Abstract
Background and Purpose
The safety and efficacy of restarting anticoagulation (AC) therapy after intracranial hemorrhage (ICH) remain unclear. We performed a systematic review and meta-analysis to summarize the associations of AC resumption with the subsequent risk of ICH recurrence and thromboembolism.
Method
We searched published medical literature to identify cohort studies involving adults with anticoagulation-associated ICH. Our predictor variable was resumption of AC. Outcome measures were thromboembolic events (stroke and/or myocardial infarction) and recurrence of ICH. After assessing study heterogeneity and publication bias, we performed a meta-analysis using random-effects models to assess the strength of association between AC resumption and our outcomes.
Results
Eight studies were eligible for inclusion in the meta-analysis, with 5,306 ICH patients. Almost all studies evaluated AC with vitamin-K antagonists. Reinitiation of AC was associated with a significantly lower risk of thromboembolic complications (pooled relative risk, 0.34; 95% confidence interval, [CI], 0.25-0.45; Q = 5.12, P for heterogeneity = 0.28). There was no evidence of increased risk of recurrent ICH after reinstatement of AC therapy, although there was significant heterogeneity among included studies (pooled relative risk, 1.01; 95% CI, 0.58-1.77; Q = 24.68, P for heterogeneity <0.001). No significant publication bias was detected in our analyses.
Conclusions
In observational studies, reinstitution of AC after ICH was associated with a lower risk of thromboembolic complications and a similar risk of ICH recurrence. Randomized clinical trials are needed to determine the true risk-benefit profile of AC resumption after ICH.
Keywords: Hemorrhagic stroke, ischemic stroke, myocardial infarction, atrial fibrillation, anticoagulantion
Atrial fibrillation increases the risk of stroke 3- to 5-fold and is implicated in about 15% of all strokes every year.1 Anticoagulation (AC) therapy has been proven to be efficacious in reducing incident stroke and systemic embolism in patients with atrial fibrillation2 and mechanical heart valves.3 However, the benefits of AC must be carefully weighed against the increased risk of intracranial hemorrhage (ICH) faced by patients receiving AC therapy.4, 5 Hence, resumption of AC after ICH poses a clinical conundrum. The absence of evidence-based guidelines to address this issue has led to wide variations in restarting AC after ICH. Premature reinstatement of AC could potentially increase recurrent ICH risk while an unnecessary delay in restarting AC could considerably increase a patient’s thromboembolic risk. Furthermore, there is also no consensus regarding the timing of reinstitution of these medications.6 Individual studies in the literature that have attempted to address this clinical challenge and have been unable to provide clear guidance on this issue because of small sample sizes and conflicting results. We performed a meta-analysis of available studies to evaluate the safety and efficacy of reinitiation of anticoagulant therapy after ICH.
Methods
We performed this study in accordance with the guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),7 and the Meta-Analysis of Observational Studies in Epidemiology (MOOSE)8 statements. The current study used publically available, de-identified published data and was exempt from approval by the Institutional Review Board.
Data Sources and Searches
We performed comprehensive searches in Ovid MEDLINE, Ovid Embase, and the Cochrane Library from the inception of each database to September 30, 2016. An English language filter was not applied. Following the initial search in Ovid MEDLINE, the search was extended to other databases. Keywords used to query the databases included: ‘intracranial hemorrhage or intracerebral hemorrhage’ and a combination of ‘anticoagulation’, ‘warfarin’, ‘atrial fibrillation’, ‘heart valve’, ‘ischemic stroke’, ‘myocardial infarction’, ‘recurrence’. Details of the search methodology are listed in the online-only Data Supplement.
Study Selection
We included studies evaluating thromboembolic complications and/or recurrence of ICH after hospitalization for ICH. The inclusion criteria for our study were: (1) studies with non-traumatic ICH as the primary inclusion criteria; (2) studies with documented outcomes of ischemic stroke, MI, and/or ICH recurrence in the follow-up period; (3) studies with clear documentation of whether or not AC was restarted; (4) adult patients ≥18 years of age; and (5) sample size ≥10 patients to avoid inclusion of case reports or small case series. We only included peer-reviewed publications in scientific journals, and not conference proceedings or abstracts since the latter typically do not provide the level of detail needed of rigorous data extraction. In case of ambiguity, we attempted to contact the corresponding author for clarification. In case of multiple publications from a single cohort of patients or institutional database, the study with the largest cohort of patients was selected to avoid duplication of data.
Data Extraction and Quality Assessment
A single investigator (S.B.M) read the title and abstract produced by the initial search, and shortlisted articles for further review. These articles were then independently reviewed by two investigators (S.B.M and A.E.M) and selected based on the inclusion criteria and quality of data. Any disagreements were resolved by a third investigator. Data were extracted using a pre-specified collection template. The following study characteristics were extracted: first author, journal of publication, year of publication, country of study origin, and study design. We also collected patient demographics including age, sex, and stroke comorbidities such as hypertension, diabetes mellitus, dyslipidemia, smoking history, coronary heart disease, atrial fibrillation, mechanical heart valve, prior stroke or transient ischemic attack. Timing of restarting of AC therapy was also recorded. Outcome data included cases of ischemic stroke, myocardial infarction, and recurrent ICH in the follow-up period.
We adapted risk of bias assessments in previously published meta-analyses on stroke risk, and generated eight specific questions to evaluate for potential selection, detection, reporting, and confounding bias.9, 10 Two readers assessed for risk of bias using this questionnaire, with disagreements in assessment resolved by a third tie-breaking evaluator.
Definitions of Outcomes
Our outcome measures were thromboembolism, defined as a composite of ischemic stroke or myocardial infarction, and recurrence of ICH. Most of the studies identified outcomes using International Classification of Diseases (ICD-9 or ICD-10) diagnosis codes. Two studies used mailed questionnaires and semi-quantitative telephone interviews to prospectively ascertain the occurrence of thromboembolic complications and ICH recurrence.11, 12
Data Synthesis and Analysis
We performed a meta-analysis to assess the association between AC resumption and thromboembolic complications and recurrence of ICH using the pooled relative risk (RR) as the effect parameter. We used a random-effects (DerSimonian-Laird) model to calculate the pooled RR, and generated forest plots to display the individual study RR and pooled RR.13 The rationale of using the more conservative random-effects model was to account for the variability in effect sizes, design, and follow-up between the individual studies. We assessed heterogeneity using the Cochrane Q test. If heterogeneity was detected, meta-regression was performed to explore underlying factors.14 The presence of publication bias was evaluated using the Begg-Mazumdar rank correlation test. Statistical analyses were performed using Stata (version 14.0, College Station, TX). All tests were two-tailed and p values <0.05 were considered significant.
Results
Study Selection and Characteristics
We screened a total of 888 titles and abstracts from which eight studies met the inclusion criteria (Supplemental Figure I). For the evaluation of thromboembolic events after ICH, we were able to include six of the eight studies, as two studies focused on ICH recurrence.15 We were able to calculate a crude RR expressing the association between anticoagulant use and the outcomes of interest in all of the selected studies. “Of the included studies, two were from Denmark15, 16, and one each from, Germany11, Belgium17, and Netherlands12, one from the United States18, and one from Canada19, while one study included patient cohorts from Sweden and Canada (Table 1).20
Table 1.
Overview of the Characteristics of Studies Included in the Meta-Analysis
| Study | Country | Design | Major Inclusion Criteria | Number of Subjects | Mean/Median Follow-up (Mo) | Outcomes |
|---|---|---|---|---|---|---|
| De Vleeshouwer 200517 | Belgium | Retrospective cohort | Patients with all subtypes of ICH-intraparenchymal, subdural and subarachnoid hemorrhages | 108 | 12 | Recurrent ICH; Thromboembolic events; functional outcome |
| Claassen 200818 | United States | Retrospective cohort | Patients with radiologically documented warfarin-associated intraparenchymal hemorrhage, INR≥1.5, discharge data | 48 | 43 | Recurrent ICH; Thromboembolic events |
| Majeed 201020 | Sweden & Canada | Retrospective cohort | Patients with any warfarin-associated ICH, data from 3 tertiary care hospitals | 234 | 34 | Recurrent ICH; Thromboembolic events |
| Yung 201219 | Canada | Retrospective cohort | Patients with intraparenchymal or subarachnoid hemorrhages, documented warfarin use, excluded trauma/tumors/surgical interventions | 284 | 12 | Primary- all-cause mortality, recurrence or expansion of intracranial bleeding. Secondary- composite of death, bleeding or thrombotic complications |
| Gathier 201312 | Netherlands | Retrospective cohort | Patients with radiologically documented AC-related intraparenchymal hemorrhage, INR≥1.1, discharge data | 38 | 42 | Primary- fatal or non-fatal radiologically confirmed cerebral infarction, and recurrent ICH; Secondary- other thrombotic sequelae |
| Nielsen 201516 | Denmark | Retrospective cohort | Patients with non-valvular atrial fibrillation only, any new incident ICH, AC treatment within 6 months of ICH | 1752 | 12 | Recurrent ICH; Thromboembolic events |
| Kuramatsu 201511 | Germany | Retrospective cohort | Patients with warfarin-associated intraparenchymal hemorrhage, and INR≥1.5 | 853 | 12 | Primary- Frequency of hematoma enlargement; Secondary-Thromboembolic events, Recurrent ICH, functional outcomes |
| Ottosen 201615 | Denmark | Retrospective cohort | Patients ≥18 years, with first-time acute spontaneous ICH, and surviving the first 30 days | 2978 | 27.6 | All-cause mortality, thromboembolic events, major bleeding, recurrent ICH |
Abbreviations: ICH, intracranial hemorrhage; INR, international normalized ratio; Mo, months
Four studies included patients only with intraparenchymal hemorrhage as the index ICH,11, 12, 15, 18 while others widened their selection criteria to include subdural and subarachnoid hemorrhages.16, 17, 19, 20 The mean age of patients was between 69 and 78 years, with the majority being men (range 56.0-63.1 years) (Supplemental Table I). Data on stroke risk factors were not available in four studies.15-17, 20
Oral Anticoagulant Therapy Indications and Reinitiation
The most common indication for AC treatment prior to the onset of ICH was atrial fibrillation (34.7-77.8%), followed by prosthetic heart valve (2.6-27.8%), venous thromboembolism (7.9-20.8%), and prior ischemic stroke (3.7-71.8%). Reinitiation of AC occurred at a median of 10-39 days (Table 2). Four studies did not report the exact timing of resumption of AC,12, 15, 16, 19 but the majority of patients were prescribed AC within the first 3 months after ICH. The AC of choice was oral vitamin K antagonist (VKA) medications in all studies, with the exception of Ottosen et al.15 and Nielsen et al.16, in which some patients were administered non-vitamin K antagonist oral anticoagulants (NOACs).
Table 2.
Overview of AC Indications and Characteristics
| Study | Indications for Anticoagulation (%)a | Received AC | AC type | Time to Restarting AC (days) | |||||
|---|---|---|---|---|---|---|---|---|---|
| NVAF | Prosthetic heart valve | VTE | Prior stroke | Recent MI | Other | ||||
| De Vleeschouwer et al17 | 56 (51.9) | 30 (27.8) | 11 (10.2) | 4 (3.7) | 2 (1.9) | 5 (4.6) | 25 (23.1) | VKA | 11 |
| Classen et al18 | 23 (47.9) | 12 (25.0) | 10 (20.8) | N/A | N/A | 3 (6.3) | 23 (47.9) | VKA (Warfarin) | 10 |
| Majeed et al20 | 135 (58.0) | 35 (15.0) | 37 (16.0) | N/A | N/A | 27 (11.0) | 45 (34.1)b | VKA (Warfarin) | 39.2 |
| Yung et al19 | 191 (67.3) | 37 (13.0) | 31 (10.9) | N/A | N/A | N/A | 91 (32.0) | VKA (Warfarin) | N/A |
| Gathier et al12 | 10 (40.0) | 2 (8.0) | 6 (24.0) | 8 (32.0) | 2 (8.0) | 4 (16.0) | 12 (48.0) | VKA | Within 2 months |
| Nielsen et al16 | 1,752 (100.0) | 0 | 0 | 0 | 0 | 0 | 509 (29.1) | VKA/NOAC | N/A |
| Kuramatsu et al11 | 664 (77.8) | 67 (7.9) | 71 (8.3) | N/A | N/A | 51 (6.0) | 172 (23.9) | VKA | 31 |
| Ottosen et al15 | 1,032 (34.7) | 78 (2.6) | 236 (7.9) | 2,139 (71.8) | 264 (8.9) | 30 (1.0) | 160 (6.3)c | VKA/NOAC | Within first 6 months |
Abbreviations: AC, anticoagulant therapy; NOAC, non-vitamin K oral anticoagulant medications; VKA, vitamin K antagonists
sum of all indications may exceed 100% since some patients had multiple indications for AC
data on 132 patients with cardiac indications who were restarted on AC was available
data was available on 2543 patients who survived.
Association between Anticoagulant Therapy and Thromboembolic Complications
We included six studies with 2,044 patients with ICH. Among these patients, AC therapy was restarted in 786 (38.4%) patients with a total follow-up for incident thromboembolic complications of 861 person-years. AC therapy was not restarted in the remaining 1,258 patients (61.6%), who were followed for a total of 1,328 person-years for thromboembolic complications. The rate of thromboembolic events in patients on AC therapy was 6.7% compared to a 17.6% for patients not restarted on AC therapy (Table 3). We observed a significant inverse association between AC therapy and the risk of thromboembolic events (pooled RR, 0.34; 95% confidence interval [CI], 0.25-0.45) (Figure 1). There was no statistically significant heterogeneity (Q = 5.12, P = 0.28) or publication bias (P = 0.55). The funnel plot for assessment of publication bias is shown in Supplemental Figure II.
Table 3.
Rates of Thromboembolic Complications and ICH recurrence
| Study | Anticoagulant Medications | No Anticoagulant Medications | ||||
|---|---|---|---|---|---|---|
| Stroke+MI (%) | ICH recurrence (%) | Total Population | Stroke+MI (%) | ICH recurrence (%) | Total Population | |
| De Vleeschouwer et al17 | 0 (0) | 1 (4.0) | 25 | 3 (3.7) | 7 (8.6) | 81 |
| Classen et al18 | 6 (26.1) | 1 (4.3) | 23 | 8 (32.0) | 0 (0) | 25 |
| Majeed et al20 | 1 (2.2) | 8 (17.8) | 45 | 18 (20.7) | 10 (11.5) | 87 |
| Yung et al19 | N/A | 14 (15.4) | 91 | N/A | 29 (15.0) | 193 |
| Gathier et al12 | 2 (16.7) | 1 (8.3) | 12 | 2 (15.4) | 0 (0) | 13 |
| Nielsen et al16 | 35 (6.9) | 36 (7.1) | 509 | 108 (21.4) | 72 (14.3) | 505 |
| Kuramatsu et al11 | 9 (5.2) | 14 (8.1) | 172 | 82 (14.9) | 36 (6.6) | 547 |
| Ottosen et al15 | N/A | 91 (8.9) | 1022 | N/A | 113 (5.8) | 1,956 |
| Total Events | 53 (6.7)a | 166 (8.7) | 1,899 | 221 (17.6)a | 267 (7.8) | 3,407 |
Abbreviations: ICH, intracranial hemorrhage; MI, myocardial infarction; N/A, not available
Studies by Yung et al. and Ottosen et al. were excluded since stroke and MI were not reported
Figure 1.
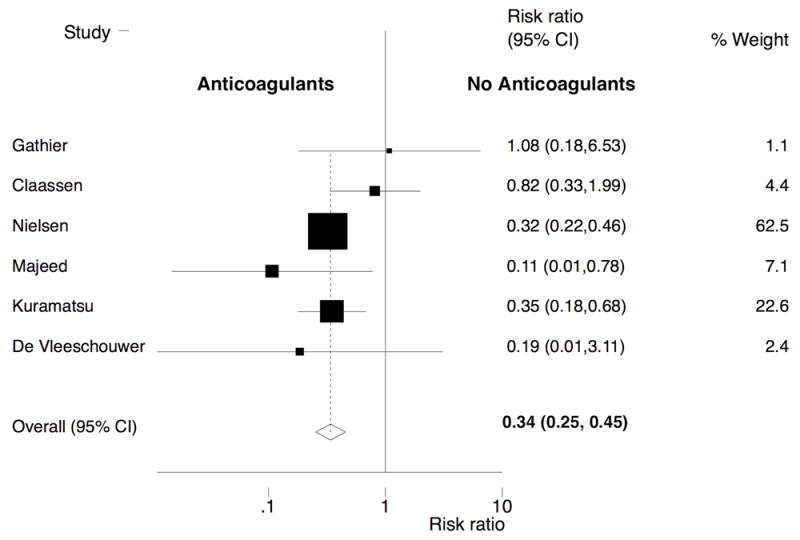
Forest plot of the association between resumption of oral anticoagulation therapy and arterial thromboembolic complications after intracranial hemorrhage. The meta-analysis was calculated using a random-effects model, with the pooled relative risk shown in the forest plot. Each square represents the point estimate of any given study’s effect size. The size of the squares is proportional to the inverse of the variance of the estimate, while the horizontal lines represent each study’s 95% confidence intervals. The diamond represents the pooled estimate with the width of the diamond representing the pooled 95% CI. Heterogeneity: Q = 5.12; P = 0.28.
Association between Anticoagulants and ICH Recurrence
Eight studies were eligible for meta-analysis of the relationship between AC therapy and ICH recurrence. These studies included 5,306 ICH patients of whom 1,899 (35.8%) were restarted on AC therapy. The total follow-up for recurrent ICH in this group restarted on AC therapy was 3,494 person-years. AC therapy was not restarted in the remaining 3,407 patients (64.2%) who were followed for a total of 7,030 person-years. Recurrence of ICH was observed in 166 (8.7%) patients on AC and in 267 (7.8%) not on antithrombotic agents. We observed no difference in ICH recurrence in the two groups (pooled RR, 1.01; 95% CI, 0.58-1.77) (Figure 2). There was evidence of significant heterogeneity (Q = 24.68, P <0.001), but no evidence of publication bias (P value for Begg-Mazumdar test = 0.55) (Supplemental Figure III).
Figure 2.
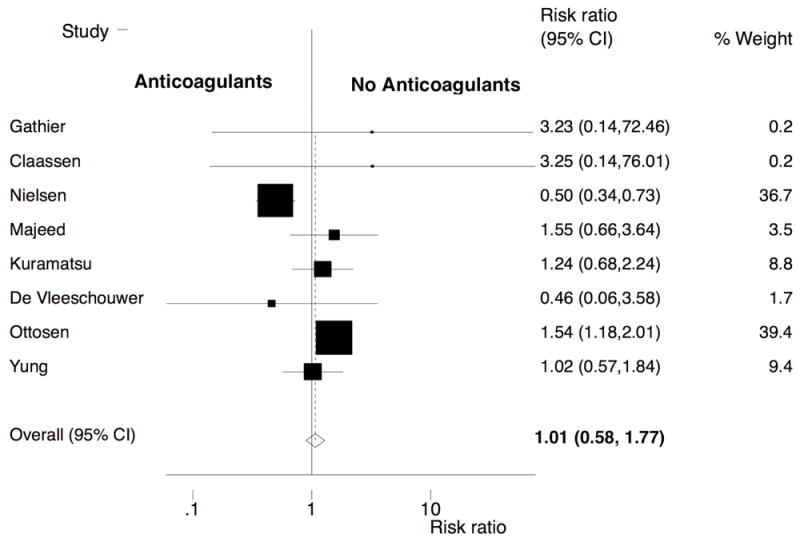
Forest plot of the association between resumption of oral anticoagulation therapy and recurrence of intracranial hemorrhage. The meta-analysis was calculated using a random-effects model, with the pooled relative risk shown in the forest plot. Heterogeneity: Q = 24.68; P <0.001.
Sensitivity Analysis
Given the significant heterogeneity in the random effects model assessing the relationship between resumption of AC therapy and ICH recurrence, we performed a meta-regression to identify factors that could have resulted in the heterogeneity. Studies were appraised based on different factors such as inclusion of intraparenchymal hemorrhages only, sample size >100 patients, clearly specified time point for resumption of AC therapy, and reinitiation with AC therapy other than VKAs. Each of these factors was assessed individually. Subgroup meta-analyses were performed using these specific criteria to evaluate for the source of heterogeneity in the overall analysis. Significant heterogeneity was found only in the meta-analysis performed limited to studies in which AC medications other than VKAs were used (p=0.006). The two studies that used NOACs were by Ottosen et al.15 and Nielsen et al.16 Following the exclusion of these studies, we constructed a random effects model to evaluate the association between reinstitution of AC medications and ICH recurrence in the remaining studies. We observed a pooled RR of 1.18 (95% CI, 0.83- 1.70; heterogeneity p = 0.82).
Given the possibility of large, heavily weighted studies skewing the pooled RR, we performed additional sensitivity analyses. For the assessment of thromboembolic risk, we excluded two studies (Kuramatsu et al.11 and Nielsen et al.16) following which the pooled RR remained significantly in favor of resumption of AC therapy (RR, 0.40; 95% CI, 0.29- 0.83; heterogeneity p = 0.13). For the assessment of ICH recurrence, the two heavily weighted studies were by Ottosen et al.15 and Nielsen et al.16 and the results are as discussed in the metaregression above.
Assessment of the Quality of Included Studies
The results from the quality assessment questionnaire are shown in Supplemental Table II. Selection bias was minimized in four studies either through random selection of patients or recruitment from a community-dwelling population.11, 15, 16, 20 The investigators were not blinded to the anticoagulant status in any of the included studies. More importantly, only three studies corrected for covariate risk factors in assessing the relationship between resumption of AC therapy and outcomes.11, 15, 16
Discussion
In this systematic review and meta-analysis of studies with more than 5,000 patients with ICH, we found that resumption of anticoagulation therapy was associated with a lower risk of thromboembolic events such as stroke and MI. In addition, there was no apparent heightened risk of ICH recurrence with administration of AC medications.
From the clinician’s standpoint, the main concern preventing reinstitution of AC is recurrence of ICH. Although, we did not find any significant difference in the rates of recurrent ICH, certain limitations of published literature may have influenced this finding. For instance, one possibility is that anticoagulation was more likely to be reinstated in patients with smaller hematomas, but lack of data on baseline hematoma volume precluded further evaluation. Moreover, not all studies provided information on hematoma location or if the ICH was a first-time bleed or recurrent hemorrhage. Our study therefore, did not account for lobar hemorrhages, which are more likely to be secondary to amyloid angiopathy and consequently have a higher rate of ICH recurrence. More broadly speaking, these specific factors are instances of confounding by indication in these observational studies, in which patients at higher perceived risk may have been less likely to be restarted on AC. These factors in addition to differences in study design and timing of resumption of AC therapy, may have accounted for the significant heterogeneity in our random effects model. These considerations underscore the importance of future randomized trials to determine optimal antithrombotic strategies after ICH.
It is notable that all studies included in our systematic review used VKAs as the medication of choice. However, a newer class of medications, NOACs, has been shown to have a significantly lower ICH risk compared to warfarin.21 Additionally, emerging data suggest that patients with NOAC-associated ICH have smaller hematomas and better functional outcomes in comparison with warfarin-associated ICH.22, 23 Although recent guidelines recommend using NOACs for non-valvular atrial fibrillation, current research is focused on identifying other potential roles in clinical conditions such as atrial fibrillation associated with valvular heart disease and patients with prosthetic heart valves.24 Thus, it is possible that the range of indications for NOAC use will expand in the future.25
Our study has shed light on additional limitations of the existing body of literature studying anticoagulant use after ICH. First, there are no randomized trials studying resumption of AC therapy after ICH. Our meta-analysis is hence subject to the intrinsic flaws from the non-blinded, retrospective observational design of the included studies. Second, there was heterogeneity in the follow-up periods in the individual studies. Third, there was variability in the selection criteria of individual studies in that some included only patients with intraparenchymal hemorrhage while others additionally included those with subarachnoid and subdural hematomas. Recurrence of subarachnoid hemorrhage irrespective of anticoagulation status is considered rare after obliteration of the culprit aneurysm26, while reoccurrence of other forms of ICH may be much higher. For example, recurrence rate for subdural is around 12%27 while that for intraparenchymal hemorrhage is ~2%28, 29 in non-anticoagulated patients, which may have significantly affected our results. We also did not have information on blood pressure control, a known predictor of ICH recurrence.30 Moreover, although warfarin was the drug of choice for AC in majority of the studies, NOACs were used in one study which may have influenced ICH recurrence.16 Fourth, reasons for reinstitution or continued withholding of AC involve a complex interplay of clinical and social factors, all of which were not available. Our study is hence subject to confounding by indication of individual studies, and may apply to patients who were deemed to be low risk for ICH recurrence. The variation in resumption of ACs may have also accounted for the wide range of thromboembolic complications observed. Additionally, the majority of the studies did not adjust for stroke/MI risk factors. As a result, our findings may have been subject to significant confounding bias. Finally, none of the studies characterized stroke subtype. Although cardioembolic strokes are more likely in non-anticoagulated patients with known atrial fibrillation or mechanical heart valves, other subtypes of stroke may have contributed to the stroke incidence.
In summary, our systematic review and meta-analysis suggests that resumption of AC therapy is associated with a lower risk of arterial thromboembolism after ICH. Moreover, there does not appear to be an increased risk of ICH recurrence. In the absence of randomized clinical trials, our results help summarize the existing literature and may serve as a guide to clinicians in making informed decisions. Furthermore, our findings will hopefully encourage further studies of the risks and benefits of anticoagulation in this population.
Supplementary Material
Acknowledgments
None
Sources of Funding
S. Murthy is supported by the American Academy of Neurology, American Brain Foundation, and the Leon Levy Neuroscience Foundation. A. Gupta is supported by the KL2TR000458 from the NIH/NCATS. B. Navi is supported by NIH grant K23NS091395 and the Florence Gould Endowment for Discovery in Stroke. D.F. Hanley was awarded significant research support through grant numbers 5U01NS062851 for Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage III and for Minimally Invasive Surgery Plus r-tPA for Intracerebral Hemorrhage Evacuation (MISTIE) III 1U01NS08082. C. Iadecola is supported by NIH grants R37NS089323-02, R01 NS034179-21, R01 NS037853-19 and R01 NS073666-04. H. Kamel is supported by NINDS grants K23NS082367, R01NS097443, and the Michael Goldberg Stroke Research Fund.
Footnotes
Conflict of Interest: None.
Disclosures: None
References
- 1.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the u.S. Adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Annals of internal medicine. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, et al. 2014 aha/acc guideline for the management of patients with valvular heart disease: A report of the american college of cardiology/american heart association task force on practice guidelines. J Thorac Cardiovasc Surg. 2014;148:e1–e132. doi: 10.1016/j.jtcvs.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty ML, Kissela B, Woo D, Kleindorfer D, Alwell K, Sekar P, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68:116–121. doi: 10.1212/01.wnl.0000250340.05202.8b. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Rodriguez LA, Gaist D, Morton J, Cookson C, Gonzalez-Perez A. Antithrombotic drugs and risk of hemorrhagic stroke in the general population. Neurology. 2013;81:566–574. doi: 10.1212/WNL.0b013e31829e6ffa. [DOI] [PubMed] [Google Scholar]
- 6.Pennlert J, Asplund K, Carlberg B, Wiklund PG, Wisten A, Asberg S, et al. Antithrombotic treatment following intracerebral hemorrhage in patients with and without atrial fibrillation. Stroke; a journal of cerebral circulation. 2015;46:2094–2099. doi: 10.1161/STROKEAHA.115.009087. [DOI] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA : the journal of the American Medical Association. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, et al. Silent brain infarction and risk of future stroke: A systematic review and meta-analysis. Stroke. 2016;47:719–725. doi: 10.1161/STROKEAHA.115.011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, et al. Carotid plaque mri and stroke risk: A systematic review and meta-analysis. Stroke. 2013;44:3071–3077. doi: 10.1161/STROKEAHA.113.002551. [DOI] [PubMed] [Google Scholar]
- 11.Kuramatsu JB, Gerner ST, Schellinger PD, Glahn J, Endres M, Sobesky J, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313:824–836. doi: 10.1001/jama.2015.0846. [DOI] [PubMed] [Google Scholar]
- 12.Gathier CS, Algra A, Rinkel GJ, van der Worp HB. Long-term outcome after anticoagulation-associated intracerebral haemorrhage with or without restarting antithrombotic therapy. Cerebrovascular diseases. 2013;36:33–37. doi: 10.1159/000351151. [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Varelas PN, Abdelhak T, Wellwood J, Benczarski D, Elias SB, Rosenblum M. The appointment of neurointensivists is financially beneficial to the employer. Neurocrit Care. 13:228–232. doi: 10.1007/s12028-010-9371-0. [DOI] [PubMed] [Google Scholar]
- 15.Ottosen TP, Grijota M, Hansen ML, Brandes A, Damgaard D, Husted SE, et al. Use of antithrombotic therapy and long-term clinical outcome among patients surviving intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2016;47:1837–1843. doi: 10.1161/STROKEAHA.116.012945. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen PB, Larsen TB, Skjoth F, Gorst-Rasmussen A, Rasmussen LH, Lip GY. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: A nationwide cohort study. Circulation. 2015;132:517–525. doi: 10.1161/CIRCULATIONAHA.115.015735. [DOI] [PubMed] [Google Scholar]
- 17.De Vleeschouwer S, Van Calenbergh F, van Loon J, Nuttin B, Goffin J, Plets C. Risk analysis of thrombo-embolic and recurrent bleeding events in the management of intracranial haemorrhage due to oral anticoagulation. Acta Chir Belg. 2005;105:268–274. doi: 10.1080/00015458.2005.11679715. [DOI] [PubMed] [Google Scholar]
- 18.Claassen DO, Kazemi N, Zubkov AY, Wijdicks EF, Rabinstein AA. Restarting anticoagulation therapy after warfarin-associated intracerebral hemorrhage. Arch Neurol. 2008;65:1313–1318. doi: 10.1001/archneur.65.10.1313. [DOI] [PubMed] [Google Scholar]
- 19.Yung D, Kapral MK, Asllani E, Fang J, Lee DS Investigators of the Registry of the Canadian Stroke N. Reinitiation of anticoagulation after warfarin-associated intracranial hemorrhage and mortality risk: The best practice for reinitiating anticoagulation therapy after intracranial bleeding (brain) study. Can J Cardiol. 2012;28:33–39. doi: 10.1016/j.cjca.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Majeed A, Kim YK, Roberts RS, Holmstrom M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke; a journal of cerebral circulation. 2010;41:2860–2866. doi: 10.1161/STROKEAHA.110.593087. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: Traditional and bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA neurology. 2013;70:1486–1490. doi: 10.1001/jamaneurol.2013.4021. [DOI] [PubMed] [Google Scholar]
- 22.Wilson D, Charidimou A, Shakeshaft C, Ambler G, White M, Cohen H, et al. Volume and functional outcome of intracerebral hemorrhage according to oral anticoagulant type. Neurology. 2016;86:360–366. doi: 10.1212/WNL.0000000000002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagii J, Tomita H, Metoki N, Saito S, Shiroto H, Hitomi H, et al. Characteristics of intracerebral hemorrhage during rivaroxaban treatment: Comparison with those during warfarin. Stroke. 2014;45:2805–2807. doi: 10.1161/STROKEAHA.114.006661. [DOI] [PubMed] [Google Scholar]
- 24.Di Biase L. Use of direct oral anticoagulants in patients with atrial fibrillation and valvular heart lesions. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yaghi S, Kamel H, Elkind MS. Potential new uses of non-vitamin k antagonist oral anticoagulants to treat and prevent stroke. Neurology. 2015;85:1078–1084. doi: 10.1212/WNL.0000000000001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simard JM, Aldrich EF, Schreibman D, James RF, Polifka A, Beaty N. Low-dose intravenous heparin infusion in patients with aneurysmal subarachnoid hemorrhage: A preliminary assessment. J Neurosurg. 2013;119:1611–1619. doi: 10.3171/2013.8.JNS1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris NA, Merkler AE, Parker WE, Claassen J, Connolly ES, Sheth KN, et al. Adverse outcomes after initial non-surgical management of subdural hematoma: A population-based study. Neurocrit Care. 2016;24:226–232. doi: 10.1007/s12028-015-0178-x. [DOI] [PubMed] [Google Scholar]
- 28.Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology. 2001;56:773–777. doi: 10.1212/wnl.56.6.773. [DOI] [PubMed] [Google Scholar]
- 29.Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2000;31:123–127. doi: 10.1161/01.str.31.1.123. [DOI] [PubMed] [Google Scholar]
- 30.Biffi A, Anderson CD, Battey TW, Ayres AM, Greenberg SM, Viswanathan A, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA. 2015;314:904–912. doi: 10.1001/jama.2015.10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


