Abstract
Many mammalian species, including humans, exhibit social behavior and form complex social groups. Mechanistic studies in animal models have revealed important roles for the endocannabinoid signaling system—consisting of G protein-coupled cannabinoid receptors and their endogenous lipid-derived agonists—in the control of neural processes that underpin social anxiety and social reward, two key aspects of social behavior. An emergent insight from these studies is that endocannabinoid signaling in specific circuits of the brain is context-dependent and selectively recruited. These insights open new vistas on the neural basis of social behavior and social impairment.
Keywords: social reward, social anxiety, autism spectrum disorder, schizophrenia, anandamide, 2-arachidonoyl-sn-glycerol
Introduction
Archeological and paleobotanical findings date the first human encounters with the cannabis plant to the early Holocene, approximately 11,000 years before present [1], when human groups living across the Eurasian continent exploited it as a source of fiber (stalks) and food (seeds), but also for the unique properties of its female flowering tops [2,3]. Eating these resin-rich flowers or inhaling their smoke produces a combination of euphoria, calmness, heightened sensation, and altered time perception [4], along with a series of medicinal effects that include stimulation of appetite and relief of pain, nausea, and spasticity [5]. Though varied, these effects are due in large part to a single chemical constituent found in the cannabis resin, the dibenzopyran derivative Δ9-tetrahydrocannabinol (THC), which binds to selective cell-surface receptors present in regions of the brain involved in the control of cognition, mood, and pain (for an overview of the endocannabinoid system, see Box 1). It is likely that early human users interpreted cannabis’ complex actions within a spiritual, rather than purely medical or recreational, frame of reference [2,3]. A survey of the ethnographic literature bears out this idea, showing that the earliest documented uses of cannabis were intimately woven into religious ritual [2]. Notably, traditional societies ranging from the Saka (Scythians) in the Eurasian steppe [6,7] to the Hindus of the Himalayan mountains [8] employed cannabis ritualistically in funerals, weddings, and holy festivals – ceremonial activities whose main objectives include the heightening of spiritual connectedness and social bonding [9]. The millenary use of cannabis in ritual practices with deep social meaning raises the possibility that THC may influence, possibly through modulation of endocannabinoid signals, the activity of neurotransmitters [10] and neural networks [11] devoted to the regulation of sociality (for an overview of the field of social neuroscience, see Box 2).
Box 1. The endocannabinoid system.
The main active constituent of cannabis, Δ9-tetrahydrocannabinol (THC), causes a mental state described as a combination of enhanced sociability, quickened mental associations, increased appetite for sweet and fatty foods, alterations in the perception of time and space, and heightened sensitivity to certain sensory stimuli (e.g., sounds or colors) [62,87]. Research over the past 30 years, since the initial discovery of cannabinoid receptors and isolation of endogenous cannabinoids (‘endocannabinoids’) [88–92], has established that endocannabinoid signaling plays important functional roles in the brain, which are related to the pharmacological effects of THC. Endocannabinoid signaling regulates circuits in the central nervous system important for stress reactivity [50], analgesia [93], and the development of reward to natural and drug stimuli [27,94].
The endocannabinoid system consists of lipid-derived messengers that act on G protein-coupled cannabinoid receptors. The CB1 receptor is the most abundant G protein-coupled receptor found in the brain, while the CB2 receptor is relatively sparse in this organ and is more abundant in immune cells such as microglia [95]. Endocannabinoid transmitters possess a unique set of properties: (i) they act as retrograde synaptic signals or local modulators to control presynaptic firing (CB1 receptors are localized presynaptically on both excitatory and inhibitory neurons); of note, 2-AG may primarily serve as a point-to-point retrograde signal, whereas anandamide may act as a local modulator [60]; (ii) as lipid mediators, the endocannabinoids are not stored in vesicles but are instead ‘demobilized’ (sequestered) in phospholipid membranes under baseline conditions to become ‘mobilized’ on demand during signaling activity [32]; (iii) while anandamide and 2-AG may work in a concerted manner, their signaling patterns are often distinct [27,93]. These properties are partially rooted in selective coupling of afferent transmitter-receptor machinery to synthetic enzymes for biochemical mobilization. For example, 2-AG is recruited by type-1 metabotropic glutamate receptors [96], type 1/3 muscarinic acetylcholine receptors [97], and type-1 orexin receptors [98]. 2-AG is produced via the hydrolysis of 1,2-diacylglycerol by diacylglycerol lipase-α (DGL-α) [96], which is coupled in a supramolecular ‘signalosome’ complex with Homer-1a and Fragile × Mental Retardation Protein (FMRP) [69]. 2-AG degradation is mediated by the serine hydrolases, monoacylglycerol lipase (MGL) and α–β domain hydrolase-6 (ABHD-6). The stimuli responsible for anandamide mobilization are less well understood, but appear to be distinct from those involved in the recruitment of 2-AG. D2-type dopamine receptors in the dorsal striatum have been shown to stimulate anandamide formation [99]. The canonical route for the synthesis of anandamide is thought to involve a phospholipase D that releases anandamide by hydrolysis of the phospholipid precursor N-arachidonoyl-phosphatidylethanolamine (N-arachidonoyl-PE). Anandamide is mainly degraded via carrier-mediated transport followed by intracellular hydrolysis, catalyzed by fatty acid amide hydrolase (FAAH).
Box 2. Social neuroscience.
Social behavior is a hallmark of many phylogenetically diverse animal species [100–102]. While animals with simple nervous systems demonstrate behaviors such as courtship, mating, parenting, and aggression [101,102], increasing neural complexity adds a greater endowment of complex social behaviors, including alliance formation, cooperative hunting, empathy and altruism [103–105]. The emerging field of social neuroscience aims to understand the neural basis of social behavior – from social-information detection and processing to integration and regulation. Here, we highlight three conceptually important findings of social neuroscience:
The adaptive value of sociality. Social behaviors are ubiquitous because they offer distinct evolutionary advantages [106,107]. Social support is protective while social isolation increases susceptibility to mental and physical illness [108–110]. Furthermore, social impairment is a central component in the psychopathology of many psychiatric disorders [4,111] and the dysphoria that accompanies them [112,113].
The existence of a social-brain network. Neuroimaging and cognitive-neuroscience experiments have identified networks that specifically process social information [11,114–118]. Molecular and optogenetic studies have found distinct circuit activity encoding representations of social states and dynamics [119–123].
The specificity and importance of oxytocin for social circuitry and behavior. Hypothalamic neurons that release the neuropeptide oxytocin respond selectively to social information, and oxytocin plays a central role in the regulation of social behaviors, such as maternal care, attachment, and social memory [55,114,124–128]. Importantly, specific neural circuitry likely mediate these effects [55,127,129–131]. Studies have validated the prosocial effects of oxytocin in humans [132–134] and elaborated its role in more complex, human-level behaviors such as trust and empathy [135,136].
In the present review, we will first describe studies of the effects of cannabis on human social behavior. These studies suggest that cannabis may temper social anxiety and enhance feelings of connectedness, but depending on dose and context may also increase aggression and isolation. To shed light on the underlying mechanisms of these discrepant actions, we will describe animal experiments that contrast the effects produced by direct activation of cannabinoid receptors versus those caused by enhancement of endocannabinoid signaling. We will briefly highlight the contribution of the endocannabinoid system to social anxiety and social reward, and then describe lines of work showing endocannabinoid abnormalities in translational models of social impairment, such as those related to schizophrenia, autism spectrum disorder (ASD) and developmental cannabinoid exposure. Lastly, we will consider how these lines of evidence collectively suggest that endocannabinoids control specific circuits of the social brain, with potentially important clinical implications.
Effects of cannabis on human social behavior
The first systematic investigations of the effects of cannabis on human social behavior were conducted in the 1970’s, in the wake of the counterculture movement that had brought the drug back into the limelight [12]. In a psychometric survey of 153 college students who were experienced cannabis users, more than 70 percent of respondents said that intoxication made them want to interact more with others, perceiving what they described as ‘a much greater sense of unity’, or ‘real social relationship’ [13]. More than 80 percent reported that cannabis use made them feel a greater degree of empathy toward others [13]. Box 3 provides a few examples of these subjective reports. Confirming these results, another study on healthy cannabis smokers in a controlled hospital setting found the participants to be more interactive, communicative, comfortable, and open toward one another, compared to non-smokers [14].
Box 3. Effects of cannabis on subjective feeling about sociality.
We transcribe here a few anecdotal reports on how cannabis affects subjective feelings regarding social relationships, connectedness, and anxiety.
“[Cannabis] was the unifying factor because [it] makes you aware of the better sides of people and, when you get high, each person himself looks like a universe and you have something to gain from the interaction.” [14]
“…a lot of them [cannabis users] say that they… are not very open with each other. The guys aren’t open with each other and they… don’t know much about each other but when they’re high a lot of their inhibitions are gone…” [137]
“I currently smoke a small amount of weed every morning before going to work and it really helps increase my ability to focus and concentrate as well as overcome some of my social interaction issues. However the key here is small amount.” [138]
“I’ve come to realize that I always face some degree of anxiety in social situations. Even when I’m with friends, I always think, ‘what should I say?’ when it gets quiet. What has helped me realize this is medicinal marijuana. When I use [it] I have no problem with socializing, I feel no anxiety; in fact, I actually seek it out. Socializing while high is as easy as sitting in a chair for me… it’s as if I understand life… my anxiety vanishes and my mind races… I have no problems keeping a convo going or even approaching total strangers with total confidence. It’s literally like I took the drug from the movie ‘Limitless’.” [138]
Users in these studies also reported that consuming cannabis made them more socially intuitive, but at the same time less able to play social games, implying that the drug might hinder skills that are required in such games [13]. Further studies in controlled small-group settings aimed to characterize the emotional and cognitive aspects of cannabis use. In one study, where each participant’s own room was delineated from a common area, cannabis use changed the distribution of social activities by decreasing time spent in verbal exchanges while increasing time spent in coactation, i.e. engaging in a shared activity such as playing a game [15,16]. In another study, small groups were subjected to a frustration stimulus to determine the effects of cannabis on within-group hostility. Each group was asked to agree on the interpretation of a short story, but was subsequently told that the interpretation was inadequate. Members of the placebo group were more hostile toward one another, which slowed task completion, whereas members of the cannabis group were less hostile and more cooperative [17]. Interestingly, the authors note that cannabis may have been emotionally disinhibitory, such that users were more willing to express their feelings. In line with the notion that cannabis suppresses hostility, a number of functional magnetic resonance imaging (fMRI) studies have found that THC use is associated with a reduction in amygdala reactivity in response to threat signals [18–20]. Perhaps distinctly from the cannabis studies described above, these changes in brain activity may have more to do with the perception rather than the expression of threat, and may alter amygdala-prefrontal connectivity with implications for overall socio-emotional network function [20].
Additional studies have examined conversation, another proxy measurement of being social. Acute or chronic cannabis use was found to have either no effect or decrease conversation, whereas psychostimulants such as amphetamines typically stimulated it [21–23]. As the authors pointed out, however, a lower tendency to engage in conversation may reflect either a negative subjective state and interaction avoidance or, alternatively, a positive subjective state of intuition, connectedness, and relaxation so that the need for speech is minimized. The latter possibility would be in line with some of the subjective feelings reported by cannabis users (Box 3, [13,17]).
While the work outlined above does not clearly delineate what aspects of social interactions may be affected by cannabis, and may not be robust [24], it suggests nevertheless that cannabis may strongly influence social interactions. Such influence could involve a range of possibly dissociable effects on the subjective emotions (e.g., empathy, calmness, disinhibition) and required skills (e.g., coactation, conversation) that contribute to sociality.
Cannabinoid receptors
The brain distribution of molecular components of the endocannabinoid system is consistent with a role in social behavior. CB1 cannabinoid receptors are highly expressed in associational regions of the frontal cortex and in subcortical structures that underpin human social-emotional functioning [11,25,26]. They are also present throughout regions implicated in the rewarding properties of natural and drug-related stimuli, including the central and basolateral amygdala, prefrontal cortex, hippocampus, dorsolateral striatum, ventral tegmental area, and, to a lesser extent, the nucleus accumbens [25,27]. Human positron emission tomography (PET) imaging studies have revealed alterations in the distribution of CB1 receptors in, for example, schizophrenia and addiction [28,29]. These regions are considered key parts of the ‘social brain,’ based on imaging and network studies (Box 2). The regional distribution of the enzymes involved in the generation and degradation of endocannabinoid transmitters is somewhat similar to the picture of CB1 receptors depicted here [27,30,31], although not necessarily duly reflective of endocannabinoid signaling [32].
Consistent with cannabis decreasing hostility in humans [17], THC was found to decrease agonistic acts in multiple mammalian species—mice, rats, and squirrel monkeys—undergoing intruder confrontation [33]. Mutant mice lacking CB1 exhibit more behaviors involving offensive aggression, as well as active and passive defensive coping behaviors, such as avoidance, freezing, and risk-assessment behaviors, suggesting CB1 receptors play a role in buffering against social stress [34,35]. This stress-modulating effect translates to control of overall time spent in direct interaction with novel conspecifics, possibly in a sex-dependent manner [36,37].
The effect on aggression appears to be complex, however, as THC may also increase defensive posturing [38], while synthetic cannabinoid agonists may enhance aggression under certain stressful conditions, as well as flight acts [39]. Moreover, cannabinoid agonists reduce interaction time in the direct social interaction test, where a novel encounter in an unfamiliar environment is considered stressful [40].
That cannabis and synthetic cannabinoid agonists can either mitigate aggression (leading to more interaction) or increase anxiety (leading to withdrawal) may reflect the biphasic nature of much of cannabinoid pharmacology [41,42]. Different cannabinoid doses under different environmental conditions, especially those with stress versus those without stress, could activate distinct patterns of endocannabinoid signaling, thereby resulting in contrasting behavioral outputs. Mutant mice lacking CB1 exhibit less direct social interactions in an unfamiliar environment, but not in a home-cage environment, suggesting that the receptor may have a more prominent role in stress reduction under adverse conditions [36,43]. Consistent with the idea of context-specific signaling effects, regional CB1 overexpression in the medial prefrontal cortex reduced interactions and increased withdrawal [44].
In sum, available evidence from animal experiments suggests that CB1 receptors are important contributors to the regulation of social behavior. This conclusion is supported by emerging translational data: a polymorphism in the CB1 receptor gene has been found to modulate social gaze in humans [45]. Moreover, the evidence recognizes that cannabinoid effects are multimodal and context-dependent. For example, a mandatory state of anxiety during a novel encounter, particularly in an unfamiliar setting, may call for a different pattern of endocannabinoid response versus a recognizable re-encounter in familiar surroundings. This context specificity highlights questions regarding the distinctive qualities of social behavior and circuit patterns regulated by endocannabinoid signaling.
Endocannabinoids
Moving our analysis from cannabinoid receptors to their endogenous ligands—anandamide and 2-arachidonoyl-sn-glycerol (2-AG)—provides a window into these questions. In rats, a novel encounter elevates anandamide levels in the striatum, compared to encounters with familiar or non-social animals [46]. Mutant mice in which genetic removal of the hydrolytic enzyme fatty acid amide hydrolase (FAAH) caused elevated levels of anandamide exhibit increased direct social interactions [47]. A plausible interpretation of these findings is that anandamide participates in the regulation of social behavior, and may dampen the social anxiety involved in these tests [48]. Trezza and colleagues further qualified the type of social behavior influenced by anandamide by examining ‘rough-and-tumble’ social play in juvenile rats, including pouncing and pinning behaviors. These authors found that play is associated with increased anandamide mobilization in the nucleus accumbens and amygdala [49]. Moreover, microinjection of the FAAH inhibitor URB597, which stops anandamide degradation and increases its levels [50], into either of these two brain regions, enhanced social play [49,51]. Endocannabinoid effects on social play may also be extended to 2-AG signaling, and may interact with opioid or dopaminergic signaling in the nucleus accumbens [52,53]. These results with an anandamide-potentiating agent stand in sharp contrast to those obtained using a direct-acting cannabinoid receptor agonist, which decreased social play [51] and other forms of direct interactions (as reviewed in the previous section).
Contextual factors may again be key determinants in the circuit role of anandamide-mediated endocannabinoid signaling. In aggressive mice, exogenously administered low-dose anandamide (0.01 or 0.1 mg-kg−1) does not significantly affect agonistic behavior, whereas a higher dose (10 mg-kg−1) decreases it; in timid mice, low-dose anandamide stimulates agonistic behavior, whereas high-dose anandamide decreases social interactions without affecting agonistic behavior [41]. In the social play model, adolescent rats respond to the FAAH inhibitor URB597 with increased play behavior in conditions of both low adverseness (familiar arena, low light) and high adverseness (unfamiliar, high light), whereas adult rats only respond in conditions of high adverseness [54].
Due to the roles of endocannabinoid signaling in the reinforcement of natural stimuli and neurotransmission in the nucleus accumbens, we hypothesized that it may play a role in the regulation of social reward, distinct from modulation of stress, that may also contribute to effects on direct interaction time (as described above). Because of the context-dependent recruitment of endocannabinoid signaling, it is crucial to clearly distinguish between the signaling of social stress versus that of social reward. To this end, in a recent study we used a model of socially conditioned place preference as a proxy for social reward [55] and selectively activated the oxytocin system, which is crucial in social bonding (Box 1). Using young cage-mate mice, we found that a relatively brief social contact (3 h) or selective chemogenetic activation of oxytocin neurons in the paraventricular nucleus of the hypothalamus stimulated anandamide mobilization in the nucleus accumbens [56] – a projection target for oxytocin neurons [55]. Oxytocin-driven anandamide signaling tightly regulates nucleus accumbens activity in its shell region (as measured using the cellular marker cFos) as well as social place preference [56]. 2-AG levels were not affected by either intervention [56]. Enhancement of anandamide activity, insofar as under the context modeled by conditioned place preference, was selective for social as opposed to high-fat-food or cocaine reward [56]. Anandamide enhancement was also selective for social- but not isolation-conditioned place preference, and had no effect on social approach [56]. These results suggest that oxytocin neurons projecting from the paraventricular nucleus to the nucleus accumbens recruit anandamide signaling, thereby encoding a circuit mechanism that influences social reward independently of stress and other natural rewards (Fig. 1).
Figure 1.
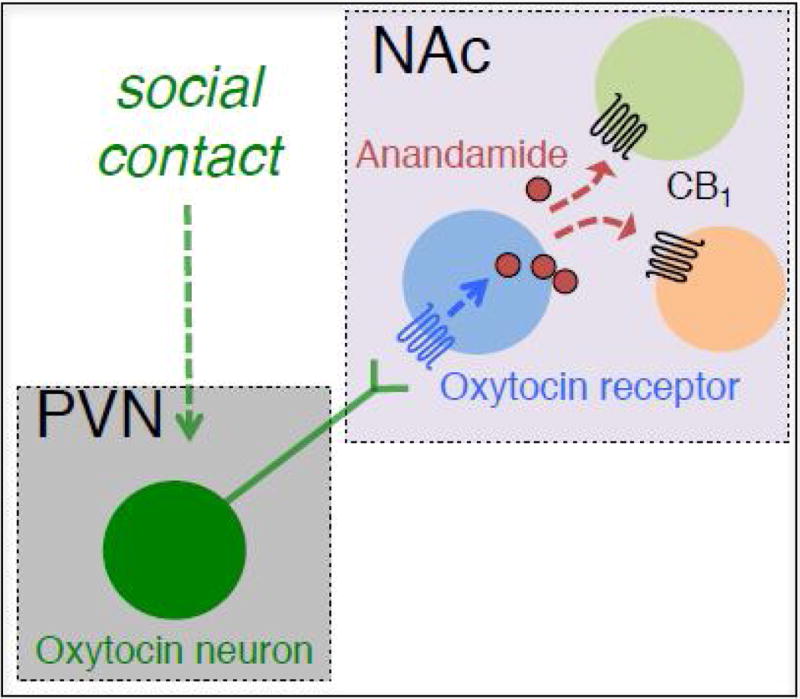
Hypothesized model for oxytocin-driven endocannabinoid signaling. Social contact activates a population of oxytocin neurons that are located in the paraventricular nucleus (PVN) of the hypothalamus and project to the nucleus accumbens (NAc) to drive endocannabinoid-mediated plasticity. Based on data in [55,56].
Does 2-AG also contribute to the regulation of social reward? Because the distribution pattern of biosynthetic and hydrolytic 2-AG enzymes varies from that of anandamide in reward pathways [27], and because of the circumstantial fact that 2-AG levels in the brain are roughly 200-fold greater than anandamide’s [32], determining how 2-AG differs from anandamide in influencing social behavior would offer valuable insights into mechanisms of differential recruitment and context dependence. To address this question, we used a transgenic mouse model with a specific forebrain reduction in 2-AG (produced by overexpression of the 2-AG- hydrolyzing enzyme, monoacylglycerol lipase [57]). We found that these transgenic mice show impaired conditioned place preference to both social and high-fat-food stimuli [58]. The non-selectivity of this effect stands in contrast to the results obtained with the anandamide-modulating manipulations described above, which selectively heighten social over high-fat-food reward [56]. Also in contrast to social contact at 3 h, prolonged social contact for 6 h was found to stimulate 2-AG mobilization without changing levels of anandamide [58]. These results argue in favor of a role for 2-AG in social reward, which may be more generalizable to other natural rewards. The collective evidence outlined thus far offers an important thematic insight: different external conditions may selectively initiate distinct patterns of endocannabinoid signaling in the brain. For example, global cannabinoid receptor activation may variably affect social interactions (e.g., suppress versus incite aggression) depending on conditions such as the state of stress, whereas selective enhancement of anandamide signaling may be largely prosocial. The anxiety associated with a novel encounter between unfamiliar adults may recruit anandamide in stress-related pathways, whereas increased social drive between familiar juveniles may recruit anandamide in reward-related pathways. Therefore, different neural circuits likely recruit specific endocannabinoid signals to reflect states of social-information processing which are qualitatively distinct. This hypothesis is further supported by two pieces of available evidence. First, familiar and unfamiliar encounters elevate anandamide to different levels in the striatum [46], and, similarly, social contact and isolation lead to qualitatively distinct regional patterns of changes in levels of endocannabinoids, i.e. not only opposite in directionality [58]. Second, a key distinction can be made between socially specific anandamide signaling, which is driven by oxytocin circuitry [56], and non-socially specific 2-AG signaling, which is not driven by oxytocin, and may also be involved in fatty-food reward [58]. Given the functional and temporal dichotomy between anandamide and 2-AG, it bears speculation that these transmitters work in concert to assign reward value to various stimuli, with anandamide primarily involved in proximal reinforcement processes and 2-AG in the consolidation of such processes.
Endocannabinoid signaling in social impairment
As endocannabinoids are key modulators of neural plasticity [59,60] and brain development [61], a variety of pathologies are thought to involve dysregulation of their signaling functions. Recently expanded lines of work have documented the occurrence of impaired endocannabinoid signaling in translational animal models of neuropsychiatric pathology where social impairment is a core feature – including schizophrenia, ASD and developmental cannabinoid exposure.
Persons with schizophrenia exhibit characteristic social withdrawal involving social anhedonia, amotivation, and acognition [4]. Cannabis smoking has been associated with an increased risk of developing psychosis, but whether this may be due to interference with endocannabinoid signaling remains controversial [62]. Seillier and colleagues investigated the model of chronic phencyclidine treatment, which in rats produces a schizophrenia-like phenotype, including reduced social interactions such as sniffing frequency/time and climbing episodes. Chronic phencyclidine treatment decreased levels of anandamide in the medial prefrontal cortex and amygdala, while increasing anandamide in the nucleus accumbens [63]. The FAAH inhibitor URB597 reversed the phencyclidine-induced social deficit, while also reducing interactions in saline-treated control rats [63,64]. Like URB597, self-administration of the cannabinoid agonist WIN55,212-2 has been reported to ameliorate phencyclidine-induced social withdrawal [65]. Decreased social interactions in phencyclidine-treated rats is mimicked by the CB1 inverse agonist AM251, and both effects are blocked by an antagonist of the cholecystokinin CCK2 receptor, whose activation has anxiogenic effects [63]. URB597 also restores phencyclidine-induced changes in prefrontal and amygdala activity (as measured using cFos) [66]. This set of data suggests that anandamide-CB1 signaling normally suppresses CCK-mediated anxiogenesis to engage social interactions, a regulation that appears to be disrupted after chronic phencyclidine treatment. It has been hypothesized that schizophrenia may be related to chronic THC treatment, which possibly disrupts cannabinoid receptor-mediated cortical inhibition of GABAergic CCK interneurons in the prefrontal cortex [67]. It remains to be determined how such CCK-mediated anxiogenesis would relate to the ego-syntonic social withdrawal or the socio-cognitive disabilities that are characteristic of schizophrenia [4].
Impairments in endocannabinoid signaling are seen as a consequence of abnormalities in synaptic maintenance and transmission associated with ASD, including neuroligins [68] as well as Fragile X mental retardation protein (FMRP) and metabotropic glutamate receptor-5 (mGluR5) [69]. ASD-related pathological insults such as valproic acid also disturb resting endocannabinoid levels and endocannabinoid system components [70]. To address whether endocannabinoid changes are only coincident with or rather directly responsible for social impairment, we focused on the role of anandamide in models of ASD-related social impairment – the BTBR and fmr1−/− mice, using the three-chambered social approach test [71]. As proof of concept, we administered the FAAH inhibitor URB597 to upregulate anandamide and found that this intervention completely restored social approach, in a CB1 receptor-dependent manner, in both mouse models. In contrast to studies done in rats [63], URB597 failed to alter the social approach of control, socially normal mice. URB597 also had no effect in the elevated plus-maze test—which assesses anxiety-like states—when administered in the low-light (less adverse) conditions used in the social approach test [71]. These results provide evidence for a direct role of anandamide signaling in ASD-related social impairment. Recent reports have confirmed the corrective, prosocial effect of FAAH inhibition across a range of studied ASD-related insults, such as in developmental exposure to valproic acid [72] and lipopolysaccharide [73]. Inappropriate developmental exposure to cannabinoid agents, on the other hand, can also disrupt the later expression of social behavior. Cannabis use in early adolescents was found to correlate with hypersensitivity to signals of threat (angry as compared to neutral faces) and higher levels of fMRI activity in the amygdala [74]. The persistence of the effect of developmental cannabinoid exposure into adulthood can be striking. Treatment with the cannabinoid agonist, WIN 55,212-2 (1.2 mg/kg) over 25 days in adolescent rats, followed by a 2-week washout, led to a persistent reduction in social interactions [75,76]. Similar protocols have replicated the effect, which is absent or less pronounced on adult administration [77–79]. Furthermore, the deficit profile appears to be sexually dimorphic, as THC induces a more complex emotional profile in female rats, including depression-like behavior, than it does in males [80,81]. Altered glutamatergic transmission in the prefrontal cortex may contribute to these changes [82]. Potentially confounding this effect could be concomitant deficits in measures of cognition such as social recognition and object recognition, as well as measures of emotional reactivity [77–79]. In addition, abnormalities in hippocampal neurogenesis [81] and the oxytocin system [83], offer the possibility of remote downstream impacts in development. These concomitant effects raise the question of whether inappropriate CB1 activation during development—i.e. by exogenous cannabinoids—might produce a generalized impairment that overlaps with social behavior or rather interferes directly with the developmental function of endocannabinoid signaling. Several results argue in favor of endocannabinoid-mediated changes that are more proximal. First, the deficits, including social, are rescuable via FAAH inhibition [81]. Second, the expression of CB1 receptors in the prefrontal cortex and striatum peaks during adolescence and decreases into adulthood – a pattern that suggests a physiological role in development [84]. Third, a mutagenesis-induced functional increase in CB1 receptor activity in the striatum prolongs the characteristically adolescent behavioral repertoire, including increased impulsivity and social play, into age normally classified as adulthood where these behaviors are absent [85]. These results suggest that endocannabinoid signaling could have a direct mediatory role in the social transition between adolescence and adulthood – a compelling hypothesis that requires more granular elaboration. These three lines of investigation—covering schizophrenia, ASD, and developmental cannabinoid overexposure—indicate that properly tuned endocannabinoid signaling is required for normal social interactions.
Concluding remarks
A growing body of studies support a distinct role for endocannabinoid signaling in the control of social behavior. Cannabis and synthetic cannabinoid receptor agonists may have varied effects, particularly under certain conditions to reduce hostility and threat perception, or during critical developmental windows to potentially effect persistent dysfunction. In contrast, anandamide-mediated signaling appears to act more selectively in reducing social anxiety and enhancing social reward. Based on translational evidence, these actions of anandamide are postulated to be important in social impairment related to (i) schizophrenia, in which tempering social anxiety might be dysfunctional, as well as in (ii) ASD, where a primary deficit may be in nucleus accumbens-regulated social reward. fMRI studies in humans supports these possible roles of anandamide, as a single nucleotide polymorphism (C385A) in the human FAAH gene is associated with decreased threat-related amygdala reactivity and increased reward-related ventral striatal reactivity [86]. In contrast to the specificity demonstrated by anandamide, the actions of 2-AG appear to be more generalizable to other natural rewards. These ongoing developments inform the promising but limited research into cannabinoid-based pharmacotherapies for neuropsychiatric conditions (see [62] for review) at a time when the legal status and public perception of cannabis are dramatically changing.
The difference between global cannabinoid receptor activation and selective endocannabinoid enhancement may be rooted in the selectivity of recruiting circuit projections, such as those of the oxytocin system (Fig. 1). Endocannabinoid signaling in processes specific to social behavior might thus be mechanistically distinguished from endocannabinoid signaling in processes that overlap with the social sphere (e.g. non-social anxiety or reward). This hypothesis addresses a core question in social neuroscience – whether a distinction can be made between social and non-social signaling [11]. The hypothesis also opens several directions for future investigations, which will be crucial to define the circuits of normal social-information processing and fluent social behavior (see Outstanding Questions). Such investigations will help us understand the contributory social factors and the social-impairment consequences of neuropsychiatric-disease states, such as schizophrenia, ASD, and drug addiction. They are also likely to provide mechanistic insights into the therapeutic actions of social bonding on mental and physical health, a key finding of social neuroscience.
Outstanding Questions.
How do patterns of endocannabinoid signaling differ in distinct social states, such as engagement, ongoing interactions, acute isolation, and prolonged isolation? The evidence as outlined and recent studies [119] suggest that chemical, temporal, and spatial specifications collectively distinguish neural representations of these states. Our own results raise the immediate question of how 2-AG is recruited and whether it cooperates with or acts independently of anandamide.
Does socially activated oxytocin signaling drive core endocannabinoid functions, such as in modulating inflammation, pain, feeding, and stress? Conversely, does endocannabinoid signaling mediate canonical actions of oxytocin, such as in maternal attachment and social recognition? There is support for these possibilities [83,139–144]. Our data suggest that more widespread oxytocin-driven endocannabinoid signaling is possible, for example in the hippocampus [56,58].
How does socially recruited, oxytocin-driven anandamide signaling interact with the reward signaling of drugs of abuse? It is possible that they might synergize with each other in certain cases while substituting for one another in others. A furthermore distinct instantiation could be a role for socially recruited endocannabinoid signaling in protectiveness/susceptibility of social support/isolation for addiction.
How does social stress distinctly activate endocannabinoid signaling relative to other forms of stress? Furthermore, what determines the response of an animal to social stress in the form of withdrawal versus that of aggression? While this review focused on the role of endocannabinoid signaling on the regulation of social behavior, there is also a line of evidence suggesting that social stress might activate endocannabinoid signaling [18,145–148]. Again, in order to identify underlying neural representations, it becomes important to distinguish between social states that might appear similar prima facie, such as social defeat [146], chronic isolation [147], and isolation from weaning [148].
Is there a role for endocannabinoid signaling in the development of the social brain, and how does exogenous cannabinoid exposure in development affect these functions? One possibility is that exogenous overactivation of cannabinoid receptors inappropriately tunes responses to early social experiences, such that later expression becomes exaggerated or attenuated. Another possibility is that exogenous cannabinoids interfere with key developmental roles of endocannabinoid signaling, such as in the transition between adolescence and adulthood.
Trends.
The crucial adaptive value of sociality is represented across evolutionary time. Modern techniques have been recently used to identify the neural circuits processing social information and regulating social behavior. The oxytocin system is now recognized as central to such socially specific signaling.
The Cannabis sativa plant has long been exploited to facilitate social bonding, and experimental studies have explored its psychotropic effects on human social behavior. Since the identification of the endocannabinoid signaling system, animal studies targeting cannabinoid receptors and transmitters (anandamide and 2-AG) have found regulatory effects, particularly in social anxiety and social reward, as well as endocannabinoid dysregulation in social impairment related to neuropsychiatric conditions.
These endocannabinoid effects are multimodal and context-dependent. Newly identified oxytocin-driven endocannabinoid signaling potentially represents a circuit-based mechanism through which selective recruitment of endocannabinoid signaling can occur, and may underlie the differential actions of anandamide and 2-AG.
Acknowledgments
Supported by NIH grant DA012413 (to D.P.) and the UC Irvine Medical Scientist Training Program and Autism Science Foundation Fellowship (to D.W.).
Glossary
- Endocannabinoid system
a lipid-derived neurotransmitter system consisting of cannabinoid receptors, endocannabinoid signaling messengers, and regulatory biosynthetic and degradative enzymes
- Social anxiety
fear of an unfamiliar conspecific, which may result in avoidance behavior
- Social reward
pleasure and incentive salience of a social stimulus, which may induce appetitive and consummatory behavior
- Autism spectrum disorder (ASD)
a set of disorders syndromically characterized by (i) deficient social reciprocity and communication and (ii) unusual, restricted, and repetitive behaviors
- Schizophrenia
a mental disorder characterized by persistent cognitive impairment, psychosis, and social anhedonia and withdrawal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Long T, et al. Cannabis in Eurasia: origin of human use and Bronze Age transcontinental connections. Vegetation History and Archaeobotany. 2016;26:245–258. [Google Scholar]
- 2.Clarke RC, Merlin MD. Cannabis: evolution and ethnobotany. University of California Press; Berkeley, Los Angeles and London: 2013. [Google Scholar]
- 3.Small E. Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization. Bot Rev. 2015;81:189–294. [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Association; 2013. [Google Scholar]
- 5.Whiting PF, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 6.Rydenko CN. Frozen tombs of Siberia: the Pazyryk burials of Iron Age horsemen 1970 [Google Scholar]
- 7.Herodotus . Herodotus: The Histories. Oxford University Press; 1998. [Google Scholar]
- 8.Sharma GK. Cannabis folklore in the Himalayas. Botanical Museum Leaflets. 1977;25:203–215. [Google Scholar]
- 9.Winkelman M, Baker JR. Supernatural as natural: A biocultural approach to religion. Routledge; 2016. [Google Scholar]
- 10.Insel TR. The Challenge of Translation in Social Neuroscience: A Review of Oxytocin, Vasopressin, and Affiliative Behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanley DA, Adolphs R. Toward a Neural Basis for Social Behavior. Neuron. 2013;80:816–826. doi: 10.1016/j.neuron.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonnes J. Hep-cats, narcs, and pipe dreams. 1Scribner; 1996. [Google Scholar]
- 13.Tart CT. Marijuana intoxication: Common experiences. Nature. 1970 doi: 10.1038/226701a0. [DOI] [PubMed] [Google Scholar]
- 14.Georgotas A, Zeidenberg P. Observations on the effects of four weeks of heavy marihuana smoking on group interaction and individual behavior. Comprehensive Psychiatry. 1979;20:427–432. doi: 10.1016/0010-440x(79)90027-0. [DOI] [PubMed] [Google Scholar]
- 15.Foltin RW, et al. Effects of smoked marijuana on social interaction in small groups. Drug Alcohol Depend. 1987;20:87–93. doi: 10.1016/0376-8716(87)90079-2. [DOI] [PubMed] [Google Scholar]
- 16.Foltin RW, Fischman MW. Effects of smoked marijuana on human social behavior in small groups. Pharmacology Biochemistry and Behavior. 1988;30:539–541. doi: 10.1016/0091-3057(88)90494-7. [DOI] [PubMed] [Google Scholar]
- 17.Salzman C, et al. Marijuana and hostility in a small-group setting. AJP. 1976;133:1029–1033. doi: 10.1176/ajp.133.9.1029. [DOI] [PubMed] [Google Scholar]
- 18.Phan KL, et al. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. Journal of Neuroscience. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornelius JR, et al. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addictive Behaviors. 2010;35:644–646. doi: 10.1016/j.addbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorka SM, et al. Cannabinoid Modulation of Amygdala Subregion Functional Connectivity to Social Signals of Threat. International Journal of Neuropsychopharmacology. 2015;18:pyu104–pyu104. doi: 10.1093/ijnp/pyu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins ST, Stitzer ML. Acute marijuana effects on social conversation. Psychopharmacology. 1986;89:234–238. doi: 10.1007/BF00310635. [DOI] [PubMed] [Google Scholar]
- 22.Babor TF, et al. Interpersonal behavior in group discussion during marijuana intoxication. International Journal of the Addictions. 1978;13:89–102. doi: 10.3109/10826087809039266. [DOI] [PubMed] [Google Scholar]
- 23.Babor TF, et al. Social effects of marijuana use in a recreational setting. International Journal of the Addictions. 1978 doi: 10.3109/10826087809039315. [DOI] [PubMed] [Google Scholar]
- 24.Galanter M, et al. Marihuana and social behavior: a controlled study. Arch Gen Psychiatry. 1974;30:518–521. doi: 10.1001/archpsyc.1974.01760100082013. [DOI] [PubMed] [Google Scholar]
- 25.Glass M, et al. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 26.Seeley WW, et al. Frontotemporal dementia: what can the behavioral variant teach us about human brain organization? Neuroscientist. 2012;18:373–385. doi: 10.1177/1073858411410354. [DOI] [PubMed] [Google Scholar]
- 27.Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2015;16:579–594. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceccarini J, et al. [(18) F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addiction Biology. 2015;20:357–367. doi: 10.1111/adb.12116. [DOI] [PubMed] [Google Scholar]
- 29.Wong DF, et al. PET Imaging of cannabinoid CB1 type receptors in healthy humans and patients with schizophrenia using [C-11]OMAR. Neuroimage. 2008;41:T51–T51. [Google Scholar]
- 30.Suarez J, et al. Distribution of diacylglycerol lipase alpha, an endocannabinoid synthesizing enzyme, in the rat forebrain. NSC. 2011;192:112–131. doi: 10.1016/j.neuroscience.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 31.Egertova M, et al. Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: A new perspective on N-acylethanolamines as neural signaling molecules. J Comp Neurol. 2008;506:604–615. doi: 10.1002/cne.21568. [DOI] [PubMed] [Google Scholar]
- 32.Piomelli D, et al. A neuroscientist’s guide to lipidomics. Nat Rev Neurosci. 2007;8:743–754. doi: 10.1038/nrn2233. [DOI] [PubMed] [Google Scholar]
- 33.Miczek KA. Delta9-tetrahydrocannabinol: antiaggressive effects in mice, rats, and squirrel monkeys. Science. 1978;199:1459–1461. doi: 10.1126/science.415367. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez-Arias M, et al. CB1 cannabinoid receptor-mediated aggressive behavior. Neuropharmacology. 2013;75:172–180. doi: 10.1016/j.neuropharm.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Litvin Y, et al. CB1 receptor signaling regulates social anxiety and memory. Genes, Brain and Behavior. 2013;12:479–489. doi: 10.1111/gbb.12045. [DOI] [PubMed] [Google Scholar]
- 36.Haller J, et al. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. European Journal of Neuroscience. 2004;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- 37.Terzian ALB, et al. Cannabinoid receptor type 1 receptors on GABAergic vs. glutamatergic neurons differentially gate sex-dependent social interest in mice. Eur J Neurosci. 2014;40:2293–2298. doi: 10.1111/ejn.12561. [DOI] [PubMed] [Google Scholar]
- 38.Cutler MG, Mackintosh JH. Cannabis and delta-9-tetrahydrocannabinol. Effects on elements of social behaviour in mice. Neuropharmacology. 1984;23:1091–1097. doi: 10.1016/0028-3908(84)90134-5. [DOI] [PubMed] [Google Scholar]
- 39.Dorr M, Steinberg H. Effects of Δ9-tetrahydrocannabinol on social behaviour in mice. Psychopharmacology. 1976;47:87–91. doi: 10.1007/BF00428707. [DOI] [PubMed] [Google Scholar]
- 40.Genn RF, et al. Unconditioned and conditioned anxiogenic effects of the cannabinoid receptor agonist CP 55,940 in the social interaction test. 2004;77:567–573. doi: 10.1016/j.pbb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Sulcova E, et al. Biphasic effects of anandamide. Pharmacology Biochemistry and Behavior. 1998;59:347–352. doi: 10.1016/s0091-3057(97)00422-x. [DOI] [PubMed] [Google Scholar]
- 42.Dewey WL. Cannabinoid pharmacology. Pharmacological Reviews. 1986;38:151–178. [PubMed] [Google Scholar]
- 43.Jacob W, et al. Endocannabinoids render exploratory behaviour largely independent of the test aversiveness: role of glutamatergic transmission. Genes, Brain and Behavior. 2009;8:685–698. doi: 10.1111/j.1601-183X.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- 44.Klugmann M, et al. AAV-mediated overexpression of the CB1 receptor in the mPFC of adult rats alters cognitive flexibility, social behavior, and emotional reactivity. Frontiers in Behavioral Neuroscience. 2011;5:37. doi: 10.3389/fnbeh.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakrabarti B, Baron-Cohen S. Variation in the human cannabinoid receptor CNR1 gene modulates gaze duration for happy faces. Mol Autism. 2011;2:10. doi: 10.1186/2040-2392-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marco EM, et al. Social encounter with a novel partner in adolescent rats: Activation of the central endocannabinoid system. Behavioural Brain Research. 2011;220:140–145. doi: 10.1016/j.bbr.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 47.Cassano T, et al. Evaluation of the emotional phenotype and serotonergic neurotransmission of fatty acid amide hydrolase-deficient mice. Psychopharmacology. 2011;214:465–476. doi: 10.1007/s00213-010-2051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.File SE, Seth P. A review of 25 years of the social interaction test. European Journal of Pharmacology. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 49.Trezza V, et al. Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. J Neurosci. 2012;32:14899–14908. doi: 10.1523/JNEUROSCI.0114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kathuria S, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2002;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 51.Trezza V, Vanderschuren LJ. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology. 2008;197:217–227. doi: 10.1007/s00213-007-1025-3. [DOI] [PubMed] [Google Scholar]
- 52.Manduca A, et al. Dopaminergic neurotransmission in the nucleus accumbens modulates social play behavior in rats. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manduca A, et al. Interacting Cannabinoid and Opioid Receptors in the Nucleus Accumbens Core Control Adolescent Social Play. Frontiers in Behavioral Neuroscience. 2016;10:858. doi: 10.3389/fnbeh.2016.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manduca A, et al. Strain- and context-dependent effects of the anandamide hydrolysis inhibitor URB597 on social behavior in rats. Eur Neuropsychopharmacol. 2014;24:1337–1348. doi: 10.1016/j.euroneuro.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Dölen G, et al. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei D, et al. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc Natl Acad Sci USA. 2015;112:14084–14089. doi: 10.1073/pnas.1509795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung KM, et al. 2-Arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metabolism. 2012;15:299–310. doi: 10.1016/j.cmet.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei D, et al. A role for the endocannabinoid 2-arachidonoyl-sn-glycerol for social and high-fat food reward in male mice. Psychopharmacology. 2016;233:1911–1919. doi: 10.1007/s00213-016-4222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 60.Piomelli D. More surprises lying ahead. The endocannabinoids keep us guessing. Neuropharmacology. 2014;76:228–234. doi: 10.1016/j.neuropharm.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harkany T, et al. The emerging functions of endocannabinoid signaling during CNS development. Trends in Pharmacological Sciences. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Wei D, Piomelli D. Cannabinoid-based drugs: potential applications in addiction and other mental disorders. Focus. 2015 doi: 10.1176/appi.focus.20150009. [DOI] [Google Scholar]
- 63.Seillier A, et al. Phencyclidine-induced social withdrawal results from deficient stimulation of cannabinoid CB1 Receptors: implications for schizophrenia. Neuropsychopharmacology. 2013;38:1816–1824. doi: 10.1038/npp.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seillier A, et al. Inhibition of fatty-acid amide hydrolase and CB1 receptor antagonism differentially affect behavioural responses in normal and PCP-treated rats. Int J Neuropsychopharmacol. 2010;13:373–386. doi: 10.1017/S146114570999023X. [DOI] [PubMed] [Google Scholar]
- 65.Spano MS, et al. Cannabinoid self-administration attenuates PCP-induced schizophrenia-like symptoms in adult rats. Eur Neuropsychopharmacol. 2010;20:25–36. doi: 10.1016/j.euroneuro.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Matricon J, et al. Distinct neuronal activation patterns are associated with PCP-induced social withdrawal and its reversal by the endocannabinoid-enhancing drug URB597. Neurosci Res. 2016 doi: 10.1016/j.neures.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zamberletti E, et al. Alterations of prefrontal cortex GABAergic transmission in the complex psychotic-like phenotype induced by adolescent delta-9-tetrahydrocannabinol exposure in rats. Neurobiol Dis. 2014;63:35–47. doi: 10.1016/j.nbd.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 68.Földy C, et al. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron. 2013 doi: 10.1016/j.neuron.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jung KM, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nature communications. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kerr DM, et al. Alterations in the endocannabinoid system in the rat valproic acid model of autism. Behavioural Brain Research. 2013;30:680–695. doi: 10.1016/j.bbr.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 71.Wei D, et al. Enhancement of anandamide-mediated endocannabinoid signaling corrects autism-related social impairment. Cannabis and Cannabinoid Research. 2016;1:81–89. doi: 10.1089/can.2015.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Servadio M, et al. Targeting anandamide metabolism rescues core and associated autistic-like symptoms in rats prenatally exposed to valproic acid. Transl Psychiatry. 2016;6:e902. doi: 10.1038/tp.2016.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doenni VM, et al. Deficient adolescent social behavior following early-life inflammation is ameliorated by augmentation of anandamide signaling. Brain, Behavior, and Immunity. 2016;58:237–247. doi: 10.1016/j.bbi.2016.07.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spechler PA, et al. Cannabis use in early adolescence: Evidence of amygdala hypersensitivity to signals of threat. Accident Analysis and Prevention. 2015;16:63–70. doi: 10.1016/j.dcn.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schneider M, et al. Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55,212-2. Behav Pharmacol. 2005;16:447–454. doi: 10.1097/00008877-200509000-00018. [DOI] [PubMed] [Google Scholar]
- 76.Schneider M, Koch M. Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology. 2005;30:944–957. doi: 10.1038/sj.npp.1300634. [DOI] [PubMed] [Google Scholar]
- 77.O’Shea M. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. Journal of Psychopharmacology. 2006;20:611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- 78.Bambico FR, et al. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol Dis. 2010;37:641–655. doi: 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 79.Schneider M, et al. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addiction Biology. 2008;13:345–357. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- 80.Rubino T, et al. Chronic |[Delta]|9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- 81.Realini N, et al. Chronic URB597 treatment at adulthood reverted most depressive-like symptoms induced by adolescent exposure to THC in female rats. Neuropharmacology. 2011;60:235–243. doi: 10.1016/j.neuropharm.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 82.Rubino T, et al. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis. 2015;73:60–69. doi: 10.1016/j.nbd.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Butovsky E, et al. Chronic exposure to Δ9-tetrahydrocannabinol downregulates oxytocin and oxytocin-associated neurophysin in specific brain areas. Molecular and Cellular Neuroscience. 2006;31:795–804. doi: 10.1016/j.mcn.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 84.Rubino T, Parolaro D. The impact of exposure to cannabinoids in adolescence: insights from animal models. Biological Psychiatry. 2016;79:578–585. doi: 10.1016/j.biopsych.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 85.Schneider M, et al. Enhanced Functional Activity of the Cannabinoid Type-1 Receptor Mediates Adolescent Behavior. Journal of Neuroscience. 2015;35:13975–13988. doi: 10.1523/JNEUROSCI.1937-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hariri AR, et al. Divergent Effects of Genetic Variation in Endocannabinoid Signaling on Human Threat- and Reward-Related Brain Function. Biological Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iversen LL. The science of marijuana 2001 [Google Scholar]
- 88.Devane WA, et al. Determination and characterization of a cannabinoid receptor in rat brain. Molecular Pharmacology. 1988;34:605–613. [PubMed] [Google Scholar]
- 89.Herkenham M, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsuda LA, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 91.Devane W, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 92.Mechoulam R, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 93.Hohmann AG, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 94.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 95.Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. British Journal of Pharmacology. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jung KM, et al. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Molecular Pharmacology. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- 97.Kano M, et al. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 98.Ho YC, et al. Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. Journal of Neuroscience. 2011;31:14600–14610. doi: 10.1523/JNEUROSCI.2671-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Giuffrida A, et al. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 100.Insel TR, Young LJ. Neuropeptides and the evolution of social behavior. Current Opinion in Neurobiology. 2000 doi: 10.1016/S0959-4388(00)00146-X. [DOI] [PubMed] [Google Scholar]
- 101.Sokolowski MB. Social interactions in “simple” model systems. Neuron. 2010;65:780–794. doi: 10.1016/j.neuron.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 102.Weitekamp CA, Hofmann HA. Evolutionary themes in the neurobiology of social cognition. Current Opinion in Neurobiology. 2014;28:22–27. doi: 10.1016/j.conb.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 103.de Waal FBM, Suchak M. Prosocial primates: selfish and unselfish motivations. Philos Trans R Soc Lond, B, Biol Sci. 2010;365:2711–2722. doi: 10.1098/rstb.2010.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Waal FBM. The Integration of Dominance and Social Bonding in Primates. The Quarterly Review of Biology. 2015;61:459–479. doi: 10.1086/415144. [DOI] [PubMed] [Google Scholar]
- 105.Soares MC, et al. Hormonal mechanisms of cooperative behaviour. Philos Trans R Soc Lond, B, Biol Sci. 2010;365:2737–2750. doi: 10.1098/rstb.2010.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alexander RD. The evolution of social behavior. Annual review of ecology and systematics 1974 [Google Scholar]
- 107.Schuelke O, et al. Social Bonds Enhance Reproductive Success in Male Macaques. Current Biology. 2010;20:2207–2210. doi: 10.1016/j.cub.2010.10.058. [DOI] [PubMed] [Google Scholar]
- 108.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985 [PubMed] [Google Scholar]
- 109.Cacioppo JT, et al. Social Neuroscience: Progress and Implications for Mental Health. Perspectives on Psychological Science. 2016;2:99–123. doi: 10.1111/j.1745-6916.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 110.Cacioppo JT, Decety J. Social neuroscience: challenges and opportunities in the study of complex behavior. Annals of the New York Academy of Sciences. 2011;1224:162–173. doi: 10.1111/j.1749-6632.2010.05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences. 2012;16:559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stein MB, Stein DJ. Social anxiety disorder. Lancet. 2008;371:1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 113.Losh M, et al. Neuropsychological Profile of Autism and the Broad Autism Phenotype. Arch Gen Psychiatry. 2009;66:518–526. doi: 10.1001/archgenpsychiatry.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- 115.Lieberman MD. Social cognitive neuroscience: a review of core processes. 2006;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. http://dx.doi.org/10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- 116.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 117.Adolphs R. The social brain: neural basis of social knowledge. 2008;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bicks LK, et al. Prefrontal cortex and social cognition in mouse and man. Front Psychol. 2015;6:693. doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matthews GA, et al. Dorsal raphe dopamine neurons represent the experience of social isolation. Cell. 2016;164:617–631. doi: 10.1016/j.cell.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gunaydin LA, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014 doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Okuyama T, et al. Ventral CA1 neurons store social memory. Science. 2016;353:1536–1541. doi: 10.1126/science.aaf7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yizhar O. Optogenetic insights into social behavior function. Biological Psychiatry. 2012;71:1075–1080. doi: 10.1016/j.biopsych.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 123.Allsop SA, et al. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Frontiers in Behavioral Neuroscience. 2014;8 doi: 10.3389/fnbeh.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 125.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 126.Marlin BJ, et al. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nat Rev Immunol. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scott N, et al. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature. 2015;525:519–522. doi: 10.1038/nature15378. [DOI] [PubMed] [Google Scholar]
- 128.Ferguson JN, et al. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 129.Knobloch HS, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 130.Owen SF, et al. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nat Rev Immunol. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chang SWC, et al. Neural mechanisms of social decision-making in the primate amygdala. Proc Natl Acad Sci USA. 2015;112:16012–16017. doi: 10.1073/pnas.1514761112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guastella AJ, et al. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 133.Rilling JK, et al. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yatawara CJ, et al. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Molecular Psychiatry. 2015 doi: 10.1038/mp.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kosfeld M, et al. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 136.Hurlemann R, et al. Oxytocin Enhances Amygdala-Dependent, Socially Reinforced Learning and Emotional Empathy in Humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Warner J, et al. “Girls Are Retarded When They’re Stoned.” Marijuana and the Construction of Gender Roles Among Adolescent Females. Sex Roles. 1999;40:25–43. [Google Scholar]
- 138.Mitchell JT, et al. “I Use Weed for My ADHD”: A Qualitative Analysis of Online Forum Discussions on Cannabis Use and ADHD. PLoS ONE. 2016;11:e0156614. doi: 10.1371/journal.pone.0156614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Russo R, et al. Central administration of oxytocin reduces hyperalgesia in mice: Implication for cannabinoid and opioid systems. Peptides. 2012;38:81–88. doi: 10.1016/j.peptides.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 140.Verty ANA, et al. Evidence for an interaction between CB1 cannabinoid and oxytocin receptors in food and water intake. Neuropharmacology. 2004;47:593–603. doi: 10.1016/j.neuropharm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 141.Schechter M, et al. Blocking the postpartum mouse dam’s CB1 receptors impairs maternal behavior as well as offspring development and their adult social–emotional behavior. Behavioural Brain Research. 2012;226:481–492. doi: 10.1016/j.bbr.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 142.Schechter M, et al. Endocannabinoid receptor deficiency affects maternal care and alters the dam’s hippocampal oxytocin receptor and brain-derived neurotrophic factor. Expression. 2013;25:898–909. doi: 10.1111/jne.12082. [DOI] [PubMed] [Google Scholar]
- 143.Oliet SH, et al. Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing. J Neurosci. 2007;27:1325–1333. doi: 10.1523/JNEUROSCI.2676-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ninan I. Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. Journal of Neurochemistry. 2011;119:324–331. doi: 10.1111/j.1471-4159.2011.07430.x. [DOI] [PubMed] [Google Scholar]
- 145.Hill MN, et al. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34:1257–1262. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dubreucq S, et al. Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology. 2012;37:1885–1900. doi: 10.1038/npp.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sciolino NR, et al. Social isolation and chronic handling alter endocannabinoid signaling and behavioral reactivity to context in adult rats. Neuroscience. 2010;168:371–386. doi: 10.1016/j.neuroscience.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Robinson SA, et al. The effect of social isolation on rat brain expression of genes associated with endocannabinoid signaling. Brain Research. 2010;1343:153–167. doi: 10.1016/j.brainres.2010.04.031. [DOI] [PubMed] [Google Scholar]


