Summary
During chemotactic signaling by Escherichia coli, the small cytoplasmic CheW protein couples the histidine kinase CheA to chemoreceptor control. Although essential for assembly and operation of receptor signaling complexes, CheW in stoichiometric excess disrupts chemotactic behavior. To explore the mechanism of the CheW excess effect, we measured the physiological consequences of high cellular levels of wild-type CheW and of several CheW variants with reduced or enhanced binding affinities for receptor molecules. We found that high levels of CheW interfered with trimer assembly, prevented CheA activation, blocked cluster formation, disrupted chemotactic ability, and elevated receptor methylation levels. The severity of these effects paralleled the receptor binding affinities of the CheW variants. Because trimer formation may be an obligate step in the assembly of ternary signaling complexes and higher-order receptor arrays, we suggest that all CheW excess effects stem from disruption of trimer assembly. We propose that the CheW-binding sites in receptor dimers overlap their trimer contact sites and that high levels of CheW saturate the receptor binding sites, preventing trimer assembly. The CheW-trapped receptor dimers seem to be improved substrates for methyltransferase reactions, but cannot activate CheA or assemble into clusters, processes that are essential for chemotactic signaling.
Keywords: chemotaxis, trimers of dimers, receptor teams, CheA
Introduction
Motile Escherichia coli cells possess an exquisitely sensitive sensory system that allows them to track chemical gradients with high precision [see (Hazelbauer et al., 2008) for a recent review]. Gradient sensing occurs through a polar array of receptor proteins known as MCPs (methyl-accepting chemotaxis proteins). MCP molecules form ternary signaling complexes with the histidine kinase CheA and with CheW, a protein that couples CheA autophosphorylation activity to receptor control. CheA in turn regulates the phosphorylation state of CheY to control the direction of rotation of the flagellar motors. These chemoreceptors achieve high sensitivity through cooperative signaling interactions, presumably promoted by physical connections between different receptors in the array. The networked receptors sense chemical gradients in temporal fashion by comparing current conditions with those averaged over the past few seconds to determine the cell’s direction of travel in the gradient. Temporal sensing relies on a sensory adaptation system that covalently modifies the receptor signaling domains in response to stimulus-induced changes in their signal output. CheR, an MCP-specific methyltransferase, adds methyl groups to receptor molecules to up-regulate clockwise (CW) rotational signals; CheB, a methylesterase, removes MCP methyl groups to down-regulate CW signals. Thus, MCP methylation level serves as a memory of chemoeffector concentrations in the recent past because it takes the cells a few seconds to update the methylation record after a concentration change.
The molecular architecture of chemoreceptor arrays is still poorly understood. However, in E. coli there is considerable evidence that receptor trimers of dimers are an important component of these higher-order complexes [see (Sourjik, 2004; Parkinson et al., 2005; Kentner et al., 2006) for reviews]. Chemoreceptors of different types can form mixed trimers, which presumably impart some of their cooperative behavior. Trimers of dimers most likely recruit CheA and CheW to form higher-order signaling teams of receptors, which in turn are somehow networked together to form the supramolecular cluster. Each successive level of receptor organization probably contributes to the highly cooperative behavior of the receptor signaling array.
The structure and function of receptor signaling teams, the basic functional unit of receptor signaling, are also poorly understood. In particular, little is known about the mechanistic role of the CheW coupling factor and how it conveys control signals from the chemoreceptors to CheA. One longstanding clue to CheW action is the dramatic disruption of chemotactic ability upon CheW overexpression. High levels of CheW interfere with MCP-mediated CheA activation both in vitro (Gegner et al., 1992; Liu et al., 1997) and in vivo (Liu & Parkinson, 1989; Sanders et al., 1989), implying that aberrant component stoichiometries might damage or disrupt the ternary signaling complex. Concomitant high level expression of MCPs, but not of CheA, alleviates much of the excess CheW effect on chemotaxis (Liu & Parkinson, 1989), demonstrating that a proper CheW-MCP stoichiometry is critical for modulation of CheA activity.
In more recent work, we showed that high levels of CheW seemed to reduce trimer-of-dimer formation, as measured by in vivo crosslinking of receptor molecules with TMEA, a cell-permeant, trifunctional, thiol-reactive reagent (Studdert & Parkinson, 2005). The cytoplasmic tips of MCP molecules promote trimer formation (Kim et al., 1999) but also correspond to the receptor region that has been implicated in CheW binding and CheA activation (Ames et al., 1996). Accordingly, the binding sites in receptor molecules that promote interactions with other receptors to form trimers and with CheW to form ternary complexes could overlap. At proper stoichiometries, CheW may only occupy on average one binding site per receptor dimer, leaving the other free to promote trimer formation. However, an excess of CheW should titrate the shared binding sites and interfere with the receptor-receptor interactions that lead to trimers of dimers.
This view of the CheW overexpression effect makes several predictions that we tested in the present study. (i) The trimer-inhibiting effect of excess CheW should be a direct effect, not dependent on the presence of other chemotaxis proteins. (ii) CheW mutants with altered binding affinity for receptor molecules should exhibit differences in their ability to prevent trimer formation. Moreover, the ability of CheW mutants to prevent trimer formation should parallel their ability to inhibit chemotaxis at high levels of expression. (iii) If trimers of dimers are building blocks of receptor arrays, as the team model holds, then excess CheW should also block assembly of receptor signaling teams and clusters. The experiments reported here confirmed each of these predictions, providing additional support for the team model of receptor signaling and for the central role of trimers of dimers in array architecture.
Results
Kinetics of trimer disruption by excess CheW
In cells containing normal levels of CheA and CheW, there is little exchange between newly-synthesized receptor molecules and the trimers of dimers that have been incorporated into chemoreceptor clusters (Studdert & Parkinson, 2005). By contrast, in cells lacking either CheA or CheW, receptor dimers readily exchange members with trimers of dimers, indicating that CheA and CheW together stabilize the higher-order organization of chemoreceptor complexes (Studdert & Parkinson, 2005). Could a sudden increase in CheW levels disrupt trimers of dimers within seemingly stable chemoreceptor clusters? To answer this question, we compared the time course of trimer decline upon CheW over-expression in cells with (UU1604) and without (UU1613) normal levels of CheA and CheW. Both strains express Tar-S364C (Tar•C) as their only chemoreceptor. The Tar•C aspartate receptor is fully functional, but forms 2- and 3-subunit crosslinking products, reflecting trimer-of-dimers organization, upon treatment of cells with the trifunctional thiol-reagent TMEA (Studdert & Parkinson, 2004; Studdert & Parkinson, 2005). To simplify the Tar•C TMEA patterns, both strains lacked the MCP methyltransferase (CheR) and methylesterase (CheB) enzymes involved in sensory adaptation, thereby clamping all Tar•C molecules in one modification state. To manipulate CheW levels and to distinguish overexpressed CheW from chromosomally-encoded CheW, the strains also carried plasmid pPA770, which encodes an IPTG-inducible variant of CheW (CheWΔ8) that lacks residues 2–9 of wild-type CheW, but has full CheW function (Studdert & Parkinson, 2005).
UU1604/pPA770 and UU1613/pPA770 were grown to mid-exponential phase and induced with 400 µM IPTG. Samples taken at various times thereafter were treated with TMEA to assess trimer of dimer levels. In the A+ W+ strain (UU1604), TMEA-crosslinked products remained at their high initial levels over the first 20 minutes of CheWΔ8 induction, then showed a decline by 40 minutes (Fig. 1A&B). By contrast, in the A− W− strain (UU1613), crosslinked Tar•C subunits had declined declined substantially by 15 minutes (Fig. 1A&B). The induced levels of CheWΔ8 were similar in both strains (Fig. 1C&D). Thus, trimers of dimers declined faster in the A− W− strain, even though the overall level of CheW was a bit lower than in the A+ W+ strain. We conclude that CheW excess probably does not “disrupt” stable receptor arrays, but rather that it interferes with trimer of dimer formation by newly-synthesized receptor molecules. Assembled receptor-CheW-CheA signaling complexes are known to be ultrastable in vitro (Erbse & Falke, 2009). In cells lacking CheA, trimers cannot form stable arrays and so undergo exchange with the pool of receptor dimers. Under these conditions, CheW excess probably inhibits trimer-of-dimer formation by trapping receptors in the dimer state. Consistent with this view, we found that after several generations of CheW excess, both CheA+ and CheA− cells showed similarly reduced levels of trimers (Studdert & Parkinson, 2005; and data not shown) indicating that the interference with trimer formation is a direct effect of CheW. Moreover, the presence of CheA does not prevent the CheW interference effect.
Fig. 1.
Time course of trimer interference by excess CheW in the presence or absence of preformed chemoreceptor clusters. Strains encoding Tar-S346C (Tar•C) and carrying plasmid pPA770 (encoding CheWΔ8) were grown to mid-log phase and induced with 400 µM IPTG. Cell samples taken at the indicated times were kept on ice with the addition of 0.1 mg/ml chloramphenicol to stop further protein synthesis, then harvested and treated with TMEA, as detailed in Methods. Host UU1613 is deleted for the cheA and cheW genes (A− W−); host UU1604 is not (A+ W+).
(A) SDS-PAGE analysis of TMEA-treated samples; Tar•C bands were visualized by immunoblotting with anti-Tsr (see Methods). Cartoons of the Tar•C crosslinking products (reading from top to bottom) indicate the positions of 3-subunit, 2-subunit, and uncrosslinked Tar•C subunits, respectively.
(B)Quantitation of crosslinking products in the gel shown in (A). Bars show the fraction of Tar•C subunits in each gel lane that were in 2- plus 3-subunit products relative to that value in the same host at 0 minutes after induction. The data points show average and standard error for three independent experiments. Note that the expected fraction of Tar•C subunits that would be crosslinked by TMEA if all receptor molecules were organized as trimers of dimers is 50% (Studdert & Parkinson, 2005) and typically falls between 45% and 55% in independent experiments of this sort (see Mowery et al., 2008).
(C)SDS-PAGE analysis of CheW proteins in the time course samples; CheW visualized by immunoblotting with anti-CheW (see Methods). CheWΔ8 lacks the first 8 residues of the CheW protein and consequently migrates faster than wild-type CheW.
(D)Quantitation of CheW bands in the gel shown in (C). The level of wild-type CheW in UU1604 samples did not change appreciably after induction of CheWΔ8 expression (see gel in (C) and data not shown). In the UU1604 samples, the amounts of wild-type CheW (produced from the chromosomal gene) and CheWΔ8 (produced from pPA770) are combined. The amount of CheWΔ8 in the UU1613 sample was normalized to that of wild-type CheW in UU1604.
Trimer disruption and the CheW-receptor binding interaction
We hypothesize that CheW inhibits trimer formation by binding to receptor dimers and masking their trimer-forming contact sites. If so, then CheW mutants with reduced or enhanced binding affinity for receptor molecules should have reduced or enhanced ability to interfere with trimer formation. Boukhvalova et al. characterized CheW mutants with different receptor affinities, which were isolated on the basis of their altered ability to inhibit chemotaxis when expressed at high levels (Boukhvalova et al., 2002a; Boukhvalova et al., 2002b). “Weak titrator” CheW mutants failed to inhibit chemotaxis when expressed at high levels and showed reduced affinity for MCPs. Conversely, a “strong titrator” CheW mutant showed enhanced ability to interfere with chemotaxis and increased affinity for receptors.
We tested derivatives of CheWΔ8 bearing weak or strong titrator lesions for their ability to interfere with trimer formation at different steady-state expression levels in a Tar•C CheR− CheB− strain (UU1598). Under these conditions, CheWΔ8 had a discernable effect on trimer levels at 40 µM IPTG induction and at 120 µM IPTG reduced TMEA crosslinking products by about 80% (Fig. 2A). CheWΔ8 bearing weak titrator lesions (E38D or G133E) had no significant effect on trimers, even at the highest inducer level, whereas CheWΔ8 with a strong titrator lesion (E154oc) reduced the TMEA crosslinking products by over 50% upon induction with only 10 µM IPTG (Fig. 2A). The steady-state levels of the weak titrator CheW proteins, measured in the same cell samples, were similar to wild-type (Fig. 2D), indicating that their lack of effect on trimer formation was not due to a change in expression or stability. CheWΔ8-E154oc, the strong titrator, exhibited about 2-fold lower intracellular levels than the wild-type (Fig. 2D). We confirmed that the E154oc protein which lacks residues 154–167 of wild-type CheW, nevertheless reacts equally well with the CheW antiserum used for immunoblotting (data not shown), so it most likely has reduced intracellular stability. This implies an even more potent ability, on a molecule-by-molecule basis, to interfere with trimer of dimers formation. These experiments demonstrate that the ability of CheWΔ8 variants to interfere with trimer of dimers formation parallels their receptor-binding affinity.
Fig. 2.
Functional consequences of weak and strong titrator CheW excess. Strains carrying pPA770 (WT) or a mutant CheW derivative (E38D, G133E, E154oc) were induced with IPTG and analyzed for trimer formation (A), chemotactic ability (B), clockwise flagellar rotation (C), and total CheW protein content (D).
(A)TMEA analysis of UU1598 (Tar•C, CheR− CheB−) strains carrying pPA770 derivatives. Crosslinking products were visualized and analyzed as described in Fig.1A & B. Crosslinking values were normalized relative to the no IPTG samples. Data points show the average and standard error of three independent experiments.
(B)Colony size on tryptone soft agar plates of UU1596 (Tar•C CheR+ CheB+) strains carrying pPA770 derivatives. Plates were incubated for 8–10 hours at 30°C. Colony diameters were normalized relative to that of cells carrying a vector control plasmid (pCJ30). The diameter of nonchemotactic pCJ30 colonies under the same conditions is ~20% of the wild-type reference diameter. Data points show the average and standard error for at least 10 colonies.
(C)Time spent in clockwise rotation by RP1616 (CheZ−) strains carrying pPA770 derivatives. Rotation patterns were analyzed by cell tethering to calculate the fraction of cell rotation time spent in CW rotation (see Methods). These CW values reflect a combination of receptor-coupled and receptor-uncoupled CheA activities (Liu & Parkinson, 1989). At normal levels of wild-type CheW, all CW rotation is due to receptor-coupled CheA; at very high levels of wild-type CheW or in the absence of CheW, CW rotation is due to the activity of uncoupled CheA. Using these limit values (80–90% and 35–50%, respectively) CW values in each experiment were adjusted to show the fraction of receptor-coupled CW rotation time. Each data point was obtained from observation of 100 rotating cells.
(D)Total CheW protein (CheW + CheWΔ8) in the UU1598 samples of panel (A), normalized relative to the level of wild-type CheW in UU1604 cells containing a vector control plasmid (pCJ30).
Effect of CheW excess on CheA activation and chemotactic ability
The team model of receptor signaling posits that trimers of dimers are integral components of ternary signaling complexes and, therefore, essential for chemotactic behavior. If CheW excess impairs chemotaxis by disrupting trimers of dimers, then the expression level at which CheW titrator mutants impair chemotaxis should parallel the level at which they block trimer formation. We tested plasmid-borne CheWΔ8 variants in a Tar•C CheR+ CheB+ strain (UU1596) for effects on chemotactic migration in tryptone soft agar plates. In all cases, the inhibition of chemotaxis closely paralleled the trimer interference effect for each variant (Fig. 2B). At the highest inducer concentration (120 µM IPTG), the weak titrator mutants produced only modest declines in chemotactic ability and no discernable effects on trimer formation (Fig. 2A&B). In contrast, the strong titrator mutant exhibited maximal inhibition of chemotaxis and trimer formation at low (20 µM) IPTG levels (Fig. 2A&B).
A corollary of the team signaling model is that receptor trimers of dimers are essential for activating and modulating CheA kinase activity, ostensibly through ternary signaling complexes. To assess their effects on CheA activation, we examined the flagellar rotation patterns of CheWΔ8 variants in a CheZ− strain (RP1616). Cells that assemble functional signaling complexes, but lack the CheY-P phosphatase CheZ, have high levels of CheA−generated CheY-P and consequently rotate their flagella predominantly in the clockwise (CW) direction. Such cells show a progressive decline in CW flagellar rotation, reflecting a drop in CheA activity, with increasing expression levels of wild type CheW (Liu & Parkinson, 1989; Sanders et al., 1989) (Fig. 2C). Cells expressing either of the weak titrator CheW variants retained a high level of receptor-coupled CW rotation, even at the highest inducer concentration. In contrast, the strong titrator variant drastically reduced receptor-coupled CW rotation by 20 µM IPTG induction (Fig. 2C).
Effect of CheW excess on receptor modification state
The signaling states and conformations of receptor molecules influence the relative activities of the MCP-modifying CheR and CheB enzymes. Receptors in a kinase-off conformation are better substrates for CheR than CheB, whereas receptors in the kinase-on state are better substrates for CheB than CheR. Thus, in response to chemotactic stimuli, kinase-off receptors undergo net methylation and kinase-on receptors undergo net demethylation to bring about sensory adaptation. The activity of CheB is also regulated by its phosphorylation state, which in turn depends on the receptor-modulated activity of CheA.
To determine whether CheW excess could influence the steady-state methylation levels of receptor molecules, we examined MCP modification patterns in a CheA− CheW− background to avoid feedback control of CheB through CheA signaling. In this way, the modification states of receptor molecules should only depend on their relative suitability as substrates for the methyltransferase and methylesterase enzymes. Compatible plasmids encoding CheWΔ8 variants and Tsr, the serine receptor, were transferred to strain UU1626 (MCP− CheA− CheW−) and the Tsr modification pattern at different CheW induction levels was assessed by electrophoretic mobility in polyacrylamide gels. We found that induction of CheWΔ8 caused a progressive shift of Tsr molecules to more highly methylated forms (Fig. 3). The strong titrator, CheW-E154oc, caused an even more pronounced shift to higher Tsr methylation states at lower inducer concentrations. The effect appeared to peak at 20 µM IPTG, but we have no explanation for that behavior (Fig. 3). The weak titrators, CheW-E38D and CheW-G133E, produced no change in Tsr band pattern at any induction level (Fig. 3). In light of the differing effects of CheWΔ8 variants on trimer formation, these findings imply that receptor dimers organized as trimers are relatively poor substrates for methylation, whereas receptor dimers are much better methylation substrates (or worse demethylation substrates).
Fig. 3.
Effect of CheW excess on Tsr methylation levels. Strain UU1626 (Tsr− Tar− Tap− Trg− Aer− CheA− CheW−) carried compatible plasmids encoding Tsr (pCS12) and CheWΔ8 (pPA770 and mutant derivatives). Tsr modification patterns were visualized in low-bis polyacrylamide gels by immunoblotting (see Methods). Faster-migrating bands represent more highly methylated species.
Effect of CheW excess on receptor clustering
Another tenet of the team model of receptor signaling is that receptor arrays or clusters represent physically networked receptor signaling teams that are based on a trimer-of-dimers organization. Given that CheW excess prevents receptors from forming trimers and ternary signaling complexes, we predicted that it would also prevent receptor clustering. To test this prediction, we analyzed the steady-state cellular distribution of a fluorescently-tagged CheR protein (YFP-CheR) in cells expressing CheWΔ8 to different extents. CheR binds to a C-terminal pentapeptide sequence (NWETF) present in both Tsr and Tar (Wu et al., 1996; Djordjevic & Stock, 1998), so YFP-CheR serves as a specific reporter for chemoreceptor localization (Shiomi et al., 2002; Kentner et al., 2006). In UU1604/pPA770 cells (Tar•C A+ W+ with plasmid-expressed CheWΔ8), YFP-CheR formed tight, polar spots under uninduced conditions, but no discernable spots when induced with 120 µM IPTG (Fig. 4A). At intermediate inducer concentrations, the fraction of cells with one or more receptor clusters progressively declined with increasing CheWΔ8 expression (Fig. 4B). The disappearance of receptor clusters at high CheWΔ8 levels is consistent with the idea that cluster assembly depends on receptor trimers of dimers. In cells lacking CheA and CheW, chemoreceptors typically form diffuse polar clusters or caps (Fig. 4A; strain UU1613, no IPTG). We found that even this localization pattern disappeared when CheWΔ8 was in excess (Fig. 4A; UU1613, 120 µM IPTG), suggesting that this form of receptor clustering is also dependent on trimer formation.
Fig. 4.
Effect of CheW excess on receptor clustering. Strains UU1604 and UU1613 carrying compatible plasmids pPA770 (CheWΔ8) and pVS102 (YFP-CheR) were induced with various concentrations of IPTG and examined for receptor clusters (see Methods).
(A)Inverted, grayscale conversions of fluorescence images showing receptor clusters (black spots). Background fluorescence makes the cell bodies visible, but fainter in cells where most of the reporter molecules are localized in clusters, e.g., upper left panel. The polar caps formed in cells lacking CheA and CheW (lower left panel) are less tightly clustered and more difficult to discern owing to higher fluorescence background in the cytoplasm.
(B)Quantitative analysis of UU1604 fluorescence images. The dashed line indicates the fraction of UU1604/pVS102 cells carrying a CheW− control plasmid (pCJ30) that had one or more receptor clusters. The CheWΔ8 levels were not explicitly quantified in this experiment.
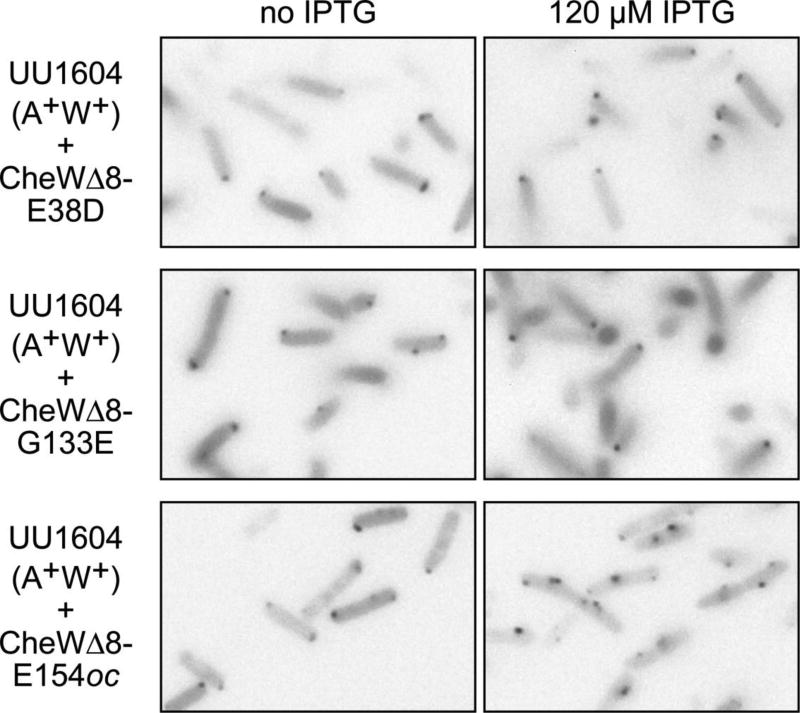
The effects of weak titrator CheW mutants on receptor clustering paralleled their effects on trimer and ternary complex assembly (Fig. 5). Neither CheWΔ8-E38D nor CheWΔ8-G133E prevented receptor clustering, even at the highest inducer concentration. However, the strong titrator CheW variant exhibited unexpected clustering behavior: At high expression levels of CheWΔ8-E154oc, the cells contained multiple clusters, often in lateral or central locations, that had an elongated or flattened appearance (Fig. 5). This unusual clustering pattern was also seen in cells lacking the kinase (UU1613; results not shown), indicating that the receptor aggregates probably arise through aberrant interactions with the strong titrator CheW protein. Because CheWΔ8-E154oc was very effective at blocking trimer formation (Fig. 2A), this result suggests that there may be two distinct receptor clustering mechanisms, one dependent on trimer formation and one that is not.
Fig. 5.
Effect of mutant CheW excess on receptor clustering. Weak and strong titrator derivatives of pPA770 were transferred to strain UU1604 carrying the compatible pVS102 (YFP-CheR) reporter plasmid, induced with various concentrations of IPTG, and examined for receptor clusters, as detailed in Fig. 4A and Methods.
Chemotactic ability and receptor clustering in a strong titrator CheW mutant
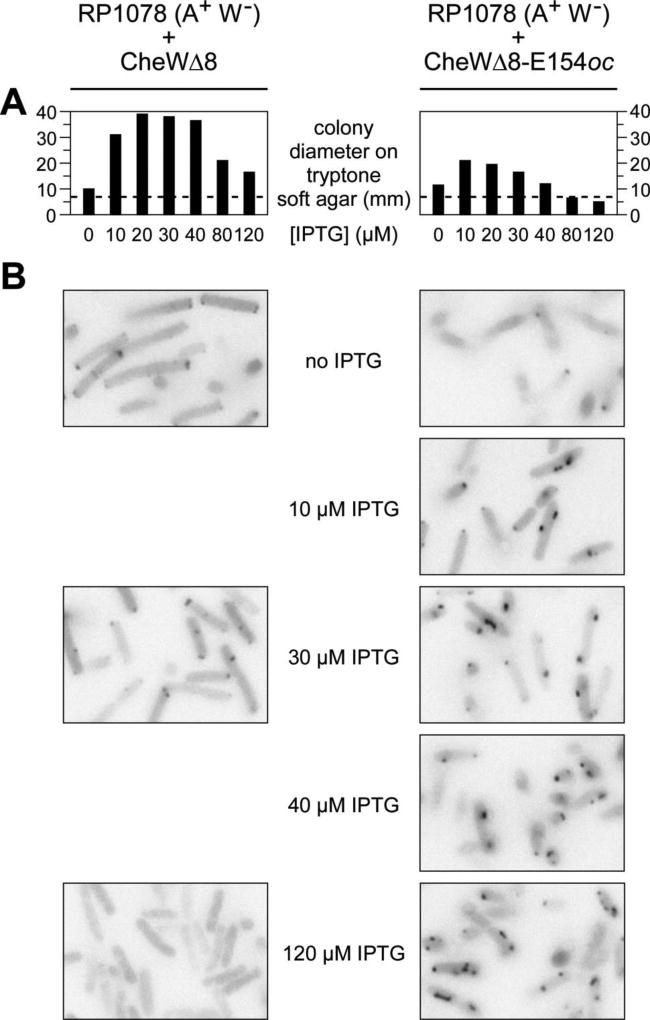
To further explore the receptor clusters formed by the strong titrator mutant, we tested the ability of CheWΔ8-E154oc to support chemotaxis and clustering in a strain (RP1078) lacking CheW, but with normal levels of receptors Tsr, Trg, and Aer, as well as all other Che components. Plasmid-encoded CheWΔ8 restored optimal chemotaxis function, assessed on soft agar plates, at about 20–30 µM IPTG (Fig. 6A). CheWΔ8-E154oc also supported chemotaxis of RP1078, but less well and with a lower inducer optimum (Fig. 6A). At 10 µM IPTG, the optimal inducer level for chemotaxis function of the strong titrator, the cells exhibited near-normal receptor clusters, frequently in polar locations, but often accompanied by prominent lateral clusters, as well (Fig. 6B; compare to CheWΔ8, 30 µM IPTG). The number of receptor clusters in the strong titrator cells increased further at higher inducer concentrations (Fig. 6B). The appearance and distribution of the strong titrator clusters also became increasingly aberrant at higher expression levels. Those clusters appeared flattened and distended, mostly in lateral locations, seldom at the poles.
Fig. 6.
Chemotactic ability and receptor clustering in the strong titrator CheW mutant. Strain RP1078 (CheW− Tar− Tap−) carrying pVS102 (YFP-CheR) and pPA770 (CheWΔ8) or its strong titrator derivative (CheWΔ8-E154oc) was tested at various IPTG concentrations for chemotactic ability (A) and receptor clustering (B).
(A)Colony size on tryptone soft agar plates incubated for 12 hours at 30°C. The dashed lines show the size of nonchemotactic colonies carrying a CheW− control plasmid (pCJ30).
(B)Fluorescence microscopy cell images as in Fig. 4A and Fig. 5.
Discussion
A mechanistic model for CheW overexpression effects
High intracellular levels of CheW have long been known to disrupt chemotactic ability (Liu & Parkinson, 1989; Sanders et al., 1989; Boukhvalova et al., 2002). We showed in this work that excess CheW disrupts a number of subsidiary chemotaxis-related functions as well, including control of receptor methylation state, activation of CheA kinase, and assembly of receptor clusters. We suggest that all of these CheW effects have the same root cause - the ability of CheW molecules to bind to receptor dimers and thereby prevent them from forming trimers of dimers (Fig. 7).
Fig. 7.
Cartoon summary of the effects of excess CheW on the formation of receptor clusters. The figure is not intended to be an accurate depiction of receptor signaling teams or clusters, whose detailed architeture is currently unknown, but rather to show how excess CheW shifts the assembly of ternary complexes off-pathway. At normal cellular levels of CheA and CheW, receptor dimers form trimers of dimers, which then bind CheA and CheW to form signaling teams that activate and modulate CheA in response to chemical stimuli. The number of trimers in a functional signaling team is unknown, but modeling studies suggest that it is more than one and possibly a variable number, depending on the modification states of the receptor molecules (Endres et al., 2008). Receptor clusters or arrays contain many signaling teams, most likely networked through their shared connections to CheA/CheW. Receptor subunits are synthesized with two of the four principal methylation sites (small circles) as glutamines (gray circles). Glutamines mimic the functional effects of methylated glutamate residues (black circles) and may persist during dimer and trimer formation, but are eventually converted to glutamates by irreversible CheB-mediated deamidation. The methylation level of arrayed receptor molecules varies with stimulus conditions, but averages 1–2 methyl groups per subunit in the absence of chemoeffectors. Receptor trimers, teams, and arrays cannot form in the presence of high levels of CheW, regardless of whether CheA is present or not. Instead, CheW appears to saturate its binding sites on receptor dimers (gray ovals), thereby preventing interactions between the receptor tips that are needed to form trimers. The isolated receptor dimers have 3–4 methyl groups per subunit, based on SDS-PAGE band patterns (see Fig. 3) and evidently are better methylation substrates (or worse demethylation substrates) than are receptor dimers organized in trimers.
We propose that the tips of receptor dimers have two identical CheW binding sites (Ames et al., 1996) that overlap with the inter-receptor contact sites that promote trimer formation (Ames et al., 2002). At low CheW levels, many receptor molecules should have at least one binding site unoccupied by CheW and those molecules would be able to initiate trimer contacts through the unoccluded site (Fig. 7). However, at higher CheW levels, both binding sites would often be occupied, reducing the opportunity for trimer contact interactions between receptors and effectively trapping receptors as dimers, leading to pleiotropic consequences (Fig. 7). Alternatively, because the CheW-binding surface on receptor molecules has not been clearly identified, CheW binding might influence trimer assembly less directly, for example, by stifling dynamic motions of the receptor tip that are needed to seed trimer formation.
The properties of CheW variants with relatively modest shifts in binding affinity for receptors (Boukhvalova et al., 2002) emphasize the central role and delicate poise of receptor-binding interactions in these CheW excess effects. Weak titrators (4- to 14-fold reduced affinity) failed to block trimer formation at any expression level tested, whereas a strong titrator (~3-fold higher affinity) interfered with trimer formation at even low expression levels. Moreover, the thresholds for CheW variant effects on chemotactic ability, receptor methylation level, and CW rotation time - a measure of CheA activation - paralleled those for trimer formation, indicating that these manifestations of CheW excess are most likely causally related.
In their seminal study of CheW binding mutants, Boukhvalova et al. reported a curious behavior of CheW-E154oc, the strong titrator (Boukhvalova et al., 2002). Their CheW binding assays used receptor molecules in membrane vesicles made from cells lacking CheA and CheW, i.e., conditions favoring dimer-trimer exchange. Under those conditions, CheW-E154oc actually enhanced the amount of wild-type CheW that bound in the same assay. This might happen because low levels of CheW-E154oc would expose new CheW-binding sites on the receptor molecules by shifting the receptor population from trimers of dimers to individual dimers, which should expose additional CheW-binding sites (Fig. 7). Alternatively, receptor-associated CheW-E154oc might bind directly to other CheW molecules, a scenario consistent with the aberrant receptor clusters they form (see below).
CheW effects on trimer formation and receptor methylation
In principle, the apparent effects of CheW excess on trimer formation could be an artifact of the TMEA crosslinking assay. For example, CheW might directly interfere with the crosslinking chemistry, distort trimer geometry, or occlude the crosslinking reporter site. Although we cannot exclude these possibilities, they seem unlikely because the thresholds for all other CheW excess effects also reflected CheW-receptor binding affinity. Moreover, we see the same CheW excess effects on trimer formation with a cysteine reporter (T433C) that is located further from the CheW-binding tip of the receptor (D.A. Massazza & C.A. Studdert, unpublished results). Finally, the trimer explanation provides the simplest explanation for the observed effects of CheW excess on receptor methylation state.
We found that CheW excess caused a dramatic increase in the steady-state methylation level of receptor molecules. This effect was CheA-independent, indicating that it did not require ternary complex formation or feedback control of CheB activity. Similar conclusions were reported by Chalah & Weis from in vitro experiments (Chalah & Weis, 2005). Thus, it appears that CheW binding shifts the methylation states of receptor molecules by directly influencing their substrate properties for the CheR and/or CheB enzymes. We suggest that isolated receptor dimers are better substrates for the CheR methyltransferase than are trimers of dimers. It may be that CheR molecules, which are normally tethered to the C-termini of Tar and Tsr molecules, have difficulty reaching methylation sites on receptor subunits that face the trimer axis (Muppirala et al., 2009). Dissolution of the trimers should make those axial sites more accessible to CheR. Consistent with this view, Boldog et al., found that the methylation rates of receptor molecules in nanodiscs were highest for nanodiscs with one or two receptors and declined at higher receptor densities, where trimers of dimers should begin to form (Boldog et al., 2006). Similarly, Besschetnova et al. showed, using templated scaffolds to control receptor densities in lipid vesicles, that receptor molecules at low density had the highest CheR methylation rates (Besschetnova et al., 2008).
It is important to note that the CheW excess effects on receptor methylation cannot be responsible for interfering with trimer or cluster formation, which we routinely measured in strains lacking CheR and CheB. Moreover, it has been shown that the methylation states of Tar and Tsr molecules do not influence their ability to form trimers of dimers (Studdert & Parkinson, 2004) or clusters (Liberman et al., 2004).
CheW effects on CheA activation
CheW is essential for CheA activation and control by receptors. Although CheW can bind directly to CheA in the absence of receptors (Gegner & Dahlquist, 1991; McNally & Matsumura, 1991), that binding interaction has little effect on CheA activity and cannot account for the CheW excess effects observed in the presence of receptors. The available evidence suggests that CheW physically couples CheA molecules to receptors, enabling allosteric control of their activity. The architecture of the ternary complex probably enhances interactions between the phosphorylation sites and ATP-binding domains of CheA dimers. A growing body of evidence indicates that higher-order receptor complexes are instrumental in activating CheA. If so, then CheW excess could prevent CheA activation simply by disfavoring trimers of dimers. Alternatively, CheW excess might block CheA incorporation into ternary complexes by saturating the available CheW-binding sites on both receptor and CheA molecules (Gegner et al., 1992).
CheW effects on receptor clustering
Receptors can form trimers of dimers in the absence of CheA or CheW, but those trimer associations are readily reversible (Studdert & Parkinson, 2005) and shifted rapidly upon CheW increases (this study). In contrast, pre-assembled receptor clusters made at normal stoichiometries of CheA and CheW were not disrupted by a sudden rise in CheW level. Rather, it took at least a cell generation of wild-type CheW excess to reduce substantially the proportion of receptor molecules in trimers. Both in vitro (Gegner & Dahlquist, 1991; Erbse & Falke, 2009) and in vivo studies (Schulmeister et al., 2008) indicate that receptor signaling complexes are stable over such timescales.
Weak titrator CheW variants had no discernable effect on receptor clustering, even at very high expression levels. We cannot say why relatively modest reductions in binding affinity for receptors have such dramatic functional consequences. Moreover, the strong titrator mutant (E154oc) exhibited unusual receptor clustering behavior, which is difficult to explain by enhanced receptor-binding affinity alone. Cells containing this CheW variant formed aberrant, CheA-independent clusters over a wide range of expression levels. Those clusters had a flattened or elongated appearance and aberrant cellular locations. The number of anomalous clusters increased in parallel with the mutant CheW expression level. It may be that receptor dimers bound to CheW-E154oc are able to aggregate, either through interactions between the receptors themselves or between their bound CheW partners.
Are receptor trimers of dimers a widespread signaling mechanism?
The CheW excess effects described in this study provide additional support for the trimer-based team model of receptor signaling in E. coli. Whether MCP-family receptors in other species operate through trimers of dimers remains an open question. However, the receptor residues involved in trimer contacts are nearly invariant over the entire MCP super-family (Alexander & Zhulin, 2007) and recent electron microscopy studies of receptor clusters in a number of other organisms are consistent with a trimer-based array organization (Briegel et al., 2008; Khursigara et al., 2008; Briegel et al., 2009). Additional experimental approaches are needed to resolve the trimer issue. CheW stoichiometry studies, in combination with TMEA crosslinking, should prove useful tools for analyzing receptor arrays in other systems.
Experimental procedures
Bacterial strains
All strains were derivatives of E. coli K12 strain RP437 (Parkinson & Houts, 1982) and carried the following genetic markers relevant to the current study: RP1078 [Δ(cheW-tap)2217] (Liu & Parkinson, 1989); RP1616 [Δ(cheZ)6725] (Liu & Parkinson, 1989); UU1596 [tar-S364C Δ(tsr)7028 Δ(trg)100] (Studdert & Parkinson, 2005); UU1598 [tar-S364C Δ(tsr)7028 Δ(trg)100 Δ(tap-cheB)2241)] (Studdert & Parkinson, 2005); UU1604 [tar-S364C Δ(tsr)7028 Δ(trg)100 Δ(tap-cheB)2241 zec::Tn10-2] (Studdert & Parkinson, 2005); UU1613 [tar-S364C Δ(tsr)7028 Δ(trg)100 Δ(tap-cheB)2234 Δ(cheA-cheW)2167 zec::Tn10-2] (Studdert & Parkinson, 2005); UU1626 [Δ(cheA-tap)2260 Δ(tsr)7028 Δ(trg)100 Δ(aer)1] (P.Mowery & J.S.Parkinson, unpublished).
Plasmids
Plasmids derived from pACYC184 (Chang & Cohen, 1978), which confers chloramphenicol resistance, were: pRR31 [salicylate-inducible expression vector] (Studdert & Parkinson, 2005); pCS12 [salicylate-inducible wild-type tsr] (Studdert & Parkinson, 2005); and pVS102 [arabinose-inducible yfp-cheR] (Kentner et al., 2006).
Plasmids derived from pBR322 (Bolivar et al., 1977), which confers ampicillin resistance, were: pCJ30 [isopropyl β-D-thiogalactopyranoside (IPTG)-inducible expression vector] (Bibikov et al., 1997); and pPA770 [IPTG-inducible cheWΔ8] (Studdert & Parkinson, 2005). pPA770 encodes a fully functional CheW protein that lacks eight N-terminal residues of wild-type CheW.
Site-directed mutagenesis
Mutations in cheWΔ8 were introduced with the QuikChange Site-Directed Mutagenesis Kit (Stratagene), using pPA770 as the template plasmid. Candidate mutants were verified by sequencing the entire cheWΔ8 coding region.
Chemotaxis assay
Cells were inoculated into tryptone semisolid agar plates (Parkinson, 1976) containing 50 µg/ml ampicillin and different amounts of IPTG inducer. Plates were incubated at 30°C for 7–10 hours.
Tethered cell assay
Flagellar rotation patterns were measured by cell tethering, essentially as described (Parkinson, 1976). Rotation profiles of cell populations were converted to clockwise (CW) time as described (Ames et al., 2002).
TMEA crosslinking assay for trimers of dimers
Cells were grown at 30°C to mid-log phase in tryptone broth (1% tryptone, 0.5% NaCl), harvested by centrifugation, and resuspended at OD600 = 2 in 10 mM potassium phosphate (pH 7.0), 0.1 mM EDTA. Cell suspensions (0.5 ml) were incubated for 5 min at 30°C and then treated with 50 µM TMEA (Pierce) for 20 sec at 30°C. Reactions were quenched by the addition of 10 mM N-ethylmaleimide. Cells were pelleted and then lysed by boiling in 50 µl of sample buffer (Laemmli, 1970). Lysate proteins were analyzed by electrophoresis in sodium dodecyl sulfate-containing polyacrylamide gels (SDS-PAGE) as described (Studdert & Parkinson, 2004) and visualized by immunoblotting with an antiserum directed against the highly conserved portion of the Tsr signaling domain (Ames & Parkinson, 1994). As secondary antibodies, we used either Cy5-labelled (Amersham) or alkaline phosphatase-conjugated (Sigma) goat anti-rabbit immunoglobulin. Cy5-labelled antibodies were detected with a Storm 840 fluorimager (Amersham); alkaline phosphatase-conjugated antibodies were developed with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (both from Sigma) and converted to grayscale images with a digital scanner. All gel images were analyzed with ImageQuant software (Amersham).
Receptor clustering assay
Receptor clusters were visualized by fluorescence light microscopy with a YFP-CheR reporter. Cells containing pVS102 and a pPA770 derivative were grown at 30°C in tryptone broth containing 25 µg/ml of chloramphenicol, 100 µg/ml of ampicillin, 0.005% L(+)-arabinose and different amounts of IPTG. Cells were collected at mid-log phase and examined essentially as described (Sourjik & Berg, 2000). Cell fields were photographed and at least 100 cells were inspected by eye to determine the proportion of individuals with one or more distinct bright spots of fluorescence indicative of a receptor cluster.
Tsr methylation state assay
UU1626 cells transformed with pCS12 plus pPA770 derivatives were grown at 30°C to mid-log phase in tryptone broth, harvested by centrifugation, washed three times with 10 mM potassium phosphate (pH 7.0), 0.1 mM EDTA and finally resuspended at OD600 = 1 in the same buffer plus 10 mM lactate, 1 mM methionine and 200 µg/ml chloramphenicol. After 15 minutes incubation at 30°C the cell suspensions (0.5 ml) were pelleted and then lysed by boiling in 50 µl of sample buffer (Laemmli, 1970). Lysate proteins were analyzed by SDS/PAGE as described (Studdert & Parkinson, 2004) and visualized by immunoblotting with anti-Tsr as described above for TMEA crosslinking.
CheW immunoblots
CheW proteins were visualized in early experiments with rabbit polyclonal CheW antiserum provided by G. Hazelbauer (University of Missouri). Later experiments were performed with a similar antiserum prepared by Natocor (Córdoba, Argentina). Bands were detected and quantified as described above for TMEA crosslinking.
Acknowledgments
We thank Jerry Hazelbauer (U. Missouri) for providing CheW antiserum and Patricia Mowery (Hobart and William Smith Colleges), Rick Stewart (U. Maryland), Peter Ames (University of Utah) and Khoosheh Gosink (University of Utah) for helpful scientific and editorial comments on the manuscript. This work was supported by a National Institutes of Health Fogarty International Research Collaboration Award (TW007216 to J.S.P. and C.A.S.), by research grant GM19559 (to J.S.P.) from the National Institute of General Medical Sciences, and by research grant 31943 (to C.A.S.) from the Agencia Nacional para la Promoción de la Ciencia y la Tecnología, Argentina. The Protein-DNA Core Facility at the University of Utah receives support from National Cancer Institute grant CA42014 to the Huntsman Cancer Institute.
References
- Alexander RP, Zhulin IB. Evolutionary genomics reveals conserved structural determinants of signaling and adaptation in microbial chemoreceptors. Proc. Natl. Acad. Sci. USA. 2007;104:2885–2890. doi: 10.1073/pnas.0609359104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames P, Parkinson JS. Constitutively signaling fragments of Tsr, the Escherichia coli serine chemoreceptor. J. Bacteriol. 1994;176:6340–6348. doi: 10.1128/jb.176.20.6340-6348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames P, Studdert CA, Reiser RH, Parkinson JS. Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2002;99:7060–7065. doi: 10.1073/pnas.092071899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames P, Yu YA, Parkinson JS. Methylation segments are not required for chemotactic signalling by cytoplasmic fragments of Tsr, the methyl-accepting serine chemoreceptor of Escherichia coli. Mol. Microbiol. 1996;19:737–746. doi: 10.1046/j.1365-2958.1996.408930.x. [DOI] [PubMed] [Google Scholar]
- Besschetnova TY, Montefusco DJ, Asinas AE, Shrout AL, Antommattei FM, Weis RM. Receptor density balances signal stimulation and attenuation in membrane-assembled complexes of bacterial chemotaxis signaling proteins. Proc. Natl. Acad. Sci. USA. 2008;105:12289–12294. doi: 10.1073/pnas.0802868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikov SI, Biran R, Rudd KE, Parkinson JS. A signal transducer for aerotaxis in Escherichia coli. J. Bacteriol. 1997;179:4075–4079. doi: 10.1128/jb.179.12.4075-4079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldog T, Grimme S, Li M, Sligar SG, Hazelbauer GL. Nanodiscs separate chemoreceptor oligomeric states and reveal their signaling properties. Proc. Natl. Acad. Sci. USA. 2006;103:11509–11514. doi: 10.1073/pnas.0604988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F, Rodriguez R, Greene PJ, Betlach MC, Heyneker HL, Boyer HW. Construction and characterization of new cloning vehicles. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- Boukhvalova MS, Dahlquist FW, Stewart RC. CheW binding interactions with CheA and Tar: Importance for chemotaxis signaling in Escherichia coli. J. Biol. Chem. 2002;277:22251–22259. doi: 10.1074/jbc.M110908200. [DOI] [PubMed] [Google Scholar]
- Briegel A, Ding HJ, Li Z, Werner J, Gitai Z, Dias DP, Jensen RB, Jensen GJ. Location and architecture of the Caulobacter crescentus chemoreceptor array. Mol. Microbiol. 2008;69:30–41. doi: 10.1111/j.1365-2958.2008.06219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel A, Ortega DR, Tocheva EI, Wuichet K, Li Z, Chen S, Muller A, Iancu CV, Murphy GE, Dobro MJ, Zhulin IB, Jensen GJ. Universal architecture of bacterial chemoreceptor arrays. Proc. Natl. Acad. Sci. USA. 2009;106:17181–17186. doi: 10.1073/pnas.0905181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalah A, Weis RM. Site-specific and synergistic stimulation of methylation on the bacterial chemotaxis receptor Tsr by serine and CheW. BMC Microbiol. 2005;5:12. doi: 10.1186/1471-2180-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ACY, Cohen SN. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J. Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic S, Stock AM. Chemotaxis receptor recognition by protein methyltransferase CheR. Nat Struct Biol. 1998;5:446–450. doi: 10.1038/nsb0698-446. [DOI] [PubMed] [Google Scholar]
- Endres RG, Oleksiuk O, Hansen CH, Meir Y, Sourjik V, Wingreen NS. Variable sizes of Escherichia coli chemoreceptor signaling teams. Mol Syst Biol. 2008;4:211–219. doi: 10.1038/msb.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbse AH, Falke JJ. The core signaling proteins of bacterial chemotaxis assemble to form an ultrastable complex. Biochem. 2009;48:6975–6987. doi: 10.1021/bi900641c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegner JA, Dahlquist FW. Signal transduction in bacteria: CheW forms a reversible complex with the protein kinase CheA. Proc. Natl. Acad. Sci. USA. 1991;88:750–754. doi: 10.1073/pnas.88.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegner JA, Graham DR, Roth AF, Dahlquist FW. Assembly of an MCP receptor, CheW, and kinase CheA complex in the bacterial chemotaxis signal transduction pathway. Cell. 1992;70:975–982. doi: 10.1016/0092-8674(92)90247-a. [DOI] [PubMed] [Google Scholar]
- Hazelbauer GL, Falke JJ, Parkinson JS. Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends. Biochem. Sci. 2008;33:9–19. doi: 10.1016/j.tibs.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner D, Thiem S, Hildenbeutel M, Sourjik V. Determinants of chemoreceptor cluster formation in Escherichia coli. Mol. Microbiol. 2006;61:407–417. doi: 10.1111/j.1365-2958.2006.05250.x. [DOI] [PubMed] [Google Scholar]
- Khursigara CM, Wu X, Subramaniam S. Chemoreceptors in Caulobacter crescentus: trimers of receptor dimers in a partially ordered hexagonally packed array. J. Bacteriol. 2008;190:6805–6810. doi: 10.1128/JB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Yokota H, Kim SH. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature. 1999;400:787–792. doi: 10.1038/23512. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liberman L, Berg HC, Sourjik V. Effect of chemoreceptor modification on assembly and activity of the receptor-kinase complex in Escherichia coli. J. Bacteriol. 2004;186:6643–6646. doi: 10.1128/JB.186.19.6643-6646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JD, Parkinson JS. Role of CheW protein in coupling membrane receptors to the intracellular signaling system of bacterial chemotaxis. Proc. Natl. Acad. Sci. USA. 1989;86:8703–8707. doi: 10.1073/pnas.86.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Levit M, Lurz R, Surette MG, Stock JB. Receptor-mediated protein kinase activation and the mechanism of transmembrane signaling in bacterial chemotaxis. EMBO J. 1997;16:7231–7240. doi: 10.1093/emboj/16.24.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally DF, Matsumura P. Bacterial chemotaxis signaling complexes: formation of a CheA/CheW complex enhances autophosphorylation and affinity for CheY. Proc. Natl. Acad. Sci. USA. 1991;88:6269–6273. doi: 10.1073/pnas.88.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muppirala UK, Desensi S, Lybrand TP, Hazelbauer GL, Li Z. Molecular modeling of flexible arm-mediated interactions between bacterial chemoreceptors and their modification enzyme. Protein Sci. 2009;18:1702–1714. doi: 10.1002/pro.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JS. cheA, cheB, and cheC genes of Escherichia coli and their role in chemotaxis. J. Bacteriol. 1976;126:758–770. doi: 10.1128/jb.126.2.758-770.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JS, Ames P, Studdert CA. Collaborative signaling by bacterial chemoreceptors. Curr. Opin. Microbiol. 2005;8:116–121. doi: 10.1016/j.mib.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Parkinson JS, Houts SE. Isolation and behavior of Escherichia coli deletion mutants lacking chemotaxis functions. J. Bacteriol. 1982;151:106–113. doi: 10.1128/jb.151.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DA, Mendez B, Koshland DJ. Role of the CheW protein in bacterial chemotaxis: overexpression is equivalent to absence. J. Bacteriol. 1989;171:6271–6278. doi: 10.1128/jb.171.11.6271-6278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulmeister S, Ruttorf M, Thiem S, Kentner D, Lebiedz D, Sourjik V. Protein exchange dynamics at chemoreceptor clusters in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2008;105:6403–6408. doi: 10.1073/pnas.0710611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi D, Zhulin IB, Homma M, Kawagishi I. Dual recognition of the bacterial chemoreceptor by chemotaxis-specific domains of the CheR methyltransferase. J. Biol. Chem. 2002;277:42325–42333. doi: 10.1074/jbc.M202001200. [DOI] [PubMed] [Google Scholar]
- Sourjik V. Receptor clustering and signal processing in E. coli chemotaxis. Trends Microbiol. 2004;12:569–576. doi: 10.1016/j.tim.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Sourjik V, Berg HC. Localization of components of the chemotaxis machinery of Escherichia coli using fluorescent protein fusions. Mol. Microbiol. 2000;37:740–751. doi: 10.1046/j.1365-2958.2000.02044.x. [DOI] [PubMed] [Google Scholar]
- Studdert CA, Parkinson JS. Crosslinking snapshots of bacterial chemoreceptor squads. Proc. Natl. Acad. Sci. USA. 2004;101:2117–2122. doi: 10.1073/pnas.0308622100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studdert CA, Parkinson JS. Insights into the organization and dynamics of bacterial chemoreceptor clusters through in vivo crosslinking studies. Proc. Natl. Acad. Sci. USA. 2005;102:15623–15628. doi: 10.1073/pnas.0506040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Li J, Li G, Long DG, Weis RM. The receptor binding site for the methyltransferase of bacterial chemotaxis is distinct from the sites of methylation. Biochem. 1996;35:4984–4993. doi: 10.1021/bi9530189. [DOI] [PubMed] [Google Scholar]