Abstract
Background
Interleukin 17 (IL-17) is produced by highly inflammatory Th17 cells and has been implicated in pathophysiology of depression. IL-17 putatively disrupts the blood brain barrier and affects dopamine synthesis whereas dopamine has been shown to decrease Th17 cell-mediated immune response. Nevertheless, whether IL-17 can predict differential treatment outcome with antidepressants modulating dopaminergic transmission is unknown.
Methods
IL-17 and other T cell and non-T cell markers (Th1, Th2 and non-T cell markers) were measured with the Bioplex Pro™ human cytokine 27-plex kit in the Combining Medications to Enhance Depression Outcomes (CO-MED) trial participants who provided baseline plasma and were treated with either bupropion plus escitalopram (bupropion-SSRI), escitalopram plus placebo (SSRI monotherapy), or venlafaxine plus mirtazapine (n=166). Differential changes in symptom severity and side-effects based on levels of IL-17 and other T and non-T cell markers were tested using a treatment-arm-by-biomarker interaction in separate repeated measures mixed model analyses. Subsequent analyses stratified by treatment arm were conducted for those markers with a significant interaction.
Results
There was a significant treatment-arm-by- IL-17 interaction for depression severity (p=0.037) but not for side-effects (p=0.28). Higher baseline IL-17 level was associated with greater reduction in depression severity (effect size=0.78, p=0.008) in the bupropion-SSRI but not the other two treatment arms. Other T and non-T cell markers were not associated with differential treatment outcomes.
Conclusion
Higher baseline levels of IL-17 are selectively associated with greater symptomatic reduction in depressed patients treated with bupropion-SSRI combination.
Keywords: Inflammation, Interleukin 17, T cells, Antidepressant, Moderator, Bupropion, Dopamine
1 Introduction
Inflammation is implicated in both the pathophysiology of depression as well as the lack of response to currently available antidepressant medications (1, 2). The role of Interleukin 17 [IL-17, initially identified in 1995 (3)] and the IL-17 producing T-helper (Th) lymphocytes [Th17, identified as distinct from the common Th1 and Th2 sub-types in 2005 (4, 5)] in systemic inflammation have gained recent attention (6, 7). Their role in pathophysiology of depression was suggested recently by Beurel et al. in animal experiments where 1) levels of Th17 cells increased in brain after learned helplessness and chronic resistant stress paradigms; 2) infusion of Th17 cells resulted in depression-like behaviors; 3) infusion of anti-IL-17 antibody or inhibition of retinoid-related orphan receptor- γT (RORγT, a transcription factor essential for differentiation of naïve CD4+ T cells to Th17 cells) with SR1001 mitigated the effects of Th17 cell infusion; and 4) RORγT knockout mice exhibited marked resistance to the learned helplessness paradigm (8). In human studies, Chen et al. found that depressed patients (n=40), as compared to control subjects (n=30), had significantly higher Th17 cells and lower regulatory T cells in peripheral circulation, along with higher levels of RORγT mRNA in peripheral blood lymphocytes (9). Elevated levels of IL-17 have also been associated with anxiety in rheumatoid arthritis patients (n=18) (10). Similarly, the role of Th17 cell mediated immune response in antidepressant treatment resistance was suggested by Hennings et al. who reported in two separate samples that lower pre-treatment levels of ROR alpha mRNA, a transcription factor also involved in differentiation of naïve CD4+ T cells into Th17 cells (6), was associated with better response to antidepressant treatment (11). Conversely, some reports with small sample sizes have failed to find significant association between peripheral IL-17 levels and depression severity (n=47) (12) or change in IL-17 levels with antidepressants [venlafaxine (n=7), paroxetine (n=6), mirtazapine (n=3), bupropion (n=3), and fluoxetine (n=2)] (13) suggesting need for studies with larger sample size.
A biological basis for the role of IL-17 in predicting differential antidepressant response may be related to its effect on central nervous system (CNS). These effects include formation of reactive oxygen species by binding of IL-17 to its receptor on endothelial cells of the blood brain barrier (BBB) (14), infiltration of peripheral immune cells (15) with potential CNS inflammation and neuronal damage (16), induction of nitric oxide synthase (NOS) (17), and increased production of nitric oxide and inflammatory cytokines by microglia (18). These inflammatory changes may result in reduced synthesis of dopamine by diversion of tetrahydrobiopterin, an essential cofactor of NOS and tyrosine hydroxylase, away from rate limiting step (conversion of tyrosine to L-3,4-dihydroxyphenylalanine) in dopamine synthesis [reviewed in detail by Miller et al. (19)]. On the contrary, increasing dopamine, in cultured human peripheral blood mononuclear cells, suppresses IL-17 levels (20). Similarly, pramipexole, a dopamine agonist, inhibits production of IL-17 in animal models of experimental autoimmune encephalitis (21). Furthermore, use of antipsychotic medications that block dopamine receptors such as amisulpiride (20), chlorpromazine, haloperidol, clozapine, and quetiapine (22) has been associated with elevated IL-17 levels in humans. Bupropion, an antidepressant medication that inhibits dopamine reuptake and stimulates presynaptic release of dopamine and norepinephrine (23–25), has been shown to reduce IL-17 mediated inflammatory response and joint swelling in the murine antigen-induced arthritis model (26). Additionally, administration of bupropion reduces the levels of pro-inflammatory cytokines produced by Th1 cells (interferon gamma and tumor necrosis factor alpha) after lipopolysaccharide activation in mice (27). In contrast, administration of SSRIs increases the levels of inflammatory cytokines produced by non-T cells (IL-1beta, IL-6) and Th1 cells (interferon gamma, and tumor necrosis factor alpha) in the frontal cortex (28). Serotonergic antidepressants, such as citalopram, predominantly suppress T cells in thymus producing IL-2 and IL-4 and not the T cells producing IL-17 (29). Taken together, these findings suggest that T cell related inflammatory markers in general and IL-17 in particular can be used to predict differential response to serotonergic vs. non-serotonergic antidepressants. Consistent with this, two recent reports found that depressed patients with a pro-inflammatory state as suggested by elevated pre-treatment levels of C-reactive protein, respond poorly to predominantly serotonergic antidepressants (such as selective serotonin reuptake inhibitors or SSRIs) as compared to non-serotonergic antidepressants that modulate dopamine neurotransmission (such as nortriptyline and bupropion) (30, 31).
The primary aim of this report is to test the hypothesis that baseline levels of IL-17 can be used to predict differential treatment outcomes with bupropion vs. other antidepressant medications. However, we also evaluated the potential role of other inflammatory cytokines in addition to IL-17, such as those related to Th1 and Th2 as well as non-T cell immune markers. Using data from the Combining Medications to Enhance Depression Outcomes (CO-MED) trial (32), which compared escitalopram plus placebo, bupropion plus escitalopram, and venlafaxine plus mirtazapine treatment arms, we 1) estimated the association of IL-17 levels with baseline clinical and sociodemographic characteristics, 2) conducted factor analyses of Th1, Th2 and non Th1/Th2 cytokines or chemokines to reduce the number of analyses, and 3) tested for differential outcomes among the three treatment arms based on pre-treatment IL-17 and other above-mentioned factor levels. In contrast to the current clinical practice of “trial and error” where antidepressant medication selection is based mostly on (33) subjective factors such as patient and provider preference, objective measurements of IL-17 levels and the subsequent response to bupropion as compared to SSRIs in depressed patients can lead to personalized medicine approaches with overall improved treatment outcomes.
2 Materials and Methods
2. 1 Study Overview
Data for this report was obtained from the CO-MED trial which has been described in detail by Rush et al. (32). Participants (n=665) were randomly assigned after stratification for site to the following treatment arms: SSRI monotherapy (escitalopram plus placebo), bupropion-SSRI combination (sustained-release [SR] bupropion plus escitalopram), and venlafaxine-mirtazapine combination (extended-release [XR] venlafaxine plus mirtazapine). The analytic sample of this report includes a sub-set of CO-MED trial participants who provided plasma samples at baseline. Baseline plasma was collected as part of a separate add-on biomarker study which was optional and required an additional consent. Hence, all subjects in this report provided a written informed consent for participation in the main trial as well as an additional optional consent for the biomarker collection. Thus, the number of plasma samples (n=166) collected at baseline was only a sub-set of the total number of CO-MED trial participants (n=665). Those participants who did not provide plasma (n=499) at baseline were younger (mean age=44.51 years vs. 42.11, p =0.03) and had lower use of statin medication (20.5% vs 13.6%, p=0.03) as compared to the analytic sample of this report. The two groups did not differ on any other baseline clinical and sociodemographic features as detailed in Supplementary Table 1. Additionally, as participation in the continuation-phase of CO-MED was censured for those participants with inadequate response (32), we restricted the analyses only to the acute-phase visits (baseline and weeks 1, 2, 4, 6, 8, 10, and 12). The CO-MED trial used broad inclusion and exclusion criteria, (fully listed at https://clinicaltrials.gov/ct2/show/NCT00590863) while recruiting from psychiatric and primary care clinics that were chosen to ensure adequate minority representation and a diverse participant group (32). The trial was reviewed and approved by the Institutional Review Boards at UT Southwestern Medical Center at Dallas, the University of Pittsburgh Data Coordinating Center, each participating regional center, and all relevant clinics.
2.2 Medications
Participants in all three treatment arms received two types of pills in single blind fashion (study personnel knew of both pill types, but participants knew only the first pill type). Dosage adjustments were made during the first 8 weeks of participation using principles of measurement based care (MBC), with dose increases permitted only if side effects were tolerable and depression severity was not adequately controlled. Dose escalation regime as well as mean doses of medications in each treatment arm have been previously described in detail by Rush et al. (32). Participants in the SSRI monotherapy treatment arm were started on escitalopram at 10 mg/day and placebo was added at week 2 as the second pill type. At the end of 12 weeks, the mean escitalopram dose was 17.6 mg/day and mean placebo dose was 1.4 pills/day. For the bupropion-SSRI combination treatment arm, participants were started on 150 mg/day of bupropion SR and titrated to 300mg/day at week 1 and escitalopram 10 mg/day was added as the second pill type at week 2. At the end of 12 weeks, mean bupropion SR dose was 324.0 mg/day and mean escitalopram dose was 14.0 mg/day. Participants in the venlafaxine-mirtazapine treatment arm were started on venlafaxine XR which was titrated from 37.5 mg/day to 150 mg/day at week 1 visit, and mirtazapine 15 mg/day was added at week 2 as the second pill type. At the end of 12 weeks, the mean venlafaxine XR dose was 207.6 mg/day and mean mirtazapine dose was 25.3 mg /day.
2.3 Assessments
At baseline, participants provided sociodemographic information. At baseline and all treatment visits, participants filled out the 16-item Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR) scale and Frequency, Intensity, and Burden of Side Effect Rating Scale (FIBSER).
Clinical and sociodemographic characteristics at baseline
These included age, gender, race, Hispanic ethnicity, onset of depression before age 18, presence of suicidal ideations at baseline, presence of rheumatoid arthritis as a comorbid medical condition, presence of anxious features (derived from the 17-item Hamilton Rating Scare for Depression, HRSD17), melancholic features, atypical features, use of non-steroidal anti-inflammatory drugs (NSAIDs), use of statin medications, and baseline depression severity.
Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR)
This commonly used scale has 16 items, each of which includes 4 choices that are scored from 0–3. A total score is calculated from nine of these 16 items (consistent with the nine criterion symptom domains of major depressive disorder or MDD) leading to a range of 0–27 (34). Both measures correlate highly (0.86–0.93) with HRSD17 (35). In previous reports, the reported Cronbach’s α of QIDS-SR has ranged from 0.86 to 0.87 (34–36). In the CO-MED trial, the QIDS-SR served as the primary depression symptom severity outcome measure.
Frequency, Intensity, and Burden of Side Effect Rating Scale (FIBSER)
This commonly used side effect rating scale was initially developed to document the frequency, intensity, and burden of side effects in the large (n=4041) multisite Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study (37). The three items of this self-report measure are rated on a scale of 0–6, with higher numbers reflecting greater severity of side effects. The Cronbach’s α of FIBSER in STAR*D ranged from 0.91–0.93 at different study visits (weeks 2, 4, 6, 9, 12, and 14) (37). The sum of three items has been used as an overall score of FIBSER (38).
2.4 Measurement of Interleukin-17 and other inflammatory biomarkers
Peripheral venous samples from CO-MED trial participants (n=166) were collected in EDTA tubes and transported overnight to the Biologic Core of the National Institute of Mental Health Repository and Genomics Resource (NIMH RGR) where plasma was extracted by centrifuging blood samples at 2500 rpm for 10 minutes at room temperature, aliquoted, and stored at −80°C. All samples for this report were obtained from the NIMH RGR core and transported to UT Southwestern on dry ice for storage at −80°C until immediately prior to assays without any freeze/thaw cycles. Levels of IL-17 and other biomarkers were measured in all samples at the same time, blinded to treatment allocation and outcomes by the Microarray Core at UT Southwestern Medical Center using the Bioplex Pro™ human cytokine standard 27-plex kit (Bio-Rad Laboratories, Hercules, CA, USA) with a Bio-plex® 200 instrument that was equipped with Bio-Plex Manager software, version 6.0 (Bio-Rad Laboratory, Hercules, CA, USA). This commonly used (39, 40) 27-plex kit measures IL-17, Th1 (interferon gamma and tumor necrosis factor alpha), Th2 (IL-4, IL-5, IL-9, and IL-13), and non Th1/Th2 (IL-1 beta, IL-1 receptor antagonist, IL-8, IL-6, and macrophage inflammatory protein (MIP) 1 alpha and beta) markers, which were interpreted only if the intra- and inter-assays coefficients of variation were less than 10% of detection limits (or precision range) specified by manufacturer. The levels of IL-17 and other immune markers are expressed in pg/ml after correcting for 4-fold dilution using the standards provided in the kit (Bio-Rad Laboratory, Hercules, CA, USA). Please see Supplementary Table 4 for the upper and lower detection limit for each marker in the 27-plex kit as well as their observed mean and standard deviations.
2.5 Statistical Analyses
We used log-transformation for biomarkers as indicated. In a multivariate analysis, we tested the association of baseline IL-17 level with clinical and sociodemographic characteristics using a general linear model. We used separate repeated measures mixed model analyses with QIDS-SR and FIBSER total score to test for treatment arm-by-baseline IL-17 interaction after controlling for select baseline covariates (age, gender, and BMI) using methods outlined by Uher et al. (31). A significant treatment arm-by-IL-17 interaction suggests that the outcomes in three treatment arms differed on the basis of baseline IL-17 levels. Hence, this was the interaction of interest in our study. We used stratified analyses for each treatment-arm to quantify the change in outcomes (QIDS-SR and FIBSER) based on IL-17 levels, consistent with the approach of Uher et al. (31). To visualize the treatment arm-by-IL-17 interaction, we plotted the estimates of dependent variable (QIDS-SR and/or FIBSER) over the course of acute-phase of CO-MED trial against the baseline plasma biomarker level.
As secondary analyses, we undertook factor analyses with Th1, Th2, and non Th1/Th2 cytokines that were interpretable (coefficients of variation <10%). For easy comparison of factor loadings across different biomarkers, we converted baseline biomarker levels into standardized score. For each group of cytokines (Th1, Th2, and non Th1/Th2), we used PROC FACTOR as implemented in SAS with varimax rotation (41). We then repeated the mixed model analyses as previously described for IL-17 using the factor scores from these analyses.
We used SAS version 9.3 for all our analyses and set the threshold of significance at p <0.05.
3 Results
Of the 665 participants in CO-MED, plasma samples were available from 166 participants which constitute the analytic sample of this report. The mean (SD) concentration of IL-17 at baseline was 25.9 (10.4) pg/ml. Participants in all three treatment arms did not differ on sociodemographic variables, except participants in the venlafaxine-mirtazapine combination were significantly younger, as shown in Table 1. In multivariate analyses, we did not find any significant effect of baseline sociodemographic and illness variables on baseline IL-17 levels (Supplementary Table 2).
Table 1.
Baseline sociodemographic and clinical characteristics of CO-MED trial participants (n = 166) who provided plasma at baseline
| Total | SSRI monotherapy | Bupropion-SSRI combination | Venlafaxine-mirtazapine combination | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 166 | 51 | 55 | 60 | ||||||
| Categorical variables | N | % | N | % | N | % | N | % | χ2 (df) | |
| Sex | 0.13 (2) | 0.94 | ||||||||
| Male | 49 | 29.5 | 16 | 31.4 | 16 | 29.1 | 17 | 28.3 | ||
| Female | 117 | 70.5 | 35 | 68.6 | 39 | 70.9 | 43 | 71.7 | ||
| Race | 4.60 (4) | 0.29 | ||||||||
| White | 107 | 64.5 | 27 | 52.9 | 39 | 70.9 | 41 | 68.3 | ||
| Black | 46 | 27.7 | 18 | 35.3 | 12 | 21.8 | 16 | 26.7 | ||
| Other | 13 | 7.8 | 6 | 11.8 | 4 | 7.3 | 3 | 5 | ||
| Hispanic ethnicity | 1.13 (2) | 0.57 | ||||||||
| No | 139 | 83.7 | 43 | 84.3 | 48 | 87.3 | 48 | 80 | ||
| Yes | 27 | 16.3 | 8 | 15.7 | 7 | 12.7 | 12 | 20 | ||
| Monthly income | 1.26 (4) | 0.87 | ||||||||
| <$2000 | 92 | 61.3 | 26 | 56.5 | 29 | 61.7 | 37 | 64.9 | ||
| $2000 – $4000 | 33 | 22 | 12 | 26.1 | 9 | 19.15 | 12 | 21.1 | ||
| >$4000 | 25 | 16.7 | 8 | 17.4 | 9 | 19.15 | 8 | 14 | ||
| Education | 4.20 (4) | 0.38 | ||||||||
| <12 years | 24 | 14.5 | 4 | 7.8 | 11 | 20.0 | 9 | 15 | ||
| 12 –15 years | 98 | 59 | 35 | 68.6 | 29 | 52.7 | 34 | 56.7 | ||
| >15 years | 44 | 26.5 | 12 | 23.5 | 15 | 27.3 | 17 | 28.3 | ||
| Anxious features | 120 | 72.3 | 33 | 64.7 | 43 | 78.2 | 44 | 73.3 | 2.45 (2) | 0.29 |
| Atypical features | 35 | 21.1 | 10 | 19.6 | 12 | 21.8 | 13 | 21.7 | 0.10 (2) | 0.95 |
| Melancholic Features | 51 | 30.7 | 14 | 27.5 | 17 | 30.9 | 20 | 33.3 | 0.45 (2) | 0.80 |
| Suicidal ideation at baseline | 94 | 56.6 | 27 | 52.9 | 31 | 56.4 | 36 | 60.0 | 0.56 (2) | 0.76 |
| Onset of depression before age 18 | 67 | 40.4 | 18 | 35.3 | 24 | 43.6 | 25 | 41.7 | 0.83 (2) | 0.66 |
| Continuous variables | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F value (df) | |
| Mean age in years | 44.5 | 12.0 | 47.0 | 11.8 | 46.3 | 12.1 | 40.8 | 11.2 | 4.92 (2) | 0.01 |
| Mean QIDS-SR | 15.6 | 4.1 | 15.7 | 3.4 | 15.0 | 4.7 | 16.0 | 4.1 | 0.97 (2) | 0.38 |
| Mean IL-17 | 103.7 | 41.4 | 105.3 | 47.5 | 106.5 | 37.6 | 99.8 | 39.6 | 1.25 (2) | 0.29 |
| Mean log IL-17 | 4.6 | 0.4 | 4.6 | 0.5 | 4.6 | 0.3 | 4.5 | 0.4 | 0.60 (2) | 0.55 |
CO-MED is Combining Medications to Enhance Depression Outcomes, SD is standard deviation, n is number, df is degrees of freedom, SSRI is selective serotonin reuptake inhibitor, QIDS-SR is Quick Inventory of Depressive Symptomatology Self-Report, and IL-17 is interleukin 17.
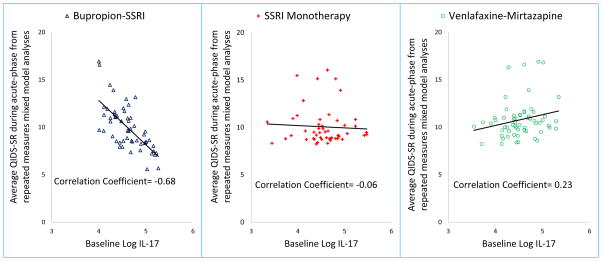
Average QIDS-SR (least square means) was obtained from repeated measures mixed model analyses of all available visits during the acute-phase of Combining Medications to Enhance Depression Outcomes (CO-MED) trial for the following three treatment arms: selective serotonin reuptake inhibitor (SSRI) monotherapy, bupropion-SSRI combination, and venlafaxine-mirtazapine combination and plotted against the log of interleukin 17 (IL-17) level at baseline.
We found a significant effect of log-IL-17-by-treatment-arm interaction for change in depression severity (F=3.36, df=2, 157, p=0.037) but not for total side-effect burden (F=1.29, df=2, 148, p=0.28) after controlling for gender, age, BMI, visit, and visit-by-treatment arm interaction, as shown in Table 2. Due to significant interaction term for QIDS-SR, we conducted subsequent analyses stratified by treatment arm and found that higher log of IL-17 levels at baseline predicted lower depression severity over the course of acute-phase treatment only in bupropion-SSRI combination treatment arm (Cohen’s d effect size=0.78, estimated difference in QIDS-SR for 1 unit change in log of IL-17 (est.)= −4.31, standard error (SE)=1.56, p=0.008) and not in SSRI monotherapy (est.= −0.21, SE=1.11, p=0.85) or venlafaxine-mirtazapine combination treatment arm (est.=0.22, SE=1.44, p=0.88), as seen in Table 3. As shown in Figure 1, we found a linear relationship where QIDS-SR over the course of acute phase decreased with increasing levels of baseline IL-17 level, only in bupropion-SSRI combination treatment (correlation coefficient=−0.68) and not in SSRI monotherapy (r-squared=−0.06) or venlafaxine-mirtazapine combination (r-squared=0.23) treatment arms.
Table 2.
Differential effect on self-reported depression severity and side-effects of baseline levels of Interleukin-17 and other inflammatory markers based on treatment arms in CO-MED trial
| Depression Severity* | Side-effects* | |||||
|---|---|---|---|---|---|---|
| F Value | df | p-Value | F Value | df | p-Value | |
| Interleukin 17 (IL-17) | ||||||
| Treatment arm | 3.22 | 2, 157 | 0.043 | 1.19 | 2, 148 | 0.31 |
| Log of baseline IL-17 level | 1.97 | 1, 157 | 0.16 | 2.96 | 1, 148 | 0.09 |
| Log-IL-17-by-treatment arm interaction | 3.36 | 2, 157 | 0.037 | 1.29 | 2, 148 | 0.28 |
| Th1 cytokines factor | ||||||
| Treatment arm | 1.07 | 2, 157 | 0.34 | 5.56 | 2, 148 | 0.005 |
| Baseline Th1 factor | 0.74 | 1, 157 | 0.39 | 0.19 | 1, 148 | 0.66 |
| Th1-factor-by-treatment arm interaction | 0.16 | 2, 157 | 0.85 | 0.09 | 2, 148 | 0.91 |
| Th2 cytokines factor | ||||||
| Treatment arm | 1.39 | 2, 156 | 0.25 | 5.90 | 2, 147 | 0.003 |
| Baseline Th2 cytokines factor | 1.00 | 1, 156 | 0.32 | 0.21 | 1, 147 | 0.65 |
| Th2-factor-by-treatment arm interaction | 0.61 | 2, 156 | 0.55 | 0.54 | 2, 147 | 0.58 |
| Non Th1/Th2 cytokines factor | ||||||
| Treatment arm | 1.10 | 2, 151 | 0.34 | 5.92 | 2, 143 | 0.003 |
| Baseline Non Th1/Th2 cytokines factor | 1.56 | 1, 151 | 0.21 | 0.05 | 1, 143 | 0.83 |
| Non-Th1/Th2-factor-by-treatment arm interaction | 0.61 | 2, 151 | 0.55 | 0.21 | 2, 143 | 0.81 |
| Th1:Th2 cytokines factor ratio | ||||||
| Treatment arm | 1.08 | 2, 156 | 0.34 | 5.06 | 2, 147 | 0.008 |
| Baseline Th1:Th2 factor ratio | 0.35 | 1, 156 | 0.56 | 1.34 | 1, 147 | 0.25 |
| Th1:Th2-factor-by-treatment arm interaction | 0.43 | 2, 156 | 0.65 | 0.32 | 2, 147 | 0.73 |
CO-MED is Combining Medications to Enhance Depression Outcomes, QIDS-SR is Quick Inventory of Depressive Symptomatology Self-Report, and FIBSER is Frequency, Intensity, and Burden of Side Effects Rating Scale, Th1 is T-helper cell type 1, Th2 is T-helper cell type 2, Th1 cytokines include interferon gamma and tumor necrosis factor alpha, Th2 cytokines include interleukin (IL) 4, IL-5, IL-9, and IL-13, non Th1/Th2 cytokines include IL-1 beta, IL-1 receptor antagonist, IL-8, IL-6, and macrophage inflammatory protein (MIP) 1 alpha and beta.
Gender, age, BMI, visit, and visit-by-treatment arm interaction were covariates in all mixed model analyses.
Table 3.
Differential effect of interleukin 17 on depression severity based on treatment arm in CO-MED trial
| QIDS-SR Estimate* | SE | F Value | df | p Value | |
|---|---|---|---|---|---|
| Log of Interleukin 17 | |||||
| SSRI monotherapy | −0.21 | 1.11 | 0.04 | 1, 46 | 0.85 |
| Bupropion-SSRI combination | −4.31 | 1.56 | 7.65 | 1, 50 | 0.008 |
| Venlafaxine-mirtazapine combination | 0.22 | 1.44 | 0.02 | 1, 55 | 0.88 |
CO-MED is Combining Medications to Enhance Depression Outcomes. Estimate obtained from solution for fixed effects in mixed model analyses and represents the estimated difference in self-reported depression severity as measured by Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR) for 1 unit difference in log of Interleukin 17 in each treatment arm.
Figure 1.
With factor analyses, we found that Th1, Th2, and non-Th1/th2 markers loaded on one factor each. See Supplementary Table 3 for detailed factor analysis results. In separate mixed model analyses for QIDS-SR, we found that treatment-arm-by-biomarker factor interactions were not statistically significant for Th1 (F=0.16, df=2, 157, p=0.85), Th2 (F=0.61, df=2, 156, p=0.55), and non-Th1/Th2 cytokines (F=0.61, df=2, 151, p=0.55), nor for Th1:Th2 ratio (F=0.43, df=2, 156, p=0.65). Similarly, treatment-arm-by-biomarker factor interaction were not statistically significant for FIBSER in separate mixed model analyses, as shown in Table 2.
4 Discussion
We have found in a large sample of depressed patients that elevated levels of IL-17 at baseline were selectively associated with greater reduction in depression severity with bupropion-SSRI combination treatment, but not treatment with SSRI monotherapy or venlafaxine-mirtazapine combination. To our knowledge, this is the first study evaluating the moderator effect of baseline levels of IL-17 on antidepressant treatment outcomes. Other inflammatory biomarkers did not predict differential treatment outcomes. We also found that IL-17 levels at baseline did not vary based on sociodemographic variables or clinical characteristics, which is consistent with findings of previous reports (12, 13).
The biological mechanism underlying the differential improvement with bupropion seen in patients with elevated IL-17 may be related its inflammatory effect on the CNS which likely reduces dopamine synthesis and hence, preferentially improves outcome with antidepressants which predominantly affect dopamine neurotransmission, such as bupropion. The effects of inflammation on dopamine are well characterized. As IL-17 is a pro-inflammatory cytokine, our novel findings complement and expand previous reports demonstrating CRP, an inflammatory biomarker, is associated with differential response to antidepressant medications (30, 31). Inflammation is associated with anhedonia in humans (42, 43) and leads to changes in brain dopamine metabolism (44) as well as reductions in effort-based motivation for reward in animal models (45). Hence, our findings are consistent with the potential role of drugs modulating dopaminergic neurotransmission in treatment of depression. For example, application of pramipexole, a dopamine receptor agonist, was reportedly effective in a recent case series of treatment resistant depression patients (46), particularly those patients with elevated IL-17 levels.
Our findings also suggest that depressed patients with low baseline IL-17 have poorer response to bupropion-SSRI treatment as compared to other treatments. This is similar to previous reports demonstrating that depressed patients with low CRP (<1 mg/L) have higher remission rates (57.1%) with SSRI monotherapy as compared to bupropion-SSRI combination (33.3%) (30). The mechanism underlying this potential reduction in SSRI responsiveness with addition of bupropion is unclear and needs to be replicated in future studies. It may be related to the anti-inflammatory effect of bupropion. Raison et al. had previously reported that depressed patients with lower levels of inflammation (CRP <5 mg/L) performed better on placebo as compared to infliximab, a tumor necrosis factor antagonist, and theorized that a minimal level of peripheral inflammation may be necessary for antidepressant response (47). Additionally, lack of association of other baseline Th1, Th2, and non-Th1/th2 cytokine with differential treatment outcomes is significant. While we used a factor analytic approach instead of analyzing individual Th1, Th2 and non-Th1/th2 factors, these findings are consistent with a recent meta-analysis by Strawbridge et al. where they found that baseline CRP, IL-6, TNF alpha, and the composite inflammatory markers were not associated with subsequent treatment outcomes (48).
As a theoretical model to guide antidepressant treatment based on inflammatory biomarkers, Martino et al. have postulated that serotonergic antidepressant medications shift the balance towards Th1 cell mediated immune response, while noradrenergic antidepressants shift the balance towards Th2 cell mediated immune response (49). However, neither does this model include potential effect(s) of antidepressants on Th17 cell mediated immune response, nor does it account for dopaminergic neurotransmission and its bidirectional relationship with inflammation.
Our results have important implications for clinical practice and research. With initial antidepressant treatment, over two-thirds of MDD patients continue to have significant depressive symptoms (50–53). No clinical variables (such as baseline depression severity, atypical features, melancholic features, and obesity) have proven useful in identifying sub-groups of MDD patients who will respond differently to currently available antidepressant medications (32, 54). Hence our findings contribute to the urgent need to identify baseline biological markers which may facilitate effective selection amongst currently available antidepressant treatments (55). Our findings also argue for future investigations of the potential moderator role of IL-17 levels when selecting atypical antipsychotic medications for antidepressant augmentation, as these medications may increase IL-17 levels (22).
Our study has several limitations. This is a secondary analysis on a subset of participants in the CO-MED trial. As identifying biological markers as moderators of treatment outcome was not the primary outcome of the CO-MED trial, we did not a priori test the power necessary to detect a moderator effect of IL-17. Additionally, the immune system is a complex interplay of multiple factors, and focusing predominantly on one marker, IL-17, may have been inadequate. Further, there was limited information available regarding the time of the day for plasma collection as well as average time from blood collection to plasma extraction, factors which may have introduced variability across samples. In light of these limitations, findings from this study should be considered preliminary and pilot in nature. Additionally, by design in the CO-MED trial, each treatment arm contained a medication with serotonergic activity which restricts the interpretation of these findings specifically to bupropion.
5 Conclusions
In conclusion, our study found that elevated levels of IL-17, a pro-inflammatory cytokine, is selectively associated with better clinical outcomes in depressed patients treated with a combination of bupropion and escitalopram as compared to those treated with either escitalopram monotherapy or a combination of venlafaxine and mirtazapine.
Supplementary Material
Acknowledgments
The authors thank the clinical staff at each clinical site for their assistance with this project; all of the study participants; and Eric Nestler, M.D., Ph.D., Carol A. Tamminga, M.D., and Jennifer Furman, Ph.D. for administrative support.
Funding
CO-MED trial was funded by NIMH under contract N01 MH-90003 to the University of Texas Southwestern Medical Center at Dallas (principal investigators, A.J. Rush and M.H. Trivedi). This work was also supported in part through the Center for Depression Research and Clinical Care at UT Southwestern (Principal Investigator: Madhukar H. Trivedi, MD) and Hersh Foundation. Forest Pharmaceuticals, GlaxoSmithKline, Organon, and Wyeth Pharmaceuticals provided medications for CO-MED trial at no cost. The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. NIMH had no role in the drafting or review of the manuscript or in the collection or analysis of the data.
Footnotes
Previous presentation: The findings of this report were presented at the 2016 Annual Meeting of Society of Biological Psychiatry.
Conflict of Interest
Drs. Jha, Minhajuddin, and Gadad as well as Ms. Mayes have no potential conflicts of interest. Dr. Greer has received research funding from NARSAD and honoraria and/or consultant fees from H. Lundbeck A/S and Takeda Pharmaceuticals International, Inc. Dr. Trivedi, is or has been an advisor/consultant and received fee from: Alkermes Inc., Allergan, Arcadia Pharmaceuticals Inc., AstraZeneca, Brintellix, BMS, Cerecor, Eli Lilly & Company, Forest Pharmaceuticals, Global Medical Education Inc., Health Research Associates, Johnson & Johnson, Lundbeck, Medscape, MSI Methylation Sciences Inc., Merck, Naurex Inc., Nestle Health Science – Pamlab Inc., One Carbon Therapeutics, Otsuka America Pharmaceuticals Inc., PamLab, Pfizer Inc., Roche, SHIRE Development, and Takeda Pharmaceuticals Inc. In addition, he has received grants/research support from: National Institute of Mental Health, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Drug Abuse, Agency for Healthcare Research and Quality, and Johnson & Johnson.
References
- 1.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews Immunology. 2015;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Z, Fanslow WC, Seldin MF, Rousseau A-M, Painter SL, Comeau MR, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3(6):811–21. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 4.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6(11):1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature immunology. 2005;6(11):1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 6.Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect. 2013;2:e60. doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nature reviews Immunology. 2008;8(5):337–48. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 8.Beurel E, Harrington LE, Jope RS. Inflammatory T Helper 17 Cells Promote Depression-like Behavior in Mice. Biological Psychiatry. 2013;73(7):622–30. doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Jiang T, Chen P, Ouyang J, Xu G, Zeng Z, et al. Emerging tendency towards autoimmune process in major depressive patients: a novel insight from Th17 cells. Psychiatry research. 2011;188(2):224–30. doi: 10.1016/j.psychres.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Ho RC-M, Mak A. The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. International Journal of Rheumatic Diseases. 2012;15(2):183–7. doi: 10.1111/j.1756-185X.2011.01673.x. [DOI] [PubMed] [Google Scholar]
- 11.Hennings JM, Uhr M, Klengel T, Weber P, Putz B, Touma C, et al. RNA expression profiling in depressed patients suggests retinoid-related orphan receptor alpha as a biomarker for antidepressant response. Translational psychiatry. 2015;5:e538. doi: 10.1038/tp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fam J, Rush AJ, Burt T, Chan ES, Siddiqui FJ, Assam PN, et al. Thyroid Autoimmune Antibodies and Major Depressive Disorder in Women. Annals of the Academy of Medicine, Singapore. 2015;44(8):284–9. [PubMed] [Google Scholar]
- 13.Kim J-W, Kim Y-K, Hwang J-A, Yoon H-K, Ko Y-H, Han C, et al. Plasma Levels of IL-23 and IL-17 before and after Antidepressant Treatment in Patients with Major Depressive Disorder. Psychiatry Investig. 2013;10(3):294–9. doi: 10.4306/pi.2013.10.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24(4):1023–34. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- 15.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–5. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waisman A, Hauptmann J, Regen T. The role of IL-17 in CNS diseases. Acta neuropathologica. 2015;129(5):625–37. doi: 10.1007/s00401-015-1402-7. [DOI] [PubMed] [Google Scholar]
- 17.Liu AC, Lee M, McManus BM, Choy JC. Induction of endothelial nitric oxide synthase expression by IL-17 in human vascular endothelial cells: implications for vascular remodeling in transplant vasculopathy. Journal of immunology (Baltimore, Md : 1950) 2012;188(3):1544–50. doi: 10.4049/jimmunol.1102527. [DOI] [PubMed] [Google Scholar]
- 18.Kawanokuchi J, Shimizu K, Nitta A, Yamada K, Mizuno T, Takeuchi H, et al. Production and functions of IL-17 in microglia. Journal of neuroimmunology. 2008;194(1–2):54–61. doi: 10.1016/j.jneuroim.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Miller AH, Haroon E, Felger JC. Therapeutic Implications of Brain-Immune Interactions: Treatment in Translation. Neuropsychopharmacology. 2017;42(1):334–59. doi: 10.1038/npp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melnikov M, Belousova O, Murugin V, Pashenkov capital Em C, Boysmallka CoCA. The role of dopamine in modulation of Th-17 immune response in multiple sclerosis. Journal of neuroimmunology. 2016;292:97–101. doi: 10.1016/j.jneuroim.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Lieberknecht V, Junqueira SC, Cunha MP, Barbosa TA, de Souza LF, Coelho IS, et al. Pramipexole, a Dopamine D2/D3 Receptor-Preferring Agonist, Prevents Experimental Autoimmune Encephalomyelitis Development in Mice. Molecular neurobiology. 2016 doi: 10.1007/s12035-016-9717-5. [DOI] [PubMed] [Google Scholar]
- 22.Himmerich H, Schonherr J, Fulda S, Sheldrick AJ, Bauer K, Sack U. Impact of antipsychotics on cytokine production in-vitro. Journal of psychiatric research. 2011;45(10):1358–65. doi: 10.1016/j.jpsychires.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, et al. Bupropion: a review of its mechanism of antidepressant activity. The Journal of clinical psychiatry. 1995;56(9):395–401. [PubMed] [Google Scholar]
- 24.Arias HR, Santamaría A, Ali SF. International Review of Neurobiology. Vol. 88. Academic Press; 2009. Chapter 9 - Pharmacological and Neurotoxicological Actions Mediated By Bupropion and Diethylpropion; pp. 223–55. [DOI] [PubMed] [Google Scholar]
- 25.Fava M, Rush AJ, Thase ME, Clayton A, Stahl SM, Pradko JF, et al. 15 Years of Clinical Experience With Bupropion HCl: From Bupropion to Bupropion SR to Bupropion XL. Primary Care Companion to The Journal of Clinical Psychiatry. 2005;7(3):106–13. doi: 10.4088/pcc.v07n0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebbinghaus M, Gajda M, Boettger MK, Schaible H-G, Bräuer R. The anti-inflammatory effects of sympathectomy in murine antigen-induced arthritis are associated with a reduction of Th1 and Th17 responses. Annals of the Rheumatic Diseases. 2012;71(2):253–61. doi: 10.1136/ard.2011.150318. [DOI] [PubMed] [Google Scholar]
- 27.Brustolim D, Ribeiro-dos-Santos R, Kast RE, Altschuler EL, Soares MB. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. International immunopharmacology. 2006;6(6):903–7. doi: 10.1016/j.intimp.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(22):9262–7. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shenoy AR, Dehmel T, Stettner M, Kremer D, Kieseier BC, Hartung HP, et al. Citalopram suppresses thymocyte cytokine production. Journal of neuroimmunology. 2013;262(1–2):46–52. doi: 10.1016/j.jneuroim.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A, et al. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology. 2017;78:105–13. doi: 10.1016/j.psyneuen.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. The American journal of psychiatry. 2014;171(12):1278–86. doi: 10.1176/appi.ajp.2014.14010094. [DOI] [PubMed] [Google Scholar]
- 32.Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. The American journal of psychiatry. 2011;168(7):689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- 33.Gelenberg AJ, Freeman MP, Markowitz JC, Rosenbaum JF, Thase ME, Trivedi MH, et al. PRACTICE GUIDELINE FOR THE Treatment of Patients With Major Depressive Disorder Third Edition. The American journal of psychiatry. 2010;167(10):1. [Google Scholar]
- 34.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 35.Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, et al. An Evaluation of the Quick Inventory of Depressive Symptomatology and the Hamilton Rating Scale for Depression: A Sequenced Treatment Alternatives to Relieve Depression Trial Report. Biological psychiatry. 2006;59(6):493–501. doi: 10.1016/j.biopsych.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychological medicine. 2004;34(1):73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- 37.Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA. Self-rated global measure of the frequency, intensity, and burden of side effects. Journal of psychiatric practice. 2006;12(2):71–9. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Schatzberg AF, DeBattista C, Lazzeroni LC, Etkin A, Greer M, Murphy J, Williams LM. ABCB1 Genetic Effects on Antidepressant Outcomes: A Report From the iSPOT-D Trial. American Journal of Psychiatry. 2015;172(8):751–9. doi: 10.1176/appi.ajp.2015.14050680. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson L, Nousiainen N, Scheinin NM, Maksimow M, Salmi M, Lehto SM, et al. Cytokine profile and maternal depression and anxiety symptoms in mid-pregnancy—the FinnBrain Birth Cohort Study. Archives of Women’s Mental Health. 2017;20(1):39–48. doi: 10.1007/s00737-016-0672-y. [DOI] [PubMed] [Google Scholar]
- 40.Leung R, Proitsi P, Simmons A, Lunnon K, Güntert A, Kronenberg D, et al. Inflammatory Proteins in Plasma Are Associated with Severity of Alzheimer’s Disease. PLOS ONE. 2013;8(6):e64971. doi: 10.1371/journal.pone.0064971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsunaga M. How to factor-analyze your data right: do’s, don’ts, and how-to’s. International Journal of Psychological Research. 2015;3(1):97–110. [Google Scholar]
- 42.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swardfager W, Rosenblat JD, Benlamri M, McIntyre RS. Mapping inflammation onto mood: Inflammatory mediators of anhedonia. Neuroscience and biobehavioral reviews. 2016;64:148–66. doi: 10.1016/j.neubiorev.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Felger JC. The Role of Dopamine in Inflammation-Associated Depression: Mechanisms and Therapeutic Implications. Current topics in behavioral neurosciences. 2016 doi: 10.1007/7854_2016_13. [DOI] [PubMed] [Google Scholar]
- 45.Vichaya EG, Hunt SC, Dantzer R. Lipopolysaccharide reduces incentive motivation while boosting preference for high reward in mice. Neuropsychopharmacology. 2014;39(12):2884–90. doi: 10.1038/npp.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fawcett J, Rush AJ, Vukelich J, Diaz SH, Dunklee L, Romo P, et al. Clinical Experience With High-Dosage Pramipexole in Patients With Treatment-Resistant Depressive Episodes in Unipolar and Bipolar Depression. The American journal of psychiatry. 2016;173(2):107–11. doi: 10.1176/appi.ajp.2015.15060788. [DOI] [PubMed] [Google Scholar]
- 47.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ. Inflammation and clinical response to treatment in depression: A meta-analysis. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2015;25(10):1532–43. doi: 10.1016/j.euroneuro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Martino M, Rocchi G, Escelsior A, Fornaro M. Immunomodulation Mechanism of Antidepressants: Interactions between Serotonin/Norepinephrine Balance and Th1/Th2 Balance. Current Neuropharmacology. 2012;10(2):97–123. doi: 10.2174/157015912800604542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, et al. Medication augmentation after the failure of SSRIs for depression. The New England journal of medicine. 2006;354(12):1243–52. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 51.Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. The New England journal of medicine. 2006;354(12):1231–42. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 52.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American journal of psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 53.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. The American journal of psychiatry. 2006;163(11):1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 54.Arnow BA, Blasey C, Williams LM, Palmer DM, Rekshan W, Schatzberg AF, et al. Depression Subtypes in Predicting Antidepressant Response: A Report From the iSPOT-D Trial. The American journal of psychiatry. 2015;172(8):743–50. doi: 10.1176/appi.ajp.2015.14020181. [DOI] [PubMed] [Google Scholar]
- 55.Toups M, Trivedi MH. Biomarkers and the future of treatment for depression. Cerebrum : the Dana forum on brain science. 2012;2012:6. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.