lure-and-kill. In: Allison JD, Carde RT, editors. Pheromone Communication in Moths: Evolution, Behavior, and Application. Univ of California Press; Oakland, CA: 2016. pp. 349–363. [Google Scholar]
- 20.Evenden M. Mating disruption of moth pests in integrated pest management a mechanistic approach. In: Allison JD, Carde RT, editors. Pheromone Communication in Moths: Evolution, Behavior, and Application. Univ of California Press; Oakland, CA: 2016. pp. 365–393. [Google Scholar]
- 21.Mescher MC, De Moraes CM. Editorial overview: Ecology: The chemical ecology of human disease transmission by mosquito vectors. Curr Opin Insect Sci. 2017;20:v–vi. doi: 10.1016/j.cois.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Bento JM, et al. How much is a pheromone worth? F1000 Res. 2016;5:1763. doi: 10.12688/f1000research.9195.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsson MC, Svensson GP. Pheromone monitoring of rare and threatened insects: Exploiting a pheromone-kairomone system to estimate prey and predator abundance. Conserv Biol. 2009;23:1516–1525. doi: 10.1111/j.1523-1739.2009.01263.x. [DOI] [PubMed] [Google Scholar]
- 24.Musa N, et al. Using sex pheromone and a multi-scale approach to predict the distribution of a rare saproxylic beetle. PLoS One. 2013;8:e66149. doi: 10.1371/journal.pone.0066149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Wildlife Federation 2017 Facts. Available at https://www.worldwildlife.org/species/giant-panda. Accessed October 10, 2017.